Abstract
While chronic lung disease causes substantial global morbidity and mortality, global estimates have primarily been based on broad assumptions. Specific country data from low-income countries such as Nepal are limited. This review assessed primary evidence on chronic respiratory disease burden among adults in Nepal. A systematic search was performed in June 2019 (updated May 2020) for studies through nine databases. High levels of heterogeneity deemed a narrative synthesis appropriate. Among 27 eligible studies identified, most were low-moderate quality with cross-sectional and retrospective study design. Chronic lung diseases identified were chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis and restrictive lung diseases. Studies were categorised as: (i) community-based, (ii) hospital-based and (iii) comorbidity-related and disease burden. Reported disease prevalence varied widely (COPD, 1.67–14.3%; asthma, 4.2–8.9%). The prevalence of airflow obstruction was higher among rural dwellers (15.8%) and those exposed to household air pollution from domestic biomass burning as opposed to liquid petroleum gas users (Odds Ratio: 2.06). Several comorbidities, including hypertension and diabetes mellitus added to the disease burden. The review shows limited literature on lung disease burden in Nepal. Publications varied in terms of overall quality. Good quality research studies with prospective cohorts related to respiratory conditions are required.
Keywords: Chronic respiratory disease, lung function, prevalence, Nepal, global lung health
Introduction
The burden of chronic lung disease worldwide is huge, both in terms of morbidity and mortality. It is increasing and the current COVID-19 pandemic is likely to add further. Just one lung non-communicable disease (NCD), chronic obstructive pulmonary disease (COPD), causes >3 million deaths per year globally, >90% of these occurring in low to middle-income countries (LMICs). 1,2 Globally identified risk factors in LMICs include tobacco smoke, household and ambient air pollution, and occupational exposure. 3 –8 The risk factors for developing chronic lung diseases will vary from country to country. The Himalayas contain several LMICs, many of which share geographical and cultural features. We chose Nepal as an exemplar Himalayan LMIC, with a population of circa 30 million and the 16th poorest country worldwide. Figures on disease prevalence for Nepal have been reported as part of the Global Burden of Disease project, these are primarily based on modelled data and are therefore dependent upon broad assumptions that may lack accuracy. 9 Reportedly, NCDs in Nepal account for 66% of mortality, of which chronic respiratory diseases are ranked as the second highest cause of death (10%), alongside cardiovascular diseases (30%) and cancer (9%). 10 The gravity of chronic respiratory diseases in Nepal was highlighted recently, with estimates second worse only to Kazakhstan for global mortality (Kazakhstan: 114.28 versus 100.75 deaths in Nepal per 100,000). 11,12
Respiratory disease greatly impacts health service demand in Nepal through hospitalisations. 13 –15 According to the 2016–2017 Department of Health services in Nepal, bronchial asthma and COPD were the second and third most common causes of outpatient department morbidity, with cardiovascular conditions as the leading cause. 12 Many patients reported having more than one long-term health condition.
Within Nepal, risk factors for chronic respiratory diseases vary according to gender and geographical location. Over 80% of the population lives in rural settings where a large proportion uses biomass as a source for heating and cooking fuel within poorly ventilated living quarters, with exposure to high levels of household air pollution. 16,17 As women generally do the cooking, this disproportionately affects them. Smoking is common, particularly in men (27% vs 6% in women) and in urban areas (30% of households); in addition, there is significant ambient, agricultural, and industrial pollution. 17 –20 These exposures do not only affect respiratory disease but also contribute to other health complications and socioeconomic burdens. 6,21 The remoteness of some rural locations hinders alternative energy sources and restricts fuel choices. Other socio-demographic factors, including illiteracy, poverty, distance and remote access to healthcare facilities, also have an adverse impact on health. 8,22
The government of Nepal has begun taking action with policies including the development of the Integrated NCDs Prevention and Control Policy of Nepal and the Multisectoral Action Plan for the Prevention and Control of Non-Communicable Diseases (2014–2020). 23 However, innovative health systemic structures and a comprehensive understanding of the burden of diseases are required for effective implementation. 24 This systematic review aims to synthesise the current available evidence across community and hospital settings on the prevalence of chronic respiratory disease (excluding cancer), respiratory symptoms, lung function and lung-related burden in Nepal.
Methods
Search strategy
Initial scoping in May 2019 through MEDLINE, Embase, Cochrane library and manually, identified no existing systematic reviews on this area.
The protocol was registered with PROSPERO (CRD42019138552). 25 Database searches were conducted in June 2019 and updated in May 2020 for publications, with no date restrictions. Nine electronic databases were searched: Ovid (MEDLINE, Embase, Global Health, CAB Abstracts), CINAHL, Scopus, Web of Science, Cochrane Library and Nepal Journal Online (NEPJOL). Grey literature searches were conducted, including Nepal Government/Health Ministry reports, World Health Organisation (WHO) data and OpenGrey. Searches were supplemented by contact with study authors where additional information was required as well as forward and backward citation tracking from included studies. A sample search strategy is shown, Appendix 1.
Study screening and selection
Study designs reporting quantitative population-level prevalence or incidence of chronic respiratory disease, lung function, respiratory symptoms or burden of lung disease in adults (individuals aged 16 years and older) living in Nepal were considered. The review included only peer reviewed publications, but grey literature set the scene in the introduction. Language was not an exclusion criterion. For this review, publications studying specific occupational exposure were excluded. Conference abstracts were excluded following screening for full-text peer-reviewed publications.
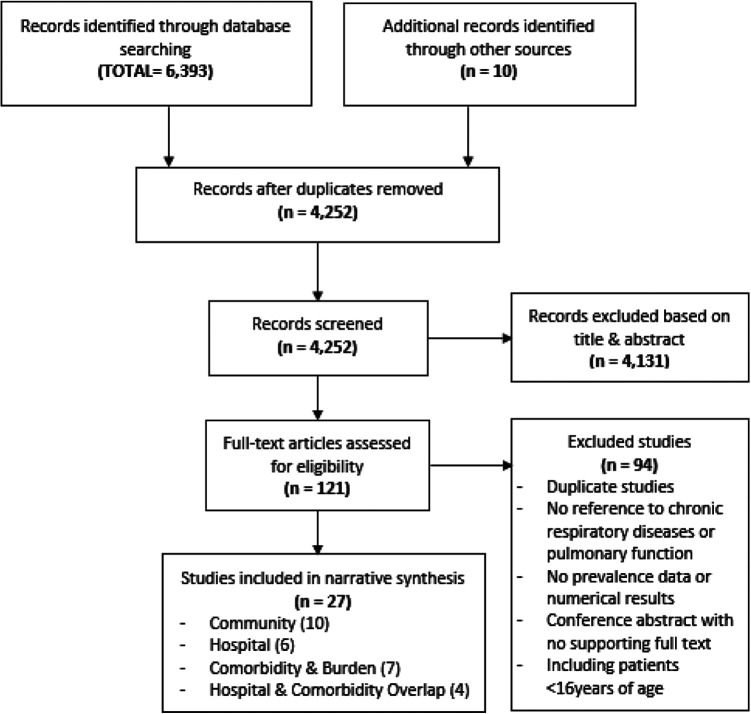
Study records identified were exported into Mendeley (London, UK), with duplicates removed. Titles and abstracts were screened by two independent reviewers (WE, CEB). Full texts of potentially eligible papers were retrieved and assessed independently by two reviewers with discrepancies resolved through discussion. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 26 flow diagram of the search and screening process is shown in Figure 1.
Figure 1.
PRISMA flow chart of included studies.
Data extraction and quality assessment
A standardised data extraction form was used for the final selection of studies. Data were extracted by one reviewer and checked for accuracy by a second reviewer. Quality of the included studies was independently assessed by two researchers from WE, ARJ and CEB using the AXIS critical appraisal checklist for cross-sectional studies. 27 In keeping with the AXIS method, the quality is not judged as a numerical scoring of the component parts and therefore has a subjective element.
Data synthesis
Studies were highly heterogeneous with huge variation used for data acquisition, analysis and reporting. Therefore the pre-planned meta-analysis was not possible (see PROSPERO: CRD42019138552), and the findings were synthesised narratively. 28 Early on, it became apparent that the review would be in three distinct sections, given the heterogeneity of sites in the manuscripts: i) community setting, ii) hospital (mostly inpatients) setting and then iii) comorbidity-related and lung-related burden of disease.
Results
Study selection
A total of 6,393 papers were identified. Followng removal of duplicates, 4,252 studies were screened by titles and abstracts, and 121 full-text studies reviewed (Figure 1), with 27 articles included (Tables 1 –3).
Table 1.
Community-related chronic respiratory disease prevalence studies.
| Author | Study design | Diseases and Symptoms | Study setting | Exposures | Disease outcomes | Respiratory and Symptoms | Lung function outcomes | Quality Range |
|---|---|---|---|---|---|---|---|---|
| Acharya et al. 37 | Cross-sectional survey | Asthma | Community older adults (≥60 years) Sample: 401 participants (Self-report) |
Not specified | Asthma : 25.4% | Not reported | Not conducted | Moderate |
| Dhimal et al. 50 | Cross-sectional | COPD | Nationwide community survey of adults >20 years Sample: 12,557 (prevalence determined in 11,277) participants (Self-report) |
Not specified |
COPD prevalence (95% CI): 11.7% (10.5–12.9) Increasing prevalence with increasing age range: 20–39 yrs: 6.7% (4.9–7.7) 40–59 yrs = 10.5% (9.1–12.1) >60 yrs = 21.5% (19.3–24.1) Male/female = 12.6% (11.2–14.1) vs 11.0% (9.6–12.4) Rural/urban = 11.7% (10.0–13.6) vs 11.7% (10.1–13.4) |
Not assessed | Not conducted | Low |
| Joshi et al. 32 | Cross-sectional | COPD Asthma symptoms Lung function |
Community rural adult kitchen dwellers Sample: 154 participants (Self-report) |
- Indoor Air Pollution - Smoking (never = NS, current = CS, previous = PS) |
-
COPD
: 1.67% - Bronchial asthma : 5.58% |
Cough (25.69%), phlegm (15.08%), breathlessness (15.08%), wheezing (11.73%) Disease prevalence based on exposure duration (hours) - Cough : <2 (8, 5.19%), 2 to <4 (27, 17.53%), ≥4 (11, 7.14%), (p < 0.05) - Phlegm : <2 (1, 0.65%), 2 to <4 (15, 9.74%), ≥4 (11, 7.14%), (p < 0.05) - Breathlessness : <2 (0, 0%), 2 to <4 (15, 9.74%), ≥4 (11, 7.14%), (p < 0.05) - Wheezing : <2 (0, 0%), 2 to <4 (10, 6.49%), ≥4 (11, 7.14%), (p < 0.05) - COPD : <2 (0, 0%), 2 to <4 (2, 1.30%), ≥4 (1, 0.65%), (p < 0.05) - Bronchial asthma : <2 (0, 0%), 2 to <4 (5, 3.25%), ≥4 (5, 3.25%), (p < 0.05) Diseases Symptoms - Cough (n = 46): NS (25.5%), CS (26.44%), PS (18.18%) (p < 0.01) - Phlegm (n = 27): NS (10.63%), CS (16.52%), PS (18.18%) (p < 0.01) - Breathlessness (n = 27): NS (10.63%), CS (15.70%), PS (27.27%) (p < 0.01) - Wheezing ( n = 21 ) : NS (10.63%), CS (11.57%), PS (18.18%) (p < 0.01) |
PEFR conducted but % predicted values not used | Low |
| Kurmi et al. 47 | Cross-sectional | Lung function | Community rural and urban population 16 years and above Sample: 1,648 participants 1,392 (valid spirometry) |
Rural:
Household Air Pollution (Biomass) Urban : Outdoor Air Pollution |
Not assessed | Not reported | Airflow obstruction comparison of biomass fuel to liquefied petroleum use: OR = 2.06 (1.16–3.67), p = 0.014 | High |
| Kurmi et al. 48 | Cross-sectional | symptoms Lung function |
Community rural and urban population 16 years and above Sample: 1,648 participants (Self-report) |
Rural:
Household Air Pollution (Biomass) Urban : Outdoor Air Pollution |
Not assessed | Male (Rural, n = 382 vs Urban, n = 380), Female (Rural, n = 463 vs Urban, n = 423) - Wheeze ever: male (23.5% vs 8.7%, p < 0.001), female (32% vs 10.3%, p < 0.001) - Chronic cough: male (4.7% vs 5.3%, p = 0.719), female (4.4% vs 4.2%, p = 0.864) - Chronic phlegm: male (3% vs 12.9%, p < 0.001), female (4% vs 5.8%, p = 0.223) - Dyspnoea : male (12% vs 2.5%, p < 0.001), female 17.8% vs 7.6%, p < 0.001) |
Airflow obstruction after exposure to Biomass (OR (95% CI, p-value) FEV1/FVC < 0.70: (male 1.94 (1.05– 3.59, p = 0.035) vs female 1.30 (0.67 –2.54, p = 0.436) FEV1/FVC < LLN: (male 1.11 (0.41–3.00, p = 0.84) vs female 1.67 (0.66– 4.23, p = 0.281) |
High |
| Pandey et al. 29 | House to house survey | Chronic bronchitis Lung function |
Community rural adults in hill region 20 years and above Sample: 2,826 participants |
Not specified |
Crude prevalence ( n = 2,826): Chronic bronchitis * (18.3%), Emphysema (3.1%), Cor pulmonale (1.5%) * Chronic bronchitis defined using the MRC definition |
Not reported | Spirometry in a representative sample of chronic bronchitis (n = 61), 35 (57.4%) had airflow obstruction, Spirometry in a representative sample of 146, 23 (15.8%) had airflow obstruction. |
Moderate |
| Prasad et al. 33 | Cross-sectional | Lung function | Community women, 30–40 years old Sample: 200 participants (Self-report) |
Smoking | Not assessed | Not reported |
Lung function test (mean ± SE) FVC (ml): Smokers (2700 ± 0.045), Non-smokers (3400 ± 0.07) FEV (ml): Smokers (2310 ± 0.03), Non-smokers (2727 ± 0.02) PEFR (l/min): Smokers (280 ± 0.09), Non-smokers (332 ± 0.06) |
Low |
| Pratali et al. 38 | Cross-sectional | COPD Lung function |
Community rural village residents 16–75 years Sample: 32 houses (78 villagers) (Lung function test) |
Biomass fuels | Bronchial obstruction: 6/78 (7.7%) of which 5/78 had a non-reversible bronchial obstruction, classed as COPD 6% | Not reported | FEF25 – 75 : <80% predicted in 54% | Moderate |
| Ranabhat et al. 20 | Cross-sectional | Asthma Symptoms |
Community rural households Sample: 157 houses (Self-report) |
Indoor Air Pollution | Asthma: 8.9% | Reported IAP related health problems based on multiple responses (n = 297) Includes Difficulty in breathing (27.3%), Productive cough (19.7%), Dry Cough (8.9%), Tearing of eyes (55.4%) |
Not conducted | Moderate |
| Shrestha and Shrestha 36 | Cross-sectional | COPD Asthma Symptoms |
Community rural and urban households Sample: 98 houses (168 villagers) (Rural, n = 140 vs Urban, n = 28) |
Indoor Air Pollution (Processed, n = 43 vs Unprocessed fuel, n = 125) |
Prevalence (Processed vs Unprocessed fuel); (Rural vs Urban)
- COPD: 14.3% (7% vs 16.8%); (16.4% vs 3.6%) - Asthma: 4.2% (0% vs 5.6%); (4.3% vs 3.7%) |
Prevalence (Processed vs Unprocessed fuel); (Rural vs Urban) Cough: 31.5% (14% vs 37.6%); (35% vs 14.3%) Phlegm: 20.2% (9.3% vs 24%); (22.9% vs 7.1%) Cough and Phlegm : 15.5% (7% vs 18.4%); (17.1% vs 7.1%) Breathlessness : 27.4% (11.6% vs 32.8%); (30% vs 14.3%) Wheezing: 23.2% (7% vs 28.8%); (26.4% vs 7.1%) Breathlessness and wheezing: 20.2% (7% vs 24.8%); (22.9% vs 7.1%) At least one respiratory symptom: 35.7% (14% vs 43.2%); (40% vs 14.3%) All respiratory symptoms: 11.9% (4.7% vs 14.4%); (15.7% vs 3.7%) |
Not conducted | Low |
Airway Obstruction (AO), Confidence Interval (CI), Chronic Obstructive Pulmonary Disease (COPD), Indoor Air Pollution (IAP), Lower Limit of Normal (LLN), Peak Expiratory Flow Rate (PEFR), Forced Expiratory Volume in one second (FEV1 ), Forced Vital Capacity (FVC), Forced Expiratory Flow at 25–75% of FVC (FEF, Standard Error (SE)).
Table 2.
Hospital-related chronic respiratory disease studies.
| Author | Study design | Disease /state | Study setting | Disease outcomes | Quality Range |
|---|---|---|---|---|---|
| Amundsen et al. 42 | Retrospective chart-review | - COPD | Hospitalised patients Sample: 1,139 patients adult non-obstetric, non-traumatic hospitalised cases (Clinical diagnosis) Duration: 4 months |
Non-communicable diseases:
332 of the 1139 of which 45% (n = 148) were
COPD
COPD accounted for 13% of all adult, non-obstetric, non-traumatic admissions Of the 148 with COPD: - Gender: male (40%), female (60%) - Location: urban (51%), village (49%) |
Moderate |
| Bhandari and Sharma 44 | Descriptive cross-sectional study | - COPD | Hospitalised COPD patients Sample: 313 patients (Clinical diagnosis) Duration: 3 years |
Of the 313 with COPD: - Gender: male (40%), female (60%) Patients presenting more in cold months, if reside closer to hospital and higher ethnic class |
Low |
| Bhandari et al. 43 | Cross-sectional study | - COPD | Hospitalised patients, 35 years and above across 28 non-specialised institutions Sample: 10,635 cases randomly selected (Clinical diagnosis) Duration: 8 months |
NCDs
accounted for 31% (n = 3294). Of the 3294 in non-specialised hospitals, COPD accounted for 43% of the NCD admissions Therefore, 14% of 10635 admissions. |
Moderate |
| Dhungel et al. 31 | Cross-sectional | - COPD | Hospitalised patients on a medical ward Sample: 1,366 patients (Clinical diagnosis) Duration: 2 months |
COPD: 17.3% | Low |
| Ghosh et al. 45 | Descriptive cross-sectional study | - COPD | Hospitalised patients of all ages to one hospital Sample: 62,446 inpatients records (Clinical diagnosis) Duration: 3 years |
COPD:
2.6% |
Low |
| Ghimire et al. 51 | Prospective observational study | - ILD | Hospitalised patients in the department Sample: 10,894 patients (Clinical diagnosis) Duration: 2 years |
ILD:
44 (0.4%) <40 years = 4 (9.1%), 40–59 years =10 (22.7%), 60–69 years = 13 (29.5%), >70 years = 17 (38.6%) Of the patients with ILD, 80% were IPF |
Low |
| Giri et al. 53 | Prospective observational study | - COPD - Asthma |
>16 year-olds presenting to the emergency department at one hospital Sample: 21,892 patients (Clinical diagnosis) Duration: 3 years |
Patients with known presenting complaint, n = 21,892; respiratory complaints, n = 2,018 (9.2%); short of breath, n = 1,572 (7.2%); COPD or asthma, n = 106 (0.5%); other respiratory, n = 340 (1.6%) | Moderate |
| Prajapati and Pradhan 49 | Descriptive cross-sectional study | - COPD - Bronchial asthma - Restrictive lung disease |
Outpatients referred for spirometry to a hospital clinic Sample: 755 patients (Clinical diagnosis) Duration: 16 months |
Diagnoses made from spirometry: COPD (31.4%), Asthma (24.2%), Restrictive lung disease (8.1%) Association between smoking history and abnormal spirometry (smokers vs non- smokers); OR (% CI), p-value COPD : (78.5% vs 21.5%); 10.43 (6.91–15.73), p < 0.0001 Asthma : (43.2% vs 56.8%); 2.17 (1.46–3.23), p < 0.0001 Restrictive disease: (47.5% vs 52.5%); 2.59 (1.46–4.58), p < 0.001 |
Moderate |
| Shankar et al. 34 | Retrospective case notes review observational study | - COPD | Hospitalised patients across several departments, aged ≥60 years to one hospital Sample: 548 patients (Clinical diagnosis) Duration: 1 year |
Data not interpretable (Conflicting reporting between results and discussion sections in relation to hospital admissions for AECOPD) |
Low |
| Shrestha et al. 54 | Descriptive cross-sectional study | - COPD - Asthma |
>18-year-old patients attending the emergency department at one hospital Sample: 1,200 patients Duration: 14 months |
107 admissions presenting with dyspnoea (9% total) History of COPD among the 107 dyspnoea admissions, n = 68 (63.3%) Asthma among dyspnoea admissions, n = 3 (2.8%) Emergency department diagnosis of 107 dyspnoea complaints: Respiratory, n = 56 (52.3%) Respiratory and cardiovascular, n = 14 (13.1%) Metabolic, n = 12 (11.2%) Respiratory with others. n = 10 (9.3%) Neuropsychiatric, n = 7 (6.7%) Cardiovascular, n = 4 (3.7%) Gastrointestinal, n = 4 (3.7%) |
Low |
Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD), Chronic Obstructive Pulmonary Disease (COPD), Non-Communicable Disease (NCD), Interstitial Lung Disease (ILD), Idiopathic Pulmonary Fibrosis (IPF).
Table 3.
Comorbidity-related and burden of chronic respiratory disease studies.
| Author | Study design | Disease | Study setting | Co-morbid disease outcomes | Symptoms and/ or other outcome associations | Quality Range |
|---|---|---|---|---|---|---|
| Bhatta et al. 30 | Retrospective observation | Bronchiectasis | Symptoms and co-morbid presence of pulmonary hypertension and cor pulmonale in patients with bronchiectasis (outpatients) Sample: 100 patients |
Pulmonary arterial hypertension and cor pulmonale (50%) | Cough (100%), haemoptysis (75%), worsening shortness of breath (70%), fever (50%), abnormal chest physical examination (75%) | Moderate |
| Dhungel et al. 31 | Cross-sectional | COPD | Co-morbid presence of cardiovascular conditions in patients with COPD (inpatients) Sample: 237 COPD patients |
Hypertension (41.3%), diabetes (5%), coronary artery disease (5.9%), dilated cardiomyopathy (4%) | Not reported | Low |
| Ghosh et al. 45 | Descriptive cross-sectional study | COPD | Mortality rate of patients admitted with COPD Sample: 62,446 inpatients records of which 1604 had COPD |
Not assessed | Inpatient mortality 6% from the 1604 patients admitted with COPD | Low |
| Ghimire et al. 51 | Prospective observational study | ILD | Co-morbid conditions, symptoms and survival rate in patients with ILD Sample: 10,894 patients of which 44 had ILD |
Gastro-oesophageal reflux disease = 20 (45.4%), Diabetes mellitus = 7 (15.9%), Hypertension = 7 (15.9%), Cor pulmonale = 6 (13.6%), Renal impairment = 5 (11.4%), IHD = 4 (9.1%), OSA = 2 (4.5%), Pulmonary tuberculosis = 2 (4.6%), Hypothyroidism = 1 (2.3%) |
Symptoms: Fever = 15 (34.1%), Cough = 43 (97.7%), Dyspnoea = 42 (95.5%), Sputum = 6 (13.6%), Chest pain = 13 (29.5%), Orthopnoea =4 (9.1%), Haemoptysis = 2 (4.5%) Survival rate - 5 months = 0.95 ± 0.03, 12 months = 0.84 ± 0.1, 24 months = 0.42 ± 0.2 |
Low |
| Giri et al. 53 | Prospective observational study | COPD Asthma |
Mortality rate in patients presenting to the emergency department with a diagnosis of COPD or asthma Sample: 21,892 patients |
Not assessed | Any presenting complaint and a diagnosis of asthma or COPD had a 90-day mortality rate of 32% | Moderate |
| Koirala et al. 52 | Cross-sectional study | COPD Asthma ILD Bronchiectasis |
Comorbidities and characteristics of hospital outpatients diagnosed with chronic respiratory disease Sample: 50 patients (COPD = 24; asthma = 18; bronchiectasis = 6; ILD = 2) |
Sleep-related breathing disorders in patients with chronic respiratory disease COPD = 15 (62.5%) Asthma = 10 (55.6%) Bronchiectasis = 3 (50%) ILD = 1 (50%) Systemic hypertension – 36%; diabetes mellitus – 16%, metabolic syndrome – 24%, heart failure – 6%, IHD – 6%. |
Not reported | Moderate |
| Hirachan et al. 46 | Cross-sectional | COPD | Evaluation of the right heart of patients with COPD attending echocardiography clinic (inpatients) Sample: 50 COPD patients |
All patients had pulmonary hypertension, 60% were severe, 28% were moderate, and 12% were mild | Significant ECG abnormality (94%), P pulmonale (90%), Atrial arrhythmias/fibrillation or Multifocal tachycardia (12%) | Moderate |
| Shrestha et al. 35 | Prospective observation | COPD | Co-morbid presence of cardiovascular conditions in patients with COPD (inpatients) Sample: 507 patients (mainly Brahmin (52.7%)) |
Selected respiratory and cardiovascular comorbidities (n = 501) Chronic cor pulmonale (27.3%) Type II respiratory failure (10.4%), Hypertension (9.9%), dilated cardiomyopathy (2.4%), valvular heart disease (1.5%), IHD (1.6%), congestive cardiac failure (3.1%), left ventricular failure (1.8%), Other cardiovascular diseases (3.3%), diabetes mellitus (3.7%) |
Not reported | Low |
| Sijapati et al. 39 | Prospective observation | COPD | Factors determining outcomes in hospitalised AECOPD Sample: 100 patients COPD stage I (8%), stage II (61%), stage III (31%) |
Hypertension (15%), diabetes mellitus (4%) and IHD (10%) | - Deaths : 20 of the 100 | Low |
| Sijapati et al. 40 | Hospital-based prospective observation | COPD Bronchiectasis |
Co-morbid presence of bronchiectasis in patients presenting with COPD (both out- and inpatients), without a former bronchiectasis history. CT done to confirm bronchiectasis. Sample: 120 patients |
COPD patients (n= 120): With bronchiectasis (COPD-B) n = 53 (44%), without bronchiectasis (COPD-nB) n = 67 (56%) |
COPD-B (n = 53), COPD-nB (n = 67) Patients with COPD plus bronchiectasis:
Had less daily breathlessness More had purulent sputum More had had an exacerbation requiring admission in last year. |
Low |
| Thapa et al. 41 | Cross-sectional | COPD | Co-morbid presence of anxiety and depression in patients with COPD patients compared to the general population (Outpatients) Sample: 198 patients |
Beck anxiety and depression inventory scale scores (mean ± SD) - COPD group: anxiety (23.76 ± 9.51), depression (27.72 ± 9.37) - General population: anxiety (8.01 ± 6.83), depression (11.60 ± 8.42) Both greater in COPD compared to general (p < 0.001) |
Not reported | Moderate |
Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD), Standard Deviation (SD), Obstructive Sleep Apnoea (OSA), Ischaemic Heart Disease (IHD), Interstitial Lung Disease (ILD), Electrocardiogram (ECG), Computed tomography (CT).
Overview of included studies
Years of publication ranged from 1984 to 2019, and all were published in English. One was published in the 1980s, 29 7 between 2000 and 2010, 30 –36 and 19 after 2011. 20,37 –54 All included studies were cross-sectional, direct observations of clinical notes, comparative and single point survey designs. 20,29 –54 The studies reported on diseases (usually self-reported), symptoms, related health conditions and lung function. Most of the studies were either of low (n = 13) or moderate (n = 12) quality, with two classed as high quality.
Some studies reported on more than one condition, with the major diseases identified being: COPD (22 studies), 31,32,34 –36,38 –54 asthma (8 studies), 20,32,36,37,49,52 –54 bronchiectasis (2 studies and as a co-morbidity in one), 30,40,52 restrictive lung diseases (3 studies), 49,51,52 and ‘chronic bronchitis’ (1 study). 29
Symptoms reported included chronic cough, phlegm or sputum production, breathlessness or shortness of breath, chest tightness, wheezing, dyspnoea and sore throat. Reporting included presence of pulmonary arterial hypertension 30 and cor pulmonale. 29,30,35,51,52
Lung function was reported in seven studies, 29,32,33,38,47,48,51 and data presented across different spirometric measures, with one reporting proportions only of patients categorised as ‘normal’, ‘obstruction’ and ‘restriction’. 51
Community-based prevalence studies
Studies included over 18,000 participants across diverse population groups (Table 1). COPD and asthma, (usually self-reported), were covered across small-medium sized studies of 78 to 401 participants, 20,32,36 –38 with one large-scale study of 12,557 participants (prevalence determined in 11,277). 50 A study of chronic bronchitis, according to the Medical Research Council definition, was based on 2,826 participants. 29
One study on both rural and urban households reported a COPD prevalence of 14.3%. 36 This was comparable with the findings of Dhimal et al., 50 which reported a nationwide prevalence of COPD of 11.7%. These findings differed from other studies, where reported COPD prevalence was 1.67% in rural adult kitchen dwellers (reported in the paper as adults who spend a large amount of the day in the kitchen), 32 and 6% COPD prevalence in rural community residents using biomass fuels. 38 Meanwhile, prevalence of chronic bronchitis of 18.3% was reported in a study with an additional 3.1% with emphysema too. 29 Asthma prevalence ranged from 4.2% to 8.9% 20,32,36 but one study reported 25.4% in adults ≥60 years. 37 Generally, chronic respiratory diseases were more prevalent in adults aged over 40 years. 37,38,50
In a general representative population, symptom-wise, one study reported cough, phlegm, breathlessness and wheezing to each have a prevalence of over 20%, and greater among populations using unprocessed fuels and living in rural regions, than in those using processed fuels and living in urban households. 36 This agreed with Ranabhat et al., 20 where reported breathlessness and productive cough were 27.3% and 19.7% of individuals living in rural communities exposed to household air pollution. Kurmi et al. 47 reported that wheeze (male, 23.5% vs 8.7%; female, 32% vs 10.3%) and dyspnoea (male, 12% vs 2.5%; female, 17.8% vs 7.6%) were more prevalent symptoms in the rural environment as a whole, whereas chronic phlegm (male, 4.7% vs 5.3%; female, 4.4% vs 4.2%) and cough (male, 3% vs 12.9%; female, 4% vs 5.8%) were more comparable between rural and urban environments. 48 Symptom reporting was also greater in current and past smokers than never smokers, with the prevalence of cough, phlegm, breathlessness and wheezing all above 11% in rural community kitchen dwellers as a whole. 32
Kurmi et al., 47,48 a large study, reported across two publications that the odds ratio of having airflow obstruction with exposure to biomass was two times higher (OR: 2.06) 47,48 compared to liquid petroleum gas users. Males (OR: 1.94; p = 0.035) were more at risk of suffering airflow obstruction after exposure to biomass (OR: 1.30; p = 0.436) when the threshold of FEV1/FVC < 0.70 was utilised but not for a threshold of FEV1/FVC < lower limit of normal (Males, OR: 1.11, p = 0.840; Females, OR: 1.67, p = 0.281). Airflow obstruction was reported in 15.8% of rural community residents using a subset representative of the overall study population, with airflow obstruction prevalent in 57.4% among those meeting the study criteria for chronic bronchitis. 29 Smokers had lower lung function in one study compared to those who reported to not smoke, although it was unclear whether this comparator group were ex- or never smokers 33 . Pratali et al. 38 found that 54% of rural community residents using biomass fuels had a FEF25 – 75 < 80% predicted. Joshi et al. 32 reported absolute peak flow and not % predicted, and therefore is largely uninterpretable.
Hospital-based studies
Ten studies, including a total of 111,188 subjects reported on the prevalence of chronic respiratory diseases in a hospital setting (Table 2). 31,34,42 –45,49,51,53,54 The majority of studies reported on COPD among hospitalised patients, 31,34,42 –45 with other studies reporting on interstitial lung disease (ILD), 51 both COPD and asthma, 53,54 and outpatient spirometry referrals. 49
Overall estimates for the proportion of admissions due to COPD varied widely from 2.6% to 14% in studies including all hospital admissions and up to 17.3% in medical wards. 31,42,43,45 Two studies reported that among hospitalised participants with an NCD, 43–45% were COPD patients. 42,43 In two studies, the majority of hospitalised COPD patients were females (60%). 42,44
One study reporting on dyspnoea admissions (this being 9% of all the admissions) at one hospital, identified that 52.3% received a respiratory diagnosis for that presentation, another 13% had a combined respiratory and cardiovascular cause, and 9.3% received a diagnosis of respiratory accompanied with other complications. 54 However, dyspnoea was also due to metabolic (11.2%), neuropsychiatric (6.7%) and cardiovascular alone (3.7%). 54 In a study where the majority of hospital admissions were attributed to injuries in young adults, respiratory complaints still accounted for 9.2% of total hospital admissions, with COPD or asthma accounting for 0.5% of total hospital admissions. 53
Ghimire et al. 51 reported a prevalence of 0.4% for ILD among patients treated in hospital for pulmonary disorders. The majority of these ILD’s were attributed to idiopathic pulmonary fibrosis (79.5%), and 68.1% of the patients were over the age of 60 years. 51
There was only one hospital-based study with spirometry for outpatients: the final diagnoses being bronchial asthma (24.2%), COPD (31.4%), and restrictive lung disease (8.1%) of those referred for testing. 49
Comorbidity-related and burden of disease
Eleven studies, with a total of 96,594 patients reported on comorbidity and burden of chronic respiratory disease. 30,31,35,39 –41,45,46,51 –53 The major chronic respiratory diseases were COPD (seven studies), 31,35,39 –41,45,46 bronchiectasis (one study), 30 ILD (one study), 51 COPD or asthma (one study), 53 or they assessed COPD, asthma, bronchiectasis and ILD together (one study) (Table 3). 52 Reported comorbidities covered other respiratory conditions, endocrine, cardiovascular disease, sleep and mental health disorders.
Common comorbidities reported in patients with COPD were hypertension ranging 9.9–41.3% of subjects, 31,35,39 diabetes mellitus: 3.7–5%, 31,35,39 ischaemic heart disease/coronary artery disease: 1.6–10%, 31,35,39 dilated cardiomyopathy: 2.4–4%, 31,35 chronic cor pulmonale: 27.3%, 35 and type II respiratory failure: 10.4%. 35 One study in patients with COPD who had echocardiography reported that all had echocardiography evidence of pulmonary hypertension, of which 60% were severe. 46 Another study reported a co-morbid presence of bronchiectasis in 44% of COPD patients. 40 Anxiety and depression were almost three times more common in COPD patients compared to the general population. 41
One study reported that patients with an initial diagnosis of ILD had a 45.4% prevalence of gastro-oesophageal reflux disease, 15.9% diabetes mellitus, 15.9% hypertension, 13.6% cor pulmonale, 11.4% renal impairment, and 9.1% ischaemic heart disease. 51 Systemic hypertension (36%), diabetes mellitus (16%), metabolic syndrome (24%), ischaemic heart disease (6%) and heart failure (6%) were prevalent across multiple chronic respiratory diseases (COPD, ILD, bronchiectasis and asthma). 52 Two studies reported on sleep disorders with Ghimire et al. 51 reporting obstructive sleep apnoea prevalence in 4.5% of ILD patients, while Koirala et al. 52 reported that 62.5% of COPD and 55.6% of asthma patients had sleep-related breathing disorders.
Two studies reported mortality rates ranging from 6–20% in patients admitted to hospital with COPD. 39,45 Another study reported a 90-day mortality rate of 32% for patients admitted to the hospital with any complication with an accompanying diagnosis of COPD or asthma. 53 The prevalence of an exacerbation requiring hospitalisation was nearly double in COPD patients with coexistent bronchiectasis compared to those without (56.6% vs 26.8%). 40 Ghimire et al. 51 reported survival rates of 95% at 5 months, 84% 1-year, and 42% 2-year following ILD diagnosis.
In patients with bronchiectasis or ILD, common symptoms experienced included cough (bronchiectasis, 100%; ILD, 97.7%), haemoptysis (bronchiectasis, 75%; ILD, 4.5%), worsening shortness of breath (bronchiectasis, 70%; ILD, 95.5%), and fever (bronchiectasis, 50%; ILD, 34.1%). 30,51
Discussion
This study has identified and synthesised the available evidence on the prevalence of chronic respiratory diseases, symptoms, lung function, comorbidities and the overall burden of lung disease in Nepal. Overall, COPD is common, both in males and females, and is associated with frequent hospital admission and a range of comorbidities. Smoking and household air pollution were found to be common risk factors. Estimates of asthma prevalence varied widely and there was no good data on the ILD prevalence.
Community-based findings
The findings highlight the high prevalence of COPD and the varied prevalence of asthma in Nepal. The global prevalence of COPD has been reported to be approximately 9.2%, 55 with other estimates suggesting age-standardised figures for prevalence of males: 3.2% and females: 2.0%. 56 In comparison, large-scale and high quality studies assessing COPD prevalence in representative samples of the overall population in LMIC’s such as China and India have shown prevalence to be 8.6% 57,58 and 4.2% respectively. 59 The prevalence estimates of 1.67% to 14.3% in Nepal vary significantly on either side of these prevalence figures, making it challenging to draw meaningful comparisons. As with COPD, asthma prevalence figures for Nepal varied widely depending on the population, rendering it difficult to interpret alongside World Health Surveys and findings from other LMICs where prevalence of diagnosed asthma have been estimated at 4.3% 60 and 2.9% respectively. 59 However, both asthma and COPD prevalence figures could be impacted by the misclassification of disease and by the lack of quality studies. To date, most publications reporting COPD diagnosis in Nepal are not based on spirometry, and where they are, they did not assess post-bronchodilator measurements. 61 Similarly, the term ‘COPD’ is not universally recognised in Nepal, and therefore there is further misclassification of patients as having asthma or other terms for chronic lung disease. This review highlights that the large proportion of the prevalence studies were of moderate to low-quality evidence and utilised diverse populations and approaches.
Two publications from the same large project met the criteria for high quality. 47,48 The use of biomass fuels as a household energy source markedly increased the risk of airflow obstruction compared to liquefied petroleum, 47 in line with the Global Burden of Disease study and other studies. 62 –65 Further, symptoms are prevalent in both urban and rural community dwellers, but particularly in rural, where there is greater use of biomass fuels leading to household air pollution. 48 These observations agree with previous findings. 66,67 However, some recent evidence contradicts the lung function observations, whereby post-bronchodilator airflow obstruction was not associated with the use of biomass fuels. 68
Hospital-based findings
In the hospital setting, the estimates of COPD and asthma also varied across studies. The reporting of hospital admissions attributed to COPD and/or asthma in Asian LMICs is scarce in the literature providing challenges in making effective comparisons. Of the available evidence, a study looking into hospital admission causes in China reported that chronic respiratory disease contributed to 22.6% of total admissions. 69 Dagenais et al. 70 further suggested that respiratory disease patients accounted for around 8.5% of global hospital admissions, but the number of first admissions attributed to respiratory disease was higher (4.9%) in high income countries compared to LMIC (1.9%). It was suggested that this was due to higher diagnosis rates and earlier treatment in high income countries. 70
Of note, two studies highlighted that most hospitalised COPD patients were female. 42,44 This conflicts evidence from other LMICs whereby hospital admissions for COPD appear to be more males, 71,72 and prevalence rates of COPD are higher in males. 58,73 This finding is of potential importance in addressing the burden of COPD in Nepal and needs to be investigated further. One possibility is the greater exposure of women to household air pollution.
It is important to note that drawing comparisons with other countries based on the low to moderate quality data are hindered by the disparities in reporting of admissions, including whether total hospital admissions, select age groups or select hospital departments and also the hospital location and access. All these factors hinder interpretation, especially when the studies tend to include large hospital centres in urban areas. This creates challenges in providing evidence relating to rural environments.
Comorbidity-related and burden findings
A high prevalence of concurrent comorbidities included other respiratory disorders, 35,40,51,52 cardiovascular disease, 31,35,39,46,51,52 diabetes, 31,35,39,51,52 anxiety and depression. 41 The estimates of prevalence varied markedly. Although these were small and discrete populations, the coexistent comorbidities concur with the literature in other countries and warrant further investigation and studies to delineate the additive burden, contribution to mortality, opportunity for earlier identification and prevention. 74,75
There was minimal literature on mortality associated with chronic respiratory disease. For COPD, in-hospital mortality rates ranged from 6–20%, 39,45 which is a wide variation in the estimate. Comparisons may be drawn with COPD in-hospital mortality rates in India, which are around 12%, 72 but further clarity is needed to distinguish a precise estimate in Nepal. Access to hospital and possible later presentation with symptoms, healthcare costs and the provision and access of core medications and treatment once at the hospital are factors. There was no population-level COPD or other chronic lung disease mortality data for Nepal.
Strengths and limitations
This review included self-reported and diagnosed lung disease, symptoms, lung function and comorbidity, allowing capture on the wider burden of respiratory disease. Outcomes needed to be contextualised to study setting, allowing for adequate reflection for future research and policy utilisation and a rounded view of respiratory disease burden in both the community and hospital while highlighting variation and a need for further research.
The main limitation was the considerable heterogeneity in the studies in terms of population sampling approach and data collection methods leading to a variety of outcome measures and a range of information. There were stark differences in study quality stemming from design and setting, which make it necessary to separate the study into three categories. The populations recruited to these studies should also be considered with several elements identified that infer heavy bias, potentially affecting the interpretation of results. For example, lack of segregated data based on the age of the population monitored meaning standardised results could not be produced, lack of spirometry especially including post-bronchodilator assessments to determine the overall prevalence, lack of reporting on medications, lifestyle factors, previous medical history (e.g. childhood respiratory complications), and socioeconomic status to help interpret the populations included. It is also important to note that no data on incidence of chronic respiratory diseases were available for reporting, and that reported symptom data were not consistent. As a result, the high variability required a narrative analysis approach with details of the population presented as opposed to a pooled estimate of prevalence and burden. Ultimately, these issues made it difficult to compare studies but have helped to provide an evidence base for future research needs assessing non-communicable lung disease burden in Nepal.
Implications for future health research and care
While unable to fully delineate the estimates of the true prevalence of respiratory disease in Nepal, the scale and burden of chronic lung disease are marked. Current research is taking a population approach to systematically identify the scale of COPD in urban Nepal. 76 Identifying the estimates and burden of disease drives the need for optimised healthcare strategies and subsequent implementation and dissemination. For example, smoking cessation to reduce tobacco exposure 77 –79 and pulmonary rehabilitation 77 have a strong evidence base in their effectiveness in managing lung diseases. Pulmonary rehabilitation takes a holistic approach to reduce breathlessness, improve function and treat multimorbidity. 80 Importantly, these are high value, low-cost interventions and will be of an increasing need for a country with an increasing life expectancy and a growing issue of NCD prevalence. Reducing exposures through smoking cessation and utilisation of alternative fuel sources alongside health promotion are key to the long-term management and prevention of chronic respiratory diseases in Nepal. However, any consideration and inclusion of public health interventions needs a full evaluation of their potential appropriateness and acceptability given the tremendous social, cultural, linguistic, economic and geographic diversity of Nepal. Important factors include the fact that a large proportion of people in Nepal live in very rural and often mountainous terrain, many are living in extreme poverty and illiteracy is high. 8,16,17,22
Conclusion
This systematic review demonstrated that there is limited published literature on the burden of lung disease and marked variation in outcome measures and populations studied. Nonetheless, chronic respiratory burden in Nepal is likely to be considerable. This is a priority area for future research. Based on the available information, there is a clear need for further studies and the need for healthcare professionals, providers and policymakers to develop effective strategies to tackle chronic respiratory diseases in Nepal. These findings are also likely to be relevant to other Himalayan LMICs.
Appendix 1
Search strategy from Scopus
(Nepal AND asthma) OR (Nepal AND bronchiectasis) OR (Nepal AND chronic bronchitis) OR (Nepal AND chronic obstructive pulmonary disease*) OR (Nepal AND emphysema) OR (Nepal AND idiopathic pulmonary fibrosis) OR (Nepal AND lung fibrosis) OR (Nepal AND interstitial lung disease*) OR (Nepal AND silicosis) OR (Nepal AND occupational lung disease*) OR (Nepal AND small airways disease*) OR (Nepal AND breathless*) OR (Nepal AND cough*) OR (Nepal AND wheez*) OR (Nepal AND dyspn*) OR (Nepal AND respiratory arrest*) OR (Nepal AND exacerbat*) OR (Nepal AND hypoxemia) OR (Nepal AND sputum) OR (Nepal AND phlegm) AND (LIMIT-TO (DOCTYPE,“ar”) OR LIMIT-TO (DOCTYPE,“cp”)) AND (LIMIT-TO (EXACTKEYWORD,“Human”)) AND (LIMIT-TO (SUBJAREA,“MEDI”) OR LIMIT-TO (SUBJAREA,“PHAR”) OR LIMIT-TO (SUBJAREA, “AGRI”) OR LIMIT-TO (SUBJAREA,“ENVI”) OR LIMIT-TO (SUBJAREA,“SOCI”) OR LIMIT-TO (SUBJAREA,“NURS”) OR LIMIT-TO (SUBJAREA,“HEAL”))
Footnotes
Author contributions: WE and ARJ are joint first authors. The original study was devised by CEB, IPH and RK. Design of the study was by CEB, IPH and WE. WE, ARJ and CEB conducted literature review and main analysis. WE and ARJ wrote the first manuscript draft. All authors contributed and critiqued the manuscript and approved the article before submission.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The systematic review was supported by the “Global Challenges Research Fund”, University of Nottingham 2018-2019. CEB & IPH report grants from the Global Challenges Research Fund during the conducting of this review. WE, ARJ, CE, RK, & OPK declare to have no conflicts of interest relating to the production of this manuscript.
ORCID iD: Alex Robert Jenkins  https://orcid.org/0000-0002-4384-2342
https://orcid.org/0000-0002-4384-2342
References
- 1. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018; 392: 1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018; 392: 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore E, Chatzidiakou L, Kuku MO, et al. Global associations between air pollutants and chronic obstructive pulmonary disease hospitalizations. a systematic review. Ann Am Thorac Soc 2016; 13: 1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Organization WH. Noncommunicable diseases and air pollution. Copenhagen: Island Press, 2019. [Google Scholar]
- 5. Shrestha SL. Time series modelling of respiratory hospital admissions and geometrically weighted distributed lag effects from ambient particulate air pollution within Kathmandu Valley, Nepal. Environ Model Assess 2007; 12: 239–251. [Google Scholar]
- 6. Saud B, Paudel G. The threat of ambient air pollution in Kathmandu, Nepal. Int J Environ Res Public Health 2018; 2018: 1504591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aryal K, Neupane S, Mehata S, et al. Non communicable diseases risk factors: STEPS Survey Nepal 2013 . Council KNHR, 2014. [Google Scholar]
- 8. Aryal KK, Mehata S, Neupane S, et al. The Burden and determinants of non communicable diseases risk factors in Nepal: findings from a nationwide STEPS survey. PloS One 2015; 10: e0134834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adhikari TB, Neupane D, Kallestrup P. Burden of COPD in Nepal. Int J Chron Obstruct Pulmon Dis 2018; 13: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Organization WH. Noncommunicable diseases country profiles 2018. Geneva: Organization WH, 2018. [Google Scholar]
- 11. Nepal ranks second in lung ailment deaths. The Himalayan Times. August 12, 2019. https://thehimalayantimes.com/kathmandu/nepal-ranks-second-in-lung-ailment-deaths (accessed 3 February 2021).
- 12. Annual Report of the Department of Health Services of the Fiscal Year 2073/74 (2016/17). Services DoH, 2018.
- 13. Pokharel B, Humagain S, Pant P, et al. Spectrum of diseases in a medical ward of a teaching hospital in a developing country. Nepal Med Coll J 2012; 8: 7–11. [Google Scholar]
- 14. Kalpana S, Sarala J. Quality of life of patients with chronic obstructive pulmonary disease in Chitwan, Nepal: a pilot study report. Int J Med Sci Public Health 2015; 4: 1235. [Google Scholar]
- 15. World Life Expectancy. Lung disease death rate by country - 2017. https://www.worldlifeexpectancy.com/cause-of-death/lung-disease/by-country/ (2017, accessed 4 February 2020).
- 16. Population Atlas of Nepal. http://cbs.gov.np/catalog/atlas/index.html (2014, accessed 30 August 2019).
- 17. Ministry of Health NNEaI. Nepal Demographic and Health Survey 2016. Kathmandu, Nepal: Ministry of Health N, 2017. [Google Scholar]
- 18. Kurmi O, Regmi PR, Pant PR. Implication of air pollution on health effects in Nepal: lessons from global research. Nepal J Epidemiol 2016; 6: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghimire D, Manna S, Shrestha E. Pulmonary function tests in transport workers within the ring road of Kathmandu Valley. Med Phoenix 2018; 3: 52–59. [Google Scholar]
- 20. Ranabhat CL, Kim C-B, Kim C-S, et al. Consequence of indoor air pollution in rural area of Nepal: a simplified measurement approach. Front Public Health 2015; 3: 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swe KT, Rahman MM, Rahman MS, et al. Cost and economic burden of illness over 15 years in Nepal: a comparative analysis. PloS One 2018; 13: e0194564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ministry of Health, Nepal. New ERA, Nepal; Nepal Health Sector Support Program (NHSSP); and ICF. 2017. Nepal Health Facility Survey 2015. Kathmandu, Nepal: Ministry of Health, Nepal. [Google Scholar]
- 23. World Health Organization GoN. Multisectoral Action Plan for the Prevention and Control of Non Communicable Diseases in Nepal (2014-2020). 2014. [Google Scholar]
- 24. Sharma SR, Page R, Matheson A, et al. Non-communicable disease prevention in Nepal: systemic challenges and future directions. Health Promot Int 2019; 26: 94–97. [DOI] [PubMed] [Google Scholar]
- 25. Ekezie W, Hall I, Bolton C. Risk factors, prevalence, and management of chronic respiratory diseases in Nepal: a systematic review, https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=138552 (accessed 15 August 2019). [DOI] [PMC free article] [PubMed]
- 26. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016; 6: e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. Universities of Exeter and Plymouth, 2006. [Google Scholar]
- 29. Pandey MR. Prevalence of chronic bronchitis in a rural community of the hill region of Nepal. Thorax 1984; 39: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhatta N, Dhakal SS, Rizal S, et al. Clinical spectrum of patients presenting with bronchiectasis in Nepal: evidence of linkage between tuberculosis, tobacco smoking and toxic exposure to biomass smoke. Kathmandu Univ Med J (KUMJ) 2008; 6: 195–203. [PubMed] [Google Scholar]
- 31. Dhungel S, Paudel B, Shah S. Study of prevalence of hypertension in chronic obstructive pulmonary disease patients admitted at Nepal Medical College and Teaching Hospital. Nepal Med Coll J 2005; 7: 90–92. [PubMed] [Google Scholar]
- 32. Joshi H, Pandeya R, Dhakal B, et al. Health impact of indoor air pollution. J Nepal Health Res Counc 2009; 7: 69–75. [Google Scholar]
- 33. Prasad B, Sahay AP, Singh AK, et al. Smoking women and their lung function tests. Kathmandu Univ Med J (KUMJ) 2003; 2: 142–144. [PubMed] [Google Scholar]
- 34. Shankar PR, Upadhyay DK, Subish P, et al. Drug utilisation among older inpatients in a teaching hospital in Western Nepal. Singapore Med J 2010; 51: 28–34. [PubMed] [Google Scholar]
- 35. Shrestha B, Dhungel S, Chokhani R. Echocardiography based cardiac evaluation in the patients suffering from chronic obstructive pulmonary disease. Nepal Med Coll J 2009; 11: 14–18. [PubMed] [Google Scholar]
- 36. Shrestha IL, Shrestha SL. Indoor air pollution from biomass fuels and respiratory health of the exposed population in Nepalese households. Int J Occup Environ Health 2005; 11: 150–160. [DOI] [PubMed] [Google Scholar]
- 37. Acharya S, Ghimire S, Jeffers EM, et al. Health care utilization and health care expenditure of Nepali older adults. Front Public Health 2019; 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pratali L, Marinoni A, Cogo A, et al. Indoor air pollution exposure effects on lung and cardiovascular health in the high Himalayas, Nepal: an observational study. Eur J Intern Med 2019; 61: 81–87. [DOI] [PubMed] [Google Scholar]
- 39. Sijapati M, Bhatta N, Khanal B, et al. Study of factors determining outcomes in the hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease (COPD). J Univers Coll Med Sci 2014; 2: 28–34. [Google Scholar]
- 40. Sijapati M, Thapa NB, Rijal R, et al. Bronchiectasis in patients with chronic obstructive pulmonary disease. J Pathol Nepal 2018; 8: 1346–1349. [Google Scholar]
- 41. Thapa N, Maharjan M, Shrestha TM, et al. Anxiety and depression among patients with chronic obstructive pulmonary disease and general population in rural Nepal. BMC Psychiatry 2017; 17: 397–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amundsen MS, Kirkeby TM, Giri S, et al. Non-communicable diseases at a regional hospital in Nepal: findings of a high burden of alcohol-related disease. Alcohol (Fayetteville, NY) 2016; 57: 9–14. [DOI] [PubMed] [Google Scholar]
- 43. Bhandari GP, Angdembe MR, Dhimal M, et al. State of non-communicable diseases in Nepal. BMC Pub Health 2014; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhandari R, Sharma R. Epidemiology of chronic obstructive pulmonary disease: a descriptive study in the mid-western region of Nepal. Int J Chron Obstruct Pulmon Dis 2012; 7: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghosh V, Lamichhane S, Thakuri SB, et al. Study on epidemiology of chronic obstructive pulmonary disease (COPD) at western regional hospital, Pokhara. J-GMC-N 2016; 9: 65–69. [Google Scholar]
- 46. Hirachan A, Maskey A, Shah RK, et al. Echocardiographic right heart study in patients with chronic obstructive pulmonary disease. N H J 2017; 14: 9–12. [Google Scholar]
- 47. Kurmi OP, Devereux GS, Smith WCS, et al. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J 2013; 41: 25–30. [DOI] [PubMed] [Google Scholar]
- 48. Kurmi OP, Semple S, Devereux GS, et al. The effect of exposure to biomass smoke on respiratory symptoms in adult rural and urban Nepalese populations. Environ Health 2014; 13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prajapati BK, Pradhan S. Prevalence of respiratory diseases according to spirometry findings among patients attending the Spirometry Department of Dhulikhel Hospital. Kathmandu Univ Med J (KUMJ) 2016; 14: 140–143. [PubMed] [Google Scholar]
- 50. Dhimal M, Karki KB, Sharma SK, et al. Prevalence of selected chronic non-communicable diseases in Nepal. J Nepal Health Res Counc 2019; 17: 394–401. [DOI] [PubMed] [Google Scholar]
- 51. Ghimire R, Ghimire A, Bimali A, et al. Clinical profile and survival rate of interstitial lung disease in a Tertiary Care Center of Eastern Nepal. Kathmandu Univ Med J (KUMJ) 2019; 17: 212–216. [PubMed] [Google Scholar]
- 52. Koirala P, Bhatta N, Ghimire RH, et al. Overlap of sleep disorders and chronic respiratory diseases: an emerging health dilemma. J. Nepal Med 2019; 2: 243–249. [Google Scholar]
- 53. Giri S, Rogne T, Uleberg O, et al. Presenting complaints and mortality in a cohort of 22 000 adult emergency patients at a local hospital in Nepal. J Glob Health 2019; 9: 020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shrestha AP, Shrestha R, Shrestha SK, et al. Prevalence of dyspnea among patients attending the emergency department of a tertiary care hospital: a descriptive cross-sectional study. JNMA J Nepal Med Assoc 2019; 57: 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28: 523–532. [DOI] [PubMed] [Google Scholar]
- 56. Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med 2018; 6: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018; 391: 1706–1717. [DOI] [PubMed] [Google Scholar]
- 59. Salvi S, Kumar GA, Dhaliwal RS, et al. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health 2018; 6: e1363–e1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC public health 2012; 12: 204–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Agusti A, Beasley R, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 Report) . 2020. [Google Scholar]
- 62. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012; 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orozco-Levi M, Garcia-Aymerich J, Villar J, et al. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J 2006; 27: 542. [DOI] [PubMed] [Google Scholar]
- 64. Köksal H, Saygı A, Sarıman N, et al. Evaluation of clinical and functional parameters in female subjects with biomass smoke exposure. Respir Care 2013; 58: 424. [DOI] [PubMed] [Google Scholar]
- 65. Ozbay B, Uzun K, Arslan H, et al. Functional and radiological impairment in women highly exposed to indoor biomass fuels. Respirology 2001; 6: 255–258. [DOI] [PubMed] [Google Scholar]
- 66. Stanković A, Nikolić M, Arandjelović M. Effects of indoor air pollution on respiratory symptoms of non-smoking women in Niš, Serbia. Multidiscip Respir Med 2011; 6: 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kiraz K, Kart L, Demir R, et al. Chronic pulmonary disease in rural women exposed to biomass fumes. Clin Invest Med 2003; 26: 243–248. [PubMed] [Google Scholar]
- 68. Amaral AFS, Patel J, Kato BS, et al. Airflow obstruction and use of solid fuels for cooking or heating: BOLD results. Am J Respir Crit Care Med 2018; 197: 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Zhang L, Li H, et al. Determinants of inappropriate admissions in county hospitals in rural china: a cross-sectional study. Int J Environ Res Public Health 2018; 15: 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet (London, England) 2020; 395: 785–794. [DOI] [PubMed] [Google Scholar]
- 71. Koul P, Dar H, Jan R, et al. Two-year mortality in survivors of acute exacerbations of chronic obstructive pulmonary disease: a North Indian study. Lung India 2017; 34: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koul PA, Nowshehr AA, Khan UH, et al. Cost of severe chronic obstructive pulmonary disease exacerbations in a high burden region in North India. Ann Glob Health 2019; 85: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis 2018; 13: 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev 2013; 22: 454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Putcha N, Drummond MB, Wise RA, et al. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med 2015; 36: 575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Siddharthan T, Pollard SL, Quaderi SA, et al. Effectiveness-implementation of COPD case finding and self-management action plans in low- and middle-income countries: global excellence in COPD outcomes (GECo) study protocol. Trials 2018; 19: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kane S, Mahal A. Cost-effective treatment, prevention and management of chronic respiratory conditions: a continuing challenge. Respirology 2018; 23: 799–800. [DOI] [PubMed] [Google Scholar]
- 78. Ward KD. Tobacco intervention research in low- and middle-income countries: lessons learned and future directions. J Smok Cessat 2016; 11: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Willemse BWM, Postma DS, Timens W, et al. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J 2004; 23: 464–476. [DOI] [PubMed] [Google Scholar]
- 80. Holland AE, Harrison SL, Brooks D. Multimorbidity, frailty and chronic obstructive pulmonary disease: are the challenges for pulmonary rehabilitation in the name? Chron Respir Dis 2016; 13: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]