Abstract
Over the past decades, a growing body of evidence has demonstrated the impact of prenatal environmental adversity on the development of the human embryonic and fetal brain. Prenatal environmental adversity includes infectious agents, medication, and substances of use as well as inherently maternal factors, such as diabetes and stress. These adversities may cause long-lasting effects if occurring in sensitive time windows and, therefore, have high clinical relevance. However, our knowledge of their influence on specific cellular and molecular processes of in utero brain development remains scarce. This gap of knowledge can be partially explained by the restricted experimental access to the human embryonic and fetal brain and limited recapitulation of human-specific neurodevelopmental events in model organisms. In the past years, novel 3D human stem cell-based in vitro modeling systems, so-called brain organoids, have proven their applicability for modeling early events of human brain development in health and disease. Since their emergence, brain organoids have been successfully employed to study molecular mechanisms of Zika and Herpes simplex virus-associated microcephaly, as well as more subtle events happening upon maternal alcohol and nicotine consumption. These studies converge on pathological mechanisms targeting neural stem cells. In this review, we discuss how brain organoids have recently revealed commonalities and differences in the effects of environmental adversities on human neurogenesis. We highlight both the breakthroughs in understanding the molecular consequences of environmental exposures achieved using organoids as well as the on-going challenges in the field related to variability in protocols and a lack of benchmarking, which make cross-study comparisons difficult.
Keywords: brain organoid, environmental programming, neurogenesis, corticogenesis, neural stem/progenitor cells
Introduction
Environmental adversities acting on the embryo or fetus may shape brain development and predispose it to neurological and psychiatric diseases later in life. Such developmental alterations upon environmental exposures are called environmental programming. The environmental exposures include maternal factors as well as external ones, such as viral infections (Faa et al., 2016; Meyer, 2019). An example of how maternal factors may affect fetal neurodevelopment is maternal malnutrition, which correlates with multiple neurodevelopmental deficits in the offspring, including changes in memory formation and motor function (Faa et al., 2016). Neurodevelopmental deficits often involve higher cognitive functions, suggesting alterations in neocortical development. The mechanisms underlying environmental programming have been difficult to investigate for several reasons. On the one hand, the correlations were found in epidemiological studies and require further perturbation experiments for causal inference. On the other hand, human intrauterine development is mostly inaccessible for perturbation studies or research into the cellular and molecular effects of environmental adversities.
The in utero development of the human nervous system includes several phases. It starts with neural induction and the formation of the neural tube, followed by the expansion of neural stem cell (NSC) pools, the subsequent generation of neurons (neurogenesis) and glial cells (gliogenesis), and, finally, the establishment and maturation of neuronal circuits. In this review, we focus on two phases: NSCs proliferation and neurogenesis in the embryonic and fetal neocortex (roughly, postconceptional weeks (PCW) 6–27) (Silbereis et al., 2016).
Mammalian model organisms have proven highly valuable to reveal mechanisms of NSCs proliferation and neurogenesis as both the neocortex itself and the intrauterine development are characteristics of mammals (Cárdenas and Borrell, 2020). Especially rodent models have catalyzed our understanding of the spatial and temporal dynamics of neocortical development. Additionally, rodents have been most extensively used to study various aspects of the environmental effects on neurogenesis. For instance, murine studies have allowed researchers to characterize the effects of maternal nicotine exposure on fetal neurogenesis (Aoyama et al., 2016). However, in contrast to the human brain, the murine brain is lissencephalic and has a restricted repertoire of NSCs (Uzquiano et al., 2018). The number of divisions that murine NSCs undergo before differentiation is also lower than in human NSCs (Lui et al., 2011). Additionally, mice as a species have different cellular dynamics, partially due to the relative instability of murine proteins compared to human ones (Rayon et al., 2020). This translates into faster cellular differentiation (Rayon et al., 2020): neurogenesis in mice is completed in a relatively short time frame (embryonic day (E) 9–14) (La Manno et al., 2020) whereas in humans it takes almost 4.5 months of pregnancy (Silbereis et al., 2016). Altogether, the differences between mouse and human brain development call for alternative model systems that recapitulate human-specific traits of neocortical development more closely. Using gyrencephalic animals, including ferret and non-human primates, is a solution to the differences between human and rodent brain development. These species have a larger variety of NSCs types than the mouse, specifically, outer radial glia (see detailed description below) (Lui et al., 2011). Additionally, primates partially recapitulate the long neurogenic period characteristic for humans (Kornack and Rakic, 1998). However, limitations for the use of these animals exist from both ethical and experimental perspectives. For instance, the experiments are difficult to scale, thus limiting experimental designs to those showing large effect size.
In some rare cases, human fetal tissue is accessible for experimentation. It provides valuable information on some species-specific mechanisms driving neurogenesis as well as particular architectural, cellular, and molecular aspects of human neurodevelopment (Pollen et al., 2015; Fiddes et al., 2018; Mayer et al., 2019; Xing et al., 2020). Specifically, human neocortical organotypic slice cultures were used to study mechanisms of Zika virus-induced microcephaly (Onorati et al., 2016). Studies with human fetal tissues pose similar ethical and experimental difficulties as studies on non-human primates. One of the main experimental challenges is that fetal neocortical organotypic slice cultures can only be maintained for several days or up to three weeks using an automated platform precluding long-term interventions (Linsley et al., 2019).
Various ex vivo systems helped to partially overcome existing limitations. For instance, primary human NSCs were used to characterize mitotic defects upon Zika virus infection (Onorati et al., 2016). However, larger experimental flexibility was achieved with the development of protocols for neural differentiation from pluripotent stem cells (PSCs), both induced PSCs and embryonic stem cells (Shi et al., 2012). Over the past years, the growing efficiency of the existing differentiation protocols allowed for large-scale screens for various neurotoxins (Pei et al., 2016; Schmidt et al., 2017). However, two-dimensional (2D) cultures lack cytoarchitectural properties of the developing brain. This limitation has recently been overcome with the development of the PSCs-derived 3D systems, so-called brain organoids (Kadoshima et al., 2013; Lancaster et al., 2013). Brain organoids combine the strengths of both in vivo and in vitro approaches: they recapitulate the cellular composition and the cytoarchitecture of the human neocortex while preserving the scalability and experimental tractability of in vitro approaches. These features have made brain organoids an attractive model system for studying the effects of environmental adversities on human neurodevelopment.
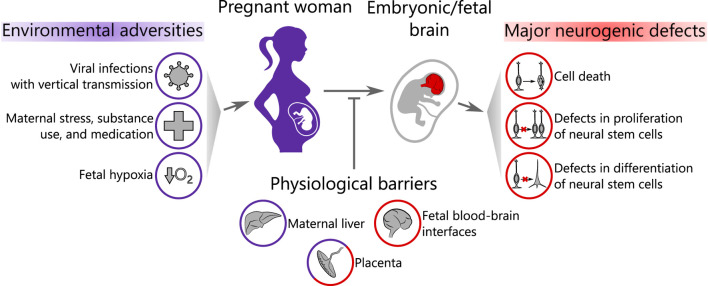
In this review, we provide an overview of the recent advances in using brain organoids to study environmental programming on human neocortical neurogenesis. We first describe how brain organoids mimic features of human neocortical development during stages of neurogenesis. Next, we will introduce the biological barriers that various compounds and infectious agents have to cross before entering the fetal brain in vivo. We will then focus on the effects of several major groups of environmental adversities on neurogenesis, which have recently been investigated using brain organoids as a model system: viral infections with vertical transmission from mother to fetus, maternal stress, substance use and medication, and, finally, fetal hypoxia (Figure 1). We conclude by discussing experimental limitations in this relatively young field and potential routes for its further development.
FIGURE 1.
Scope of the review. Environmental adversities affect the pregnant woman and may pass through a series of biological barriers to the embryonic/fetal brain causing neurogenic defects. Purple color represents the maternal compartment and red color corresponds to the embryo/fetus.
Major Populations of Cortical Neural Stem Cells Can be Found in Brain Organoids
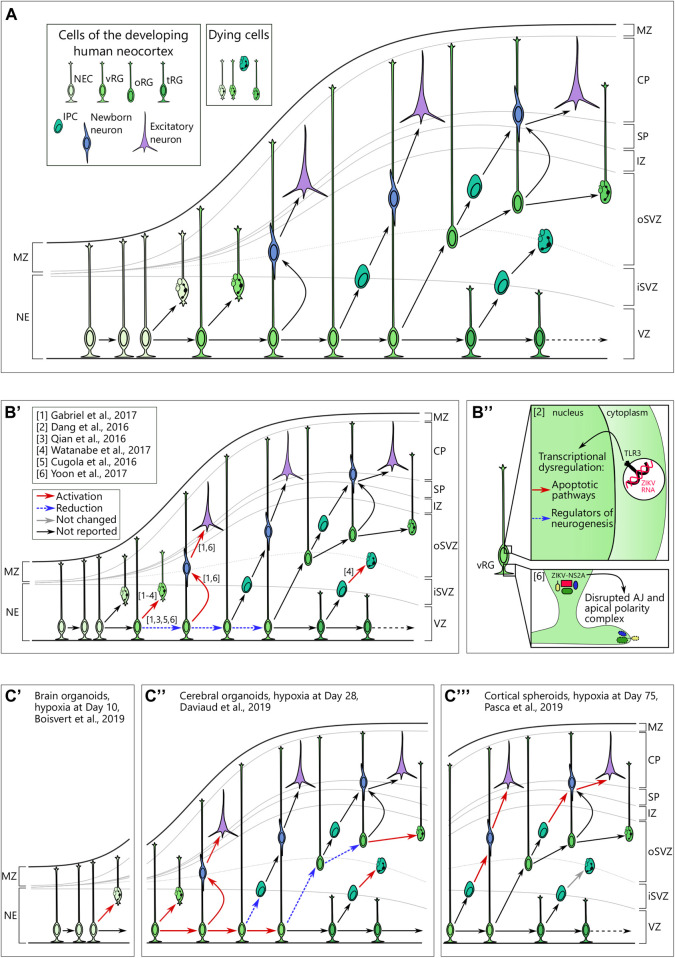
Throughout mammalian brain development, specific populations of stem cells sequentially produce a vast diversity of neurons in a predefined order (Telley et al., 2019; Sagner et al., 2020). Neocortical development in humans largely follows the same logic (Figure 2A). The first population of NSCs in the telencephalon emerges at neurulation (around PCW4) and is named neuroepithelial cells (NEC). These cells divide predominantly in a symmetrical manner to expand the pool of proliferating cells (Subramanian et al., 2017). Upon replacement of tight junctions with adherens junctions at the apical polarity complex, NECs transform to neural precursor/progenitor cells (NPC) of the ventricular zone, the so-called ventricular or apical radial glia (vRG) cells (Uzquiano et al., 2018). The cells with vRG properties emerge around PCW6-8 (Subramanian et al., 2017). They are capable of both symmetric and asymmetric divisions that either promote self-renewal of this population or generate transit-amplifying/intermediate progenitor cells (IPC) and newborn neurons (Noctor et al., 2004), respectively. vRG processes span from the ventricular to the pial surface and provide a scaffold for migrating neurons (Noctor et al., 2001). Starting from PCW10, vRGs give rise to a unique population of NPCs that is almost exclusively present in the neocortex of gyrencephalic mammals, the so-called outer or basal radial glia (oRG) (Smart et al., 2002; Fietz et al., 2010; Hansen et al., 2010; Reillo et al., 2011; Wang et al., 2011). oRGs are localized in the inner and outer subventricular zone (iSVZ, oSVZ) and predominantly divide asymmetrically to generate daughter oRG and an IPC or a neuron (Hansen et al., 2010; Betizeau et al., 2013). Later, around PCW15, vRG cells lose their connection to the pial surface and transform into truncated radial glial (tRG) cells, while still giving rise to excitatory neurons (Nowakowski et al., 2016b).
FIGURE 2.
(A) Cellular view of the normal human neocortical development. Human neocortical development starts from neuroepithelial cells (NEC), which further transform to ventricular radial glia (vRG). Both NEC and vRG have nuclei next to the ventricle (neuroeplithelium (NE) and ventricular zone (VZ), respectively) and a basal process spanning through the cortical wall and marginal zone (MZ) to reach the pial surface. vRG can either divide symmetrically to generate two vRGs or differentiate to produce excitatory neurons or basal neural progenitor cells. Basal progenitor cells reside in the inner (iSVZ) and outer (oSVZ) subventricular zones and include intermediate progenitor cells (IPC) and outer radial glia (oRG). Both types of basal neural progenitor cells can self-amplify or differentiate into excitatory neurons. Later in development, vRG lose connection to the pial surface and transform into truncated radial glia (tRG) while still generating basal progenitors and excitatory neurons. Newborn neurons of different origins migrate along the radial fibers through the intermediate zone (IZ) and subplate (SP) and take their place in the cortical plate (CP). A low level of cell death in neural cells is normal over neurogenesis. (B) Brain organoids infected with Zika virus reveal cellular mechanisms of microcephaly. B’. Multiple studies suggest preferential infection of neural progenitor cells over neurons. Infection in vRG leads to the apoptotic cell death, attenuation of proliferation as well as shift to direct neurogenesis. (B’’) upper panel. Zika virus (ZIKV) activates Toll-like receptor 3 in vRGs that leads to transcriptional deregulation with activation of apoptotic pathways and inhibition of regulators of neurogenesis. Adapted from Dang et al. (2016). (B’’) lower panel. NS2A, a protein of ZIKV envelop, binds the components of adherens junctions (AJ) in the cytoplasm and prevents them from forming functional AJ and apical polarity complex. Reprinted from Yoon et al. 2017 with permission from Elsevier. Copyright (2017). (C) Hypoxic exposure in brain organoids at different time points results in distinct defects on the cellular level. (C’) Hypoxic exposure (1% O2, 72 h) at day 10 of organoid differentiation results in the increased cell death, presumably, of NECs. (C’’) When hypoxia (3% O2, 24 h) is applied to brain organoids at day 28, it results in the immediate cell death across the cortical wall followed by proliferation in vRG. Subsequently, vRG tend to differentiate into neurons at the expense of generation of both IPCs and oRG, which results in the decreased number of these cells 14 days after hypoxic exposure. (C’’’) Cortical spheroids that are exposed to hypoxia (1% O2, 48 h) at day 75 of differentiation show decreased numbers of IPCs resulting from premature differentiation but not from cell death in these cells.
The first neurons of the developing neocortex, the so-called Cajal-Retzius cells, originate from the cortical hem as well as from other sources at the early stages of neurogenesis (Meyer, 2010). This heterogeneous neuronal population resides in the marginal zone (MZ) and controls migration and laminar positioning of the subsequent excitatory neurons through secretion of the extracellular matrix glycoprotein Reelin (Meyer, 2010). The excitatory neurons of layers II-VI are generated from RG cells as well as from IPCs in an inside-out manner in which deeper layers are generated before upper layers. Thus, layer VI neurons are being born and migrate to their final location first followed by layer V neurons and, finally, by layer II-IV neurons. A more detailed review on in utero human cortical neurogenesis can be found elsewhere (Lui et al., 2011; Taverna et al., 2014; Silbereis et al., 2016; Uzquiano et al., 2018).
Human PSC-derived brain organoids recapitulate both temporal and spatial components of the neocortical development. Cerebral organoids at days 10–15 after seeding show tight junctions at the apical side of the SOX2-positive neural rosettes reminiscent of NECs in the developing human brain (Benito-Kwiecinski et al., 2021). At week 5 of differentiation, cortical organoids possess mitotic NECs that are later replaced by vRGs (Subramanian et al., 2017). Both vRGs and oRGs and IPCs can be readily identified in the majority of cerebral and neocortical organoids protocols (Quadrato et al., 2017; Kanton et al., 2019; Pollen et al., 2019; Velasco et al., 2019). Additionally, NSCs in brain organoids mimic the cell cycle characteristics of primate NSCs rather than murine NSCs (Kornack and Rakic, 1998; Mora-Bermúdez et al., 2016). Furthermore, the differentiation trajectory from stem cell-like toward neuronal identity correlates between many organoid protocols and human fetal neocortex (Camp et al., 2015; Pollen et al., 2019; Tanaka et al., 2020) as well as temporal dynamics of this differentiation (Tanaka et al., 2020). This results in the generation of both lower (V-VI) and upper (II-IV) layer excitatory neurons in a sequential manner. The separation of the markers of these neuronal layers reflects maturation of the organoids and it has become partially feasible with the improvement of organoid protocols (Qian et al., 2020).
In this review, we summarize studies employing different types of brain organoids as a model system. The so-called “cerebral organoids” are generated according to the protocol by Lancaster et al. (2013) in its original or a modified version. At the first stages of this protocol, PSCs are seeded at densities that allow the formation of embryoid bodies, which are further subjected to neural induction at day 6 after seeding. From day 11 in culture, a change in media composition ensures neuronal differentiation and maturation (Lancaster et al., 2013). As a result, cerebral organoids develop a large variety of regional identities of the brain, including neocortical, choroid plexus and hindbrain (Lancaster et al., 2013; Lancaster and Knoblich, 2014). This protocol is therefore referred to as “unguided” (Qian et al., 2019). In order to specifically induce the formation of a particular brain region, organoids may be subjected to patterning factors, the so-called morphogens. Such organoid differentiation protocols are referred to as “guided” protocols (Qian et al., 2019). Morphogens used to guide dorsal forebrain identity include TGF-β, Wnt and BMP pathway inhibitors (Kadoshima et al., 2013; Paşca et al., 2015; Velasco et al., 2019) while BMP and Wnt pathway activators are required for induction of choroid plexus identity (Pellegrini et al., 2020b). A more detailed review of the different organoid differentiation protocols can be found elsewhere (Qian et al., 2019; Khakipoor et al., 2020).
Maternal and Fetal Barriers Protect Fetal Brain From the Effects of Environmental Adversities
Although human brain organoids closely recapitulate human neocortical development, their use for modeling the effects of environmental adversities on fetal neurodevelopment requires validation. Specifically, the developing fetal brain is protected from the environment by multiple barriers that are absent in an in vitro setting. These barriers include maternal protective mechanisms, such as inactivation of xenobiotics in the liver, as well as placental barrier and fetal blood-brain interfaces (Dorman et al., 2001; Ek et al., 2012; Tetro et al., 2018).
The placenta plays a dual role in fetal neurodevelopment. On the one hand, it transports maternal hormones, nutrients and oxygen to the fetus (Tetro et al., 2018). On the other hand, it protects the fetus from xenobiotics and other potentially harmful compounds derived from maternal blood through the selective influx of various compounds from maternal to fetal circulation, enzymatic inactivation of xenobiotics, and their efflux from fetal to maternal blood (Tetro et al., 2018). Trophoblast and capillary endothelial cells take over the main barrier function (Tetro et al., 2018).
Fetal blood-brain interfaces include the blood-brain barrier (BBB), blood-cerebrospinal fluid (CSF) and meningeal barrier as well as a fetus-specific brain-CSF barrier (Ek et al., 2012; Strazielle and Ghersi-Egea, 2013; Goasdoué et al., 2017). The primary barrier function of the fetal BBB is performed by the endothelial cells and is established upon vascularization of the neocortex (Ek et al., 2012). Endothelial cells express tight junction proteins starting from PCW8 (Møllgård and Saunders, 1975) and protect the fetal brain from protein entry starting from PCW10 (Virgintino et al., 2000). It is not clear when fetal endothelial cells gain similar transport properties as the adult ones (Ek et al., 2012). Fully functional endothelial cells are permissive to gases, lipophilic molecules, and a subset of small molecules (<400 Da) (Ross et al., 2020). Additionally, they are capable of carrier-mediated, receptor-mediated, and active transport (Ross et al., 2020).
The fetal blood-CSF barrier is formed by epithelial choroid plexus (ChP) cells. ChP cells are bound together with tight junctions located apically as early as PCW8 (Møllgård and Saunders, 1986). Interestingly, the blood-CSF barrier both in fetal development and adulthood is permissive to plasma protein entry to the CSF (Strazielle and Ghersi-Egea, 2013). The barrier and transport functions of the ChP complement its synthetic and secretory function. Embryonic CSF produced by ChP cells can maintain proliferative divisions in NPCs in the ex vivo setting through a combination of instructive cues, including insulin-like growth factor 2 (IGF2) in rats (Lehtinen et al., 2011). Interestingly, both barrier function of the ChP and its secretome can be recapitulated in vitro with ChP organoids (Pellegrini et al., 2020b).
Like ChP and endothelial cells, cells of the arachnoid that form the meningeal barrier also possess tight junctions (Ek et al., 2012). Additionally, the fetal brain contains a specific barrier that is not found in adults, the so-called brain-CSF barrier (Møllgård et al., 1987; Whish et al., 2015). It is formed by the “strap” junctions between the vRGs before their differentiation to ependymal cells (Møllgård et al., 1987; Whish et al., 2015). This brain-CSF barrier contains components of adherens and tight junctions and does not allow molecules as small as 268 Da to enter brain parenchyma at E17 in mice (Whish et al., 2015). Together, these studies suggest that the fetal brain is protected from entry of large and/or water-soluble molecules unless specific transport mechanisms exist.
Despite advances in understanding barrier properties at the tissue level, less is known about molecular transport and xenobiotic efflux machinery in the developing brain. However, recent studies indicate that the major efflux pump of the BBB, P-glycoprotein (P-gp), is expressed as early as PCW8-10 in fetal capillaries and increases with gestational age and postnatally (Schumacher and Mollgård, 1997; Daood et al., 2008; Lam et al., 2015; De Trizio et al., 2020). P-gp expression in the fetal BBB is altered by glucocorticoids (Petropoulos et al., 2010) and selective serotonin reuptake inhibitors (Bhuiyan et al., 2012). Thus, environmental adversities may not only affect the developing brain by reaching it but also by changing the barrier functions that protect it.
Viral Infections With Vertical Transmission From Mother to Fetus
There is a limited number of infectious diseases that can be transmitted from mother to embryo or fetus through either transplacental or intrauterine routes including the well-known ToRCH pathogens (toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, and others). It has been difficult to study the effects of these pathogens on neurodevelopment in animal models due to the high species specificity of the infectious agents. The applicability of brain organoids to studying these diseases was first shown during the Zika virus outbreak that led to microcephaly cases predominantly in Brazil starting in 2015. Since then, brain organoids have been used to model various viral infections and even to test and develop therapeutic strategies (Watanabe et al., 2017; Xu et al., 2019).
Zika Virus Causes Massive Cellular Death in Neural Precursor/Progenitor Cells as Well as Structural Disorganization of Proliferative Zones
The recent breakout of Zika virus showed multiple abnormalities in the developing fetuses of infected mothers. Indeed, the first-trimester human placenta is not only permissive to the viral infection but also supports its replication (Pereira, 2018; Pique-Regi et al., 2020). Among other defects, Zika virus induces severe microcephaly in fetuses through preferential infection of NPCs as seen in primary tissue (Onorati et al., 2016; Retallack et al., 2016). The potential cause of the microcephalic phenotype was replicated in induced PSC-derived neurospheres where Zika virus showed a productive infection of NPCs and caused severe size defects (Cugola et al., 2016). Following this initial finding, brain organoids infected by Zika virus showed impaired growth in multiple studies (Dang et al., 2016; Garcez et al., 2016; Watanabe et al., 2017). The restricted growth was accompanied by increased cell death, and disrupted proliferative zones and cortical layering (Qian et al., 2016; Wells et al., 2016; Watanabe et al., 2017) (Figure 2B’). Although microcephaly can be readily detected in a murine model of Zika virus infection (Cugola et al., 2016), brain organoids proved to be a versatile experimental platform. Particularly, several independent studies have shown tropism of the Zika virus for oRGs (Qian et al., 2016; Watanabe et al., 2017). Moreover, using brain organoids, several studies revealed the molecular mechanisms of Zika virus toxicity in NPCs. Infected NPCs showed a perturbed centrosomal structure, which was accompanied by abnormal division planes and premature differentiation (Gabriel et al., 2017). Another study indicates the specific activation of the immune receptor Toll-like receptor 3 (TLR3) upon Zika virus infection as the upstream mechanism of premature differentiation (Dang et al., 2016) (Figure 2B’’). Activation of the anti-viral immune response in NPCs was corroborated in another study (Watanabe et al., 2017) suggesting the robustness of the experimental results between the different studies. Moreover, Watanabe and colleagues tested potential treatment strategies (Watanabe et al., 2017). One antibiotic, duramycin, targeted the viral lipid membrane to prevent its interaction with the suggested cell entry receptors for Zika virus AXL, TYRO3, MER, and TIM1 and partially rescued the effect of Zika infection on organoids (Watanabe et al., 2017). However, another study showed that knockout of AXL alone, the presumptive principal cell entry receptor for Zika virus (Nowakowski et al., 2016a), did not attenuate pathological phenotype in cerebral organoids upon Zika virus infection (Wells et al., 2016).
Among other potential mechanisms that were validated both in murine and brain organoid models, there was disruption of adherens junctions in the vRGs leading to premature differentiation and, potentially, to aberrant newborn neuron migration along radial fibers (Yoon et al., 2017) (Figure 2B’’’). Brain organoid studies also hinted at the mechanism of infection, showing tropism of viral particles toward aquaporin-1 (AQP1)-positive cells which may have represented ChP cells (Watanabe et al., 2017). A recent meta-study on modeling Zika virus infection in brain organoids has shown a high correlation between the results from different research groups and employing different organoid differentiation protocols (Sutarjono, 2019). The consensus is that Zika virus reduces proliferation in NPCs, induces selective cell death in NPCs, and, finally, decreases the size of the organoid (Sutarjono, 2019). Later, 3D image analysis of the morphology of the organoids infected by Zika revealed a decreased number of VZ-like areas as well as the number of SOX2-positive RGs and TBR1-positive layer VI neurons (Albanese et al., 2020). However, the same analysis found a decrease in size of ventricle-like cavities, which is inconsistent with clinical observations of ventriculomegaly characteristic to Zika virus-induced microcephaly (De Fatima Vasco Aragao et al., 2016; Albanese et al., 2020). Ventriculomegaly in humans is associated with the massive loss of neural tissue (De Fatima Vasco Aragao et al., 2016), which is recapitulated in brain organoids. Altogether, these discrepancies highlight the necessity to critically assess of features of human brain development that can be modeled with brain organoids.
In summary, brain organoids have helped to reveal the particular vulnerability of the NPCs to the Zika virus infection, the cell tropism and molecular alterations that may explain microcephaly in patients. Finally, during the emergence of Zika virus-related studies, it was shown that brain organoids can be used as a platform for drug screening (Watanabe et al., 2017; Xu et al., 2019).
Cytomegaloviral Infection of Neural Precursor/Progenitor Cells Leads to Severe Microcephaly in the Developing Fetus
Cytomegalovirus (CMV) is a threat to the developing human embryo or fetus upon transmission from the mother. Primary CMV infection during pregnancy results in the 40% chance of vertical transmission (Enders et al., 2011). A recent study has shown expression of CMV cell entry receptors in placental cells (Pique-Regi et al., 2020). The syncytiotrophoblast cells, which form the fetal part of placental barrier, are permissive to viral infection and replication in the first and second trimester (Fisher et al., 2000). This may lead to viral infection of multiple cell types within the embryo, including NSCs (Baggiani et al., 2020). Infection of murine embryos with murine CMV showed that ventricular and subventricular zones are the most affected (Tsutsui et al., 2005). In vitro, CMV infection of primary human NSCs impaired their proliferation with expression of viral genes persisting in glial but not neuronal differentiation (Cheeran et al., 2005). Another study indicates impaired proliferation and neuronal differentiation as well as increased cell death in primary human NPCs upon CMV infection (Odeberg et al., 2006). Recent experiments employing human brain organoids showed impaired organoid growth after CMV infection mimicking clinical observations of microcephaly (Sun et al., 2020). Sun and colleagues further confirmed previous suggestions on the CMV cell entry route through PDGFRa and EGFR (Sun et al., 2020). Additionally, the authors found a partial colocalization of TBR2-positive IPCs with the CMV protein IE1 (immediate-early 1) (Sun et al., 2020). Furthermore, recently Brown and coworkers showed massive changes in the morphology of the organoids upon CMV infection, including the presence of regions of necrosis, cysts as well as an overall decrease in cellularity (Brown et al., 2019). Additionally, the authors noticed the disorganization of both Nestin and Tuj1-positive areas suggesting that the primary effect on NPCs also leads to disrupted neurogenesis (Brown et al., 2019). Employing 3D engineered neural tissue, various transcriptional programs were found to be differentially expressed as infection progressed (Cosset et al., 2015). Three days after infection, genes related to lipid metabolism were upregulated, followed by an upregulation of developmental genes and genes involved in inflammatory response at day 5 and 7 after infection, respectively. The authors found hardly any PAX6-positive cells to be infected (Cosset et al., 2015). In their hands, the infected population consisted of the cells adjacent to the neural rosettes and positive for doublecortin (Cosset et al., 2015), a marker of newborn neurons (Ayanlaja et al., 2017). Taken together, a plethora of studies in animal models, primary human NSCs and organoids converge on CMV primarily infecting NSCs with subsequent deficits in neuronal and glial differentiation thus mimicking clinical observations.
In Utero Herpes Simplex Virus Infection Leads to Massive Neurodevelopmental Abnormalities
The herpes simplex virus (HSV) family consists of two members, HSV-1 and HSV-2. According to the WHO data for 2016, HSV-1 is carried by ∼64% of the world population under 50 years old whereas the prevalence of the HSV-2 infection is ∼13% among people between 15 and 49 years old (James et al., 2020). The majority of cases of HSV vertical transmission from mother to child happens intrapartum leading to so-called neonatal herpes, which can cause severe complications and fatality of ∼60% if left untreated (Looker et al., 2017). Intrauterine infection is rare and controversies remain about the route of transmission from mother to fetus (Avgil and Ornoy, 2006; Avila et al., 2020). Intrauterine infection causes severe multiorgan abnormalities that are often lethal (Avgil and Ornoy, 2006). Abnormalities may include skin scarring, microcephaly, hydranencephaly, and ophthalmic manifestations (Hutto et al., 1987). In adults, neurons of both the central and peripheral nervous system can be infected by the HSV family with possible viral quiescence and reactivation later in life (Berger and Houff, 2008). The mechanisms of latency and reactivation remain poorly understood due to the human-specific nature of the infection leading to controversial results coming from animal experiments (D’Aiuto et al., 2019). Recently, an organoid differentiation strategy that is distinct from the original PSC-derived organoid protocols was employed to study the mechanisms of HSV-1 infection, quiescence, and reactivation in neuronal cells (D’Aiuto et al., 2019). Specifically, induced PSCs were differentiated into NPCs in adherent culture followed by the differentiation of the NPCs in a 3D environment (D’Aiuto et al., 2019). While modeling quiescent infection in a 3D environment is important for better understanding of the mechanisms of the host-virus interaction, the results obtained for the lytic infection in brain organoids may also have implications from the neurodevelopmental perspective. Specifically, the authors showed the degeneration of neurites as well as cell-cell fusion followed by generation of neuronal syncytia (D’Aiuto et al., 2019). In concordance with this finding, syncytia formed by various neural cells can also be observed in the murine organotypic slice cultures upon HSV-1 infection (Ecob-Johnston and Whetsell, 1979). It has been suggested that HSV use cell-cell fusion to avoid the “cell-free” spread of viral particles and thus evade interaction with host immune system (Carmichael et al., 2018). It remains to be investigated whether the intrauterine HSV infection-associated neural damage can be attributed to cell-cell fusion. Another study employed cerebral organoids at day 15 of differentiation to study the effects of HSV-1 infection (Qiao et al., 2020). The authors found a relative decrease of Nestin and SOX2 expression, which was accompanied by a decrease of the expression of Tuj1 and in the thickness of the cortical plate-like structure (Qiao et al., 2020). However, the authors did not report colocalization of the cell type markers with viral proteins, therefore no cell type-specific assessment of the phenotype induced by HSV-1 exposure can be performed (Qiao et al., 2020). The early time point in organoid differentiation suggests that HSV-1 may have affected NSCs. This hypothesis has been corroborated by a recent preprint, which finds that HSV-1 infection resulted in the highest viral load in NPCs in the cerebral organoids enriched for neocortical cellular identities at day 60 of differentiation (Lancaster et al., 2017; Rybak-Wolf et al., 2021). Cell type-specific differential gene expression analysis showed upregulation of the cellular stress- and infectious disease-associated genes in both RGs and IPCs 3 days after infection (Rybak-Wolf et al., 2021). Additionally, IPCs showed a downregulation of the pathways associated with cell division (Rybak-Wolf et al., 2021). Apart from cell type-specific changes following HSV-1 infection, the authors demonstrated a global increase in poly(A) tail lengths and preferential choice of the distal 3’ adenylation site of mRNA molecules (Rybak-Wolf et al., 2021). Both measurements may be interpreted as restructuring of the translational machinery to favor viral replication but not host translation and mRNA turnover (Rybak-Wolf et al., 2021). Altogether, the results of these studies suggest that in utero HSV-1 infection may affect both NSCs and neurons and explain some of the clinical manifestations, such as microcephaly.
SARS-CoV-2 Appears to Cross Placental Barrier and Infect Particular Types of Neural Cells During Fetal Development
Clinical observations from adult patients infected with the novel SARS-CoV-2 suggest that the disease may have neurological manifestations (Khan and Gomes, 2020). Although the mechanisms of neural damage in adults are of great clinical importance, the potential damage to the fetal brain during in utero development upon maternal infection should also be investigated. Indeed, several reports suggest transplacental transmission of the novel coronavirus from mother to fetus (Algarroba et al., 2020; Ashary et al., 2020; Hosier et al., 2020; Patanè et al., 2020; Vivanti et al., 2020) while others provide evidence against this possibility (Pique-Regi et al., 2020). The prevailing opinion, however, is that the two major SARS-CoV-2 cell entry mediators (angiotensin-converting enzyme 2, ACE2, and the S protein priming protease TMPRSS2) are expressed in the syncytiotrophoblasts cells and the extravillous trophoblasts during the first and the second trimester of pregnancy, respectively, (Ashary et al., 2020) and support virus entry into these cells (Hosier et al., 2020; Patanè et al., 2020). Moreover, bulk expression of both ACE2 and TMPRSS2 decreases in the human placenta as pregnancy progresses, showing a particular vulnerability of the fetus during the first half of gestation (Bloise et al., 2020). After crossing the placental barrier, SARS-CoV-2 can be found in the umbilical cord at midgestation (Hosier et al., 2020) and potentially reach the fetal brain through infection of the ChP as shown in ChP organoids (Jacob et al., 2020; Pellegrini et al., 2020a). In addition to the potential entry of viral particles into the brain, infection of ChP per se results in its severe malfunctioning where synthetic, secretory, barrier and transport functions are affected (Jacob et al., 2020). If ChP is permissive to SARS-CoV-2 brain entry, the question is which cell types within the brain may be affected by the virus. Several brain organoid studies show infection of NSCs (Mesci et al., 2020; Song et al., 2020; Zhang et al., 2020), neurons (Jacob et al., 2020; Mesci et al., 2020; Ramani et al., 2020; Song et al., 2020; Zhang et al., 2020; Andrews et al., 2021), and even astrocytes (Jacob et al., 2020; Mesci et al., 2020; Andrews et al., 2021) by the novel coronavirus. Considering neurogenesis, the next focus will be on the potential infection of NSCs and newborn neurons. Reports suggest tropism of SARS-CoV-2 to NPCs with viral proteins colocalizing with SOX2-positive neural rosettes (Song et al., 2020) and NPC marker Nestin (Mesci et al., 2020; Zhang et al., 2020). However, other reports do not support this observation (Andrews et al., 2021; Ramani et al., 2020). This discrepancy cannot be readily attributed to the protocol for organoid differentiation, their age, incubation time with the virus and the viral dose (Table 1) highlighting the need for further corroboration by independent studies with larger numbers of replications (see also Brain Organoid Models Have Started to Reveal Cellular and Molecular Mechanisms of Environmental Insults and Technological Advances Drive Progress in Brain Organoid Research and Increase Interpretability Across Studies). Interestingly, some studies report apoptotic cell death not only in the infected cells but also in the non-infected neighboring cells within brain organoids (Mesci et al., 2020; Ramani et al., 2020; Song et al., 2020). The proportion of apoptotic cells correlates with the proportion of SARS-CoV-2 infected cells suggesting non-cell autonomous toxicity of the virus, which may be attributed to the hypermetabolic state of infected cells and their ability to create a local hypoxic environment (Song et al., 2020). Additionally, the authors report shifts in cell type composition upon coronavirus infection with neuronal cells over- and transient progenitor cell underrepresented suggesting premature differentiation in the infected organoids (Song et al., 2020). Collectively, these studies indicate that SARS-CoV-2 infection may be potentially hazardous for the developing brain at peak neurogenesis due to permissiveness of the placental barrier and infection of ChP followed by viral entry into neural cells. The observed cell death and metabolic alterations upon SARS-CoV-2 exposure in brain organoids provide the potential for long-lasting neurodevelopmental defects in children after maternal infection. Additionally, systemic effects should also be investigated. Specifically, pregnant women are considered a risk group for developing severe symptoms upon SARS-CoV-2 infection (Allotey et al., 2020). Activation of maternal immune response has, in turn, been repeatedly correlated with an increased risk for neurodevelopmental disorders in the offspring (Meyer, 2019; Allswede et al., 2020).
TABLE 1.
Comparison between different studies focusing on the cell type tropism of SARS-CoV-2.
| Publication | Organoid protocol and age upon infection | Viral strain, time of exposure and MOI | Infection | Viral production | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NSCs | Neurons | Astrocytes | Choroid plexus | NSCs | Neurons | Astrocytes | Choroid plexus | |||
| Jacob et al. (2020) | Cortical, age not reported | SARS-CoV-2 USA-WA1/2020, 8 h, MOI 0.1–0.05 | N/A | + | + | + | − | − | − | + |
| Zhang et al. (2020) | Cerebral, Day 35 | N/A, 72 h, N/A | + | + | N/A | N/A | + | N/A | N/A | N/A |
| Ramani et al. (2020) | Cerebral, Day 15 or 60 | SARS-CoV-2 NRW-42, 2 days, MOI 1.8 × 10−4 for day 15, 8.8 × 1005 for day 60 | − | + | N/A | N/A | − | − | N/A | N/A |
| Mesci et al. (2020) | Cortical, Day 52 | SARS-CoV-2 isolated from a patient in Washington State, 1 week, MOI 2.5 | + | + | + | N/A | N/A | N/A | N/A | N/A |
| Song et al. (2020) | Cerebral, Week 9 | SARS-CoV-2 isolate USA-WA1/2020, 24 or 96 h, MOI 1 | + | + | N/A | N/A | + | + | N/A | N/A |
| Andrews et al. (2021) | Cortical, Week 5, 10, 16 or 22 | SARS-CoV-2 isolate USA-WA1/2020, 2 h, MOI 0.5 | + | + | + | N/A | − | N/A | + | N/A |
+, stands for positive; −, for negative; N/A, not investigated; MOI, multiplicity of infection.
Maternal Stress, Medication, and Substance Use
Glucocorticoids Disrupt Neural Precursor/Progenitor Cells Differentiation
Maternal stress has been repeatedly correlated with diverse neurodevelopmental abnormalities in the fetus with penetration into adolescence and adulthood (Krontira et al., 2020). However, human studies are mostly observational. This study design makes causal inference complicated due to the variety of confounding factors and the inability to analyze objective measurements of maternal stress (Dipietro, 2012). In this review, we will focus on the known molecular players that define maternal stress, the glucocorticoid (GC) hormones, and their effects on cortical neurogenesis.
GCs, being lipid-soluble molecules, readily cross the placental barrier and blood-brain interfaces (Benediktsson et al., 1997; Krontira et al., 2020). Although 50–90% of the main human GC hormone cortisol is inactivated in the human placenta by 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2) (Mulder et al., 2002), chronic stress or inflammation, as well as maternal alcohol consumption, reduce placental activity of this enzyme (Howerton and Bale, 2012). It is, therefore, likely that maternal cortisol reaches the embryonic and fetal brain. In addition to natural glucocorticoids, their synthetic analogs (e.g. dexamethasone, betamethasone) are prescribed to pregnant women, for example to correct the risk (or the diagnosis) of fetal congenital adrenal hyperplasia at midgestation (Nimkarn and New, 2007). The placental deactivation of synthetic GCs is less efficient than that of natural GCs (Seckl, 1997). The receptors for both natural and synthetic GCs include intracellular GC receptors (GRs) and mineralocorticoid receptors (Seckl, 1997). According to a recently published preprint, the gene coding for GR, NR3C1, is expressed in human brain organoids from day 17 to day 158 of organoid development together with the whole molecular machinery that is needed for the response to GCs (Cruceanu et al., 2020). NR3C1 expression is enriched in NPCs and GR can be identified in the nuclei of the cells forming neural rosettes (Cruceanu et al., 2020). Functionally, organoids are capable of upregulating GR-dependent gene expression upon acute exposure to dexamethasone (Cruceanu et al., 2020). Moreover, single-cell transcriptomic analysis indicates differential expression of genes responsible for fate determination in the developing brain, including HES6 and PAX6 upon dexamethasone treatment (Cruceanu et al., 2020). Gene ontology analysis showed enrichment for cell differentiation, head development, neurogenesis, neuron differentiation, and nervous system development in both neurons and NPCs, suggesting that GC exposure in the developing brain may interfere with neuronal differentiation (Cruceanu et al., 2020).
In addition to the direct effects of GCs on fetal brain development, they also affect the placenta. For instance, GCs decrease the transport of glucose to the fetal compartment of the placenta resulting in intrauterine growth restriction and upregulation of hypoxia-related genes at E12.5 in mice (Mueller and Bale, 2008). Thus, the effects of maternal stress may be attributed not only to the glucocorticoids but also to fetal response to nutrient deficiency and hypoxia (see below).
Ethanol May Promote Premature Differentiation of Neural Precursor/Progenitor Cells in the Developing Human Brain
Maternal alcohol consumption can cause long-lasting deficits in fetal neurodevelopment leading to an increased risk for the development of psychiatric disorders (Ross et al., 2015). These deficits, also known as fetal alcohol spectrum disorder, have a global prevalence of 0.15% (Popova et al., 2017). Alarmingly, this number is 2.6 times higher in Europe suggesting a higher burden for the healthcare system (Popova et al., 2017). The severity of symptoms can vary from subtle neurobehavioral abnormalities to structural changes, including microcephaly (Ross et al., 2015). In the most extreme scenario, continuous drinking throughout pregnancy or binge drinking can cause fetal alcohol syndrome, which is characterized by both physical and neurobehavioral abnormalities (Ross et al., 2015). Importantly, ethanol, as a small molecule, readily crosses biological membranes, thus reaching the fetal brain. Recent data on primary human NPCs and NPCs-derived immature neurons suggests their particular vulnerability to increasing ethanol concentrations through the induction of apoptosis by alternative splicing of MCL-1, a member of the BCL2 protein family (Donadoni et al., 2019). Continuous exposure to ethanol (50 mM, 23 mg/dl) for 20 days starting from day 10 of differentiation, induces apoptosis in cerebral organoids and leads to a reduction of SOX2-positive cells while increasing the Tuj1-positive cell number (Zhu et al., 2017). Thus, NPCs either undergo cell death or prematurely differentiate into neurons upon ethanol exposure. Among the genes that were deregulated by ethanol exposure were cell adhesion molecules (Zhu et al., 2017). This observation has been functionally validated by attenuated neurite outgrowth in sliced organoids exposed to ethanol (Zhu et al., 2017).
Another study focused on acute ethanol exposure for 6 h of 2 month old cerebral organoids, where apoptosis was induced at ethanol concentrations of 115 mg/dl. This concentration is comparable to the blood ethanol concentration upon binge drinking (80 mg/dl, corresponding roughly to four glasses of wine over less than 2 h) (Arzua et al., 2020). In addition to apoptosis induction, the authors noticed ultrastructural changes, such as abnormal mitochondria cristae and glycogen foci (Arzua et al., 2020). The abnormal morphology of mitochondria was accompanied by functional changes in energy metabolism (Arzua et al., 2020). More changes were found at the transcriptional level with microarray analysis showing a deregulation of groups of genes involved in cell proliferation, nervous system development, and neurological diseases (Arzua et al., 2020). Given the complexity of cell type composition in cerebral organoids at 2 months of differentiation (Lancaster et al., 2013), these results provide an interesting starting point for further experiments on cell type-specific events.
Nicotine, Cannabis, and Cocaine Interfere With Neurogenesis, as Seen in Brain Organoids
Prenatal nicotine exposure has been related to long-lasting changes in the developing brain (Faa et al., 2016). Specifically, it may affect neurogenesis, as seen in mice (Aoyama et al., 2016). Epidemiological reports indicate 15% of pregnant women smoke during pregnancy in the United States (Oh et al., 2017). Thirteen percent of newborns in the United Kingdom were exposed to environmental tobacco smoke and 36% to maternal smoking antenatally in 2000–2001 (Ward et al., 2007). Nicotine is well-known to cross the placenta and enter the fetal circulation in humans (Luck et al., 1985). In a recent study Wang and colleagues applied nicotine to brain organoids for 5 days during the early stages of neuroepithelial expansion and found that nicotine induced cell death as well as an increased proportion of Tuj1-positive cells suggesting premature differentiation of NPCs (Wang et al., 2018). Additionally, the expression of forebrain markers PAX6 and FOXG1 was depleted (Wang et al., 2018). Nicotine exposure at a later time point in organoid development resulted in the specific depletion of TBR1-positive layer VI neurons whereas the proportion of CTIP2-positive layer V neurons was increased (Wang et al., 2018). These findings indicate that nicotine exposure in critical periods of fetal brain development may disrupt proper cortical layering (Wang et al., 2018).
Like ethanol and nicotine, the major psychoactive component of cannabis, Δ-9-tetrahydrocannabinol (THC), readily crosses the placenta and enters the fetal circulation as seen in pregnant rats (Hutchings et al., 1989). However, it does not induce major developmental defects in the fetus (Warner et al., 2014). Its perceived safety leads to 1–5% of pregnant women using it in the general population with this number reaching 16.5% in pregnant adolescents (15–17 years old) (data reported for France and the United States) (Saurel-Cubizolles et al., 2014; Warner et al., 2014). However, mild longitudinal effects of this drug should not be overlooked. Epidemiological data indicate alterations in certain aspects of executive and visuoperceptual functioning in the adolescent offspring of mothers using cannabis during pregnancy (Huizink, 2014). Animal studies demonstrate protracted behavioral deficits in rat pups that were prenatally exposed to the synthetic cannabinoid receptor agonist WIN 55,212–2 (Mereu et al., 2003). Recent studies in cerebral organoids found that THC induced relative upregulation of PAX6 while downregulating neuronal markers CTIP2 and Tuj1 (Ao et al., 2020). Additionally, the expression of cannabinoid receptor CB1 was downregulated in organoids exposed to THC (Ao et al., 2020). CB1 is normally expressed in the embryonic and fetal brain (Díaz-Alonso et al., 2012; Nowakowski et al., 2017) and is known to be involved in the regulation of the balance between proliferation and neurogenesis in different brain areas, including cerebral cortex (Díaz-Alonso et al., 2012) consistent with the findings in cerebral organoids.
Cocaine is the third most commonly used substance and is being taken by 0.3% of women above the age of 12 in the United States (Ross et al., 2015). The European Monitoring Center for Drugs and Drug Addiction reports that 0.2–2.3% of adults (15–64 years old) used cocaine in 2018 in different countries, with the highest burden for Spain and the United Kingdom (EMCDDA, 2018). 1 Cocaine is considered as a teratogen for the developing fetus, thus presenting a major concern for the healthcare system (Ross et al., 2015). It can be detected in the human placenta at PCW10 (Joya et al., 2010) and in the amniotic fluid at PCW14 (Apple and Roe, 1990). Cocaine is highly lipophilic and crosses the BBB, and accumulates in the adult brain (Farrar and Kearns, 1989) suggesting a similar effect in the fetus. Rodent and non-human primate studies have shown that maternal cocaine intake during the vulnerable window of neurogenesis causes structural abnormalities in the neocortex of the offspring (Lidow et al., 2001; Lidow and Song, 2001; Lee et al., 2011). Cytochrome P450 (CYP450) enzymes, which are the first to metabolize cocaine in the neural cells, were shown to have a causal role in the disturbance of neurogenesis in rats, where they induce reactive oxygen species (ROS)-dependent oxidative endoplasmic reticulum (ER) stress (Lee et al., 2008). The expression of CYP450 enzymes varies between species, making brain organoids an attractive model system for recapitulating human-specific abnormalities of corticogenesis upon cocaine exposure (Lee et al., 2017). Indeed, a dorsal forebrain organoid model identified expression of two members of CYP3A P450 subfamily, CYP3A5 and CYP3A43 (Lee et al., 2017). In the same study, periodic application of cocaine during the proliferative phase of organoid development resulted in the accumulation of ROS that was accompanied by the inhibited proliferation of NPCs and their premature differentiation (Lee et al., 2017). Finally, the neuronal output was reduced in cocaine-treated organoids (Lee et al., 2017). Knockdown of CYP3A5 reversed the pathological phenotype induced by cocaine (Lee et al., 2017). Interestingly, CYP3A enzymes are shown to metabolize ∼50% of all currently used therapeutic drugs suggesting their potential involvement in neocortical abnormalities caused by other drugs (Lee et al., 2017). In addition to the direct effects of cocaine on the fetal brain, it causes severe vasoconstriction in the mother leading to reduced blood flow in the placenta (Farrar and Kearns, 1989). This, in turn, results in restricted fetal growth and may also add fetal hypoxia to the compound effect of cocaine use by a pregnant woman (Farrar and Kearns, 1989).
The Effects of Hypoxia on Neurogenesis are Time- and Dose-Specific
Oxygen pressure in the umbilical vein blood of the developing fetus at midgestation is half of the arterial blood of the mother (22–32 vs. 80–100 mmHg) showing that a hypoxic environment is natural for the developing fetus (Soothill et al., 1986). While physiological hypoxia is necessary for normal fetal development (Giaccia, 2004), several maternal factors may alter the delicate oxygen balance. Among them are certain medications, drugs, and high altitude (Jensen and Moore, 1997). Irrespective of the cause, the effects of hypoxia on fetal brain development depend on the severity of exposure and time window during development and vary between brain regions. The differential vulnerability of the fetal brain to hypoxia at different stages of development is recapitulated in organoids (Boisvert et al., 2019; Daviaud et al., 2019; Paşca et al., 2019). Indeed, hypoxic exposure in 10-day old brain organoids significantly reduces cortical marker expression (i.e. FOXG1, CTIP2, TBR1) later in the organoid development (Boisvert et al., 2019). In contrast, cortical spheroids exposed to hypoxic insult at day 75 of differentiation show mild changes, which are restricted to a specific cell type, the IPCs (Paşca et al., 2019). While taking into account different organoid protocols employed in these studies, we may roughly correlate these two time points with the development of the human brain at the early neuroepithelial stage and at midgestation (Paşca et al., 2019; Benito-Kwiecinski et al., 2021), respectively.
In addition to altered cortical marker gene expression, hypoxia induced apoptotic program in 10 day old brain organoids (Boisvert et al., 2019) (Figure 2C’). Considering that at this step of differentiation cerebral organoids consist almost exclusively of NECs (Benito-Kwiecinski et al., 2021), induction of apoptosis may be attributed to this cell type. Therefore, reduced cortical marker expression may be a secondary effect to the loss of NECs. Both apoptotic program induction and loss of cortical marker expression could be partially rescued by treatment with the antibiotic minocycline (Boisvert et al., 2019). Interestingly, minocycline has shown potential for the treatment of some neurodevelopmental diseases (Zhu et al., 2014; Mattei et al., 2017). Indeed, its administration to murine pups presented with maternal immune activation rescues the behavioral phenotype by normalizing the inflammatory status of microglial cells (Zhu et al., 2014; Mattei et al., 2017). However, this antibiotic is prohibited in pregnancy starting from PCW18 due to fetal toxicity related to skeletal development (Bevelander et al., 1960) and may thus have a limited potential to be used in humans.
Another study investigated the response to hypoxia in 4 week old cerebral organoids, where an immediate effect on apoptosis and DNA damage could be seen in the VZ-like area (Daviaud et al., 2019) (Figure 2C’’). The authors report a decreased number of TBR2-positive IPCs and FAM107A-positive oRGs 14 days after hypoxic exposure. Furthermore, the authors show alterations in the VZ-like zone, including the persistent loss of the proliferation marker Ki-67 and a shift in cleavage plane angles toward oblique or vertical suggesting self-renewal of vRG cells at the expense of indirect neurogenesis (Daviaud et al., 2019).
Interestingly, according to a study by Pasca and colleagues cell type-specific effects of hypoxia differed in 75 day old cortical spheroids from those at 4 weeks as reported by Daviaud and colleagues. Pasca and colleagues found that the number of RG cells marked by PAX6 was not altered upon hypoxic exposure (1% O2, 48 h) (Paşca et al., 2019). In contrast, they noticed specific depletion of TBR2-positive IPCs (Paşca et al., 2019) (Figure 2C’’’). RNA sequencing revealed the correlation between genes related to hypoxia and unfolded protein response (UPR), which marks ER stress (Paşca et al., 2019). The specific vulnerability of IPCs to ER stress was confirmed by loss-of-function and rescue experiments pointing toward a causal relationship between ER stress and IPCs depletion (Paşca et al., 2019). The depletion of IPCs was found to be caused by an increase in cell cycle exit (p27-positive cells), rather than induction of apoptosis (Paşca et al., 2019). ER stress and cell cycle exit in IPCs was followed by an increase in CTIP2-positive neurons suggesting premature differentiation of IPCs driven by UPR (Paşca et al., 2019).
In summary, brain organoids show differential vulnerability of particular cell types to hypoxic exposure applied at different time points of differentiation (Figure 2C). When applied early in the course of differentiation, hypoxia causes apoptosis in the VZ followed by potentially secondary effects on the numbers of basal progenitors (IPCs and oRGs) (Daviaud et al., 2019) and reduced expression of cortical markers (Boisvert et al., 2019). In contrast, hypoxic exposure at a later stage of organoid development leads to the loss of proliferative capacity in IPCs due to upregulation of UPR without affecting vRGs (Paşca et al., 2019).
Brain Organoid Models Have Started to Reveal Cellular and Molecular Mechanisms of Environmental Insults
In the past years, brain organoids have been increasingly used for modeling the effects of environmental adversities on human neurogenesis (Table 2). Although organoid protocols differ in their speed of development, the majority of them are enriched for NPCs, particularly for vRGs, until day 50 of differentiation. Accordingly, the majority of the environmental insults listed in the Table 2 target these cells. Interestingly, environmental insults on vRGs often lead to either their death or premature differentiation resulting in the secondary loss of other NSCs and neurons. It was recently shown that murine vRGs have differential sensitivity to the environmental factors as a function of developmental age (Telley et al., 2019). Murine vRGs progress from an “introverted” state, when the cells do not express receptors to sense their surrounding at E12, to an “extraverted” state, when the cells are capable of responding to environmental cues later in development (Telley et al., 2019). In light of this finding, it would be interesting to analyze progressive changes in the transcriptome of vRGs within human brain organoids in order to decipher their sensitivity to the environmental factors.
TABLE 2.
Summary of the neurogenic defects induced by environmental adversities modeled in brain organoids.
| Group of environmental adversity | Environmental adversity | Publication | Organoid protocol | Regional identity, age of the organoid at the start of experiment | Major findings |
|---|---|---|---|---|---|
| Viral infections with vertical transmission | Zika virus | Gabriel et al. (2017) | Gabriel et al. (2017) | Brain, Day 9 | Infection of NPCs followed by either apoptosis or premature differentiation due to defect in centriole assembly |
| Dang et al. (2016) | Lancaster et al. (2013) | Cerebral, Day 10 | Restricted growth | ||
| TLR3 mediates transcriptional dysregulation of apoptosis and regulators of neurogenesis | |||||
| Qian et al. (2016) | Qian et al. (2016) | Cortical, Day 14 or 80 | Infection in NPCs, including oRGs, as well as in IPCs and immature neurons | ||
| Disrupted proliferation in the VZ-like areas | |||||
| Decreased neuronal output and increased size of ventricle-like cavities | |||||
| Watanabe et al. (2017) | Watanabe et al. (2017) | Cerebral, Day 21 | Restricted growth | ||
| Activation of innate immune response promoting cell death | |||||
| Albanese et al. (2020) | Lancaster et al. (2013) | Cerebral, Day 21 | Restricted growth | ||
| Decreased number of VZ-like areas | |||||
| Reduced number of vRGs accompanied with reduced neuronal output | |||||
| Wells et al. (2016) | Wells et al. (2016) | Cerebral, Day 24 | Knockout of AXL does not protect VZ-like areas from viral infection and apoptosis | ||
| Cugola et al. (2016) | Lancaster et al. (2013) | Cerebral, Day 28 | Disruption of proliferative zones | ||
| Decreased neuronal number and increased apoptosis | |||||
| Garcez et al. (2016) | Lancaster et al. (2013) | Cerebral, Day 35 | Restricted growth | ||
| Yoon et al. (2017) | Qian et al. (2016) | Cortical, Day 45 | Disrupted apical polarity complex in vRGs, disrupted adherens junctions leading to premature differentiation | ||
| Cytomegalovirus | Brown et al. (2019) | Lancaster et al. (2013) with modifications | Cerebral, Day 0 | Decreased cellularity | |
| Regions of necrosis and cysts | |||||
| Disrupted VZ-like areas and radial scaffold | |||||
| Sun et al. (2020) | Lancaster et al. (2013) with modifications | Cerebral, Day 30 | Restricted growth | ||
| Decreased proliferation in the VZ-like areas | |||||
| Increased apoptosis adjacent to the VZ-like areas | |||||
| PDGFRa and EGFR are potential viral entry receptors | |||||
| Infection in TBR2-positive IPCs | |||||
| Decreased neuronal output | |||||
| Upregulated immune response and downregulated metabolism-related gene expression | |||||
| Cosset et al. (2015) | Preynat-Seauve et al. (2009) | Engineered neural tissue, age not reported | Upregulated lipid metabolism and inflammation-related genes | ||
| Infection in doublecortin-positive newborn neurons but not in PAX6-positive NPCs | |||||
| Qiao et al. (2020) | Lancaster et al. (2013) with modifications | Cerebral, Day 15 | Decreased expression of SOX2 and Nestin | ||
| Decreased thickness of CP-like structures and decreased expression of neuronal markers | |||||
| D’Aiuto et al. (2019) | D’Aiuto et al. (2019) | Brain, age not reported | Infection in MAP2-positive neurons with formation of neuronal syncytia | ||
| Rybak-Wolf et al. (2021) | Lancaster et al. (2017) | Cerebral, enriched for dorsal forebrain cellular identities, Day 60 | Infection in different cell types with highest viral load in NPCs | ||
| Cell type-specific changes in transcriptional profile | |||||
| Global elongation of poly(A) tails and preferential use of distal 3’UTR in mRNA molecules | |||||
| SARS-CoV-2 | Ramani et al. (2020) | Gabriel et al. (2016), a modification of Lancaster et al. (2013) | Cerebral, Day 15 or 60 | Little to no infection in organoids inoculated with the virus on Day 15 of differentiation, significant infection in Day 60 organoids | |
| Infection in Tuj1-positive neurons | |||||
| Apoptotic neuronal cell death due to aberrant tau localization | |||||
| Zhang et al. (2020) | Lancaster et al. (2013) | Cerebral, Day 35 | Infection in Nestin-positive NPCs and Tuj1-positive neurons | ||
| Productive infection of the organoid | |||||
| Mesci et al. (2020) | Trujillo et al. (2019) | Cortical, Day 52 | Infection and increased apoptosis rate in Nestin-positive NPCs, MAP2-positive neurons and GFAP-positive astrocytes | ||
| The phenotype is reversed by sofosbuvir | |||||
| Song et al. (2020) | Lancaster et al. (2013) | Cerebral, Week 9 | Productive infection in SOX2-positive NPCs and MAP2-positive neurons but not in GFAP-positive astroglia | ||
| ACE2 protein localization to MAP2-positive neurons and close to the VZ-like cavities | |||||
| Overall increased apoptosis rate within the organoid irrespective of the infection status of the cell | |||||
| Hypermetabolic state of the infected cells and overall downregulation of catabolic processes | |||||
| Andrews et al. (2021) | Pollen et al. (2019) | Cortical, Week 5, 10, 16, or 22 | Infection in double-GFAP,AQP4-positive astrocytes and rare infection of NeuN-positive neurons at Week 22 | ||
| No infection in SOX2-positive NPCs at Week 5 and 10 | |||||
| No ACE2 protein expression detected | |||||
| Jacob et al. (2020) | Qian et al. (2016) | Cortical, age not reported | Infection in doublecortin-positive neurons | ||
| Maternal stress, medication, and substance use | Glucocorticoids (dexamethasone) | Cruceanu et al. (2020) | Lancaster et al. (2013) | Cerebral, Day 45 | Cerebral organoids express the molecular machinery for response to glucocorticoids starting from Day 17 of differentiation |
| GR expression is enriched in NPCs | |||||
| Altered expression profile indicates that dexamethasone interferes with neuronal differentiation | |||||
| Ethanol | Zhu et al. (2017) | Zhu et al. (2017), an organ-on-chip modification of Lancaster et al. (2013) | Cerebral, Day 10 | Apoptosis induction | |
| Decreased SOX2-posivive NPC number | |||||
| Increased Tuj1-posivive neuron number | |||||
| Decreased expression of cell adhesion molecules | |||||
| Arzua et al. (2020) | Lancaster et al. (2013) | Cerebral, Day 60 | Apoptosis induction in NeuN-positive neurons but not in S100B-positive astrocytes | ||
| Altered energy metabolism and mitochondrial function | |||||
| Altered gene expression profile including genes related to neurodevelopment and neurological diseases | |||||
| Nicotine | Wang et al. (2018) | Wang et al. (2018), an organ-on-chip modification of Lancaster et al. (2013) | Cerebral, Day 11 | Induction of apoptosis | |
| Increased proportion of Tuj1-positive neurons indicating premature differentiation | |||||
| Decreased expression of forebrain markers PAX6 and FOXG1 | |||||
| Cannabis | Ao et al. (2020) | Ao et al. (2020), a microfluidic modification of Lancaster et al. (2013) | Cerebral, Day 3 | Increased PAX6-positive NPCs number and increased thickness of VZ-like areas indicating increased proliferation of NPCs | |
| Decreased expression of neuronal markers Tuj1 and CTIP2 | |||||
| Downregulated CB1 expression | |||||
| Cocaine | Lee et al. (2017) | Lee et al. (2017) | Cortical, Day 32 | Decreased PAX6-positive NPCs number | |
| Increased migration of BrdU-positive neurons indicating premature differentiation | |||||
| Increased ROS formation | |||||
| Fetal hypoxia | Fetal hypoxia | Boisvert et al. (2019) | Boisvert et al. (2019) | Brain, Day 10 | Induction of apoptotic program |
| Decreased expression of cortical markers FOXG1, CTIP2, TBR1 | |||||
| Both the induction of apoptotic program and decreased cortical marker expression may be reverted by minocycline | |||||
| Daviaud et al. (2019) | Lancaster et al. (2013) | Cerebral, Day 28 | Immediate cell death in VZ-like areas and increased CTIP2-positive neuron number | ||
| Delayed decrease in TBR2-positive IPCs and FAM107A-positive oRGs | |||||
| Self-renewal divisions in aRGs at the expense of indirect neurogenesis | |||||
| Paşca et al. (2019) | Sloan et al. (2018) | Cortical spheroid, Day 75 | No cell death observed | ||
| PAX6-positive NPCs number not altered | |||||
| Decreased number of TBR2-positive IPCs due to premature cell cycle exit and differentiation |
Finally, the diversity of the existing protocols (Table 2) requires particular attention when comparing and extrapolating results from other studies and designing experiments. The following prerequisites may provide a roadmap for the experimental design when modeling environmental adversities on human neurogenesis with brain organoids:
1. Brain organoids should recapitulate the main stages of (early) brain development in a timely manner and display characteristic cytoarchitectural features of the developing brain. The benchmarking of the organoid differentiation should be clearly reported in the publication;
2. Environmental insults should have a form that reaches the fetus in utero meaning that the fetus should not be protected from it through one of the previously discussed barriers (i.e. placenta and blood-brain interfaces);
3. In order to be able to draw solid conclusions, the experimental design should include several cell lines, several batches of organoid differentiation and a sufficient number of technical replicates within each batch. For neurodevelopmental phenotypes with differential gender penetrance, both male and female PSC lines should be included in the study.
Technological Advances Drive Progress in Brain Organoid Research and Increase Interpretability Across Studies
Recent Developments Aim at Overcoming Limitations of Organoid Models
Brain organoids are a promising model system, but do not fully recapitulate human brain development in vivo. First, they lack the cells not originating from neuroectoderm, such as those of the vasculature and microglia (Khakipoor et al., 2020). Specifically, the vasculature is important for the proper modeling of fetal brain hypoxia with brain organoids. Brain vasculature is known to have a pivotal role in switching the NPCs from proliferative state to neurogenic one by resolving hypoxia (Lange et al., 2016). The first attempts to recapitulate interactions between brain organoid tissue and vascular cells in vitro were made in 2018 (Pham et al., 2018) and further advanced since then (Cakir et al., 2019; Shi et al., 2020). Vasculature-like tubes within brain organoids express tight junction proteins and exhibit BBB-like functions (Cakir et al., 2019). The use of such organoids will advance the neurotoxicity research. Additionally, the introduction of vascular-like cells helped to reduce the size of the hypoxic core in the organoids (Cakir et al., 2019) which is a valuable improvement in light of the recent finding that glycolytic and ER stress impairs cell subtype specification in brain organoids (Bhaduri et al., 2020).
Microglial cells are brain resident macrophages that originate from the yolk sac (Utz et al., 2020). They are likely important players in translating environmental adversities to abnormalities in fetal brain development. To date, the majority of studies have focused on the role of environmental factors in shaping synapse pruning and circuit maturation by microglia (Salter and Stevens, 2017). However, microglial cells can be found in the human forebrain as early as PCW4.5 where they reside within and next to the VZ (Monier et al., 2007). Early microglia plays an active role in regulating neurogenesis in the primate neocortex (Cunningham et al., 2013). Moreover, human microglia at PCW11 starts acquiring a more mature phenotype including the activation of environment-sensing programs (Kracht et al., 2020). It is, therefore, likely that microglial cells can sense the local environment starting from the midgestation and adapt their interactions with the NSCs accordingly. Human brain organoids can be colonized with induced PSC-derived microglial progenitors, which differentiate within the organoid (Abud et al., 2017; Fagerlund et al., 2020). We suggest that in order to model environmental adversities with an inflammatory component more faithfully in vitro, the use of such brain organoid-microglia co-cultures is beneficial.
As mentioned before, insufficient oxygen and nutrient supply due to large distances not overcome by diffusion may impact differentiation trajectories of the NSCs within the organoid (Bhaduri et al., 2020). To overcome this limitation, several groups have proposed to maintain the organoids in slice cultures (Qian et al., 2020; Giandomenico et al., 2021). Particularly, slicing of the organoids was shown to sustain the neurogenic capacity of the NSCs for longer, thus allowing the generation of distinct cortical layers (Qian et al., 2020). Alternatively, the organoids may be cultivated on the bio-compatible microfilaments allowing for higher area-to-volume ratio (Lancaster et al., 2017). Further improvement may be achieved by using organ-on-chip approaches that allow better control over the biochemical environment of the organoid. Organ-on-chip methods have been applied to study the effects of nicotine (Wang et al., 2018), cadmium (Yin et al., 2018), and ethanol (Zhu et al., 2017) exposure on brain organoids.
We suggest that modeling environmental effects on fetal brain development would benefit from combining placental barrier, blood-brain interfaces and brain organoids in an in vitro setting. This would enhance our understanding of the mechanisms of viral infections and could serve as a versatile platform for developmental neurotoxicity testing. This might be a possibility in the near future since in vitro models of the placental barrier (Blundell et al., 2016; Muoth et al., 2016), the BBB (Wang et al., 2017) and the blood-CSF barrier (Monnot and Zheng, 2012; Pellegrini et al., 2020b) have been developed recently.
The Scalability of Brain Organoids Poses a Variety of Challenges and New Opportunities
One of the major assets of brain organoids as a model system is their scalability. Indeed, depending on the protocol, one researcher can maintain hundreds of organoids in parallel and automation approaches that are currently being developed could further allow high-throughput experiments (Sirenko et al., 2019; Renner et al., 2020). However, the scaled generation of organoids requires appropriate readout and data analysis options. These include 3D imaging as well as a variety of omics approaches and large-scale genetic screens.
Tissue clearing protocols have recently been adapted for brain organoids allowing immunohistochemistry to be combined with whole-mount imaging (Albanese et al., 2020; Renner et al., 2020). Whole-mount microscopy allows the unbiased estimation of cell counts within the organoid and evaluation of tissue cytoarchitecture (Albanese et al., 2020). However, the organoids lack a stereotyped anatomical arrangement which precludes “tissue atlas”-based volumetric analysis (Albanese et al., 2020). Instead, “atlas-free” analysis strategies must be employed, which rely on artificial neural networks (Albanese et al., 2020). In addition to the analysis of marker expression, the scaled generation of organoids requires careful automatic monitoring from bright field images or the use of reporter cell lines to closely follow organoid differentiation trajectories without endpoint analysis, for example accounting for unsuccessful differentiation (Bagley et al., 2017; Kegeles et al., 2020).
The development of organoid generation protocols coincided with the evolution of single-cell transcriptomics. This overlap resulted in a series of works comparing single-cell signatures of neural cells within brain organoids to those in the developing human brain (Camp et al., 2015; Pollen et al., 2019; Velasco et al., 2019; Bhaduri et al., 2020; Tanaka et al., 2020). Omics approaches, and especially single-cell omics approaches, require tailored data analysis toolboxes (Lähnemann et al., 2020). Additionally, in order to characterize cellular identity and function in a holistic manner, the combination of several modalities at the single-cell level, while challenging, is becoming increasingly feasible. We have recently developed a method to combine calcium imaging with single-cell transcriptomics in the developing human neocortex revealing changes in physiological features alongside transcriptomic changes as neurons differentiate (Mayer et al., 2019). Another modality, that is often combined with single-cell transcriptomics, is single-cell epigenomics (Amiri et al., 2018). Recently, cell type-specific chromatin accessibility analysis in long-term organoid culture revealed an epigenetic switch resembling the transition from pre- to postnatal development in humans (Trevino et al., 2020). Finally, a proof-of-principle study showed that bulk proteomic approach can be applied to resolve the effects of a psychedelic analogue of serotonin on brain organoids (Dakic et al., 2017). The integration of several data modalities also requires specific data analysis techniques. These unique challenges can be approached from different perspectives, including facilitation of data analysis with artificial intelligence-based approaches (Badai et al., 2020).
Altogether, the advances in single-cell omics could allow to decipher cell type-specific events upon the exposure to an environmental factor. Indeed, single-cell transcriptomics has recently helped to reveal sex-specific changes in the neuronal development in mice upon maternal immune activation (Kalish et al., 2021). Importantly, environmental exposures are likely to have long-lasting effects on developmental outcomes also through epigenetic mechanisms (Schaevitz and Berger-Sweeney, 2012; Svrakic et al., 2013). Therefore, we propose that analyzing how environmental exposures affect epigenetic features of specific cell types together with single-cell transcriptomics will be leading to new insights.
The epigenetic component is likely one of the players defining the effect of environmental exposures on the fetal neurodevelopment. Another side of the coin is the genetic predisposition for a neurodevelopmental disorder. Together, genetic predisposition followed by an environmental adversity may increase the risk to develop a neurodevelopmental disorder. This idea, the so-called “double-hit” hypothesis, may explain the development of complex disorders like autism spectrum disorder (ASD) (Schaevitz and Berger-Sweeney, 2012; Schaafsma et al., 2017). To study the genetic component of ASD and other neurodevelopmental disorders and its interaction with the environmental adversity, genetic screening applications can be used. The first step in this direction was made by employing CRISPR–lineage tracing at cellular resolution in heterogeneous tissue (CRISPR-LICHT) technology to study genetic risk factors for primary microcephaly (Esk et al., 2020). Here two complementary techniques, namely inducible CRISPR-Cas9-mediated gene editing and dual DNA barcoding were combined to allow lineage tracing from individual embryonic stem cells used for organoid generation (Esk et al., 2020). This study thus provides the foundation for single-cell tracing of the proliferative and neurogenic capacity of various NSCs with different genetic backgrounds in the future (Esk et al., 2020).
Discussion
Recently, organoid research has allowed increasing insights into the effect of environmental insults on brain development on a cellular and molecular level. In light of the new developments in organoid research both at the level of organoid protocols (such as inclusion of vasculature, microglia, and bioengineering) and readouts (multimodal omics approaches, physiological readouts, whole-mount stainings), we expect even greater impacts in the years ahead.
For the future, we suggest that it will be important to integrate the perspectives on environmental programming of different disciplines further to generate breakthroughs in this research field that has widespread medical and societal implications. We propose that this may be achieved by tighter collaborations between scientists working on in vitro models, on animal models, and performing studies in humans from a systems neuroscience and medical point of view.
Acknowledgments
We would like to thank Elizabeth Crouch, Ingeborg Krägeloh-Mann, Theresa Kagermeier, Zeynep Yentür, Jasmin Treu, Lea Fischer and Elisabeth Gustafsson for critical feedback on the manuscript. We thank Michael Pelzer (Presentation Research Center/Project Knowledge Design at University of Tübingen) for continuous support in graphical design.
Footnotes
Author Contributions
KS drafted the article, reviewed the relevant literature, made substantial contributions to conception and design, interpreted the data, and approved the final version. SM drafted the article, made substantial contributions to conception and design, interpreted the data, revised the article critically, and approved the final version.
Funding
This review was supported in parts by a 2018 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to Simone Mayer, by the State Postgraduate grant from the Ministry of Science, Research and the Arts of the State of Baden-Württemberg, Germany to Kseniia Sarieva as well as the Hertie Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
- NSC
neural stem cell
- PCW
postconceptional week
- PSC
pluripotent stem cell
- NEC
neuroepithelial cell
- NPC
neural precursor/progenitor cell
- vRG
ventricular radial glia
- IPC
transit-amplifying/intermediate progenitor cell
- oRG
outer radial glia
- iSVZ
inner subventricular zone
- oSVZ
outer subventricular zone
- tRG
truncated radial glia
- IZ
intermediate zone
- SP
subplate
- CP
cortical plate
- MZ
marginal zone
- TGF-β
tumor growth factor β
- BMP
bone morphogenetic protein
- BBB
blood-brain barrier
- CSF
cerebrospinal fluid
- ChP
choroid plexus
- ToRCH pathogens
toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, and others
- AQP1
aquaporin 1
- TLR3
Toll-like receptor 3
- AXL
AXL receptor tyrosine kinase
- TYRO3
tyrosine-protein kinase receptor
- MER
proto-oncogene tyrosine-protein kinase
- TIM1
T-cell immunoglobulin and mucin domain 1 or hepatitis A virus cellular receptor 1
- SOX2
(sex determining region Y)-box 2 protein
- TBR1
T-box brain transcription factor 1
- CMV
cytomegalovirus
- PDGFRa
platelet derived growth factor receptor alpha
- EGFR
epidermal growth factor receptor
- TBR2
T-box brain transcription factor 2, alternative name EOMES
- IE1
immediate early gene of cytomegalovirus
- PAX6
paired box protein 6
- HSV
herpes simplex virus
- ACE2
angiotensin-converting enzyme 2
- TMPRSS2
transmembrane protease, serine 2
- GC
glucocorticoid
- 11βHSD2
11β-hydroxysteroid dehydrogenase type 2
- GR
glucocorticoid receptor
- NR3C1
nuclear receptor subfamily 3 Group C member 1, gene coding for GR
- HES6
Hes Family BHLH transcription factor 6
- MCL-1
induced myeloid leukemia cell differentiation protein
- BCL2
B-cell lymphoma 2 protein
- FOXG1
Forkhead box G1 protein
- CTIP2
alternative name BCL11B, B-cell lymphoma/leukemia 11B protein
- THC
Δ-9-tetrahydrocannabinol
- Tuj1
neuron-specific class III beta-tubulin
- CB1
cannabinoid receptor type 1
- CYP450
cytochrome P450
- CYP3A
cytochrome P450 family 3 subfamily A
- ROS
reactive oxygen species
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- ASD
autism spectrum disorder
- CRISPR
clustered regularly interspaced short palindromic repeats
- CRISPR-LICHT
CRISPR-lineage tracing at cellular resolution in heterogeneous tissue
References
- Abud E. M., Ramirez R. N., Martinez E. S., Healy L. M., Nguyen C. H. H., Newman S. A., et al. (2017). iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 94 (2), 278–293.e9. 10.1016/j.neuron.2017.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A., Swaney J. M., Yun D. H., Evans N. B., Antonucci J. M., Velasco S., et al. (2020). Multiscale 3D Phenotyping of Human Cerebral Organoids. Sci. Rep. 10 (1), 21487. 10.1038/s41598-020-78130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algarroba G. N., Rekawek P., Vahanian S. A., Khullar P., Palaia T., Peltier M. R., et al. (2020). Visualization of Severe Acute Respiratory Syndrome Coronavirus 2 Invading the Human Placenta Using Electron Microscopy. Am. J. Obstet. Gynecol. 223 (2), 275–278. 10.1016/j.ajog.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. (2020). Clinical Manifestations, Risk Factors, and Maternal and Perinatal Outcomes of Coronavirus Disease 2019 in Pregnancy: Living Systematic Review and Meta-Analysis. BMJ 370, m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allswede D. M., Yolken R. H., Buka S. L., Cannon T. D. (2020). Cytokine Concentrations Throughout Pregnancy and Risk for Psychosis in Adult Offspring: A Longitudinal Case-Control Study. Lancet Psychiatry 7 (3), 254–261. 10.1016/s2215-0366(20)30006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri A., Coppola G., Scuderi S., Wu F., Roychowdhury T., Liu F., et al. (2018). Transcriptome and Epigenome Landscape of Human Cortical Development Modeled in Organoids. Science 362 (6420), eaat6720. 10.1126/science.aat6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M. G., Mukhtar T., Eze U. C., Simoneau C. R., Perez Y., Mostajo-Radji M. A., et al. (2021). Tropism of SARS-CoV-2 for Developing Human Cortical Astrocytes. bioRxiv. 10.1101/2021.01.17.427024 [DOI] [Google Scholar]
- Ao Z., Cai H., Havert D. J., Wu Z., Gong Z., Beggs J. M., et al. (2020). One-Stop Microfluidic Assembly of Human Brain Organoids to Model Prenatal Cannabis Exposure. Anal. Chem. 92 (6), 4630–4638. 10.1021/acs.analchem.0c00205 [DOI] [PubMed] [Google Scholar]
- Aoyama Y., Toriumi K., Mouri A., Hattori T., Ueda E., Shimato A., et al. (2016). Prenatal Nicotine Exposure Impairs the Proliferation of Neuronal Progenitors, Leading to Fewer Glutamatergic Neurons in the Medial Prefrontal Cortex. Neuropsychopharmacology, 41 (2), 578–589. 10.1038/npp.2015.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple F. S., Roe S. J. (1990). Cocaine-Associated Fetal Death In Utero. J. Anal. Toxicol. 14 (4), 259–260. 10.1093/jat/14.4.259 [DOI] [PubMed] [Google Scholar]
- Arzua T., Yan Y., Jiang C., Logan S., Allison R. L., Wells C., et al. (2020). Modeling Alcohol-Induced Neurotoxicity Using Human Induced Pluripotent Stem Cell-Derived Three-Dimensional Cerebral Organoids. Transl. Psychiatry 10 (1), 347. 10.1038/s41398-020-01029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashary N., Bhide A., Chakraborty P., Colaco S., Mishra A., Chhabria K., et al. (2020). Single-Cell RNA-seq Identifies Cell Subsets in Human Placenta that Highly Expresses Factors Driving Pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 8, 783. 10.3389/fcell.2020.00783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgil M., Ornoy A. (2006). Herpes Simplex Virus and Epstein-Barr Virus Infections in Pregnancy: Consequences of Neonatal or Intrauterine Infection. Reprod. Toxicol. 21 (4), 436–445. 10.1016/j.reprotox.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Avila E. C., Finger-Jardim F., Gonçalves C. V., da Hora V. P., Soares M. A., Martínez A. M. B. (2020). High Incidence of Herpes Simplex Virus-1 in Cord Blood and Placenta Infection of Women in Southern Brazil. Rev. Bras. Ginecol. Obstet. 42 (1), 5–11. 10.1055/s-0039-1700794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanlaja A. A., Xiong Y., Gao Y., Ji G., Tang C., Abdikani Abdullah Z., et al. (2017). Distinct Features of Doublecortin as a Marker of Neuronal Migration and Its Implications in Cancer Cell Mobility. Front. Mol. Neurosci. 10, 199. 10.3389/fnmol.2017.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badai J., Bu Q., Zhang L. (2020). Review of Artificial Intelligence Applications and Algorithms for Brain Organoid Research. Interdiscip. Sci. Comput. Life Sci. 12 (4), 383–394. 10.1007/s12539-020-00386-4 [DOI] [PubMed] [Google Scholar]
- Baggiani M., Dell’Anno M. T., Pistello M., Conti L., Onorati M. (2020). Human Neural Stem Cell Systems to Explore Pathogen-Related Neurodevelopmental and Neurodegenerative Disorders. Cells 9 (8), 1893. 10.3390/cells9081893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J. A., Reumann D., Bian S., Lévi-Strauss J., Knoblich J. A. (2017). Fused Cerebral Organoids Model Interactions Between Brain Regions. Nat. Methods 14 (7), 743–751. 10.1038/nmeth.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson R., Calder A. A., Edwards C. R. W., Seckl J. R. (1997). Placental 11β-Hydroxysteroid Dehydrogenase: A Key Regulator of Fetal Glucocorticoid Exposure. Clin. Endocrinol. 46 (2), 161–166. 10.1046/j.1365-2265.1997.1230939.x [DOI] [PubMed] [Google Scholar]
- Benito-Kwiecinski S., Giandomenico S. L., Sutcliffe M., Riis E. S., Freire-Pritchett P., Kelava I., et al. (2021). An Early Cell Shape Transition Drives Evolutionary Expansion of the Human Forebrain. Cell 184, 2084–2102.e19. 10.1016/j.cell.2021.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J. R., Houff S. (2008). Neurological Complications of Herpes Simplex Virus Type 2 Infection. Arch. Neurol. 65 (5), 596–600. 10.1001/archneur.65.5.596 [DOI] [PubMed] [Google Scholar]
- Betizeau M., Cortay V., Patti D., Pfister S., Gautier E., Bellemin-Ménard A., et al. (2013). Precursor Diversity and Complexity of Lineage Relationships in the Outer Subventricular Zone of the Primate. Neuron 80 (2), 442–457. 10.1016/j.neuron.2013.09.032 [DOI] [PubMed] [Google Scholar]
- Bevelander G., Nakahara H., Rolle G. K. (1960). The Effect of Tetracycline on the Development of the Skeletal System of the Chick Embryo. Dev. Biol. 2 (3), 298–312. 10.1016/0012-1606(60)90011-7 [DOI] [PubMed] [Google Scholar]
- Bhaduri A., Andrews M. G., Mancia Leon W., Jung D., Shin D., Allen D., et al. (2020). Cell Stress in Cortical Organoids Impairs Molecular Subtype Specification. Nature 578 (7793), 142–148. 10.1038/s41586-020-1962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan M., Petropoulos S., Gibb W., Matthews S. G. (2012). Sertraline Alters Multidrug Resistance Phosphoglycoprotein Activity in the Mouse Placenta and Fetal Blood–Brain Barrier. Reprod. Sci. 19 (4), 407–415. 10.1177/1933719111424438 [DOI] [PubMed] [Google Scholar]
- Bloise E., Zhang J., Nakpu J., Hamada H., Dunk C. E., Li S., et al. (2020). Expression of Severe Acute Respiratory Syndrome Coronavirus 2 Cell Entry Genes, Angiotensin-Converting Enzyme 2 and Transmembrane Protease Serine 2, in the Placenta across Gestation and at the Maternal-Fetal Interface in Pregnancies Complicated by Preterm. Am. J. Obstet. Gynecol. 224 (3), 298.e1–298.e8. 10.1016/j.ajog.2020.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell C., Tess E. R., Schanzer A. S. R., Coutifaris C., Su E. J., Parry S., et al. (2016). A Microphysiological Model of the Human Placental Barrier. Lab Chip 16 (16), 3065–3073. 10.1039/c6lc00259e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert E. M., Means R. E., Michaud M., Madri J. A., Katz S. G. (2019). Minocycline Mitigates the Effect of Neonatal Hypoxic Insult on Human Brain Organoids. Cell Death Dis. 10 (4), 325. 10.1038/s41419-019-1553-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Rana P. S. J. B., Jaeger H. K., O’Dowd J. M., Balemba O. B., Fortunato E. A. (2019). Human Cytomegalovirus Compromises Development of Cerebral Organoids. J. Virol. 93 (17), e00957–19. 10.1128/jvi.00957-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas A., Borrell V. (2020). Molecular and Cellular Evolution of Corticogenesis in Amniotes. Cell Mol. Life Sci. 77 (8), 1435–1460. 10.1007/s00018-019-03315-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B., Xiang Y., Tanaka Y., Kural M. H., Parent M., Kang Y.-J., et al. (2019). Engineering of Human Brain Organoids With a Functional Vascular-Like System. Nat. Methods 16 (11), 1169–1175. 10.1038/s41592-019-0586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp J. G., Badsha F., Florio M., Kanton S., Gerber T., Wilsch-Bräuninger M., et al. (2015). Human Cerebral Organoids Recapitulate Gene Expression Programs of Fetal Neocortex Development. Proc. Natl. Acad. Sci. 112 (51), 15672–15677. 10.1073/pnas.1520760112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J. C., Yokota H., Craven R. C., Schmitt A., Wills J. W. (2018). The HSV-1 Mechanisms of Cell-To-Cell Spread and Fusion are Critically Dependent on Host PTP1B. PLOS Pathog. 14 (5), e1007054. 10.1371/journal.ppat.1007054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran M. C. J., Hu S., Ni H. T., Sheng W., Palmquist J. M., Peterson P. K., et al. (2005). Neural Precursor Cell Susceptibility to Human Cytomegalovirus Diverges along Glial or Neuronal Differentiation Pathways. J. Neurosci. Res. 82 (6), 839–850. 10.1002/jnr.20682 [DOI] [PubMed] [Google Scholar]
- Cosset É., Martinez Y., Preynat-Seauve O., Lobrinus J.-A., Tapparel C., Cordey S., et al. (2015). Human Three-Dimensional Engineered Neural Tissue Reveals Cellular and Molecular Events Following Cytomegalovirus Infection. Biomaterials 53, 296–308. 10.1016/j.biomaterials.2015.02.094 [DOI] [PubMed] [Google Scholar]
- Cruceanu C., Dony L., Krontira A. C., Fischer D. S., Roeh S., Di Giaimo R., et al. (2020). Cell-Type Specific Impact of Glucocorticoid Receptor Activation on the Developing Brain. bioRxiv. 10.1101/2020.01.09.897868 [DOI] [PubMed] [Google Scholar]
- Cugola F. R., Fernandes I. R., Russo F. B., Freitas B. C., Dias J. L. M., Guimarães K. P., et al. (2016). The Brazilian Zika Virus Strain Causes Birth Defects in Experimental Models. Nature 534 (7606), 267–271. 10.1038/nature18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. L., Martinez-Cerdeno V., Noctor S. C. (2013). Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J. Neurosci. 33 (10), 4216–4233. 10.1523/jneurosci.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aiuto L., Bloom D. C., Naciri J. N., Smith A., Edwards T. G., McClain L., et al. (2019). Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived From Induced Pluripotent Stem Cells. J. Virol. 93 (9), e00111–19. 10.1128/jvi.00111-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakic V., Minardi Nascimento J., Costa Sartore R., Maciel R. M., de Araujo D. B., Ribeiro D. B., et al. (2017). Short Term Changes in the Proteome of Human Cerebral Organoids Induced by 5-MeO-DMT. Sci. Rep. 7 (1), 12863. 10.1038/s41598-017-12779-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J., Tiwari S. K., Lichinchi G., Qin Y., Patil V. S., Eroshkin A. M., et al. (2016). Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids Through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 19 (2), 258–265. 10.1016/j.stem.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daood M., Tsai C., Ahdab-Barmada M., Watchko J. (2008). ABC Transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) Expression in the Developing Human CNS. Neuropediatrics 39 (4), 211–218. 10.1055/s-0028-1103272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviaud N., Chevalier C., Friedel R. H., Zou H. (2019). Distinct Vulnerability and Resilience of Human Neuroprogenitor Subtypes in Cerebral Organoid Model of Prenatal Hypoxic Injury. Front. Cell Neurosci. 13, 336. 10.3389/fncel.2019.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fatima Vasco Aragao M., Van Der Linden V., Brainer-Lima A. M., Coeli R. R., Rocha M. A., Sobral Da Silva P., et al. (2016). Clinical Features and Neuroimaging (CT and MRI) Findings in Presumed Zika Virus Related Congenital Infection and Microcephaly: Retrospective Case Series Study. BMJ 353, i1901. 10.1136/bmj.i1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Trizio I., Errede M., D'Amati A., Girolamo F., Virgintino D. (2020). Expression of P-Gp in Glioblastoma: What we Can Learn From Brain Development. Curr. Pharm. Des. 26 (13), 1428–1437. 10.2174/1381612826666200318130625 [DOI] [PubMed] [Google Scholar]
- Díaz-Alonso J., Guzmán M., Galve-Roperh I. (2012). Endocannabinoids via CB 1 Receptors Act as Neurogenic Niche Cues During Cortical Development. Philos. Trans. R. Soc. B: Biol. Sci. 367 (1607), 3229–3241. 10.1098/rstb.2011.0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro J. A. (2012). Maternal Stress in Pregnancy: Considerations for Fetal Development. J. Adolesc. Health 51 (2), S3–S8. 10.1016/j.jadohealth.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadoni M., Cicalese S., Sarkar D. K., Chang S. L., Sariyer I. K.. (2019). Alcohol Exposure Alters Pre-mRNA Splicing of Antiapoptotic Mcl-1L Isoform and Induces Apoptosis in Neural Progenitors and Immature Neurons. Cell Death Dis 10 (6), 447. 10.1038/s41419-019-1673-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman D. C., Allen S. L., Byczkowski J. Z., Claudio L., Fisher J. E., Fisher J. W., et al. (2001). Methods to Identify and Characterize Developmental Neurotoxicity for Human Health Risk Assessment. III: Pharmacokinetic and Pharmacodynamic Considerations. Environ. Health Perspect. 109 (Suppl. 1), 101–111. 10.1289/ehp.01109s110110.2307/3434851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecob-Johnston M. S., Whetsell W. O. (1979). Host-Cell Response to Herpes Virus Infection in Central and Peripheral Nervous Tissue In Vitro . J. Gen. Virol. 44 (3), 747–757. 10.1099/0022-1317-44-3-747 [DOI] [PubMed] [Google Scholar]
- Ek C. J., Dziegielewska K. M., Habgood M. D., Saunders N. R. (2012). Barriers in the Developing Brain and Neurotoxicology. NeuroToxicology 33 (3), 586–604. 10.1016/j.neuro.2011.12.009 [DOI] [PubMed] [Google Scholar]
- EMCDDA (2018). Statistical Bulletin 2018 — Prevalence of Drug Use. Available at: https://www.emcdda.europa.eu/data/stats2018/gps_en.
- Enders G., Daiminger A., Bäder U., Exler S., Enders M. (2011). Intrauterine Transmission and Clinical Outcome of 248 Pregnancies With Primary Cytomegalovirus Infection in Relation to Gestational Age. J. Clin. Virol. 52 (3), 244–246. 10.1016/j.jcv.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Esk C., Lindenhofer D., Haendeler S., Wester R. A., Pflug F., Schroeder B., et al. (2020). A Human Tissue Screen Identifies a Regulator of ER Secretion as a Brain-Size Determinant. Science 370 (6519), 935–941. 10.1126/science.abb5390 [DOI] [PubMed] [Google Scholar]
- Faa G., Manchia M., Pintus R., Gerosa C., Marcialis M. A., Fanos V. (2016). Fetal Programming of Neuropsychiatric Disorders. Birth Defects Res. C Embryo Today 108 (3), 207–223. 10.1002/bdrc.21139 [DOI] [PubMed] [Google Scholar]
- Fagerlund I., Dougalis A., Shakirzyanova A., Gomez-Budia M., Konttinen H., Ohtonen S., et al. (2020). Microglia Orchestrate Neuronal Activity in Brain Organoids. bioRxiv. 10.1101/2020.12.08.416388 [DOI] [Google Scholar]
- Farrar H. C., Kearns G. L. (1989). Cocaine: Clinical Pharmacology and Toxicology. J. Pediatr. 115 (5), 665–675. 10.1016/s0022-3476(89)80640-7 [DOI] [PubMed] [Google Scholar]
- Fiddes I. T., Lodewijk G. A., Mooring M., Bosworth C. M., Ewing A. D., Mantalas G. L., et al. (2018). Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 173 (6), 1356–1369.e22. 10.1016/j.cell.2018.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz S. A., Kelava I., Vogt J., Wilsch-Bräuninger M., Stenzel D., Fish J. L., et al. (2010). OSVZ Progenitors of Human and Ferret Neocortex are Epithelial-Like and Expand by Integrin Signaling. Nat. Neurosci. 13 (6), 690–699. 10.1038/nn.2553 [DOI] [PubMed] [Google Scholar]
- Fisher S., Genbacev O., Maidji E., Pereira L. (2000). Human Cytomegalovirus Infection of Placental Cytotrophoblasts In Vitro and In Utero: Implications for Transmission and Pathogenesis. J. Virol. 74 (15), 6808–6820. 10.1128/jvi.74.15.6808-6820.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E., Wason A., Ramani A., Gooi L. M., Keller P., Pozniakovsky A., et al. (2016). CPAP Promotes Timely Cilium Disassembly to Maintain Neural Progenitor Pool. EMBO J. 35 (8), 803–819. 10.15252/embj.201593679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E., Ramani A., Karow U., Gottardo M., Natarajan K., Gooi L. M., et al. (2017). Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 20 (3), 397–406.e5. 10.1016/j.stem.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Garcez P. P., Loiola E. C., Madeiro Da Costa R., Higa L. M., Trindade P., Delvecchio R., et al. (2016). Zika Virus Impairs Growth in Human Neurospheres and Brain Organoids. Science 352 (6287), 816–818. 10.1126/science.aaf6116 [DOI] [PubMed] [Google Scholar]
- Giaccia A. J. (2004). The Biology of Hypoxia: The Role of Oxygen Sensing in Development, Normal Function, and Disease. Genes Dev. 18 (18), 2183–2194. 10.1101/gad.1243304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico S. L., Sutcliffe M., Lancaster M. A. (2021). Generation and Long-Term Culture of Advanced Cerebral Organoids for Studying Later Stages of Neural Development. Nat. Protoc. 16 (2), 579–602. 10.1038/s41596-020-00433-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goasdoué K., Miller S. M., Colditz P. B., Björkman S. T. (2017). Review: The Blood-Brain Barrier; Protecting the Developing Fetal Brain. Placenta 54, 111–116. 10.1016/j.placenta.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Lui J. H., Parker P. R. L., Kriegstein A. R. (2010). Neurogenic Radial Glia in the Outer Subventricular Zone of Human Neocortex. Nature 464 (7288), 554–561. 10.1038/nature08845 [DOI] [PubMed] [Google Scholar]
- Hosier H., Farhadian S. F., Morotti R. A., Deshmukh U., Lu-Culligan A., Campbell K. H., et al. (2020). SARS–CoV-2 Infection of the Placenta. J. Clin. Invest. 130 (9), 4947–4953. 10.1172/jci139569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton C. L., Bale T. L. (2012). Prenatal Programing: At the Intersection of Maternal Stress and Immune Activation. Horm. Behav. 62 (3), 237–242. 10.1016/j.yhbeh.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink A. C. (2014). Prenatal Cannabis Exposure and Infant Outcomes: Overview of Studies. Prog. Neuropsychopharmacology Biol. Psychiatry 52, 45–52. 10.1016/j.pnpbp.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Hutchings D. E., Martin B. R., Gamagaris Z., Miller N., Fico T. (1989). Plasma Concentrations of Delta-9-Tetrahydrocannabinol in Dams and Fetuses Following Acute or Multiple Prenatal Dosing in Rats. Life Sci. 44 (11), 697–701. 10.1016/0024-3205(89)90380-9 [DOI] [PubMed] [Google Scholar]
- Hutto C., Arvin A., Jacobs R., Steele R., Stagno S., Lyrene R., et al. (1987). Intrauterine Herpes Simplex Virus Infections. J. Pediatr. 110 (1), 97–101. 10.1016/s0022-3476(87)80298-6 [DOI] [PubMed] [Google Scholar]
- Jacob F., Pather S. R., Huang W.-K., Zhang F., Wong S. Z. H., Zhou H., et al. (2020). Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 27 (6), 937–950.e939. 10.1016/j.stem.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C., Harfouche M., Welton N. J., Turner K. M., Abu-Raddad L. J., Gottlieb S. L., et al. (2020). Herpes Simplex Virus: Global Infection Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 98, 315–329. 10.2471/blt.19.237149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen G. M., Moore L. G. (1997). The Effect of High Altitude and Other Risk Factors on Birthweight: Independent or Interactive Effects?. Am. J. Public Health 87 (6), 1003–1007. 10.2105/ajph.87.6.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joya X., Pujadas M., Falcón M., Civit E., Garcia-Algar O., Vall O., et al. (2010). Gas Chromatography–Mass Spectrometry Assay for the Simultaneous Quantification of Drugs of Abuse in Human Placenta at 12th Week of Gestation. Forensic Sci. Int. 196 (1–3), 38–42. 10.1016/j.forsciint.2009.12.044 [DOI] [PubMed] [Google Scholar]
- Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M., et al. (2013). Self-Organization of Axial Polarity, Inside-Out Layer Pattern, and Species-Specific Progenitor Dynamics in Human ES Cell-Derived Neocortex. Proc. Natl. Acad. Sci. 110 (50), 20284–20289. 10.1073/pnas.1315710110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish B. T., Kim E., Finander B., Duffy E. E., Kim H., Gilman C. K., et al. (2021). Maternal Immune Activation in Mice Disrupts Proteostasis in the Fetal Brain. Nat. Neurosci. 24 (2), 204–213. 10.1038/s41593-020-00762-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanton S., Boyle M. J., He Z., Santel M., Weigert A., Sanchís-Calleja F., et al. (2019). Organoid Single-Cell Genomic Atlas Uncovers Human-Specific Features of Brain Development. Nature 574 (7778), 418–422. 10.1038/s41586-019-1654-9 [DOI] [PubMed] [Google Scholar]
- Kegeles E., Naumov A., Karpulevich E. A., Volchkov P., Baranov P. (2020). Convolutional Neural Networks Can Predict Retinal Differentiation in Retinal Organoids. Front. Cell Neurosci. 14, 171. 10.3389/fncel.2020.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakipoor S., Crouch E. E., Mayer S. (2020). Human Organoids to Model the Developing Human Neocortex in Health and Disease. Brain Res. 1742, 146803. 10.1016/j.brainres.2020.146803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Gomes J. (2020). Neuropathogenesis of SARS-CoV-2 Infection. eLife 9, e59136. 10.7554/elife.59136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack D. R., Rakic P. (1998). Changes in Cell-Cycle Kinetics During the Development and Evolution of Primate Neocortex. Proc. Natl. Acad. Sci. 95 (3), 1242–1246. 10.1073/pnas.95.3.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracht L., Borggrewe M., Eskandar S., Brouwer N., Chuva De Sousa Lopes S. M., Laman J. D., et al. (2020). Human Fetal Microglia Acquire Homeostatic Immune-Sensing Properties Early in Development. Science 369 (6503), 530–537. 10.1126/science.aba5906 [DOI] [PubMed] [Google Scholar]
- Krontira A. C., Cruceanu C., Binder E. B. (2020). Glucocorticoids as Mediators of Adverse Outcomes of Prenatal Stress. Trends Neurosci. 43 (6), 394–405. 10.1016/j.tins.2020.03.008 [DOI] [PubMed] [Google Scholar]
- La Manno G., Siletti K., Furlan A., Gyllborg D., Vinsland E., Langseth C. M., et al. (2020). Molecular Architecture of the Developing Mouse Brain. bioRxiv. 10.1101/2020.07.02.184051 [DOI] [PubMed] [Google Scholar]
- Lähnemann D., Köster J., Szczurek E., McCarthy D. J., Hicks S. C., Robinson M. D., et al. (2020). Eleven Grand Challenges in Single-Cell Data Science. Genome Biol., 21 (1), 31. 10.1186/s13059-020-1926-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Baello S., Iqbal M., Kelly L. E., Shannon P. T., Chitayat D., et al. (2015). The Ontogeny of P-Glycoprotein in the Developing Human Blood–Brain Barrier: Implication for Opioid Toxicity in Neonates. Pediatr. Res. 78 (4), 417–421. 10.1038/pr.2015.119 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Knoblich J. A. (2014). Generation of Cerebral Organoids From Human Pluripotent Stem Cells. Nat. Protoc. 9 (10), 2329–2340. 10.1038/nprot.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., et al. (2013). Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 501 (7467), 373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M. A., Corsini N. S., Wolfinger S., Gustafson E. H., Phillips A. W., Burkard T. R., et al. (2017). Guided Self-Organization and Cortical Plate Formation in Human Brain Organoids. Nat. Biotechnol. 35 (7), 659–666. 10.1038/nbt.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Turrero Garcia M., Decimo I., Bifari F., Eelen G., Quaegebeur A., et al. (2016). Relief of Hypoxia by Angiogenesis Promotes Neural Stem Cell Differentiation by Targeting Glycolysis. EMBO J. 35 (9), 924–941. 10.15252/embj.201592372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-T., Chen J., Hayashi T., Tsai S.-Y., Sanchez J. F., Errico S. L., et al. (2008). A Mechanism for the Inhibition of Neural Progenitor Cell Proliferation by Cocaine. PLoS Med. 5 (6), e117. 10.1371/journal.pmed.0050117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-T., Chen J., Worden L. T., Freed W. J. (2011). Cocaine Causes Deficits in Radial Migration and Alters the Distribution of Glutamate and GABA Neurons in the Developing Rat Cerebral Cortex. Synapse 65 (1), 21–34. 10.1002/syn.20814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-T., Chen J., Kindberg A. A., Bendriem R. M., Spivak C. E., Williams M. P., et al. (2017). CYP3A5 Mediates Effects of Cocaine on Human Neocorticogenesis: Studies Using an In Vitro 3D Self-Organized hPSC Model with a Single Cortex-like Unit. Neuropsychopharmacology 42 (3), 774–784. 10.1038/npp.2016.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen M. K., Zappaterra M. W., Chen X., Yang Y. J., Hill A. D., Lun M., et al. (2011). The Cerebrospinal Fluid Provides a Proliferative Niche for Neural Progenitor Cells. Neuron 69 (5), 893–905. 10.1016/j.neuron.2011.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow M. S., Song Z.-M. (2001). Primates Exposed to Cocaine In Utero Display Reduced Density and Number of Cerebral Cortical Neurons. J. Comp. Neurol. 435 (3), 263–275. 10.1002/cne.1028 [DOI] [PubMed] [Google Scholar]
- Lidow M. S., Bozian D., Song Z.-M. (2001). Cocaine Affects Cerebral Neocortical Cytoarchitecture in Primates Only if Administered During Neocortical Neuronogenesis. Dev. Brain Res. 128 (1), 45–52. 10.1016/s0165-3806(01)00139-0 [DOI] [PubMed] [Google Scholar]
- Linsley J. W., Tripathi A., Epstein I., Schmunk G., Mount E., Campioni M., et al. (2019). Automated Four-Dimensional Long Term Imaging Enables Single Cell Tracking within Organotypic Brain Slices to Study Neurodevelopment and Degeneration. Commun. Biol. 2, 115. 10.1038/s42003-019-0411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker K. J., Magaret A. S., May M. T., Turner K. M. E., Vickerman P., Newman L. M., et al. (2017). First Estimates of the Global and Regional Incidence of Neonatal Herpes Infection. Lancet Glob. Health 5 (3), e300–e309. 10.1016/s2214-109x(16)30362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck W., Nau H., Hansen R., Steldinger R. (1985). Extent of Nicotine and Cotinine Transfer to the Human Fetus, Placenta and Amniotic Fluid of Smoking Mothers. Dev. Pharmacol. Ther. 8, 384–395. 10.1159/000457063 [DOI] [PubMed] [Google Scholar]
- Lui J. H., Hansen D. V., Kriegstein A. R. (2011). Development and Evolution of the Human Neocortex. Cell 146 (1), 18–36. 10.1016/j.cell.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D., Ivanov A., Ferrai C., Jordan P., Guneykaya D., Buonfiglioli A., et al. (2017). Maternal Immune Activation Results in Complex Microglial Transcriptome Signature in the Adult Offspring That is Reversed by Minocycline Treatment. Transl. Psychiatry 7 (5), e1120. 10.1038/tp.2017.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S., Chen J., Velmeshev D., Mayer A., Eze U. C., Bhaduri A., et al. (2019). Multimodal Single-Cell Analysis Reveals Physiological Maturation in the Developing Human Neocortex. Neuron 102 (1), 143–158.e7. 10.1016/j.neuron.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G., Fa M., Ferraro L., Cagiano R., Antonelli T., Tattoli M., et al. (2003). Prenatal Exposure to a Cannabinoid Agonist Produces Memory Deficits Linked to Dysfunction in Hippocampal Long-Term Potentiation and Glutamate Release. Proc. Natl. Acad. Sci. 100 (8), 4915–4920. 10.1073/pnas.0537849100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesci P., Macia A., Saleh A., Martin-Sancho L., Yin X., Snethlage C., et al. (2020). Sofosbuvir Protects Human Brain Organoids against SARS-CoV-2. bioRxiv. 10.1101/2020.05.30.125856 [DOI] [Google Scholar]
- Meyer G. (2010). Building a Human Cortex: the Evolutionary Differentiation of Cajal-Retzius Cells and the Cortical Hem. J. Anat. 217 (4), 334–343. 10.1111/j.1469-7580.2010.01266.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. (2019). Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci. 42 (11), 793–806. 10.1016/j.tins.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Møllgård K., Saunders N. (1975). Complex Tight Junctions of Epithelial and of Endothelial Cells in Early Foetal Brain. J. Neurocytol. 4 (4), 453–468. 10.1007/BF01261375 [DOI] [PubMed] [Google Scholar]
- Møllgård K., Saunders N. R. (1986). The Development of the Human Blood-Brain and Blood-CSF Barriers. Neuropathol. Appl. Neurobiol. 12 (4), 337–358. 10.1111/j.1365-2990.1986.tb00146.x [DOI] [PubMed] [Google Scholar]
- Møllgård K., Balslev Y., Lauritzen B., Saunders N. R. (1987). Cell Junctions and Membrane Specializations in the Ventricular Zone (Germinal Matrix) of the Developing Sheep Brain: A CSF-Brain Barrier. J. Neurocytol. 16 (4), 433–444. 10.1007/bf01668498 [DOI] [PubMed] [Google Scholar]
- Monier A., Adle-Biassette H., Delezoide A.-L., Evrard P., Gressens P., Verney C. (2007). Entry and Distribution of Microglial Cells in Human Embryonic and Fetal Cerebral Cortex. J. Neuropathol. Exp. Neurol. 66 (5), 372–382. 10.1097/nen.0b013e3180517b46 [DOI] [PubMed] [Google Scholar]
- Monnot A. D., Zheng W. (2012). Culture of Choroid Plexus Epithelial Cells and In Vitro Model of Blood–CSF Barrier. Epithelial Cell Culture Protocols: Second Edition, In Editors Randell S. H. & Fulcher M. L. Humana Press, 13–29. 10.1007/978-1-62703-125-7_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Bermúdez F., Badsha F., Kanton S., Camp J. G., Vernot B., Köhler K., et al. (2016). Differences and Similarities Between Human and Chimpanzee Neural Progenitors During Cerebral Cortex Development. Elife 5, e18683. 10.7554/elife.18683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B. R., Bale T. L. (2008). Sex-Specific Programming of Offspring Emotionality After Stress Early in Pregnancy. J. Neurosci. 28 (36), 9055–9065. 10.1523/jneurosci.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder E. J. H., Robles De Medina P. G., Huizink A. C., Van Den Bergh B. R. H., Buitelaar J. K., Visser G. H. A. (2002). Prenatal Maternal Stress: Effects on Pregnancy and the (Unborn) Child. Early Hum. Dev. 70 (1–2), 3–14. 10.1016/s0378-3782(02)00075-0 [DOI] [PubMed] [Google Scholar]
- Muoth C., Wichser A., Monopoli M., Correia M., Ehrlich N., Loeschner K., et al. (2016). A 3D Co-Culture Microtissue Model of the Human Placenta for Nanotoxicity Assessment. Nanoscale 8 (39), 17322–17332. 10.1039/c6nr06749b [DOI] [PubMed] [Google Scholar]
- Nimkarn S., New M. I. (2007). Prenatal Diagnosis and Treatment of Congenital Adrenal Hyperplasia. Horm. Res. 67 (2), 53–60. 10.1159/000096353 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Flint A. C., Weissman T. A., Dammerman R. S., Kriegstein A. R. (2001). Neurons Derived from Radial Glial Cells Establish Radial Units in Neocortex. Nature 409 (6821), 714–720. 10.1038/35055553 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Martínez-Cerdeño V., Ivic L., Kriegstein A. R. (2004). Cortical Neurons Arise in Symmetric and Asymmetric Division Zones and Migrate through Specific Phases. Nat. Neurosci. 7 (2), 136–144. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Nowakowski T. J., Pollen A., Di Lullo E., Sandoval-Espinosa C., Bershteyn M., Kriegstein A. R. (2016a). Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell 18 (5), 591–596. 10.1016/j.stem.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski T. J., Pollen A., Sandoval-Espinosa C., Kriegstein A. R. (2016b). Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 91 (6), 1219–1227. 10.1016/j.neuron.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski T. J., Bhaduri A., Pollen A. A., Alvarado B., Mostajo-Radji M. A., Di Lullo E., et al. (2017). Spatiotemporal Gene Expression Trajectories Reveal Developmental Hierarchies of the Human Cortex. Science 358 (6368), 1318–1323. 10.1126/science.aap8809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg J., Wolmer N., Falci S., Westgren M., Seiger A. K., Söderberg-Nauclér C. (2006). Human Cytomegalovirus Inhibits Neuronal Differentiation and Induces Apoptosis in Human Neural Precursor Cells. J. Virol. 80 (18), 8929–8939. 10.1128/jvi.00676-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S., Reingle Gonzalez J. M., Salas-Wright C. P., Vaughn M. G., Dinitto D. M. (2017). Prevalence and Correlates of Alcohol and Tobacco Use Among Pregnant Women in the United States: Evidence from the NSDUH 2005–2014. Prev. Med. 97, 93–99. 10.1016/j.ypmed.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Onorati M., Li Z., Liu F., Sousa A. M. M., Nakagawa N., Li M., et al. (2016). Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 16 (10), 2576–2592. 10.1016/j.celrep.2016.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca A. M., Sloan S. A., Clarke L. E., Tian Y., Makinson C. D., Huber N., et al. (2015). Functional Cortical Neurons and Astrocytes From Human Pluripotent Stem Cells in 3D Culture. Nat. Methods 12 (7), 671–678. 10.1038/nmeth.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca A. M., Park J.-Y., Shin H.-W., Qi Q., Revah O., Krasnoff R., et al. (2019). Human 3D Cellular Model of Hypoxic Brain Injury of Prematurity. Nat. Med. 25 (5), 784–791. 10.1038/s41591-019-0436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanè L., Morotti D., Giunta M. R., Sigismondi C., Piccoli M. G., Frigerio L., et al. (2020). Vertical Transmission of Coronavirus Disease 2019: Severe Acute Respiratory Syndrome Coronavirus 2 RNA on the Fetal Side of the Placenta in Pregnancies with Coronavirus Disease 2019–positive Mothers and Neonates at Birth. Am J Obstet Gynecol MFM 2 (3), 100145. 10.1016/j.ajogmf.2020.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Peng J., Behl M., Sipes N. S., Shockley K. R., Rao M. S., et al. (2016). Comparative Neurotoxicity Screening in Human iPSC-Derived Neural Stem Cells, Neurons and Astrocytes. Brain Res. 1638, 57–73. 10.1016/j.brainres.2015.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L., Albecka A., Mallery D. L., Kellner M. J., Paul D., Carter A. P., et al. (2020a). SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 27 (6), 951–961.e5. 10.1016/j.stem.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L., Bonfio C., Chadwick J., Begum F., Skehel M., Lancaster M. A. (2020b). Human CNS Barrier-Forming Organoids with Cerebrospinal Fluid Production. Science 369 (6500), eaaz5626. 10.1126/science.aaz5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. (2018). Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 5 (1), 273–299. 10.1146/annurev-virology-092917-043236 [DOI] [PubMed] [Google Scholar]
- Petropoulos S., Gibb W., Matthews S. G. (2010). Developmental Expression of Multidrug Resistance Phosphoglycoprotein (P-gp) in the Mouse Fetal Brain and Glucocorticoid Regulation. Brain Res. 1357, 9–18. 10.1016/j.brainres.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Pham M. T., Pollock K. M., Rose M. D., Cary W. A., Stewart H. R., Zhou P., et al. (2018). Generation of Human Vascularized Brain Organoids. NeuroReport 29 (7), 588–593. 10.1097/wnr.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique-Regi R., Romero R., Tarca A. L., Luca F., Xu Y., Alazizi A., et al. (2020). Does the Human Placenta Express the Canonical Cell Entry Mediators for SARS-CoV-2?. Elife 9, e58716. 10.7554/elife.58716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen A. A., Nowakowski T. J., Chen J., Retallack H., Sandoval-Espinosa C., Nicholas C. R., et al. (2015). Molecular Identity of Human Outer Radial Glia during Cortical Development. Cell 163 (1), 55–67. 10.1016/j.cell.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen A. A., Bhaduri A., Andrews M. G., Nowakowski T. J., Meyerson O. S., Mostajo-Radji M. A., et al. (2019). Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 176 (4), 743–756.e17. 10.1016/j.cell.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S., Lange S., Probst C., Gmel G., Rehm J. (2017). Estimation of National, Regional, and Global Prevalence of Alcohol Use during Pregnancy and Fetal Alcohol Syndrome: A Systematic Review and Meta-Analysis. Lancet Glob. Health 5 (3), e290–e299. 10.1016/s2214-109x(17)30021-9 [DOI] [PubMed] [Google Scholar]
- Preynat-Seauve O., Suter D. M., Tirefort D., Turchi L., Virolle T., Chneiweiss H., et al. (2009). Development Of Human Nervous Tissue Upon Differentiation Of Embryonic Stem Cells in Three-Dimensional Culture. Stem Cells 27 (3), 509–520. 10.1634/stemcells.2008-060010.1002/stem.20080600 [DOI] [PubMed] [Google Scholar]
- Qian X., Nguyen H. N., Song M. M., Hadiono C., Ogden S. C., Hammack C., et al. (2016). Brain-Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 165 (5), 1238–1254. 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Song H., Ming G.-L. (2019). Brain Organoids: Advances, Applications and Challenges. Development 146 (8), dev166074. 10.1242/dev.166074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Su Y., Adam C. D., Deutschmann A. U., Pather S. R., Goldberg E. M., et al. (2020). Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell 26 (5), 766–781.e9. 10.1016/j.stem.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Guo M., Shang J., Zhao W., Wang Z., Liu N., et al. (2020). Herpes Simplex Virus Type 1 Infection Leads to Neurodevelopmental Disorder-Associated Neuropathological Changes. PLOS Pathog. 16 (10), e1008899. 10.1371/journal.ppat.1008899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G., Nguyen T., Macosko E. Z., Sherwood J. L., Min Yang S., Berger D. R., et al. (2017). Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 545 (7652), 48–53. 10.1038/nature22047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Müller L., Ostermann P. N., Gabriel E., Abida-Islam P., Müller-Schiffmann A., et al. (2020). SARS -CoV-2 Targets Neurons of 3D Human Brain Organoids. EMBO J. 39 (20), e106230. 10.15252/embj.2020106230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayon T., Stamataki D., Perez-Carrasco R., Garcia-Perez L., Barrington C., Melchionda M., et al. (2020). Species-Specific Pace of Development Is Associated with Differences in Protein Stability. Science 369 (6510), eaba7667. 10.1126/science.aba7667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reillo I., De Juan Romero C., García-Cabezas M., Borrell V. (2011). A Role for Intermediate Radial Glia in the Tangential Expansion of the Mammalian Cerebral Cortex. Cereb. Cortex 21 (7), 1674–1694. 10.1093/cercor/bhq238 [DOI] [PubMed] [Google Scholar]
- Renner H., Grabos M., Becker K. J., Kagermeier T. E., Wu J., Otto M., et al. (2020). A Fully Automated High-Throughput Workflow for 3D-Based Chemical Screening in Human Midbrain Organoids. Elife 9, e52904. 10.7554/elife.52904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack H., Di Lullo E., Arias C., Knopp K. A., Laurie M. T., Sandoval-Espinosa C., et al. (2016). Zika Virus Cell Tropism in the Developing Human Brain and Inhibition by Azithromycin. Proc. Natl. Acad. Sci. 113 (50), 14408–14413. 10.1073/pnas.1618029113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. J., Graham D. L., Money K. M., Stanwood G. D. (2015). Developmental Consequences of Fetal Exposure to Drugs: What we Know and What we Still Must Learn. Neuropsychopharmacology 40 (1), 61–87. 10.1038/npp.2014.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. M., Kim C., Allen D., Crouch E. E., Narsinh K., Cooke D. L., et al. (2020). The Expanding Cell Diversity of the Brain Vasculature. Front Physiol 11, 600767. 10.3389/fphys.2020.600767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak-Wolf A., Wyler E., Legnini I., Loewa A., Glažar P., Kim S. J., et al. (2021). Neurodegeneration in Human Brain Organoids Infected with Herpes Simplex Virus Type 1. bioRxiv. 10.1101/2021.03.05.434122 [DOI] [Google Scholar]
- Sagner A., Zhang I., Watson T., Lazaro J., Melchionda M., Briscoe J. (2020). Temporal Patterning of the central Nervous System by a Shared Transcription Factor Code. bioRxiv. 10.1101/2020.11.10.376491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M. W., Stevens B. (2017). Microglia Emerge as central Players in Brain Disease. Nat. Med. 23 (9), 1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Saurel-Cubizolles M. J., Prunet C., Blondel B. (2014). Cannabis Use During Pregnancy in France in 2010. BJOG 121 (8), 971–977. 10.1111/1471-0528.12626 [DOI] [PubMed] [Google Scholar]
- Schaafsma S. M., Gagnidze K., Reyes A., Norstedt N., Månsson K., Francis K., et al. (2017). Sex-Specific Gene–Environment Interactions Underlying ASD-Like Behaviors. Proc. Natl. Acad. Sci. 114 (6), 1383–1388. 10.1073/pnas.1619312114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaevitz L. R., Berger-Sweeney J. E. (2012). Gene-Environment Interactions and Epigenetic Pathways in Autism: The Importance of One-Carbon Metabolism. ILAR J. 53 (3–4), 322–340. 10.1093/ilar.53.3-4.322 [DOI] [PubMed] [Google Scholar]
- Schmidt B. Z., Lehmann M., Gutbier S., Nembo E., Noel S., Smirnova L., et al. (2017). In Vitro Acute and Developmental Neurotoxicity Screening: an Overview of Cellular Platforms and High-Throughput Technical Possibilities. Arch. Toxicol. 91 (1), 1–33. 10.1007/s00204-016-1805-9 [DOI] [PubMed] [Google Scholar]
- Schumacher U., Mollgård K. (1997). The Multidrug-Resistance P-Glycoprotein (Pgp, MDR1) is an Early Marker of Blood-Brain Barrier Development in the Microvessels of the Developing Human Brain. Histochem. Cell Biol. 108 (2), 179–182. 10.1007/s004180050159 [DOI] [PubMed] [Google Scholar]
- Seckl J. R. (1997). 11β-Hydroxysteroid Dehydrogenase in the Brain: A Novel Regulator of Glucocorticoid Action?. Front. Neuroendocrinol. 18 (1), 49–99. 10.1006/frne.1996.0143 [DOI] [PubMed] [Google Scholar]
- Shi Y., Kirwan P., Livesey F. J. (2012). Directed Differentiation of Human Pluripotent Stem Cells to Cerebral Cortex Neurons and Neural Networks. Nat. Protoc. 7 (10), 1836–1846. 10.1038/nprot.2012.116 [DOI] [PubMed] [Google Scholar]
- Shi Y., Sun L., Wang M., Liu J., Zhong S., Li R., et al. (2020). Vascularized Human Cortical Organoids (vOrganoids) Model Cortical Development In Vivo . PLoS Biol. 18 (5), e3000705. 10.1371/journal.pbio.3000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis J. C., Pochareddy S., Zhu Y., Li M., Sestan N. (2016). The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 89 (2), 248–268. 10.1016/j.neuron.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko O., Parham F., Dea S., Sodhi N., Biesmans S., Mora-Castilla S., et al. (2019). Functional and Mechanistic Neurotoxicity Profiling Using Human iPSC-Derived Neural 3D Cultures. Toxicol. Sci. 167 (1), 58–76. 10.1093/toxsci/kfy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan S. A., Andersen J., Paşca A. M., Birey F., Paşca S. P. (2018). Generation and Assembly of Human Brain Region–Specific Three-Dimensional Cultures. Nat. Protoc. 13 (9), 2062–2085. 10.1038/s41596-018-0032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart I. H. M., Dehay C., Giroud P., Berland M., Kennedy H. (2002). Unique Morphological Features of the Proliferative Zones and Postmitotic Compartments of the Neural Epithelium Giving Rise to Striate and Extrastriate Cortex in the Monkey. Cereb. Cortex 12 (1), 37–53. 10.1093/cercor/12.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A. V., Skriabine S., et al. (2020). Neuroinvasion of SARS-CoV-2 in Human and Mouse Brain. bioRxiv. 10.1101/2020.06.25.169946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soothill P. W., Nicolaides K. H., Rodeck C. H., Gamsu H. (1986). Blood Gases and Acid-Base Status of the Human Second-Trimester Fetus. Obstet. Gynecol. 68 (2), 173–176. [PubMed] [Google Scholar]
- Strazielle N., Ghersi-Egea J. F. (2013). Physiology of Blood–Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules. Mol. Pharm. 10 (5), 1473–1491. 10.1021/mp300518e [DOI] [PubMed] [Google Scholar]
- Subramanian L., Bershteyn M., Paredes M. F., Kriegstein A. R. (2017). Dynamic Behaviour of Human Neuroepithelial Cells in the Developing Forebrain. Nat. Commun. 8 (1), 14167. 10.1038/ncomms14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Chiuppesi F., Chen X., Wang C., Tian E., Nguyen J., et al. (2020). Modeling Human Cytomegalovirus-Induced Microcephaly in Human iPSC-Derived Brain Organoids. Cell Rep. Med. 1 (1), 100002. 10.1016/j.xcrm.2020.100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutarjono B. (2019). Can we Better Understand How Zika Leads to Microcephaly? A Systematic Review of the Effects of the Zika Virus on Human Brain Organoids. J. Infect. Dis. 219 (5), 734–745. 10.1093/infdis/jiy572 [DOI] [PubMed] [Google Scholar]
- Svrakic D. M., Zorumski C. F., Svrakic N. M., Zwir I., Cloninger C. R. (2013). Risk Architecture of Schizophrenia: The Role of Epigenetics. Curr. Opin. Psychiatry 26 (2), 188–95. 10.1097/YCO.0b013e32835d8329 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Cakir B., Xiang Y., Sullivan G. J., Park I.-H. (2020). Synthetic Analyses of Single-Cell Transcriptomes From Multiple Brain Organoids and Fetal Brain. Cell Rep. 30 (6), 1682–1689.e3. 10.1016/j.celrep.2020.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E., Götz M., Huttner W. B. (2014). The Cell Biology of Neurogenesis: Toward an Understanding of the Development and Evolution of the Neocortex. Annu. Rev. Cell Dev. Biol. 30 (1), 465–502. 10.1146/annurev-cellbio-101011-155801 [DOI] [PubMed] [Google Scholar]
- Telley L., Agirman G., Prados J., Amberg N., Fièvre S., Oberst P., et al. (2019). Temporal Patterning of Apical Progenitors and Their Daughter Neurons in the Developing Neocortex. Science 364 (6440), eaav2522. 10.1126/science.aav2522 [DOI] [PubMed] [Google Scholar]
- Tetro N., Moushaev S., Rubinchik-Stern M., Eyal S. (2018). The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res. 35 (4), 71. 10.1007/s11095-017-2286-0 [DOI] [PubMed] [Google Scholar]
- Trevino A. E., Sinnott-Armstrong N., Andersen J., Yoon S.-J., Huber N., Pritchard J. K., et al. (2020). Chromatin Accessibility Dynamics in a Model of Human Forebrain Development. Science 367 (6476), eaay1645. 10.1126/science.aay1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo C. A., Gao R., Negraes P. D., Gu J., Buchanan J., Preissl S., et al. (2019). Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 25 (4), 558–569.e7. 10.1016/j.stem.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui Y., Kosugi I., Kawasaki H. (2005). Neuropathogenesis in Cytomegalovirus Infection: Indication of the Mechanisms Using Mouse Models. Rev. Med. Virol. 15 (5), 327–345. 10.1002/rmv.475 [DOI] [PubMed] [Google Scholar]
- Utz S. G., See P., Mildenberger W., Thion M. S., Silvin A., Lutz M., et al. (2020). Early Fate Defines Microglia and Non-Parenchymal Brain Macrophage Development. Cell 181 (3), 557–573.e18. 10.1016/j.cell.2020.03.021 [DOI] [PubMed] [Google Scholar]
- Uzquiano A., Gladwyn-Ng I., Nguyen L., Reiner O., Götz M., Matsuzaki F., et al. (2018). Cortical Progenitor Biology: Key Features Mediating Proliferation versus Differentiation. J. Neurochem. 146 (5), 500–525. 10.1111/jnc.14338 [DOI] [PubMed] [Google Scholar]
- Velasco S., Kedaigle A. J., Simmons S. K., Nash A., Rocha M., Quadrato G., et al. (2019). Individual Brain Organoids Reproducibly Form Cell Diversity of the Human Cerebral Cortex. Nature 570, 523–527. 10.1038/s41586-019-1289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D., Robertson D., Benagiano V., Errede M., Bertossi M., Ambrosi G., et al. (2000). Immunogold Cytochemistry of the Blood–Brain Barrier Glucose Transporter GLUT1 and Endogenous Albumin in the Developing Human Brain. Dev. Brain Res. 123 (1), 95–101. 10.1016/s0165-3806(00)00086-9 [DOI] [PubMed] [Google Scholar]
- Vivanti A. J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., et al. (2020). Transplacental Transmission of SARS-CoV-2 Infection. Nat. Comm. 11 (1), 3572. 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tsai J.-W., Lamonica B., Kriegstein A. R. (2011). A New Subtype of Progenitor Cell in the Mouse Embryonic Neocortex. Nat. Neurosci. 14 (5), 555–561. 10.1038/nn.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. I., Abaci H. E., Shuler M. L. (2017). Microfluidic Blood-Brain Barrier Model Provides In Vivo-Like Barrier Properties for Drug Permeability Screening. Biotechnol. Bioeng. 114 (1), 184–194. 10.1002/bit.26045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang L., Zhu Y., Qin J. (2018). Human Brain Organoid-on-a-Chip to Model Prenatal Nicotine Exposure. Lab Chip 18 (6), 851–860. 10.1039/c7lc01084b [DOI] [PubMed] [Google Scholar]
- Ward C., Lewis S., Coleman T. (2007). Prevalence of Maternal Smoking and Environmental Tobacco Smoke Exposure During Pregnancy and Impact on Birth Weight: Retrospective Study Using Millennium Cohort. BMC Public Health 7 (1), 81. 10.1186/1471-2458-7-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner T. D., Roussos-Ross D., Behnke M. (2014). It’s Not Your Mother’s Marijuana. Clin. Perinatol. 41 (4), 877–894. 10.1016/j.clp.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Buth J. E., Vishlaghi N., De La Torre-Ubieta L., Taxidis J., Khakh B. S., et al. (2017). Self-Organized Cerebral Organoids With Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 21 (2), 517–532. 10.1016/j.celrep.2017.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. F., Salick M. R., Wiskow O., Ho D. J., Worringer K. A., Ihry R. J., et al. (2016). Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids From Zika Virus Infection. Cell Stem Cell 19 (6), 703–708. 10.1016/j.stem.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Whish S., Dziegielewska K. M., Møllgård K., Noor N. M., Liddelow S. A., Habgood M. D., et al. (2015). The Inner CSF-Brain Barrier: Developmentally Controlled Access to the Brain via Intercellular Junctions. Front. Neurosci. 9, 16. 10.3389/fnins.2015.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L., Kalebic N., Namba T., Vaid S., Wimberger P., Huttner W. B. (2020). Serotonin Receptor 2A Activation Promotes Evolutionarily Relevant Basal Progenitor Proliferation in the Developing Neocortex. Neuron 108 (6), 1113–1129.e6. 10.1016/j.neuron.2020.09.034 [DOI] [PubMed] [Google Scholar]
- Xu Y.-P., Qiu Y., Zhang B., Chen G., Chen Q., Wang M., et al. (2019). Zika Virus Infection Induces RNAi-Mediated Antiviral Immunity in Human Neural Progenitors and Brain Organoids. Cell Res. 29 (4), 265–273. 10.1038/s41422-019-0152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F., Zhu Y., Wang Y., Qin J. (2018). Engineering Brain Organoids to Probe Impaired Neurogenesis Induced by Cadmium. ACS Biomater. Sci. Eng. 4 (5), 1908–1915. 10.1021/acsbiomaterials.8b00160 [DOI] [PubMed] [Google Scholar]
- Yoon K.-J., Song G., Qian X., Pan J., Xu D., Rho H.-S., et al. (2017). Zika-Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins. Cell Stem Cell 21 (3), 349–358.e6. 10.1016/j.stem.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.-Z., Chu H., Han S., Shuai H., Deng J., Hu Y.-F., et al. (2020). SARS-CoV-2 Infects Human Neural Progenitor Cells and Brain Organoids. Cell Res. 30 (10), 928–931. 10.1038/s41422-020-0390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Zheng Y., Liu Y., Zhang X., Zhao J. (2014). Minocycline Alleviates Behavioral Deficits and Inhibits Microglial Activation in the Offspring of Pregnant Mice After Administration of Polyriboinosinic–Polyribocytidilic Acid. Psychiatry Res. 219 (3), 680–686. 10.1016/j.psychres.2014.06.046 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wang L., Yin F., Yu Y., Wang Y., Shepard M. J., et al. (2017). Probing Impaired Neurogenesis in Human Brain Organoids Exposed to Alcohol. Integr. Biol. 9 (12), 968–978. 10.1039/c7ib00105c [DOI] [PubMed] [Google Scholar]