Abstract
Developmental dysplasia of the hip comprises a broad spectrum of abnormalities in hip development, of variable severity. Besides physical examination, ultrasound is the preferred imaging modality for screening for developmental dysplasia of the hip in children aged younger than six months. The Graf method is the most widely used ultrasound technique for infant hips; a stepwise approach will be shown in this article. Furthermore, the process of dynamic ultrasound imaging will be explained as well as the use of transinguinal ultrasound in infants wearing a spica cast. There is no consensus on the best way to screen for developmental dysplasia of the hip, which is probably the reason why different screening programs exist throughout Europe, as will be discussed in this article. The use of universal versus selective ultrasound remains a controversy, as does the timing. Is it better to perform sonography in all newborn infants like in Germany and Austria? Or should we examine only the infants with clinical hip instability or risk factors (breech position, positive family history), like in the UK and the Netherlands? This article reviews the epidemiology, static and dynamic ultrasound techniques in screening for developmental dysplasia of the hip, and differences in screening programs throughout Europe. Set aside the uncertainties about whom and when to screen, it needs to be emphasized that ultrasound screening for developmental dysplasia of the hip is important, since the disease is initially occult and easier to treat when identified early. In this way, the radiologist can aid in preventing serious disability of the hip.
Keywords: ultrasound, screening, hip, pediatric, DDH
Introduction
Developmental dysplasia of the hip (DDH) occurs in infancy and comprises a broad spectrum of abnormalities, ranging from mild instability to dislocation. Dysplasia includes deformity of the acetabulum, the femoral head, or both: the acetabulum is too shallow, and the development of the femoral head is delayed, causing it to be smaller and aspherical. The exact mechanism of pathogenesis is not clear, but there seems to be a co-dependent system in which a normal shape of the acetabulum stimulates normal spherical development and central position of the femoral head, and vice versa.
Ultrasound of the neonatal hip plays a role in screening, diagnosis, and (monitoring of) treatment of DDH. This article reviews the epidemiology, static and dynamic ultrasound techniques in screening for DDH, and differences in screening programs throughout Europe.
Developmental dysplasia of the hip
The incidence of DDH varies from 1.5 to 20 per 1,000 births, depending on the criteria used for diagnosis and the population studied(1). In certain populations, a higher incidence of DDH was found due to cradling traditions: In the Navaho Indian tribes, in Japan, and in Turkey hips in newborns were tightly bound in extension and adduction, causing restricted motion of the hips and predisposing infants to DDH(2–4). At present, safe swaddling and carrying is advised, with hips in flexion and abduction(2,5).
Girls are two to seven times more likely to be affected by the condition than boys; 75–80% of affected infants are of the female sex(6–10). This is probably due to ligamentous laxity caused by maternal and fetal estrogens. Also, a higher number of estrogen receptors might play a role(11). In 60% of cases, DDH affects the left hip, since the most common left occiput anterior (LOA) position of the fetus in utero leaves the left leg with limited space for abduction. The left hip is pressed against the spine of the mother, which makes it less mobile. In 20% of cases, the right hip is affected, leaving another 20% of newborns with bilateral involvement(12,13). Besides female sex, the two strongest risk factors for developing DDH include breech position in utero and a positive family history(14,15). Breech position increases the risk of DDH by 2–24 times for both girls and boys(2,7–10,12,16). First-degree relatives (parent or sibling with DDH) increases the risk 12-fold(10,14,17,18).
Minor risk factors are widely mentioned but remain disputable. Most of the minor risk factors are related to limited space in utero, the so-called “packaging effects” among which are first born, oligohydramnios, high birth weight, and post maturity(12).
Clinical features of DDH
DDH is asymptomatic in infancy; however, when the child starts walking, symptoms emerge including limp, waddle, abnormal gait, and leg length discrepancy, which are mostly pain free. The average walking age is delayed by one month, which is clinically insignificant, since there is a wide variability in walking age and all are within the expected time(19). Late consequences of undetected DDH include pain and early osteoarthritis, frequently leading to premature hip replacement therapy.
Abnormalities in physical examination include limited hip abduction, leg length discrepancy, and thigh or buttock fold asymmetry(2). The most sensitive clinical test to detect hip instability is the Ortolani maneuver, in which abducting the infant’s hip causes the dislocated femoral head to relocate into the acetabulum with a palpable clunk(20,21).
Ultrasound techniques DDH: static evaluation
Static ultrasound imaging of the newborns hips according to Graf’s method is widely performed(22,23). It is important to carry out this examination in a standardized and reproducible manner:
Equipment. Examination is performed with a high-frequency linear array probe. Optionally, one could make use of a special cradle, so that the newborns lie still.
Positioning. With the infant lying on its side, the hip should be placed in natural position (15–20° flexion) or in 90° flexion. The transducer is positioned on the lateral side, with 10–15° posterior rotation (of the superior edge of the transducer), such that the infant’s hip is depicted in coronal view (Fig. 1).
Fig. 1.
Ultrasound of a normal Graf 1 hip: A. Landmarks on standard imaging plane 1 – bony acetabular rim, 2 – triradiate cartilage, 3 – labrum; B. Measurement of alpha–angle as the angle between baseline and acetabular line (landmarks 1 and 2) and measurement of beta–angle as the angle between baseline and inclination line (landmarks 1 and 3)
Reference imaging plane. A standard image for taking measurements needs to be obtained (Fig. 2), in which the iliac bone should be a straight horizontal line. Three anatomical landmarks are recognized:
Fig. 2.
Staging of DDH according to Graf: A. Graf 2A; B. Graf 2B; C. Graf 2C; D. Graf D; E. Graf 3; F. Graf 4. * 1 – bony acetabular rim, 2 – triradiate cartilage, 3 – labrum. ** In F. Graf 4 not the optimal probe position for depicting the triradiate cartilage was chosen, so landmark 2 is not depicted
the bony acetabular rim,
the triradiate cartilage: a hypoechoic zone between the acetabulum, ischial bone and iliac bone, representing the deepest point of the acetabulum,
the center of the labrum.
Measurements. 1.) Firstly, we describe the depth/steepness of the osseous acetabular roof by measuring the Graf α-angle. This is the angle between a horizontal line through the lateral side of the iliac bone (baseline, Fig. 2) and a line through the bony acetabular rim and the triradiate cartilage (acetabular line, between landmarks 1 and 2, Fig. 2). A Graf α-angle <60° is abnormal, and means that the osseous acetabulum is too shallow, which predisposes to femoral dislocation. It is important to realize that the bony acetabular rim is not always sharp, but can also be blunt or rounded, especially in patients younger than three months old. In such cases, the landmark has to be placed at the point where the bony acetabulum becomes concave, which is not always on the iliac baseline. 2.) Secondly, we describe the positioning of the femoral head: whether it is centrally located within the acetabulum or in an eccentric position. 3.) Thirdly, we describe the position of the labrum by measuring the Graf β-angle. This is the angle between the bony acetabular rim and the cartilaginous acetabular labrum (inclination line between landmarks 1 and 3, Fig. 2). The inclination line should pass the middle of the labrum, meaning through its strongest echo. A Graf β-angle >55° is considered abnormal, and means the labrum is elevated due to femoral dislocation. It needs to be emphasized that the three lines rarely cross at one point (Fig. 2)(22).
Reporting. A standard report should preferably include the items mentioned in Tab. 1.
Tab. 1.
Graf classification scale for developmental dysplasia of the hip
| Type | Alpha angle | Acetabular modelling | Bony acetabular rim | Beta angle | Labrum | Position of femoral head | Age |
|---|---|---|---|---|---|---|---|
| 1A | >60° | good | sharp | <55° | good femoral coverage | central | all |
| 1B | >60° | good | slightly rounded | <55° | good femoral coverage | central | all |
| 2A | 50–59° | deficient | rounded | <55° | coverage femoral head | central | <3 mo |
| 2B | 50–59° | deficient | rounded | <55° | coverage femoral head | central | >3 mo |
| 2C | 43–49° | severely deficient | rounded/flat | >55 but <77° | still coverage femoral head | stable or unstable | all |
| D | 43–49° | severely deficient | rounded/flat | >77° | still coverage femoral head | decentering | all |
| 3 | <43° | poor | flat | labrum pressed upwards | eccentric | all | |
| 4 | <43° | poor | flat | labrum pressed downwards/disappears | eccentric | all |
Different stages of abnormalities and their associated ultrasound findings are graded according to the Graf system and range from grade 1 (no dysplasia) to grade 4 (dislocation), as summarized in Tab. 2(22). In a normal “mature” hip (Graf 1), the α-angle is higher than 60°, and the femoral head is located centrally in the acetabulum. In a Graf 2 hip, the α-angle is between 50 and 59°, and the femoral head is located centrally within the acetabulum. At ages below three months, a natural course in hip development may still show increasing α-angles, categorized as an immature hip (Graf 2A); after three months of age the slightly shallow acetabulum is due to a delay in development (Graf 2B). When the α-angle is between 43 and 49°, the hip can become unstable. If there is still a normal position of the cartilage labrum (β-angle <77°), this is referred to as Graf 2C dysplasia. In Graf D dysplasia, the hip is decentered; the cartilage labrum is pressed upwards, increasing the β-angle (>77°). In Graf 3 and 4 dysplasia, the α-angle is <43°, and the hips have an eccentric lateral position. In Graf 3, the labrum is pressed upwards because the subluxated femoral head pushes it away. In a Graf 4 situation, the femoral head is dislocated over the labrum; it is pushed down, or even disappears from the ultrasound field of view.
Tab. 2.
Example of standard report in ultrasound of DDH
| Acetabular modelling: good/adequate/deficient/poor |
| Bony acetabular rim: sharp/rounded/flat |
| Alpha angle (°) |
| Femoral head: ossification no/symmetric/asymmetric |
| Femoral head position: centric – eccentric |
| Beta angle (°) |
Probe positioning can substantially influence the measured α-angle, as shown in a recent publication by Jaremko et al.(24). The authors performed 3D ultrasound of newborn hips and reconstructed images for a wide variety of different hand positions. The study showed that a normal hip might mimic a dysplastic one with α-angles below 60° depending on the operator’s performance; therefore, the highest acquired α-angle should be determined(1,25). Furthermore, one may assess normal α-angles in dislocated hips, so it is highly advocated to always look beyond the α-angle, and also take into account the position and coverage of the femoral head and the position of the cartilaginous labrum.
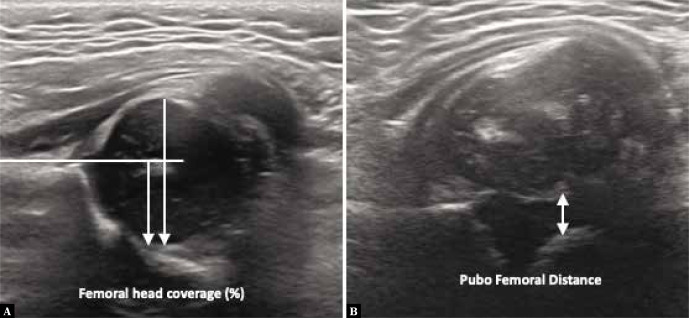
Difficulties in probe positioning in combination with multiple categories of the Graf classification can make the Graf method appear complex and confusing, especially for inexperienced radiographers. As an alternative to the angles, Morin et al. focused on the importance of femoral head coverage by the bony acetabulum (Fig. 3)(26). They showed that 58% femoral coverage on ultrasound was always correlated with normal acetabular angles on X-ray. In the literature, this approach is sometimes slightly inadequately referred to as the “50%-rule”: the bony acetabulum should cover 50% (one half or more) of the femoral head(27). Another easy-to-learn ultrasound screening technique involves measuring the distance between the pubic bone and the femoral head: the pubofemoral distance (PFD, Fig. 3), which has a good reproducibility. When using a cutoff point of PFD >6 mm at an age of >1 month old, a sensitivity of 100% to the diagnosis of DDH is reached(28). This screening technique is used in some European countries such as France, Belgium, Luxembourg and Switzerland. It is followed by further ultrasound evaluation performed by an experienced operator when the PFD is abnormal.
Fig. 3.
Measuring DDH beyond angles, using A. Femoral head coverage (in %, where >58% is the reference value for a normal hip) and B. Pubofemoral distance (in mm, where <6 mm is the reference for a normal hip)
Ultrasound techniques DDH: Dynamic evaluation
The morphology of the acetabulum and femoral head is best assessed at rest. When hip stability has to be evaluated, stress maneuvers with dynamic ultrasound can be performed. Two techniques described in the literature include lateral dynamic ultrasound (LDUS),(29) and anterior dynamic ultrasound (ADUS)(30). The most common application of LDUS in Europe is as an addition to static Graf imaging. Hips can be potentially unstable in Graf type 2C and more severe types of dysplasia; then dynamic US can be performed(22).
The dynamic LDUS examination is performed as follows: in the lateral/coronal transducer position with hip in flexion: 1.) observe while abducting and adducting the hip, and 2.) apply gentle stress to assess stability, while adducting the thigh (Barlow/posterior push maneuver). Examples of LDUS can be seen in Fig. 4. If the femoral head moves away from the posterior acetabulum with gentle stress, the hip is unstable. Furthermore, the percentage change in femoral head coverage with pressure can be analyzed.
Fig. 4.
Lateral dynamic ultrasound procedure showing a Graf 3 hip during Barlow/posterior pressure maneuver with the femoral head moving away from the medial side of the acetabulum
It is important to realize that, when using the Graf classification, all hip joints are classified without stress provocation. For example, a Graf 2C hip that is unstable with dynamic imaging is classified as 2C unstable, not as a Graf D. Also, a Graf 3 hip that can be dislocated further with dynamic imaging is called a Graf 3, not a Graf 4(22).
ADUS is performed with a transinguinal probe position during the Barlow maneuver. This enables the operator to quantify (in millimeters) the movement of the hip within the acetabulum.
Ultrasound techniques DDH: transinguinal evaluation
US can be used for screening purposes but also for monitoring active treatment in patients with dysplastic hips. The goal of DDH treatment is to obtain and maintain concentric reduction of the femoral head in the acetabulum. For infants younger than six months old, this can be managed by wearing an abduction (Pavlik) brace, with hips in flexion and abduction. After unsuccessful Pavlik treatment, a closed reduction of the femoral head into the acetabulum will be performed by the orthopedic surgeon. In our practice, we perform closed reductions under procedural sedation on the pediatric ward instead of general anesthesia in the operating room. After the radiologist has confirmed the proper position of the hip with transinguinal ultrasound, the hips are plastered in a spica cast(31–33). Regular follow-up in the cast is done by transinguinal ultrasound.
During transinguinal ultrasound, an axial image is obtained at the level of the pubic bone. In this view, the pubic bone and the femoral head should be in the same level (Fig. 5). If the hip is dislocated, the femoral head moves posteriorly and is no longer visualized in the expected level, so only the femoral neck is appreciated.
Fig. 5.

Normal anterior transinguinal ultrasound of the hip in a spica cast, showing the pubic bone in line with the femoral neck
Screening programs
Screening for DDH is important, as the disease is occult at first, but may lead to serious disability on the long-term. Moreover, it is easily treated when detected at an early stage. For over 80 years, screening based on clinical examination has been highly recommended(20,21). Unfortunately, there is no international consensus concerning the application of ultrasound as a screening tool for DDH(34). There is no agreement as to whether one should perform ultrasound on all newborns (universal screening) or only on those with risk factors or clinical instability of the hip (selective screening)(34,35). Furthermore, the optimal timing of the ultrasound examination is not clear yet. This leads to considerable variation within Europe, as displayed in Tab. 3, predominantly based on differences in the incidence of DDH and organization of healthcare systems. In the German-speaking countries and Italy, universal ultrasound screening is performed at an early age. In the Netherlands, selective ultrasound screening is performed when the infant is three months old if risk factors are present, or earlier if there is clinical instability of the hips at a physical examination which is performed at one week, one month, and three months old at the children’s healthcare center(36). Although there is no consensus regarding the optimal timing in different screening programs, it is advisable not to perform screening ultrasound before six weeks (unless there is clinical dislocation), since physiological laxity due to maternal estrogens will resolve by six weeks of age(37).
Tab. 3.
Different screening programs across Europe for the detection of DDH in newborns
| Country | Type of ultrasound screening | Timing (week) |
|---|---|---|
| The Netherlands | selective | 12 |
| Belgium | selective | <12 |
| France | selective | <4 |
| Portugal | selective | 6–8 |
| Italy | universal | 4–12 |
| Austria | universal | 1 6–8 |
| Switzerland | universal | <5 |
| Germany | universal | Risk factor + 1–2 Risk factor – 4–5 |
| Sweden | selective | 1–12 |
| Norway | selective | 1 |
| Hungary | selective | <6 |
| Slovenia | universal | Risk factor + 1–2 Risk factor – 6 |
| Slovakia | universal | 1–12 |
| United Kingdom | selective | 6 |
| Ireland | selective | 6 |
* Information on different screening programs presented with help from Young club members of the European Society of Skeletal Radiology (ESSR)
Good-quality studies comparing different screening programs are scarce(34). Two studies from Austria and Germany showed a decrease in surgery rates and complications, and also in costs, since the introduction of universal ultrasound screening, when compared with no ultrasound screening at all.(38,39). There are three studies that compared universal versus selective ultrasound screening; all found no significant differences in early (subluxation/dislocation) or late (acetabular dysplasia degenerative changes at skeletal maturity) outcome measures(40–42). However, a lower rate of re-examinations and treatment in the selective screening group was described: treatment was necessary in 1.8%, versus 3.4% in the universal group(40,42). A Cochrane review on this topic failed to reveal any clear recommendations: for ethical reasons, there are no studies comparing ultrasound screening with no screening at all. Furthermore, the randomized controlled trials that were conducted were underpowered in the events of late DDH or surgery; preferably a follow-up of 50 years is needed.
Conclusion
Ultrasound screening for DDH is important, as the disease is initially occult, and easier to treat when identified early. There is no international consensus on the ultrasound screening for DDH specifying who should be selected for screening and at what age. This results in major differences in screening programs throughout Europe; mainly based on the incidence of DDH and healthcare system organization. All in all, despite the controversies described in this article, it can be stated that in the “best practice” screening programs ultrasound evaluation is used to prevent serious disability of the hip in the long-term.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Starr V, Ha BY: Imaging update on developmental dysplasia of the hip with the role of MRI. AJR Am J Roentgenol 2014; 203: 1324–1335. [DOI] [PubMed] [Google Scholar]
- 2.Schwend RM, Shaw BA, Segal LS: Evaluation and treatment of developmental hip dysplasia in the newborn and infant. Pediatr Clin North Am 2014; 61: 1095–1107. [DOI] [PubMed] [Google Scholar]
- 3.Yamamuro T, Ishida K: Recent advances in the prevention, early diagnosis, and treatment of congenital dislocation of the hip in Japan. Clin Orthop Relat Res 1984: 34–40. [PubMed]
- 4.Kutlu A, Memik R, Mutlu M, Kutlu R, Arslan A: Congenital dislocation of the hip and its relation to swaddling used in Turkey. J Pediatr Orthop 1992; 12: 598–602. [PubMed] [Google Scholar]
- 5.International Hip Dysplasia Institute : Understanding hip dysplasia. Pobrane z: https://hipdysplasia.org/developmental-dysplasia-of-the-hip/hip-healthy-swaddling/.
- 6.Loder RT, Skopelja EN: The epidemiology and demographics of hip dysplasia. ISRN Orthop 2011: 238607. [DOI] [PMC free article] [PubMed]
- 7.de Hundt M, Vlemmix F, Bais JMJ, Hutton EK, de Groot CJ, Mol BWJ. et al. : Risk factors for developmental dysplasia of the hip: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2012; 165: 8–17. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz-Neira CL, Paolucci EO, Donnon T: A meta-analysis of common risk factors associated with the diagnosis of developmental dysplasia of the hip in newborns. Eur J Radiol 2012; 81: e344–e351. [DOI] [PubMed] [Google Scholar]
- 9.Woodacre T, Ball T, Cox P: Epidemiology of developmental dysplasia of the hip within the UK: refining the risk factors. J Child Orthop 2016; 10: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swarup I, Penny CL, Dodwell ER: Developmental dysplasia of the hip: An update on diagnosis and management from birth to 6 months. Curr Opin Pediatr 2018; 30: 84–92. [DOI] [PubMed] [Google Scholar]
- 11.Desteli EE, Pişkin A, Gülman AB, Kaymaz F, Köksal B, Erdoĝan M: Estrogen receptors in hip joint capsule and ligamentum capitis femoris of babies with developmental dysplasia of the hip. Acta Orthop TraumatolTurc 2013; 47: 158–161. [DOI] [PubMed] [Google Scholar]
- 12.Kotlarsky P, Haber R, Bialik V, Eidelman M: Developmental dysplasia of the hip: What has changed in the last 20 years? World J Orthop 2015; 6: 886–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guille JT, Pizzutillo PD, MacEwen GD: Development dysplasia of the hip from birth to six months. J Am Acad Orthop Surg 2000; 8: 232–242. [DOI] [PubMed] [Google Scholar]
- 14.Shaw BA, Segal LS, Section on Orthopaedics: Evaluation and referral for developmental dysplasia of the hip in infants. Pediatrics 2016; 138: e20163107. [DOI] [PubMed] [Google Scholar]
- 15.Yang S, Zusman N, Lieberman E, Goldstein RY: Developmental dysplasia of the hip, Pediatrics 2019; 143: e20181147. [DOI] [PubMed] [Google Scholar]
- 16.Bache CE, Clegg J, Herron M: Risk factors for developmental dysplasia of the hip: ultrasonographic findings in the neonatal period. J Pediatr Orthop B 2002; 11: 212–218. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DA, Mineau G, Kerber RA, Viskochil DH, Schaefer C, Roach JW: Familial predisposition to developmental dysplasia of the hip. J Pediatr Orthop 2009; 29: 463–466. [DOI] [PubMed] [Google Scholar]
- 18.Carroll KL, Schiffern AN, Murray KA, Stevenson DA, Viskochil DH, Toydemir R. et al. : The occurrence of occult acetabular dysplasia in relatives of individuals with developmental dysplasia of the hip. J Pediatr Orthop 2016; 36: 96–100. [DOI] [PubMed] [Google Scholar]
- 19.Kamath SU, Bennet GC: Does developmental dysplasia of the dip cause a delay in walking? J Pediatr Orthop 2004; 24: 265. [DOI] [PubMed] [Google Scholar]
- 20.Ortolani M: Un segno poco noto e la sua importanza per la diagnosi precoce di pre lUSGsazione congenita dell’anca. Pediatria 1937; 45: 129–136. [Google Scholar]
- 21.Mubarak SJ: In search of Ortolani: The man and the method. J Pediatr Orthop 2015; 35: 210–216. [DOI] [PubMed] [Google Scholar]
- 22.Graf R, Lercher K, Scott S, Spiess T: Essentials of infant hip sonography. According to GRAF. Stolzape Sonocenter 2017.
- 23.Graf R, Mohajer M, Plattner F: Hip sonography update. Quality-management, catastrophes – tips and tricks. Med Ultrason 2013; 15: 299–303. [DOI] [PubMed] [Google Scholar]
- 24.Jaremko JL, Mabee M, Swami VG, Jamieson L, Chow K, Thompson RB: Potential for change in US diagnosis of hip dysplasia solely caused by changes in probe orientation: Patterns of alpha-angle variation revealed by using three-dimensional US. Radiology 2014; 273: 870–878. [DOI] [PubMed] [Google Scholar]
- 25.Kang YR, Koo J: Ultrasonography of the pediatric hip and spine. Ultrasonography 2017; 36: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morin C, Harcke HT, MacEwen GD: The infant hip: real-time US assessment of acetabular development. Radiology 1985; 157: 673–677. [DOI] [PubMed] [Google Scholar]
- 27.Harcke HT, Pruszczynski B: Hip ultrasound for developmental dysplasia: the 50% rule. Pediatr Radiol 2017; 47: 817–821. [DOI] [PubMed] [Google Scholar]
- 28.Tréguier C, Chapuis M, Branger B, Bruneau B, Grellier A, Chouklati K. et al. : Pubo-femoral distance: an easy sonographic screening test to avoid late diagnosis of developmental dysplasia of the hip. Eur Radiol 2013; 23: 836–844. [DOI] [PubMed] [Google Scholar]
- 29.Harcke HT, Grissom LE: Performing dynamic sonography of the infant hip. Am J Roentgenol 1990; 155: 837–844. [DOI] [PubMed] [Google Scholar]
- 30.Andersson JE, Funnemark PO: Neonatal hip instability: screening with anterior-dynamic ultrasound method. J Pediatr Orthop 1995; 15: 322–324. [DOI] [PubMed] [Google Scholar]
- 31.Beek FJA, Nievelstein RJ, Pruijs HE, de Jong PA, Sakkers RJB: Transinguinal sonographic determination of the position of the femoral head after reposition and follow-up in a spica cast. Pediatr Radiol 2010; 40: 1794–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberhardt O, Zieger M, Langendoerfer M, Wirth T, Fernandez FF: Determination of hip reduction in spica cast treatment for DDH: a comparison of radiography and ultrasound. J Child Orthop 2009; 3: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grissom LE, Harcke HT, Kumar SJ, Basset GS, MacEwen GD: Ultrasound evaluation of hip position in the pavlik harness. J Ultrasound Med 1988; 7: 1–6. [DOI] [PubMed] [Google Scholar]
- 34.Shorter D, Hong T, Osborn DA: Cochrane review: screening programmes for developmental dysplasia of the hip in newborn infants. Evid Based Child Health 2013; 8: 11–54. [DOI] [PubMed] [Google Scholar]
- 35.Mahan ST, Katz JN, Kim YJ: To screen or not to screen? A decision analysis of the utility of screening for developmental dysplasia of the hip. J Bone Joint Surg Am 2009: 91: 1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Federatie Medisch Specialisten : DDH (dysplastische heupontwikkeling) bij kinderen onder één jaar. 2020. Available from: https://richtlijnendatabase.nl/richtlijn/ddh_dysplastische_heupontwikkeling_bij_kinderen_onder_n_jaar/startpagina_-_ddh.html. [Google Scholar]
- 37.American Institute of Ultrasound in Medicine, American College of Radiology : AIUM practice guideline for the performance of an ultrasound examination for detection and assessment of developmental dysplasia of the hip. J Ultrasound Med 2009; 28: 114–119. [DOI] [PubMed] [Google Scholar]
- 38.Thallinger C, Pospischill R, Ganger R, Radler C, Krall C, Grill F: Long-term results of a nationwide general ultrasound screening system for developmental disorders of the hip: the Austrian hip screening program. J Child Orthop 2014; 8: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Kries R, Ihme N, Oberle D, Lorani A, Stark R, Altenhofen L. et al. : Effect of ultrasound screening on the rate of first operative procedures for developmental hip dysplasia in Germany. Lancet 2003; 362: 1883–1887. [DOI] [PubMed] [Google Scholar]
- 40.Rosendahl K, Markestad T, Lie RT: Ultrasound screening for developmental dysplasia of the hip in the neonate: the effect on treatment rate and prevalence of late cases. Pediatrics 1994; 94: 47–52. [PubMed] [Google Scholar]
- 41.Holen KJ, Tegnander A, Bredland T, Johansen OJ, Saether OD, Eik-Nes SH. et al. : Universal or selective screening of the neonatal hip using ultrasound? J Bone Joint Surg Br 2002; 84: 886–890. [DOI] [PubMed] [Google Scholar]
- 42.Laborie LB, Engesaeter IO, Lehmann TG, Eastwood DM, Engesaeter LB, Rosendahl K. et al. : Screening strategies for hip dysplasia: long-term outcome of a randomized controlled trial. Pediatrics 2013; 132: 492–501. [DOI] [PubMed] [Google Scholar]