Abstract
Patients who relapse or are refractory after first-line therapy for diffuse large B-cell lymphoma (DLBCL) frequently have poor prognoses, especially when they are not candidates for autologous stem cell transplant (ASCT). Tafasitamab is a humanized monoclonal anti-CD19 antibody that has recently been approved by the FDA in combination with lenalidomide for the treatment of relapsed/refractory (R/R) DLBCL in patients who are not eligible for ASCT. Tafasitamab has an Fc region which has been modified to have an increased affinity for Fcγ receptors, to potentiate antibody-dependent cellular cytotoxicity and antibody-dependent cell-mediated phagocytosis. Here, we review the development, mode of action and clinical data for tafasitamab in combination with lenalidomide in R/R DLBCL, and discuss the various ways in which this novel antibody could be utilized in the treatment sequence to improve clinical outcomes for patients with DLBCL.
Keywords: anti-CD19, clinical data, diffuse large B-cell lymphoma, DLBCL, lenalidomide, mode of action, monoclonal antibody, real-world evidence, tafasitamab
Introduction
As the most common aggressive non-Hodgkin’s lymphoma (NHL) subtype, diffuse large B-cell lymphoma (DLBCL) accounts for approximately 40% of NHL cases, with a median age at diagnosis of 66 years, and initial treatment is of curative intent.1,2 First-line treatment predominantly consists of six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy, which is ultimately successful in 50–70% of patients. 3 However, the outlook for patients who are refractory to or relapse following R-CHOP is not promising; although younger and fit patients can receive salvage chemotherapy and autologous stem cell transplant (ASCT), its benefit is limited. 4 Elderly patients are frequently ineligible for high-dose chemotherapy and ASCT due to considerable toxicity, which is an important consideration given the aging patient population and age at diagnosis. 5 Over the past 5 years, attention has turned from salvage chemotherapy to immunologic approaches to provide additional, effective alternatives for patients with relapsed/refractory (R/R) DLBCL.
CD19 is a transmembrane protein expressed on B-cells from early in their development and is highly conserved throughout their maturation; it is one of the most reliable surface markers of B-cells. 6 In normal B-cell development, CD19 has a role in modulating B-cell receptor (BCR) and independent developmental signaling thresholds, and influences both antigen-independent and immunoglobulin-induced B-cell activation, via protein kinases including Src, Ras, Abl, Btk, adapter molecules and PI3K.6,7 In malignant B-cells, CD19 may be an essential contributor to the chronic activation of BCR and CD40 signaling to drive B-cell lymphomagenesis, survival and proliferation. 8 CD19 engagement is also known to augment Myc levels and amplify Myc functioning in murine lymphoma cells. 9
CD19 has emerged as a valuable target in DLBCL, being expressed more broadly than CD20 (the target for rituximab) in B-NHL, and is expressed in patients with CD20 downregulation following rituximab exposure. 10 Several different approaches have been developed to exploit CD19 on B-cells in patients with R/R DLBCL over the past 5 years, including chimeric antigen receptor T-cell therapy (CAR-T), bispecific antibodies which localize T-cells to CD19, antibody-drug conjugates which deliver a cytotoxic payload to CD19-bearing cells and now tafasitamab in combination with lenalidomide.11–15
Tafasitamab development, structure, mechanism of action (MOA) and early clinical data
Initial attempts to exploit CD19 via murine anti-human CD19 monoclonal antibodies (mAbs), with or without linked toxins, were met with limited success, partly as a result of CD19 internalization following antibody binding and the development of human anti-murine antibodies during treatment.10,16 The second generation of CD19-targeting antibodies utilized computational algorithms and high-throughput screening to design and select antibodies with specific engineered Fc variant regions to enhance immune effector functions, including antibody-dependent cell-mediated cytotoxicity (ADCC). 17 Immune effector functions are triggered via the interaction of CD19-bound mAb Fc with effector cell Fcγ receptors (FcγRs), resulting in immune responses including natural killer (NK) cell activation, cytotoxic attack and the release of inflammatory mediators.17,18 Tafasitamab is one such engineered mAbs, which incorporates S239D and I332E mutations 17 into the Fc region of humanized anti-CD19 immunoglobulin G. 18 The S239D/I332E combination demonstrated preclinical enhancement of affinity for FcγRIIIa when engineered into mAbs for a variety of targets. 17 These effects were replicated with a S239D/I332E in a humanized anti-CD19 mAb, which demonstrated highly enhanced ADCC against several lymphoma and leukemia cell lines in addition to increased antibody-dependent cell-mediated phagocytosis (ADCP) and antiproliferative activity in murine xenograft models. 18 These effects were further investigated in chronic lymphocytic leukemia (CLL) patient cells, revealing the importance of enhanced activation of NK-cells as immune effectors, 19 as well as superior ADCC against acute lymphoblastic lymphoma (ALL) patient blast cells, compared with alemtuzumab, rituximab and ofatumumab, 20 again with a significant role for NK-cells. 21
Tafasitamab monotherapy was initially investigated with encouraging efficacy in a phase I dose-escalation study in patients with R/R CLL. 22 No maximum tolerated dose was identified, and tafasitamab was well tolerated at the highest (recommended phase II) dose studied (12 mg/kg each week, with an additional dose on day 4 of cycle 1). The most common adverse events (AEs) observed were Grade 1–2 infusion reactions in 67% of patients, with the most common Grade 3–4 hematologic AEs (neutropenia and thrombocytopenia) occurring in ⩽10% of patients; there was no evidence of immunogenicity. The early efficacy signals reported were a partial response (PR) rate of 67%, and a stable disease rate of 33%; the PR rate was 30% by International Workshop on Chronic Lymphocytic Leukemia 2008 criteria (including response by computed tomography). 22
Clinical activity with tafasitamab monotherapy was also observed in patients with R/R NHL across indolent and aggressive subtypes, including DLBCL, in a phase II study. 23 Response rates of 20–30% were observed across subtypes, with an objective response rate (ORR) of 25.7% [95% confidence interval (CI) = 12.5–43.3] in 9 out of 35 patients with DLBCL [seven PR and two complete responses (CRs)], with a median duration of response (DoR) of 20.1 months (95% CI = 1.1–not reached). 23 Interestingly, in this study, an exploratory post hoc analysis found that progression-free survival (PFS) was longer for patients with a baseline peripheral NK-cell count above a threshold of 100 cells/μl. 24
Lenalidomide MOA and activity in DLBCL
Lenalidomide is a thalidomide derivative immunomodulatory drug with noted anti-tumor activity across a range of hematologic malignancies. 25 It has long been acknowledged to have various immunologic effects related to enhancing anti-tumor NK- and T-cell activity, altering the balance of pro- and anti-inflammatory cytokines in the tumor microenvironment (TME), inhibition of angiogenesis, and, to a lesser extent, induction of cell cycle arrest and apoptosis. 25 Lenalidomide has several direct effects on malignant B-cells, including increased cell cycle arrest in the G0–G1 phase, decreased cellular proliferation and downregulated expression of checkpoint inhibitors (including PD-L1). 26 In the TME, lenalidomide stimulates the proliferation and activation of NK-cells, enhancing NK-cell-mediated cytotoxicity and ADCC as a result of enhanced FcγR signaling from bound antibodies such as rituximab. In addition, lenalidomide stimulates the activation and proliferation of T-cells (CD8+ and CD4+) and improves immune synapse formation between malignant B-cells, antigen-presenting cells and effector cells, including NK- and T-cells. Lenalidomide also decreases the production of pro-inflammatory cytokines from T-cells (e.g. TNF-α, IL-1, IL-6 and IL-2) and increases the production of anti-inflammatory IL-10, thereby making the TME less supportive of tumor growth, metastasis and chemoresistance. 26
In a pooled analysis of early trials of lenalidomide monotherapy in heavily pre-treated patients with R/R DLBCL (N = 134), therapy was associated with an ORR of 26% (including 9% CRs) and a median DoR of 6.0 months; a separate retrospective analysis of cell-of-origin found that lenalidomide was more effective in non-germinal center B cell DLBCL than germinal center B (GCB) DLBCL. 27 In terms of safety, the most common Grade ⩾3 AEs generally associated with lenalidomide monotherapy are neutropenia (~30–40%) and thrombocytopenia (~20%), with lower rates of leukopenia (7–14%) and anemia (6–9%). 27
Combination therapy with lenalidomide in DLBCL has been more successful. Lenalidomide has been utilized in combination with rituximab in R/R DLBCL in phase II studies including 32 28 and 23 patients 5 with moderate efficacy, including an ORR of 28% and 35%, respectively, and CR rates of 22% and 30%, respectively. R-lenalidomide may also be feasible as a bridge to stem cell transplant; in the former study, five of nine responders with DLBCL went on to achieve a CR with stem cell transplant. 28 In untreated DLBCL, despite promising phase II results in unselected DLBCL patients, 29 a phase III, randomized study assessing R-CHOP with or without lenalidomide failed to show a benefit for R-CHOP plus lenalidomide in patients with activated B-cell DLBCL (assessed using gene expression profiling). 30 However, lenalidomide maintenance for 24 months in elderly patients who responded to first-line R-CHOP was found to provide significant improvements in PFS compared with placebo [N = 650; PFS not reached (NR) versus 58.9 months; hazard ratio (HR) = 0.708 (95% CI = 0.537–0.933); p = 0.0135], but with no significant change in overall survival (OS). 31 In aggressive B-cell lymphomas, lenalidomide combined with the anti-CD20 mAb obinutuzumab prompted the reversal of immature NK phenotypes and an increased expression of NK-activating receptors. 32
Rationale for combining tafasitamab and lenalidomide in DLBCL
The rationale for combining tafasitamab and lenalidomide is based on the stimulation and proliferation of NK-cells by lenalidomide, coupled with the amplification of NK-cell-mediated ADCC by tafasitamab. 33 DLBCL is a disease well-suited to this approach, and low circulating NK-cell levels have been associated with short event-free survival following chemotherapy. 34 Low NK-cell count at diagnosis (<100/μl) did not affect initial response to therapy, but was associated with significantly poorer PFS and OS outcomes following R-CHOP in a subgroup of DLBCL patients with non-GCB type disease (there was no significant difference with baseline NK count in patients with GCB-type disease), in a retrospective analysis of 72 patients receiving first-line treatment [HR for PFS and OS = 6.03 (95% CI = 1.79–13.55; p < 0.001) and 3.75 (95% CI = 1.32–7.72; p = 0.005), respectively]. 35 More recently, a retrospective analysis including 1287 patients with DLBCL who received CHOP chemotherapy plus either obinutuzumab or rituximab in the GOYA trial found that a baseline peripheral NK-cell count of <100/μl was associated with significantly shorter PFS (HR = 1.36; 95% CI = 1.01–1.83; p = 0.04), and that low tumor NK-cell gene expression was associated with shorter PFS (HR = 1.95; 95% CI = 1.22–3.15; p < 0.01) in patients who received obinutuzumab plus CHOP. 36 Additionally, in a study comparing samples from 36 patients with newly-diagnosed DLBCL with 20 healthy controls, NK-cells from the DLBCL patients were found to have reduced expression of FcγIIIR and reduced degranulation activity when challenged with rituximab-coated tumor cells. 37 As a result, clinical response to rituximab-based therapy could be affected by impaired rituximab-mediated NK-cell cytotoxicity. 37
Other observations support the importance of NK-cells in the TME from the perspective of treating DLBCL. In single droplet microfluidic analyses of individual interactions between NK-cells and target B-lymphoma cells, increased NK-cell cytotoxicity was observed with decreased proximity and uninterrupted contact time. 38 The TME is a crucial regulator of tumor development, and NK-cell inhibition and dysfunction are recognized as important mechanisms of tumor cell escape.39,40 In a prognostic gene model of immune cell infiltration and prognosis in DLBCL, the largest cellular factor in the TME that contributed to prognosis was the ratio of activated to resting NK-cells. Higher proportions of activated NK-cells were associated with poorer OS outcomes, indicating NK-cell dysfunction in the TME, and point to reprogramming of activated NK-cells as an important contributor to tumor growth and metastasis. 40
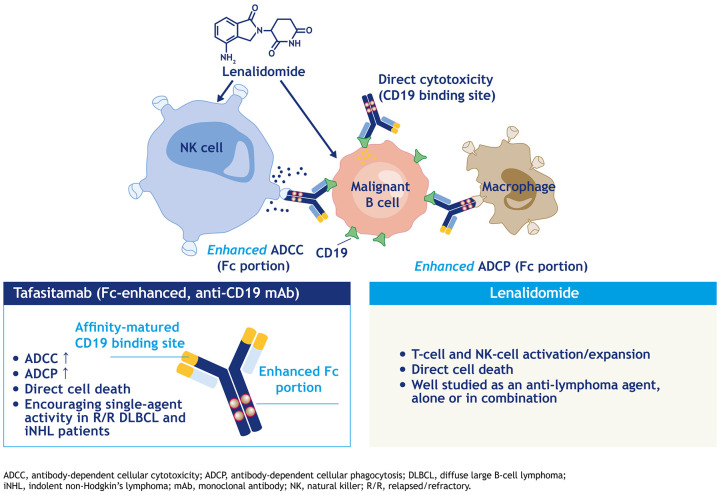
Through these observational data, the augmentation of NK-cell activity with lenalidomide and the simultaneous exploitation of tafasitamab’s FcγR modifications to further enhance ADCC, ADCP and NK-cell localization to tumor cells via CD19 binding (Figure 1) represents a rational and novel combination strategy worth being explored in DLBCL.
Figure 1.
Combination mechanism of action of tafasitamab and lenalidomide. 41
Tafasitamab plus lenalidomide in DLBCL: L-MIND and RE-MIND
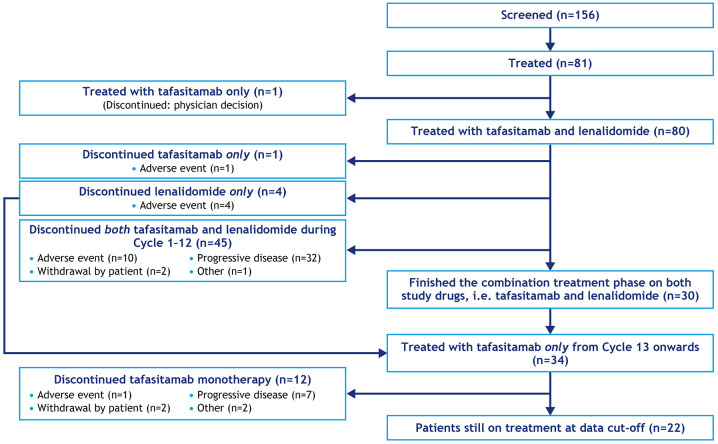
The combination strategy for tafasitamab plus lenalidomide is under investigation in the single-arm, open-label phase II L-MIND study (NCT02399085). 33 Eighty-one patients with R/R DLBCL who had received 1–3 prior regimens, including at least one anti-CD20 regimen, and who were not candidates for high-dose chemotherapy and subsequent ASCT, were enrolled. Patients had a median age of 72 years and a median of 2 prior lines of therapy (range 1–4); all had received R-CHOP (or equivalent chemoimmunotherapy). Nearly half (46%) of patients were aged >70 years, and three-quarters (75%) had stage III/IV disease; 47% of patients had immunohistochemistry-confirmed GCB-type disease. Few patients (11%) had received prior ASCT. Primary refractory disease was present in 19% of cases, with 42.0% and 44.4% of patients refractory to rituximab and last line of therapy, respectively. Although presence of known double- and triple-hit lymphoma was an exclusion criterion for L-MIND, two patients (one double-hit and one triple-hit) were found to have these alterations after enrollment. 33
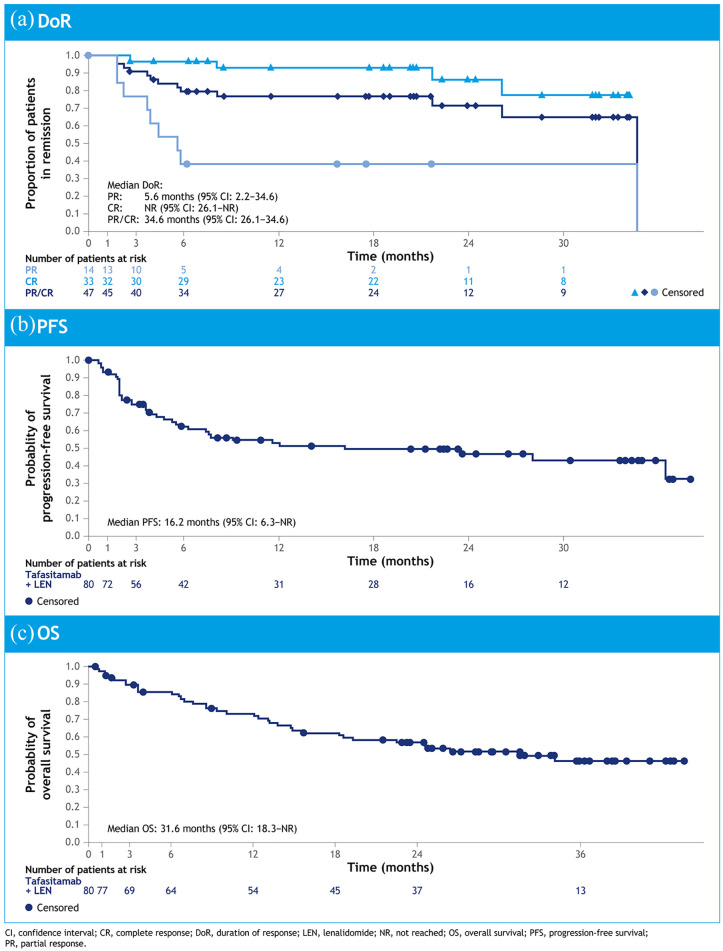
Eighty patients received combination therapy with tafasitamab 12 mg/kg intravenously once weekly and lenalidomide 25 mg/day orally on days 1–21 for up to 12 28-day cycles, followed by tafasitamab monotherapy (every 2 weeks in patients with stable disease or better) until disease progression (one patient of the 81 enrolled did not receive both agents; 33 Figure 2). At long-term follow-up (⩾24 months after last patient enrolled), 42 the ORR was 57.5% (n = 46/80), including CR in 40.0% of patients (n = 32/80) (Table 1). Median DoR was 34.6 months (95% CI = 26.1–34.6); median OS was 31.6 months (18.3–NR); and median PFS was 12.1 months (6.3–NR). Median DoR was not reached in patients with CR (95% CI = 26.1–NR months), and consistent ORRs were seen in subgroups of patients with primary refractory (53.3) and last-therapy refractory (60.0%) disease [Figure 3(d)]. 42 Responses were also observed in the two patients with double- or triple-hit lymphoma (PR and CR, respectively). 33
Figure 2.
L-MIND schema. 43
Table 1.
ORR and CRR in the primary and long-term analyses of L-MIND.
| Tafasitamab plus lenalidomide N = 80 b |
||
|---|---|---|
| Primary analysis Data cut-off: 30 November 201833 |
Follow-up analysis Data cut-off: 30 November 201942 |
|
| Best objective response, n (%) | ||
| CR | 34 (43) | 32 (40) |
| PR | 14 (18) | 14 (18) |
| ORR – CR + PR; n (%) (95% CI) a | 48 (60) (48–71) | 46 (58) (45.9–68.5) |
| Median DoR – IRC; months (95% CI) | 21.7 (21.7–NR) | 34.6 (26.1–34.6) |
| Median PFS – IRC; months (95% CI) | 12.1 (5.7–NR) | 12.1 (6.3–NR) |
| Median OS, months (95% CI) | NR (18.3–NR) | 31.6 (18.3–NR) |
Using the two-sided 95% Clopper–Pearson exact method based on a binomial distribution.
One patient received tafasitamab only and was excluded from 81 enrolled patients.
CI, confidence interval; CR, complete response; CRR, complete response rate; DoR, duration of response; IRC, independent review committee; NR, not reached; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response.
Figure 3.
Kaplan–Meier curves for (a) duration of response, (b) PFS and (c) OS. 43
Analysis of time-to-event endpoints [Figure 3(a) to (c)] was encouraging, with the Kaplan–Meier curve for PFS including a potential plateau section after around 12 months [Figure 3(b)], for approximately 40% of patients. 43 As expected, 24-month OS was shorter in patients with primary refractory disease, although ORR in refractory subgroups was consistent with the rest of the population [Figure 4(a)]. Of the refractory subgroups, primary refractoriness appeared to have a negative impact on both 24-month DoR and 24-month OS, while refractoriness to last therapy had little impact on 24-month DoR [Figure 4(b) and (c)]. Of the other patient subgroups analyzed, the only characteristic to stand out as a consistent marker of poor prognosis was intermediate–high or high-risk International Prognostic Index score at baseline. 42 Patients who received tafasitamab plus lenalidomide as second-line therapy experienced an improved ORR [67.5% (95% CI = 50.9–81.4) versus 47.5% (95% CI = 31.5–63.9)] and 24-month OS [67.9% (95% CI = 50.4–80.3) versus 46.3% (95% CI = 29.8–61.3)] compared with those who received the combination as third-or-later-line therapy. 42
Figure 4.
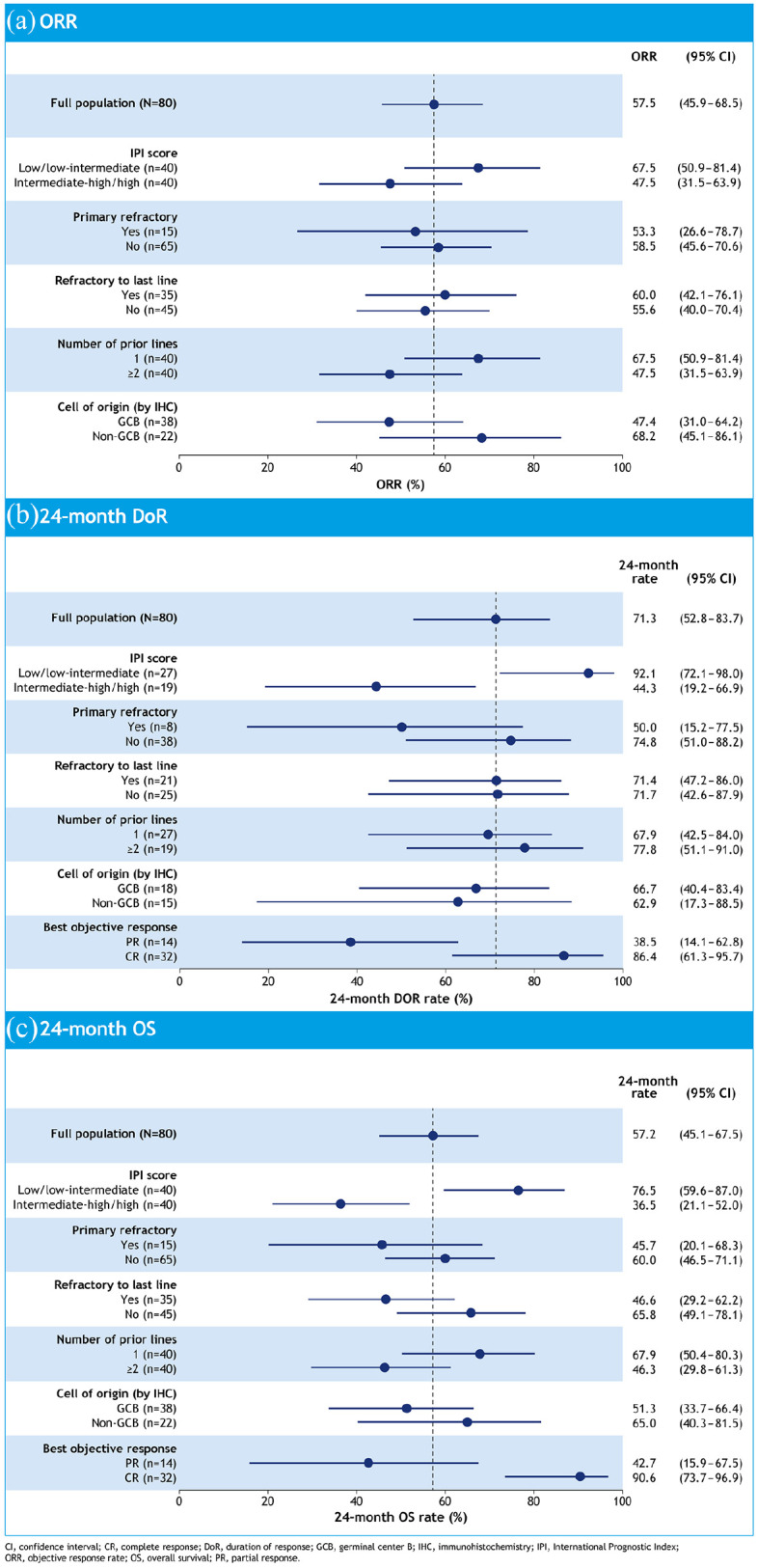
Forest plots for (a) ORR, (b) 24-month duration of response and (c) 24-month OS. 42
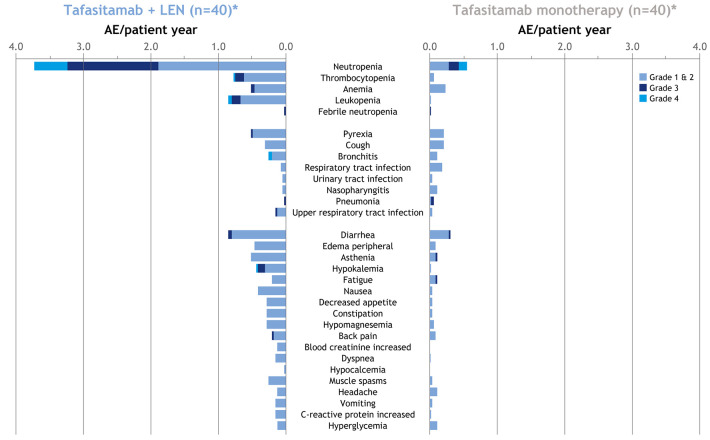
At long-term follow-up, AEs were consistent with the primary analysis 33 and with the toxicity profiles of each drug. The primary hematologic AEs were neutropenia (49.4% Grade ⩾3) and thrombocytopenia (17.3% Grade ⩾3), which were both manageable with standard therapy. The incidence of hematologic and non-hematologic AEs declined in the tafasitamab monotherapy phase, following cessation of lenalidomide (Figure 5). Several patients discontinued one or both agents during the combination phase (50 patients; 60%). The majority of discontinuations (32/50; 64%) resulted from disease progression, and 15 patients (18.8%) discontinued one or both drugs due to AEs [the remaining discontinuations were owing to patient withdrawal (n = 2) and other (n = 1)]. Of the 34 patients who received tafasitamab for >12 months (30 patients who completed 12 months’ lenalidomide and four patients who discontinued lenalidomide early but received tafasitamab for ⩾12 months in total), there was one discontinuation due to AEs (2.9%), seven (20.6%) owing to disease progression, two (5.9%) patient withdrawals and two discontinuations for other reasons (5.9%). 43
Figure 5.
AEs per patient-year during combination and monotherapy phases. 43
*n = 40 includes 30 patients who completed 12 cycles of tafasitamab plus lenalidomide and continued tafasitamab monotherapy and 10 patients who discontinued lenalidomide but continued tafasitamab monotherapy.
AE, adverse event; LEN, lenalidomide.
Although data from L-MIND are encouraging, as a single-arm study it cannot delineate the contribution of tafasitamab to the efficacy of combination therapy. Following discussions with the Food and Drug Administration (FDA) to estimate the added efficacy of combination therapy, the observational real-world retrospective cohort RE-MIND (NCT04150328) study of lenalidomide monotherapy was conducted to generate a patient-level matched comparator for the L-MIND study.44,45 In this real-world evidence approach, a pool of 140 patients with R/R DLBCL were eligible for matching by receiving lenalidomide monotherapy with a starting dose of 25 mg/day, having at least 6 months’ follow-up data and fulfilling eligibility criteria aligned with L-MIND. The retrospective monotherapy cohort was then balanced for nine prognostically important baseline covariates to represent patients similar to those enrolled in L-MIND, using estimated propensity score-based nearest neighbor 1:1 matching. Cohorts of 76 patients were identified from each study (five patients from L-MIND were not eligible for matching but were included in sensitivity analyses) and outcomes were compared. A significantly higher ORR (primary endpoint) of 67.1% (combination therapy) versus 34.2% (lenalidomide monotherapy; p < 0.0001) was predicted, together with significant increases in OS and PFS for combination therapy (Table 2). 45 Efficacy data for the lenalidomide monotherapy cohort in RE-MIND were similar to published prospective trial data with lenalidomide monotherapy,45–48 making the RE-MIND cohort a realistic comparator for L-MIND. Although not a substitute for a conventional randomized study, real-word evidence makes an important contribution to drug development and clinical research. The RE-MIND study design included multiple predefined sensitivity analyses to detect and mitigate sources of bias, including repeat analyses of the data with more stringent patient-matching criteria, and additional prognostic factors (e.g. Eastern Cooperative Oncology Group performance status) as balancing covariates, all of which supported the primary analysis. 45
Table 2.
Predicted outcomes with tafasitamab plus lenalidomide and lenalidomide monotherapy in the RE-MIND study. 45 .
| Predicted outcome | Tafasitamab plus lenalidomide cohort L-MIND regimen; n = 76 |
Lenalidomide monotherapy cohort RE-MIND; n = 76 |
Comparison (95% CI); p-value |
|---|---|---|---|
| ORR, % (95% CI) | 67.1 (55.4–77.5) | 34.2 (23.7–46.0) | OR = 3.9 (1.9–8.1); p < 0.0001 |
| CRR, % (95% CI) | 39.5 (28.4–51.4) | 13.2 (6.5–22.9) | – |
| Median OS, months a | NR | 9.4 | HR = 0.499 (0.317–0.785); p = 0.0026 |
| Median PFS, months b | 12.1 | 4.0 | HR = 0.463 (0.307–0.698); p = 0.0002 |
Median follow-up for OS = 21.5 months (tafasitamab + lenalidomide) and 20.9 months (lenalidomide).
Median follow-up for PFS = 19.7 months (tafasitamab + lenalidomide) and 12.6 months (lenalidomide).
CI, confidence interval; CRR, complete response rate; HR, hazard ratio; NR, not reached; OR, odds ratio; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
Therefore, tafasitamab plus lenalidomide is an effective option in patients with R/R DLBCL who are not eligible for ASCT, with tafasitamab making a clinically significant contribution to the efficacy of the combination. Tafasitamab was approved by the FDA in combination with lenalidomide for the treatment of patients with R/R DLBCL not eligible for ASCT on the strength of overall response data from L-MIND. 49 This indication is approved under accelerated approval based on overall response rate, and continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Beyond evaluation with lenalidomide monotherapy, comparisons with more routinely used treatment regimens, such as rituximab-lenalidomide, would provide greater context for a role for tafasitamab plus lenalidomide in the treatment of patients with R/R DLBCL; however, large randomized data sets comparing standard systemic treatment approaches in R/R DLBCL are lacking. To further assess the clinical utility of tafasitamab plus lenalidomide within the broader treatment landscape for R/R DLBCL, another observational retrospective cohort study, RE-MIND2 (NCT04697160), will compare the efficacy outcomes of the L-MIND cohort with those of matched patient populations treated with systemic NCCN/ESMO guideline listed regimens administered in routine clinical care.
Open questions for CD19-directed therapy in DLBCL: treatment sequencing and compatibility with CAR-T
The availability of multiple therapies that target the CD19 receptor, including tafasitamab, CAR-T products and the antibody–drug conjugate loncastuximab tesirine, raises the important question of treatment sequencing. Given that exposure to rituximab has long been associated with B-cell CD20 modulation and consequent resistance to subsequent CD20-based therapy,50,51 it is possible that CD19-targeted therapies may be affected in a similar manner. Reduced CD19 expression following CAR-T therapy has been observed as a tumor escape mechanism resulting in relapse, likely as a result of genetic post-translational mechanisms rather than modulated CD19 expression, and may preclude further CD19-targeted therapy. 52 In patients with B-ALL, relapse with loss of CD19 antigen following CD19-targeted CAR-T therapy has been linked to a range of genetic mutations in the exons coding for the transmembrane portion of CD19, which would necessitate the use of alternative targets for further therapy. 53
Data for other CD19-targeted therapies have been more promising: in a study of 14 patients with DLBCL who received loncastuximab tesirine, CD19 positivity by immunohistochemistry was not affected by treatment, with CD19-directed CAR-T therapy possible after a median 120 days from antibody-drug conjugate failure. 14 Extensive loss of CD19 expression has not been associated with tafasitamab in CLL cells. 18 Limited clinical data support the maintenance of CD19 expression following tafasitamab treatment, with one report of a CR to CAR-T therapy after participation in the L-MIND study. 33
Sequencing of therapies in R/R DLBCL is a developing field now that several alternatives exist outside of traditional chemotherapy. Tafasitamab–lenalidomide and antibody–drug conjugates are regimens that have the advantage of being immediately available ‘off the shelf’ without the manufacturing and pre-conditioning process necessary for CAR-T, and could, therefore, be an option for patients with highly active disease for whom immediate treatment is vital. The continuous tafasitamab–lenalidomide regimen is a departure from more usual fixed-duration regimens in DLBCL, but continuous therapy is not unusual in other fields of oncology and it is likely that long-term efficacy and tolerability will ultimately determine their acceptability to patients.
Tafasitamab–lenalidomide could have several places in the treatment paradigm for R/R DLBCL: for the treatment of transplant-ineligible patients, as a bridge to ASCT, and, which may also be worth exploring, as a bridge to CAR-T. While tafasitamab has been shown to not impair CAR-T activity in vitro, 54 the duration of CD19-masking by tafasitamab in vivo should be investigated as it may hinder an immediate switch to a different CD19-targeted therapy immediately post-tafasitamab administration. Further research into the detection of CD19 masking is necessary; staining by fluorescence-activated cell sorting may not be capable of detecting tafasitamab-masked CD19 and immunohistochemistry methods may return false positives due to the detection of the intracellular CD19 domain. New staining methods may, therefore, be required to conclusively determine the duration of tafasitamab occupancy of cell surface CD19.
Future potential for tafasitamab in DLBCL
R-CHOP has been the standard first-line treatment for DLBCL for over two decades, despite several attempts to improve on it with a variety of approaches. 55 The FIRST-MIND study incorporates either tafasitamab alone or tafasitamab–lenalidomide alongside R-CHOP as a first-line therapy for DLBCL. Preliminary safety data published at ASH 2020 support the feasibility of this regimen in terms of toxicity, with an expected increased incidence of neutropenia and thrombocytopenia in patients who received lenalidomide. 56 Efficacy data are imminent.
Other combinations with tafasitamab are also a logical approach: the B-MIND study is a randomized, phase II/III study of rituximab in combination with either tafasitamab or bendamustine in patients with R/R DLBCL who are ineligible for ASCT, and is currently recruiting (NCT02763319 57 ). Future combinations of tafasitamab–lenalidomide with bispecific anti-CD20 antibodies, or antibody-drug conjugates, would also be logical steps to enhance responses to simultaneously target CD19 and CD20, and may help prevent disease relapse.
In conclusion, tafasitamab plus lenalidomide is an effective option for patients with R/R DLBCL who are not eligible for ASCT, and tafasitamab itself is a promising combination partner for other therapies. This is an exciting time in the field of DLBCL; while the optimum sequence for CD19-targeting therapies following R-CHOP has yet to be determined, the last 5 years have at least provided several new avenues to explore, including further potential opportunities to improve on R-CHOP as first-line therapy.
Footnotes
Conflict of interest statement: GS reports non-financial support from MorphoSys, during the conduct of the study; other from MorphoSys, other from BMS, other from AbbVieˇ, other from Janssen, other from Merck, other from Novartis, other from Gilead/Kite, other from Epizyme, other from Celgene/BMS, other from Roche/Genentech, other from Takeda, other from Autolus, other from Genmab, other from Velosbio, outside the submitted work; in addition, GS has a patent WO2012010561A1 issued. MT has nothing to disclose. JD reports non-financial support from MorphoSys, during the conduct of the study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing assistance was provided by Ori Bowen of Syneos Health and funded by MorphoSys.
Contributor Information
Johannes Düll, Medizinische Klinik und Poliklinik II, Universitätsklinik Würzburg, Josef-Schneider-Straße 2, Würzburg, 97080, Germany.
Max Topp, Medizinische Klinik und Poliklinik II, Universitätsklinik Würzburg, Würzburg, Germany.
Gilles Salles, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
References
- 1. Solimando AG, Annese T, Tamma R, et al. New insights into diffuse large B-cell lymphoma pathobiology. Cancers (Basel) 2020; 12: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Cancer Institute. SEER cancer stat facts: diffuse large B-cell lymphoma [Internet], https://seer.cancer.gov/statfacts/html/dlbcl.html (2019, accessed 4 January 2021).
- 3. Coiffier B, Ementine Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure – what to do? Hematol Am Soc Hematol Educ Progr 2016; 2016: 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017; 130: 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zinzani PL, Pellegrini C, Gandolfi L, et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 2 trial. Clin Lymphoma Myeloma Leuk 2011; 11: 462–466. [DOI] [PubMed] [Google Scholar]
- 6. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol 2012; 1: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujimoto M, Fujimoto Y, Poe JC, et al. CD19 regulates Src family protein tyrosine kinase activation in B lymphocytes through processive amplification. Immunity 2000; 13: 47–57. [DOI] [PubMed] [Google Scholar]
- 8. Hojer C, Frankenberger S, Strobl LJ, et al. B-cell expansion and lymphomagenesis induced by chronic CD40 signaling is strictly dependent on CD19. Cancer Res 2014; 74: 4318–4328. [DOI] [PubMed] [Google Scholar]
- 9. Chung EY, Psathas JN, Yu D, et al. CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis. J Clin Invest 2012; 122: 2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makita S, Tobinai K. Antibody therapy targeting CD19 for B-cell non-Hodgkin’s lymphoma. Ann Oncol 2018; 29: 1086–1089. [DOI] [PubMed] [Google Scholar]
- 11. Abramson JS, Ghosh N, Smith SM. ADCs, BiTEs, CARs, and small molecules: a new era of targeted therapy in non-Hodgkin lymphoma. Am Soc Clin Oncol Educ Book 2020; 40: 302–313. [DOI] [PubMed] [Google Scholar]
- 12. Watkins MP, Bartlett NL. CD19-targeted immunotherapies for treatment of patients with non-Hodgkin B-cell lymphomas. Expert Opin Investig Drugs 2018; 27: 601–611. [DOI] [PubMed] [Google Scholar]
- 13. Hoy SM. Tafasitamab: first approval. Drugs 2020; 80: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 14. Thapa B, Caimi PF, Ardeshna KM, et al. CD19 antibody-drug conjugate therapy in DLBCL does not preclude subsequent responses to CD19-directed CAR T-cell therapy. Blood Adv 2020; 4: 3850–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coiffier B, Thieblemont C, de Guibert S, et al. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol 2016; 173: 722–730. [DOI] [PubMed] [Google Scholar]
- 16. Katz BZ, Herishanu Y. Therapeutic targeting of CD19 in hematological malignancies: past, present, future and beyond. Leuk Lymphoma 2014; 55: 999–1006. [DOI] [PubMed] [Google Scholar]
- 17. Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A 2006; 103: 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horton HM, Bernett MJ, Pong E, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res 2008; 68: 8049–8057. [DOI] [PubMed] [Google Scholar]
- 19. Awan FT, Lapalombella R, Trotta R, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood 2010; 115: 1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rafiq S, Cheney C, Mo X, et al. XmAb-5574 antibody demonstrates superior antibody-dependent cellular cytotoxicity as compared with CD52-and CD20-targeted antibodies in adult acute lymphoblastic leukemia cells. Leukemia 2012; 26: 1720–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kellner C, Zhukovsky EA, Pötzke A, et al. The Fc-engineered CD19 antibody MOR208 (XmAb5574) induces natural killer cell-mediated lysis of acute lymphoblastic leukemia cells from pediatric and adult patients. Leukemia 2013; 27: 1595–1598. [DOI] [PubMed] [Google Scholar]
- 22. Woyach JA, Awan F, Flinn IW, et al. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood 2014; 124: 3553–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jurczak W, Zinzani PL, Hess G, et al. A phase IIa, open-label, multicenter study of single-agent tafasitamab (MOR208), an Fc-optimized anti-CD19 antibody, in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma: long-term follow-up, final analysis. Blood 2019; 134: 4078. [Google Scholar]
- 24. Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol 2018; 29: 1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batoo SA, Hernandez-Ilizaliturri FJ. The emerging role of lenalidomide in the management of lymphoid malignancies. Ther Adv Hematol 2011; 2: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol 2015; 33: 2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Witzig TE, Nowakowski GS, Habermann TM, et al. A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Ann Oncol 2015; 26: 1667–1677. [DOI] [PubMed] [Google Scholar]
- 28. Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia 2013; 27: 1902–1909. [DOI] [PubMed] [Google Scholar]
- 29. Castellino A, Chiappella A, LaPlant BR, et al. Lenalidomide plus R-CHOP21 in newly diagnosed diffuse large B-cell lymphoma (DLBCL): long-term follow-up results from a combined analysis from two phase 2 trials. Blood Cancer J 2018; 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vitolo U, Witzig TE, Gascoyne RD, et al. ROBUST: first report of phase III randomized study of lenalidomide/R-CHOP (R2 -CHOP) vs placebo/R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Hematol Oncol 2019; 37: 36–37. [Google Scholar]
- 31. Thieblemont C, Tilly H, Gomes da, Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2017; 35: 2473–2481. [DOI] [PubMed] [Google Scholar]
- 32. Vo DN, Alexia C, Allende-Vega N, et al. NK cell activation and recovery of NK cell subsets in lymphoma patients after obinutuzumab and lenalidomide treatment. Oncoimmunology 2017; 7: e1409322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020; 21: 978–988. [DOI] [PubMed] [Google Scholar]
- 34. Plonquet A, Haioun C, Jais JP, et al. Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2-3 diffuse large B-cell lymphoma. Ann Oncol 2007; 18: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 35. Kim JK, Chung JS, Shin HJ, et al. Influence of NK cell count on the survival of patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood Res 2014; 49: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klanova M, Oestergaard MZ, Trneny M, et al. Prognostic impact of natural killer cell count in follicular lymphoma and diffuse large b-cell lymphoma patients treated with immunochemotherapy. Clin Cancer Res 2019; 25: 4632–4643. [DOI] [PubMed] [Google Scholar]
- 37. Danielou-Lazareth A, Henry G, Geromin D, et al. At diagnosis, diffuse large B-cell lymphoma patients show impaired rituximab-mediated NK-cell cytotoxicity. Eur J Immunol 2013; 43: 1383–1388. [DOI] [PubMed] [Google Scholar]
- 38. Sarkar S, Sabhachandani P, Ravi D, et al. Dynamic analysis of human natural killer cell response at single-cell resolution in B-Cell Non-Hodgkin Lymphoma. Front Immunol 2017; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tamma R, Ranieri G, Ingravallo G, et al. Inflammatory cells in diffuse large B cell lymphoma. J Clin Med 2020; 9: 2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou H, Zheng C, Huang DS. A prognostic gene model of immune cell infiltration in diffuse large B-cell lymphoma. PeerJ 2020; 8: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burke JM, André M, Cheson BD, et al. A phase Ib, open-label, randomized study to assess safety and preliminary efficacy of tafasitamab (MOR208) or tafasitamab + lenalidomide in addition to R-CHOP in patients with newly diagnosed diffuse large B-cell lymphoma: the first-mind trial. Blood 2019; 134: 2877. [Google Scholar]
- 42. Maddocks KJ, Duell J, González-Barca E, et al. Long-term subgroup analyses from L-mind, a phase II study of tafasitamab (MOR208) combined with lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Blood 2020; 136: 19–21. [Google Scholar]
- 43. Salles G, Duell J, Gonzalez-Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. EHA Libr, p. 293691; EP1201, https://library.ehaweb.org/eha/2020/eha25th/293691/gilles.salles.long-term.outcomes.from.the.phase.ii.l-mind.study.of.tafasitamab.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dl-mind (2020, accessed 17 June 2020).
- 44. Zinzani PL, Rodgers T, Marino D, et al. RE-MIND study: comparison of tafasitamab + lenalidomide (L-MIND) vs lenalidomide monotherapy (real-world data) in transplant-ineligible patients with relapsed/refractory diffuse large B-cell lymphoma. Abstract S238 presented at EHA on 11-21 June 2020, p. S238, https://library.ehaweb.org/eha/2020/eha25th/295058/p.zinzani.re-mind.study.comparison.of.tafasitamab.2B.lenalidomide.28l-mind29.vs.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D3%2Ace_id%3D1766%2Aot_id%3D23235%2Amarker%3D756 (2020, accessed 17 June 2020).
- 45. Nowakowski G, Rodgers T, Marino D, et al. RE-MIND study: a propensity score-based 1:1-matched comparison of tafasitamab + lenalidomide (L-MIND) versus lenalidomide monotherapy (real-world data) in transplant-ineligible patients with relapsed/refractory diffuse large B-cell lymphoma. J Clin Oncol 2020; 38: abstract 8020 and poster 353. [Google Scholar]
- 46. Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2008; 26: 4952–4957. [DOI] [PubMed] [Google Scholar]
- 47. Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol 2011; 22: 1622–1627. [DOI] [PubMed] [Google Scholar]
- 48. Czuczman MS, Trneˇný M, Davies A, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 2017; 23: 4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. FDA. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma, https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-tafasitamab-cxix-diffuse-large-b-cell-lymphoma (accessed 30 September 2020).
- 50. Taylor RP, Lindorfer MA. Antigenic modulation and rituximab resistance. Semin Hematol 2010; 47: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dahal LN, Huang C-Y, Stopforth RJ, et al. Shaving is an epiphenomenon of type I and II anti-CD20–mediated phagocytosis, whereas antigenic modulation limits type I monoclonal antibody efficacy. J Immunol 2018; 201: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shalbani H, Kraft IL, Wang H-W, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica 2018; 103: e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 2018; 24: 1504–1506. [DOI] [PubMed] [Google Scholar]
- 54. Horvei P, Sakemura R, Cox MJ, et al. Targeting of CD19 By tafasitamab does not impair CD19 directed chimeric antigen receptor T cell activity in vitro. Blood 2019; 134: 2859. [Google Scholar]
- 55. Narkhede M, Cheson BD. Tafasitamab: monoclonal antibody targeting CD19 treatment of B-cell malignancies. Drugs Future 2020; 45: 641–651. [Google Scholar]
- 56. Belada D, Nowakowski GS, Bergua Bergues JM, et al. A phase Ib, open-label, randomized study to assess safety and preliminary efficacy of tafasitamab (MOR208) or tafasitamab + lenalidomide in addition to R-CHOP in patients with newly diagnosed diffuse large B-cell lymphoma: analysis of the safety run-in Ph. Blood 2020; 136: 3028. [Google Scholar]
- 57. Nowakowski GS, Belada D, Molina L, et al. B-MIND: MOR208 plus bendamustine (BEN) versus rituximab (RTX) plus BEN in patients with relapsed or refractory (R-R) diffuse large B-cell lymphoma (DLBCL): an open-label, randomized phase II/III trial. J Clin Oncol 2017; 35: TPS7571. [Google Scholar]