Abstract
Morton’s neuroma is a painful lesion of the interdigital nerve, usually at the third intermetatarsal space, associated with fibrotic changes in the nerve, microvascular degeneration, and deregulation of sympathetic innervation. Patients usually present with burning or sharp metatarsalgia at the dorsal or plantar aspect of the foot. The management of Morton’s neuroma starts with conservative measures, usually with limited efficacy, including orthotics and anti-inflammatory medication. When conservative treatment fails, a series of minimally invasive ultrasound-guided procedures can be employed as second-line treatments prior to surgery. Such procedures include infiltration of the area with a corticosteroid and local anesthetic, chemical neurolysis with alcohol or radiofrequency thermal neurolysis. Ultrasound aids in the accurate diagnosis of Morton’s neuroma and guides the aforementioned treatment, so that significant and potentially long-lasting pain reduction can be achieved. In cases of initial treatment failure, the procedure can be repeated, usually leading to the complete remission of symptoms. Current data shows that minimally invasive treatments can significantly reduce the need for subsequent surgery in patients with persistent Morton’s neuroma unresponsive to conservative measures. The purpose of this review is to present current data on the application of ultrasound for the diagnosis and treatment of Morton’s neuroma, with emphasis on the outcomes of ultrasound-guided treatments.
Keywords: Morton’s neuroma, ultrasound, injection, chemical neurolysis, radiofrequency ablation
Introduction
Morton’s neuroma (MN) was first described by Civinini in 1835 as a “neural ganglial swelling of the foot sole”(1). However, it received its current name years later, in 1876, from Thomas George Morton who reported a “painful affection of the foot” occurring particularly at the fourth metatarsal after wearing high-heeled shoes or without any identifiable cause(2). Morton treated his patients at the Philadelphia Polyclinic by amputating the affected toe, with considerable success in pain reduction(2). Microscopic changes in MN include demyelination and fibrotic lesions with epineural hylinization of the interdigital nerve, vascular degeneration, and increase in sympathetic nerve fibers, typically at the third intermetatarsal space (IMS) along with interneural edema, and less frequently at the second and rarely at the fourth IMS(3–5).
Patients with MN present with significant burning or sharp metatarsalgia either on the plantar or dorsal side of the foot, which can be associated with numbness of the toes. Interestingly, it can also been found in 30% of asymptomatic volunteers(6) and 17% of patients can associate the onset of symptoms with forefoot trauma(7). Pain is elicited during weight-bearing in cases where the respective IMS is loaded, particularly during activities which involve walking and running, as well as when wearing high-heeled shoes(7,8). History and clinical examination can raise the suspicion of MN. The thumb index finger squeeze test is the most accurate clinical test. However, a variety of forefoot disorders can mimic MN, eliciting similar pain, including synovitis, metatarsal stress injuries, plantar plate tears, metatarsophalangeal joint laxity, and Freiberg’s disease. Therefore, imaging by means of ultrasound (US), achieving 98% accuracy, can confirm the diagnosis and provide immediate pain relief when combined with percutaneous injections(8). The treatment of Morton’s neuroma traditionally starts with conservative measures including the use of orthotic devices, metatarsal pads, and analgesics(9,10). For patients not responding to mild conservative measures, injection of corticosteroids with the addition of a local anesthetic, chemical neurolysis with alcohol or radiofrequency ablation can be performed as minimally invasive measures prior to surgery(11,12). US-guided interventions for the treatment of musculoskeletal diseases are being increasingly incorporated into everyday clinical practice, and US-guided management of MN combines accurate imaging diagnosis with immediate pain relief(13). The aim of this review is to present current data on the application of US-guided treatment of Morton’s neuroma as a minimally invasive technique prior to open surgery.
US diagnosis of MN
US is the imaging modality of choice for the diagnosis of MN due to its high soft tissue resolution, lack of radiation exposure, wide availability, and real-time correlation between symptoms and imaging findings(14,15). In addition, US enables both diagnosis and treatment during the same session. US-guided injections provide immediate alleviation of pain, which also serves as an indirect confirmation of the diagnosis(16,17). In US images, MN typically appears as a well-circumscribed, relatively mobile at direct pressure, hypoechoic lesion (compared to the muscle) within the normal fatty tissue of the IMS (Fig. 1)(18,19). However, a small proportion (<15%) of MN appear anechoic or with a mixed echotexture(18). Identification of MN by means of US requires sagittal images from the dorsal side of the foot with simultaneous application of pressure at the web space. This forces the tissues of the space to spread, separating the layers anterior (mobile under pressure) and deep (static under pressure) to the intermetatarsal ligament. Other approaches (e.g. transverse or plantar) can also be used, especially during US-guided injection, but sagittal images captured from the dorsal side of the foot are mostly described in the literature for diagnostic purposes(19). MN can be located either dorsal or plantar to the plantar aspect of the metatarsal head(18). In a study of 27 cases of MN, all of them were found to have a length less than 20 mm(18). However, their length may also be smaller than 5 mm, especially in cases of asymptomatic MN which may as well be undetectable by means of US(6,18,20). The sensitivity and specificity of MRI is similar to those of US in the diagnosis of Morton’s neuroma, however the low cost, short duration of the examination, and possibility of dynamic evaluation and treatment, render US as the modality of choice for the diagnosis of Morton’s neuroma(14). In fact, according to the guidelines of the European Society of Skeletal Radiology, as revised in 2017, US is considered equivalent to MR imaging for the diagnosis of Morton’s neuroma(21).
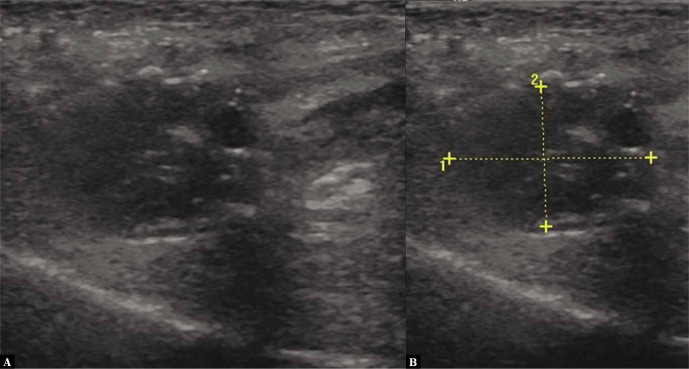
Fig. 1.
A 46-year-old female patient, avid runner, with a 6-month history of pain during running. Dorsal sagittal ultrasonographic image showing a hypoechoic Morton’s neuroma in the 3d intermetatarsal space (left) measuring 20 × 14 mm (right)
US-guided treatment technique
Prior to the commencement of the procedure, informed consent is always obtained from the patient, and complications are explained in detail including infection, damage to adjacent tissues, plantar fascia contraction, liponecrosis, flushing, post-treatment flare, and the possibility of treatment failure. The patient is positioned supine, and the foot is firmly positioned on the examination table to restrict movement and allow stable needle/probe placement through the dorsal aspect of the foot (Fig. 2).
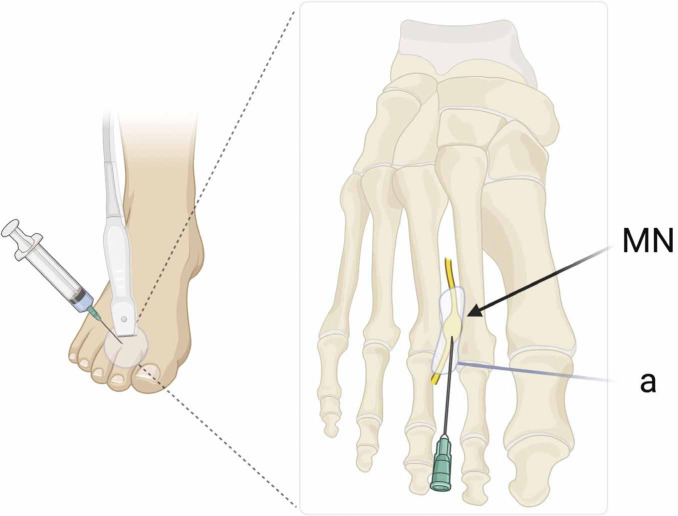
Fig. 2.
Schematic representation of US-guided treatment of Morton’s neuroma. MN: Morton’s neuroma; a: area of infiltration surrounding the neuroma at the intermetatarsal space (created with biorender.com)
The procedure starts with a diagnostic scan with the use of a linear 7–12 MHz US probe to confirm the presence of MN and select the optimal access point. Usually, the needle or radiofrequency ablation probe is ideally positioned at the dorsal aspect of the foot (Fig. 3). Sterilization of the area is of utmost importance, which can be performed with chlorhexidine- or iodophor-containing solutions. The US transducer is sterilized with alcohol-free disinfectants and immediately enclosed in a sterile probe cover.
Fig. 3.
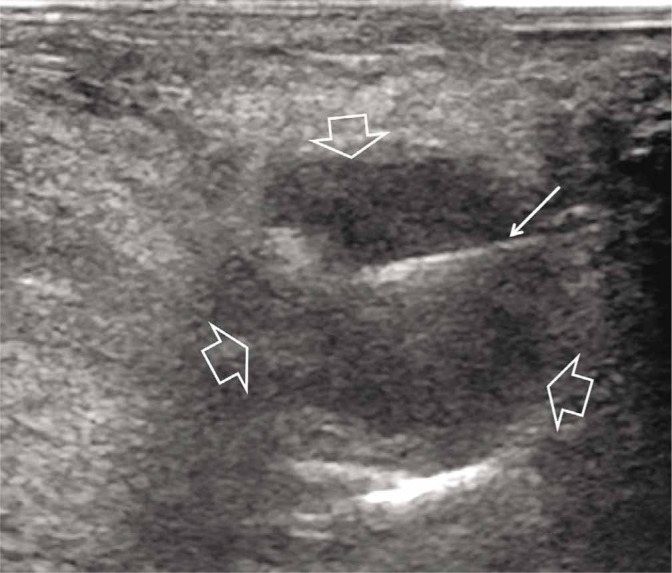
A 55-year-old female patient with a 1-year history of pain and burning during walking. Sagittal with dorsal approach US scan of the 3d intermetatarsal space showing a 12 mm hypoechoic Morton’s neuroma (open arrows) and in-plane demonstration of the needle (thin arrow)
Based on the literature and the authors’ experience, 25G needles are used for regional anesthesia and for the administration of corticosteroid or alcohol solutions. A wide variety of corticosteroid – local anesthetic combinations have been reported in the literature. In our institution, 1 mL of a corticosteroid, such as beta-methasone acetate 3 mg/mL (or equivalent), and 2 mL of a medium- to long-acting local anesthetic, typically 0.5% Bupivacaine, are used.
Following the removal of the needle and prior to the application of pressure and bandaging, a post-injection scan is performed. The patient is advised to rest for at least three days and use appropriate orthotics. In cases of corticosteroid injections or alcohol neurolysis, a second treatment round is advised when pain remission is not satisfactory within 2 weeks.
Outcomes of US-guided treatment of MN
A variety of US-guided procedures have been used for the treatment of MN, with the most popular being injections of corticosteroids and local anesthetic, chemical neurolysis, and radiofrequency ablation(22–25). Treatment choice largely depends on the experience of the treating physician, with surgeons preferring surgical management over less invasive options. However, it is accepted nowadays that minimally invasive US-guided procedures should precede operative management since they provide complete pain relief in the majority of MN cases(26). In addition, it has been shown that US-guided procedures offer superior results to non-guided interventions, including more efficient pain reduction, less complications, and reduced referrals for further surgical management(27).
The use of corticosteroids for the treatment of MN has been widely studied, with variable results. The general consensus holds that corticosteroids provide sufficient short-term relief, but only one third of patients benefit from long-term (>2 years) pain alleviation(28), being superior to conservative measures such as metatarsal pads(29). According to Lizano-Diez et al., the addition of a corticosteroid to injections performed for the treatment of MN does not improve pain or function compared to a local anesthetic alone(24), whereas Matthews et al. found evidence of limited pain reduction following corticosteroid injections(30). However, the fact that injections in this study were not performed under US-guidance may have a significant impact on the accurate targeting of the injection and the distribution of drugs at the area. The size of MN has been inversely correlated with the success of corticosteroid treatment, since 84% of MN with a length >6.3 mm did not achieve pain remission after the first treatment session(31). Repeated corticosteroid infiltration can be performed upon failure of the initial treatment. However, multiple corticosteroid infiltrations should be avoided because of the risk of fat atrophy and adjacent joint capsule degeneration and rupture(3,32,33).
According to a recent meta-analysis, the treatment of MN by corticosteroid injections is less effective than US-guided chemical neurolysis in terms of permanent pain relief and patient satisfaction. On the other hand, chemical neurolysis had similar results to surgical neurectomy(25) and has been shown to provide short-term relief in up to 89% of patients(34–36). However, it has been demonstrated that in the long run approximately 30% of patients undergoing alcohol neurolysis will need a repeat infiltration, and 30% will require surgical therapy(3,37). Nonetheless, recent results show that the injection of 2.5 mL of 70% ethanol under fluoroscopic and electroneurographic guidance can achieve significant long-lasting pain reduction for up to 5 years(38).
Radiofrequency (RF) thermal neurolysis has been used as an alternative to corticosteroid, anesthetic and alcohol injections in patients where the aforementioned therapies have failed. For the purposes of RF treatment, a probe is inserted under US or fluoroscopy guidance at the site of maximum pain, and temperature is raised at 85oC for 90 seconds in an attempt to sever adjacent nerve endings by denaturing proteins and destroying their myelin sheaths(39). According to Moore et al., RF treatment of MN offered complete pain remission in 83%, and no detectable benefit in 17% of patients at 1 month follow-up(40). Masala et al. used US-guided RF ablation to treat patients with MN after other conservative measures had failed and pain persisted for at least 6 months, achieving a mean VAS score of 2.1/10 and 1.7/10 at 2 and 6 months, respectively(22). Similar results were reported at 8 months by Shah et al.(41), confirming the long-term pain relief achieved by means of targeted US-guided RF ablation of MN. Surprisingly, no robust long-term data exists on the efficacy of RF treatment of MN.
Conclusions
US is the imaging modality of choice for the diagnosis of MN, while also enabling guided treatment. US-guided procedures including corticosteroid infiltration, chemical neurolysis and radiofrequency thermal neurolysis are viable alternatives to surgical treatment, offering high rates of complete remission of symptoms in patients where conservative management has failed, prior to surgery. Further research is needed to determine the long-term outcomes of radiofrequency thermal neurolysis, and placebo-controlled trials are needed to clearly define the long-term benefits and complications of these treatments compared to open surgery.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Pasero G, Marson P: Filippo Civinini (1805–1844) and the discovery of plantar neuroma. Reumatismo 2011; 58: 319–322. [DOI] [PubMed] [Google Scholar]
- 2.Morton TS: Metatarsalgia (Morton’s painful affection of the foot), with an account of six cases cured by operation. Ann Surg 1893; 17: 680–699. [PMC free article] [PubMed] [Google Scholar]
- 3.Di Caprio F, Meringolo R, Shehab Eddine M, Ponziani L: Morton’s interdigital neuroma of the foot: a literature review. Foot Ankle Surg 2018; 24: 92–98. [DOI] [PubMed] [Google Scholar]
- 4.Morscher E, Ulrich J, Dick W: Morton’s intermetatarsal neuroma: morphology and histological substrate. Foot Ankle Int 2000; 21: 558–562. [DOI] [PubMed] [Google Scholar]
- 5.Kay D, Bennett GL: Morton’s neuroma. Foot Ankle Clin 2003; 8: 49–59. [DOI] [PubMed] [Google Scholar]
- 6.Pfirrmann CWA, Zanetti M, Hodler J: Joint magnetic resonance imaging: Normal variants and pitfalls related to sports injury. Radiol Clin North Am 2002; 40: 167–180. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia M, Thomson L: Morton’s neuroma – current concepts review. J Clin Orthop Trauma 2020; 11: 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahadevan D, Venkatesan M, Bhatt R, Bhatia M: Diagnostic accuracy of clinical tests for Morton’s neuroma compared with ultrasonography. J Foot Ankle Surg 2015; 54: 549–553. [DOI] [PubMed] [Google Scholar]
- 9.Thomson L, Aujla RS, Divall P, Bhatia M: Non-surgical treatments for Morton’s neuroma: a systematic review. Foot Ankle Surg 2020; 26: 736–743. [DOI] [PubMed] [Google Scholar]
- 10.McKean KA: Neurologic running injuries. Neurol Clin 2008; 26: 281–296. [DOI] [PubMed] [Google Scholar]
- 11.Lento PH, Strakowski JA: The use of ultrasound in guiding musculoskeletal interventional procedures. Phys Med Rehabil Clin N Am 2010; 21: 559–583. [DOI] [PubMed] [Google Scholar]
- 12.Abreu E, Aubert S, Wavreille G, Gheno R, Canella C, Cotten A: Peripheral tumor and tumor-like neurogenic lesions. Eur J Radiol 2013; 82: 38–50. [DOI] [PubMed] [Google Scholar]
- 13.Sconfienza LM, Adriansen M, Albano D, Allen G, Aparisi Gómez MP, Bazzocchi A. et al. : Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR) – part I, shoulder. Eur Radiol 2020; 30: 903–913. [DOI] [PubMed] [Google Scholar]
- 14.Blankenbaker DG, De Smet AA: The role of ultrasound in the evaluation of sports injuries of the lower extremities. Clin Sport Med 2006; 25: 867–897. [DOI] [PubMed] [Google Scholar]
- 15.Martinoli C, Tagliafico A, Bianchi S, Bodner G, Padua L, Schenone A. et al. : Peripheral nerve abnormalities. Ultrasound Clin 2007; 2: 655–667. [Google Scholar]
- 16.Mak MS, Chowdhury R, Johnson R: Morton’s neuroma: review of anatomy, pathomechanism, and imaging. Clin Radiol 2020; 76: 235.e15–235.e23. [DOI] [PubMed] [Google Scholar]
- 17.Finlay K, Friedman L: Ultrasonography of the lower extremity. Orthop Clin North Am 2006; 37: 245–275. [DOI] [PubMed] [Google Scholar]
- 18.Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT: Sonography of Morton’s neuromas. AJR Am J Roentgenol 2000; 174: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 19.De Maeseneer M, Madani H, Lenchik L, Brigido MK, Shahabpour M, Marcelis S. et al. : Normal anatomy and compression areas of nerves of the foot and ankle: US and MR imaging with anatomic correlation. Radiographics 2015; 35: 1469–1482. [DOI] [PubMed] [Google Scholar]
- 20.Pollak RA, Bellacosa RA, Dornbluth NC, Strash WW, Devall JM: Sonographic analysis of Morton’s neuroma. J Foot Surg 1992; 31: 534–537. [PubMed] [Google Scholar]
- 21.Sconfienza LM, Albano D, Allen G, Bazzocchi A, Bignotti B, Chianca V. et al. : Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol 2018; 28: 5338–5351. [DOI] [PubMed] [Google Scholar]
- 22.Masala S, Cuzzolino A, Morini M, Raguso M, Fiori R: Ultrasound-guided percutaneous radiofrequency for the treatment of Morton’s neuroma. Cardiovasc Interv Radiol 2018; 41: 137–144. [DOI] [PubMed] [Google Scholar]
- 23.Chuter GSJ, Chua YP, Connell DA, Blackney MC: Ultrasound-guided radiofrequency ablation in the management of interdigital (Morton’s) neuroma. Skelet Radiol 2013; 42: 107–111. [DOI] [PubMed] [Google Scholar]
- 24.Lizano-Díez X, Ginés-Cespedosa A, Alentorn-Geli E, Pérez-Prieto D, González-Lucena G, Gamba C. et al. : Corticosteroid injection for the treatment of Morton’s neuroma: a prospective, double-blinded, randomized, placebo-controlled trial. Foot Ankle Int 2017; 38: 944–951. [DOI] [PubMed] [Google Scholar]
- 25.Lu VM, Puffer RC, Everson MC, Gilder HE, Burks SS, Spinner RJ: Treating Morton’s neuroma by injection, neurolysis, or neurectomy: a systematic review and meta-analysis of pain and satisfaction outcomes. Acta Neurochir 2021; 163: 531–543. [DOI] [PubMed] [Google Scholar]
- 26.Hassouna HZ, Singh D: The variation in the management of Morton’s metatarsalgia. Foot 2005; 15: 149–153. [Google Scholar]
- 27.Morgan P, Monaghan W, Richards S: A systematic review of ultrasound-guided and non-ultrasound-guided therapeutic injections to treat Morton’s neuroma. J Am Pod Med Assoc 2014; 104: 337–348. [DOI] [PubMed] [Google Scholar]
- 28.Peters PG, Adams SB, Schon LC: Interdigital neuralgia. Foot Ankle Clin 2011; 16: 305–315. [DOI] [PubMed] [Google Scholar]
- 29.Adams WR 2nd: Morton’s neuroma. Clin Pod Med Surg 2010; 27: 535–545. [DOI] [PubMed] [Google Scholar]
- 30.Matthews BG, Hurn SE, Harding MP, Henry RA, Ware RS: The effectiveness of non-surgical interventions for common plantar digital compressive neuropathy (Morton’s neuroma): a systematic review and meta-analysis. J Foot Ankle Res 2019; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YH, Lee JW, Choi GW, Kim HJ: Risk factors and the associated cutoff values for failure of corticosteroid injection in treatment of Morton’s neuroma. Int Orthop 2018; 42: 323–329. [DOI] [PubMed] [Google Scholar]
- 32.Peck E, Finnoff JT, Smith J: Neuropathies in runners. Clin Sport Med 2010; 29: 437–457. [DOI] [PubMed] [Google Scholar]
- 33.Santiago FR, Muñoz PT, Pryest P, Martínez AM, Olleta NP: Role of imaging methods in diagnosis and treatment of Morton’s neuroma. World J Radiol 2018; 10: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franson J, Baravarian B: Intermetatarsal compression neuritis. Clin Podiatr Med Surg 2006; 23: 569–578. [DOI] [PubMed] [Google Scholar]
- 35.Gross CE, Lin J: Injection therapy in the management of musculoskeletal injuries: foot and ankle. Oper Techn Sport Med 2012; 20: 185–191. [Google Scholar]
- 36.Hyer CF, Mehl LR, Block AJ, Vancourt RB: Treatment of recalcitrant intermetatarsal neuroma with 4% sclerosing alcohol injection: a pilot study. J Foot Ankle Surg 2005; 44: 287–291. [DOI] [PubMed] [Google Scholar]
- 37.Valisena S, Petri GJ, Ferrero A: Treatment of Morton’s neuroma: a systematic review. Foot Ankle Surg 2018; 24: 271–281. [DOI] [PubMed] [Google Scholar]
- 38.Pabinger C, Malaj I, Lothaller H, Samaila E, Magnan B: Improved injection technique of ethanol for Morton’s neuroma. Foot Ankle Int 2020; 41: 590–595. [DOI] [PubMed] [Google Scholar]
- 39.Cione JA, Cozzarelli J, Mullin CJ: A retrospective study of radiofrequency thermal lesioning for the treatment of neuritis of the medial calcaneal nerve and its terminal branches in chronic heel pain. J Foot Ankle Surg 2009; 48: 142–147. [DOI] [PubMed] [Google Scholar]
- 40.Moore JL, Rosen R, Cohen J, Rosen B: Radiofrequency thermoneurolysis for the treatment of Morton’s neuroma. J Foot Ankle Surg 2012; 51: 20–22. [DOI] [PubMed] [Google Scholar]
- 41.Shah R, Ahmad M, Hanu-Cernat D, Choudhary S: Ultrasound-guided radiofrequency ablation for treatment of Morton’s neuroma: initial experience. Clin Radiol 2019; 74: 815.e9–815.e13. [DOI] [PubMed] [Google Scholar]