Abstract
Background:
Testosterone levels have been used to examine infant boys’ vulnerability to health and developmental problems, following the general theories of gender differences and the theory of extreme male brain of autism.
Objectives:
As testosterone is a representative androgen hormone and is higher in preterm than full-term infants, we used this steroid to determine if hypothalamic pituitary hormones, testosterone, and cortisol, were related to physical growth, health, and development of very-low-birthweight (VLBW, BW < 1,500 g) infants.
Method:
For this comparative longitudinal study, 40 VLBW infants were recruited from a neonatal intensive care unit of a tertiary medical center. Data were collected from medical record reviews, questionnaires, and assessments of infant development at 6, 12, and 24 months. We collected saliva at the three time points and measured hormones using enzyme-immunoassays.
Results:
General and generalized mixed models showed that a 1pg/ml increment of testosterone was related to a −0.42% decrease in body weight, a −0.18% decrease in length, and a −0.10% decrease in head circumference. Cortisol levels were not associated with any outcome variable. The interactions between testosterone and time on physical growth and socioemotional development also occurred.
Discussion:
Elevated testosterone levels can be a biological risk factor for poor infant growth and development. Theories about the effects of elevated prenatal testosterone could be useful in predicting health and developmental outcomes among VLBW infants. Research beyond the first 2 years will be needed as infants show more socioemotional and behavioral problems as they grow older.
Keywords: hormonal biomarkers, very-low-birthweight infants, infant development, infant physical growth, gender differences, testosterone, cortisol
The health and development of preterm infants have been an important issue in pediatric research as prevalence of preterm birth is relatively high in the United States. The rate of preterm birth decreased between 2009 (10.1%) and 2014 (9.6%), but rose for the fourth year in 2018 (10.02%; Hamilton et al., 2019; Martin & Oysterman, 2018). Preterm infants who are very-low-birthweight infants (VLBW, birthweight < 1,500 gm) present with more health and developmental problems than normal birthweight infants (NBW; birthweights ≥ 2,500 gm), and these problems are more common in boys (Hintz et al., 2006). Boys are more likely to be born prematurely than girls and show higher neonatal mortality and morbidity rates than girls (Hintz et al., 2006). Also, in the general population, boys are slower in language, fine-motor, and personal-social development during their first years than girls (Whitehouse, Mattes, Maybery, Sawyer, et al., 2012).
Gender differences in infant health and development have been studied widely subsequent to introduction of general gender-differences theories and the extreme male brain theory of autism (Baron-Cohen, 2002; Geschwind, 1987). These well-established theories explain that the exposure to elevated prenatal androgen is a biological risk factor for brain and neurobehavioral development. The extreme male brain theory of autism emphasizes that elevated prenatal testosterone increases the prevalence of autism spectrum disorder in males and that male brain is associated with less empathy, which is often related with fewer skills such as affect and interaction with people (Baron-Cohen, 2002).
It is unethical to manipulate testosterone levels in humans during pregnancy, but animal studies found that rats exposed to dexamethasone in utero had low birthweight and catch-up growth within the first month after birth; that male adult offspring of spiny mice exposed to high-glucocorticoids (dexamethasone) at mid-pregnancy had elevated testosterone levels and high body weight; and that male and testosterone-propionate treated female rats had smaller brain weight and showed poorer auditory processing and spatial learning than untreated female rats following early hypoxic-ischemic injury, which affected more and longer such deficits in males (Hill et al., 2011; O’Regan et al., 2004; Quinn et al., 2014). Human studies have shown that perinatal androgen exposure, as indicated by elevated testosterone levels in amniotic fluid and fetal and cord blood, is associated with greater aggression, more tumbling and rough play, and higher rates of attention deficient hyperactivity disorder and autistic spectrum disorder as compared to infants with lower testosterone levels (Saenz & Alexander, 2013).
Exposure to elevated testosterone levels is also associated with delays in language, motor, and socioemotional development (Schaadt et al., 2015; Whitehouse, Mattes, Maybery, Sawyer, et al., 2012). The role of testosterone in cognitive and language development has been investigated in normal, full-term infants (Schaadt et al., 2015). In the general population, bioavailable (i.e., free or unbound) testosterone levels in umbilical cord blood were negatively associated with language development in boys but positively associated with language development in girls from birth to three years of age (Whitehouse, Mattes, Maybery, Sawyer, et al., 2012). Similarly, elevated testosterone in cord blood was negatively associated with expressive vocabulary development in boys, but not in girls, at two years of age (Hollier et al., 2013). However, little is known about its role in health and development in VLBW infant despite the finding that testosterone levels are significantly higher in VLBW than in healthy, full-term infants (Kuiri-Hanninen et al., 2011).
Unlike testosterone levels, the role of cortisol in health and development have been widely investigated. High pre- and postnatal cortisol levels are found to be a risk factor for smaller gestation and birthweight and developmental delays (Iwata et al., 2019). Cortisol levels in mothers and infants are dependent on each other (Khoury et al., 2016) and higher in mothers of boys throughout pregnancy (Bosquet et al., 2019). Elevated levels of maternal cortisol induced an increase in the level of fetal cortisol, and this increase functioned as a trigger for increasing the fetal testosterone levels (Sarkar et al., 2008). Mothers with increased cortisol levels had significantly higher prevalence of low birthweight birth if they had male newborns but not female newborns (Flom et al., 2018).
VLBW infants who were exposed to multiple noxious stimuli in the NICU showed high cortisol levels, especially infants with gestational ages smaller than 28 weeks (D’Agata et al., 2019). Repeated noxious stimuli might increase allostatic load and cause problematic health and development. Allostatic load is the physiologic response to repeated stress and increases cortisol levels through alteration in hypothalamic-pituitary-adrenal (HPA) axis upon failing to adapt to the stressors (McEwen, 2005). Preterm infants with altered HPA have more medical complications including bronchopulmonary dysplasia, intraventricular hemorrhage, necrotizing enterocolitis, and retinopathy of prematurity (Moore et al., 2014). They also have more neurodevelopmental problems (Casavant et al., 2019).
To the best of our knowledge, no study has been conducted to examine the associations of salivary testosterone and cortisol concurrently with infant health and development in VLBW infants over the first two years of life. The purpose of this study was to examine how hormonal biomarkers, testosterone, and cortisol levels are concurrently and longitudinally associated with physical growth (weight, length, and head circumference), health (presence of technology-dependence and common health problems), and development (cognitive, motor, language, and socioemotional) among VLBW infants. Based on theories of gender differences and the extreme male brain theory of autism as well as our previous studies (Baron-Cohen, 2002; Cho et al., 2012, 2017; Geschwind, 1987), we hypothesized that testosterone would be a better predictor than cortisol for infant outcomes during the first two years of life. To test our hypothesis, we examined the associations between salivary testosterone and cortisol levels and infant outcomes at 6, 12, and 24 months of age corrected (CA) for prematurity. These time points capture significant neurodevelopmental milestones during infancy.
As end products of HPA and hypothalamic-pituitary-gonadal (HPG) axes, testosterone and cortisol are intimately related to each other in hormonal cascades, partial production from adrenals, and stress responses. We examined both steroid hormones as potential biomarkers in this study. Since protein-unbound testosterone and cortisol are physiologically relevant to the human species and these steroids in saliva are largely free but strongly correlated with total steroid levels (protein-bound testosterone and cortisol) in the blood (r = 0.67–0.95; Fang et al., 2017; Gunnala et al., 2015), we measured the two steroid hormones in saliva.
Methods
We used a comparative longitudinal research design. We collected data through review of infants’ medical records, reports from mothers, biochemical measurement, and standardized infant developmental assessment.
Participants and Setting
A convenience sample of 40 VLBW infants were recruited from a neonatal intensive care unit (NICU) of a tertiary medical center in the southern US. Infants were recruited if they were: (a) 6–7 days old to avoid infants less likely to survive; (b) less than 32 weeks of gestational age (GA); and (c) less than 1,500 gm at birth. The infants were excluded if they: (a) had congenital deformities that might affect health and developmental outcomes; (b) had history of substance exposure such as positive urine test; or (c) would be adopted after discharge from the hospital. After 3 infants died before 40 weeks post menstrual age (PMA), all data and samples were collected from 34, 34, and 31 infants at 6, 12, and 24 months CA for prematurity, respectively.
Measures
During infant hospitalization, we collected demographic information, birth record, and neonatal history from the infant’s medical record. We measured physical growth, health problems, and developmental outcomes at three time points (6, 12, and 24 months CA). We measured salivary testosterone and cortisol levels at the same time points using enzyme immunoassays.
Infant demographic information
Demographic data included gender, anthropometric measurement (weight, length, and head circumference) at birth, gestational age, race, 1- and 5-minute Apgar scores, whether the infant received cardiopulmonary resuscitation at birth, and type of health insurance. Data on the neonatal history including presence of medical complications and technology-dependence (TD; oxygen, apnea monitor or pulse oximetry, gastrostomy-tube, ventilator, tracheostomy, and medications such as chlorothiazide and propranolol) were collected at 40 weeks PMA or when the infant was discharged from the NICU, which ever was earlier.
Infant physical growth and health
Data on infant physical growth and health were obtained during the visits to either the Newborn Follow-Up Clinic (if the infant’s BW < 1,000 gm) or the Research Project Office (if the infant’s BW between 1,000 and < 1,500 gm) at 6, 12, and 24 months CA. Physical growth measurement (weight, length, and head circumference) was completed by the research nurse. Infant health was assessed through the infant’s medical history and neurodevelopmental examination by a developmental pediatrician or her designee such as occupational-physical therapist, presence of technology-dependence, and number of common health problems such as gastroesophageal reflux, diarrhea, ear infections, constipation, wheezing between visits by the mother’s report.
Infant development
We assessed infant cognitive, motor, and language development at three time points (6, 12, and 24 months infant CA). We obtained infant socioemotional developmental problems from the mother’s report at three time points.
Infant cognitive, motor, and language development
We used the Bayley Scales of Infant and Toddler Development-Third edition (BSID-III; Bayley, 2006) to assess infant cognitive, motor, and language development. This instrument was administered by a pediatric psychologist or his designee, post-doctoral fellow or graduate student. The means for each category is 100, with a standard deviation of 15 (Bayley, 2006). A score <70 is considered to be a developmental delay in each domain. The internal consistency of the BSID-III was higher for the special groups than for the healthy groups (.86–.98). The BSID-III is highly correlated with Wechsler Preschool Primary Scale of Intelligence III, Preschool Language Scale−4, Peabody Developmental Motor Scale−2, and Adaptive Behavioral Assessment System II (Bayley, 2006).
Infant socioemotional development
We used the Ages & Stages questionnaires, Social-Emotional (ASQ: SE; Squires et al., 2002), to assess infant socioemotional developmental problems. The ASQ: SE was developed to identify socioemotional development problems in infants. The questionnaire includes seven behavioral areas: (1) self-regulation, (2) compliance, (3) communication, (4) adaptive functioning, (5) autonomy, (6) affect, and (7) interaction with people. The ASQ: SE was standardized on a national random sample of 3,014 preschool-age children and their families across eight age intervals from 6 months through 60 months. The internal consistency of the ASQ: SE was reported as .67–.91 (Squires et al., 2002). The possible total score ranged from 0 to 205 at 6 months, 0 to 235 at 12 months, and 0 to 275 at 24 months with a higher score indicating more developmental problems. The cut-off score indicating the developmental problem was 45 at 6 months, 48 at 12 months, and 50 at 24 months (Squires et al., 2002).
Hormonal biomarkers
Salivary testosterone and cortisol levels in VLBW infants were determined with enzyme immunoassay procedures using commercially obtained assay kits (Salimetrics, LLC, State College, PA; https://www.salimetrics.com). All samples were collected between 8:00 am to noon to reduce diurnal variations in testosterone and cortisol levels as reported in the literature (i.e., higher in the AM than in the PM; Mezzullo et al., 2017). The intra- and inter-assay coefficients of variation, which express the precision and repeatability of the assay were 2.5% and 5.6% in testosterone and 3.3% and 3.7% on cortisol.
Procedure
This study was approved by the Institutional Review Board at the university. On daily basis, a research nurse identified eligible infants for the study through the NICU admission log. After maternal consent, a research nurse collected baseline data at the NICU. When the infant was discharged from the NICU at 40 weeks PMA, the research nurse and research assistant coded the presence of medical complications and technology-dependence from the medical records, updated contact information and scheduled the follow-up visits at 6, 12, and 24 months CA.
Data Collection at 6, 12, and 24 Months CA
During the visits at 6, 12, and 24 months CA, the research nurse measured physical growth parameters (weight, length, and head circumference) and plotted the parameters as age- and gender-appropriate growth groups on the CDC growth charts. The infant was weighed on a battery-operated, electronic scale with a capacity of 20 kg and accuracy within 10 gm. Length was measured on a collapsible board with increments to the nearest 0.25 cm. Head circumference was determined with a disposable tape that measured to the nearest 0.25 cm. The assessment of infant cognitive, motor, and language development required about 60 minutes and the assessment of infant socioemotional development required about 10 minutes.
We collected infant’s saliva (1.0 ml) by using a portable suction with low pressure (<100 mmHg). Cotton and commercial collection devices interfere with the results of the testosterone assays but not the cortisol assays. For example, testosterone levels were lower when using synthetic Salivettes, while the levels were higher when using cotton Salivettes (Büttler et al., 2018). Thus, saliva was collected using 1-cc sterile plastic syringes with a blunt end attached to the suction tube. Infant’s saliva was sampled 30 min before or after any oral intake to reduce interference with drinks and foods. Since testosterone release is episodic, we collected three saliva samples at least 15-minute apart. Three saliva samples in three 1-cc syringes were transferred to the lab on ice and stored in a −80 °C freezer without centrifugation until assayed. During the enzyme immunoassays, three saliva samples were combined and measured twice in the same assay to obtain the most accurate values. The assays were completed at the pediatric endocrinologist’s lab by a lab technician who was blinded for infant information.
Data Analysis
We used descriptive statistics to present infant demographic characteristics, neonatal health history, and the levels of testosterone and cortisol at the three time points. We calculated the repeated measures correlation coefficient to quantify the linear relationship between two variables (Bakdash & Marusich, 2017). The general linear mixed model was the main analytical method to examine whether the levels of testosterone and cortisol were longitudinally associated with infant’s physical growth (weight, length, and head circumference) and development (cognitive, motor, language, and socioemotional development) across three time points. As the number of technology-dependence variables and the number of common health problems were counting data, we additionally applied the generalized linear mixed models to handle such non-continuous dependent variables by defining them with a Poisson distribution. The correlation of repeated measures was also taken into account in both models. We transformed all continuous dependent variables by a logarithm function because most of their distributions were skewed.
A small data set with repeated measurement may lead power issues and biased estimations in the analysis. Thus, we reanalyzed data using a Bayesian method in conjunction with information prior distributions to solve those issues (de Schoot et al., 2015). We calculated the Bayes factor (BF, Dienes, 2014), where a BF > 2.44 is equivalent to a p-value < 0.05, using Studio version 1.1.463 (R Development Core Team, Vienna, Austria). Descriptive statistics were performed in SAS v9.4 (SAS Institute, Cary, NC).
Results
Infant’s Demographic Characteristics and Hormonal Biomarkers
The means of birthweight, length, and head circumference for the 40 infants were 1,026 gm, 36 cm, and 25 cm. Mean of gestational age was 29 weeks. Forty two percent were boys, 60% were African Americans, and 63% received the special supplemental nutrition program for women, infants, and children (WIC). Means of medical complications and technology-dependence at 40 weeks PMA were 3.8 and 0.1. As shown in Table 1, of the 37 infants at 40 weeks PMA (3 newborns died), most infants were not dependent on medical technology, except for one infant on continuous oxygen with an apnea monitor or pulse oximeter and two infants were on medications such as chlorothiazide and propranolol to improve heart function. Table 2 shows summary statistics for testosterone, cortisol, physical growth, development, and health of the infants at the different time points, revealing an increase in the average body weight, length, head circumference, motor development, and socioemotional developmental problems over 24 months. On the other hand, testosterone and technology-dependence decreased from 6 months to 24 months. Cortisol, cognitive, language development, and common health problems did not have any time-related changes. Testosterone and cortisol levels did not longitudinally differ by gender.
Table 1.
Infant Demographic Characteristics at Enrollment and 40 Weeks Postmenstrual Age.
| Variable | N | Min | Max | Mean(SD) or % |
|---|---|---|---|---|
| Gender: Male (%) | 40 | 42 | ||
| Bodyweight at birth (gm) | 40 | 450 | 1,460 | 1,026 (276) |
| Length at birth (cm) | 40 | 25.0 | 42.5 | 35.5 (3.8) |
| Head circumference (cm) | 40 | 20.0 | 28.7 | 25.3 (2.2) |
| Gestational age (weeks) | 40 | 25.0 | 31.5 | 28.7 (1.7) |
| Race: African American (%) | 40 | 57.5 | ||
| 1-min Apgar score | 40 | 1 | 8 | 4.5 (2.4) |
| 5-min Apgar score | 40 | 1 | 9 | 7.1 (1.7) |
| CPR at birth (0–6) | 40 | 1 | 4 | 2.4 (0.8) |
| Public-assistance: WIC (%) | 40 | 63 | ||
| Medical complications at | 37 | 1 | 6 | 3.8 (1.4) |
| 40 weeks postmenstrual age (0–16) | ||||
| Technology-dependence at 40 weeks | 37 | 1 | 3 | 0.1 (0.5) |
| postmenstrual age (0-6) | ||||
| Oxygen | 37 | 2.7 | ||
| Apnea monitor | 37 | 2.7 | ||
| Gastrostomy-tube | 37 | 0.0 | ||
| Ventilator | 37 | 0.0 | ||
| Tracheostomy | 37 | 0.0 | ||
| Medications | 37 | 5.4 |
Note. Min = minimum; Max = maximum; SD = standard deviation; CPR (0-6) = number of cardiopulmonary resuscitation treatment received at birth including oxygen, bagging and mask, continuous positive airway pressure (CPAP), intubation, chest compression, and epinephrine; WIC = Women, Infants, and Children Food and Nutrition Service.
Table 2.
Levels of Testosterone, Cortisol, Physical Growth (Bodyweight, Length, & Head Circumference), Development (Cognitive, Motor, Language, & Socioemotional), and Health (Technology-Dependence & Common Health Problems) at 6, 12, and 24 Months Corrected Age.
| 6 Months | 12 Months | 24 Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | N | Min | Max | Mean | SD |
| Testost | 33 | 19.6 | 96.8 | 48.8 | 17.6 | 34 | 13.3 | 91.4 | 34.1 | 18.3 | 28 | 6.0 | 61.7 | 19.6 | 12.9 |
| Cortisol | 34 | 0.1 | 0.7 | 0.2 | 0.2 | 33 | 0.0 | 1.1 | 0.2 | 0.2 | 28 | 0.0 | 0.6 | 0.2 | 0.1 |
| BW | 32 | 5340.0 | 9880.0 | 7093.8 | 1192.6 | 34 | 6720.0 | 12360.0 | 8951.5 | 1473.8 | 31 | 8820.0 | 21500.0 | 11409.4 | 2522.4 |
| LT | 32 | 57.6 | 69.0 | 63.9 | 2.8 | 34 | 67.0 | 78.5 | 72.5 | 3.1 | 31 | 77.5 | 90.0 | 84.2 | 3.6 |
| HC | 32 | 40.4 | 46.0 | 42.8 | 1.5 | 34 | 42.8 | 50.0 | 45.5 | 1.6 | 31 | 44.0 | 51.5 | 47.3 | 1.6 |
| Cog | 32 | 60.0 | 125.0 | 91.7 | 15.1 | 34 | 65.0 | 130.0 | 99.6 | 13.5 | 30 | 55.0 | 120.0 | 95.8 | 13.1 |
| Lang | 32 | 74.0 | 115.0 | 95.2 | 10.6 | 34 | 56.0 | 129.0 | 99.1 | 16.0 | 30 | 47.0 | 132.0 | 91.1 | 17.0 |
| Motor | 32 | 52.0 | 127.0 | 91.3 | 18.1 | 34 | 55.0 | 121.0 | 97.3 | 12.0 | 30 | 64.0 | 136.0 | 98.3 | 14.8 |
| SE | 32 | 0.0 | 100.0 | 24.8 | 18.0 | 34 | 10.0 | 85.0 | 33.1 | 19.0 | 30 | 10.0 | 150.0 | 54.7 | 36.7 |
| TD | 32 | 0.0 | 2.0 | 0.7 | 0.6 | 33 | 0.0 | 1.0 | 0.6 | 0.5 | 30 | 0.0 | 1.0 | 0.5 | 0.5 |
| CHP | 32 | 1.0 | 8.0 | 4.5 | 1.8 | 34 | 1.0 | 10.0 | 4.4 | 1.9 | 30 | 2.0 | 8.0 | 5.1 | 1.9 |
Note. Min = minimum; Max = maximum; SD = standard deviation; Testost = testosterone; BW = bodyweight; LT = length; HC = head circumference; Cog = cognitive development; Lang = language development; Motor = motor development; SE = socioemotional developmental problems; TD = technology-dependence; CHP = common health problems.
Correlations among Hormonal Biomarkers, Physical Growth, Health, and Development Over the 2 Years After Birth
As shown in Table 3, repeated measures correlation coefficients reveal that testosterone across three time points was significantly and negatively correlated with body weight, length, head circumference and socioemotional developmental problems. Cortisol was not correlated to any variable. Physical growth variables (body weight, length, and head circumference) were positively correlated with one another. Health variables (technology-dependence and common health problem) were also positively correlated with each other. Developmental variables (cognitive, motor, language, and socioemotional development) were not correlated, except that cognitive development was positively correlated with language and motor development, and socioemotional developmental problems were negatively correlated with language development.
Table 3.
Correlations among Salivary Testosterone and Cortisol Levels, Physical Growth (Bodyweight, Length, & Head Circumference), Development (Cognitive, Motor, Language, & Socioemotional), and Health (Technology-Dependence & Common Health Problems) Among VLBW Infants During the 2 Years After Birth.
| Testost | Cortisol | BW | LT | HC | Cog | Lang | Motor | SE | TD | CHP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Testost | 1.00 | 0.14 | −0.65*** | −0.78*** | −0.76*** | −0.22 | 0.12 | −0.29 | −0.44** | 0.19 | −0.26 |
| Cortisol | 1.00 | −0.12 | −0.11 | −0.19 | −0.14 | −0.10 | −0.19 | 0.08 | −0.03 | −0.12 | |
| BW | 1.00 | 0.93*** | 0.91*** | 0.07 | −0.17 | 0.13 | 0.50*** | −0.14 | 0.18 | ||
| LT | 1.00 | 0.96*** | 0.13 | −0.18 | 0.23 | 0.58*** | −0.14 | 0.20 | |||
| HC | 1.00 | 0.21 | −0.09 | 0.11 | 0.50*** | −0.14 | 0.17 | ||||
| Cog | 1.00 | 0.47*** | 0.51*** | −0.09 | −0.10 | 0.11 | |||||
| Lang | 1.00 | 0.08 | −0.40* | −0.11 | −0.04 | ||||||
| Motor | 1.00 | −0.01 | −0.02 | 0.03 | |||||||
| SE | 1.00 | −0.12 | 0.23 | ||||||||
| TD | 1.00 | 0.46*** | |||||||||
| CHP | 1.00 |
Note. Testost = testosterone; BW = bodyweight; LT = length; HC = head circumference; Cog = cognitive development; Lang = language development; Motor = motor development; SE = socioemotional developmental problems; TC = technology-dependent; CHP = common health problems. *p < .05, **p < .01, ***p < .001.
Associations of the Levels of Testosterone and Cortisol with Infant Physical Growth, Health, and Development by Using Mixed Models
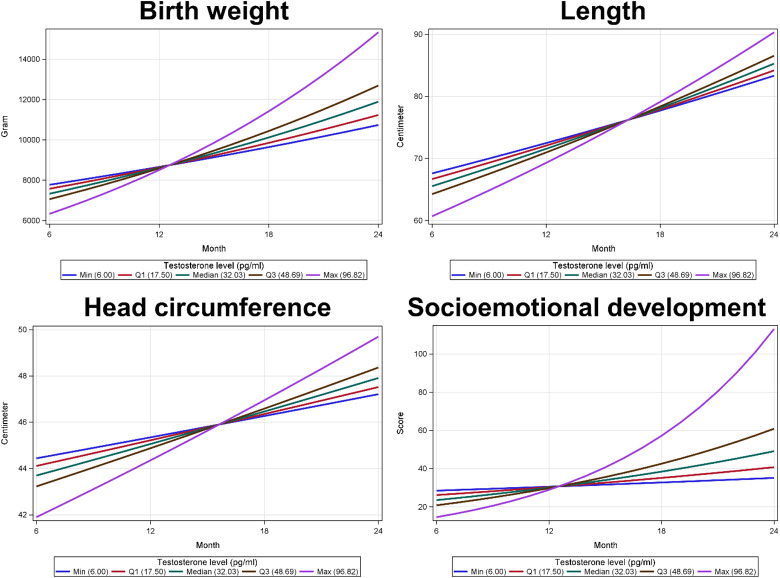
Using advanced modeling approaches of the general and generalized mixed models, as shown in Table 4, we found that testosterone was significantly associated with the infant’s body weight, length, and head circumference, while cortisol was not associated with any infant outcome variable. When testosterone level was lower by 1 pg/ml, the infant’s mean body weight, mean length, and mean head circumference were higher by 0.43% (95% confidence interval [CI] = 0.76, 0.11; p = 0.0108), 0.19% (95% CI = 0.30, 0.08; p = 0.0013), and 0.11% (95% CI = 0.16, 0.05; p = 0.0003), respectively. In addition, testosterone levels had a significant positive interaction with time on the three physical growth variables, as time increased by 1 month, the average of infant’s body weight, length, and head circumference significantly increased per one ng/dL change of testosterone levels. For example, as infants aged 1 month, the average of body weight increased 0.03% (95% CI = 0.01, 0.06; p = 0.0066) when testosterone level increased one ng/dL. This positive interaction term does not contradict the negative association between testosterone and physical growth because the associations between testosterone and physical growth variables became positive over time (Figure 1). Testosterone levels also had a significant positive interaction with time on the socioemotional developmental problems, as time increased by 1 month, the average of socioemotional developmental problems significantly increased per one ng/dL change of testosterone levels. For example, as infants aged 1 month, the average of socioemotional developmental problems increased 0.113% (95% CI = 0.002, 0.220; p = 0.0452) when testosterone level increased one ng/dL.
Table 4.
Associations of Salivary Testosterone and Cortisol Levels with Physical Growth (Bodyweight, Length, & Head Circumference), Development (Cognitive, Motor, Language, & Socioemotional), and Health (Technology-Dependence & Common Health Problems) among VLBW Infants over the 2 Years Using General and Generalized Linear Mixed Models.
| Effect | Change% | 95% CI | p | Effect | Change% | 95% CI | p |
|---|---|---|---|---|---|---|---|
| Body weigh t | |||||||
| Testosterone | −0.43 | (−0.76, −0.11) | 0.0108 | Cortisol | −3.34 | (−29.08, 31.73) | 0.8269 |
| Time | 1.60 | (0.72, 2.49) | 0.0006 | Time | 2.62 | (2.11, 3.12) | <.0001 |
| Testosterone × Time | 0.03 | (0.01, 0.06) | 0.0096 | Cortisol × Time | −0.07 | (−2.13, 2.03) | 0.9473 |
| Length | |||||||
| Testosterone | −0.19 | (−0.30, −0.08) | 0.0013 | Cortisol | −2.98 | (−11.78, 6.70) | 0.5274 |
| Time | 1.10 | (0.81, 1.39) | <.0001 | Time | 1.44 | (1.27, 1.62) | <.0001 |
| Testosterone × Time | 0.01 | (0.003, 0.02) | 0.0077 | Cortisol × Time | 0.31 | (−0.40, 1.02) | 0.3925 |
| Head circumference | |||||||
| Testosterone | −0.11 | (−0.16, −0.05) | 0.0003 | Cortisol | −3.33 | (−9.29, 3.02) | 0.2917 |
| Time | 0.30 | (0.17, 0.42) | <.0001 | Time | 0.50 | (0.38, 0.61) | <.0001 |
| Testosterone × Time | 0.01 | (0.003, 0.01) | 0.0007 | Cortisol × Time | 0.17 | (−0.31, 0.65) | 0.4864 |
| Cognitive development | |||||||
| Testosterone | −0.23 | (−0.61, 0.15) | 0.2238 | Cortisol | −4.65 | (−40.38, 52.52) | 0.8407 |
| Time | −0.32 | (−1.24, 0.61) | 0.4924 | Time | 0.18 | (−0.68, 1.05) | 0.6788 |
| Testosterone × Time | 0.01 | (−0.02, 0.04) | 0.3720 | Cortisol × Time | −0.10 | (−3.88, 3.82) | 0.9572 |
| Language development | |||||||
| Testosterone | −0.23 | (−0.65, 0.19) | 0.2750 | Cortisol | −2.72 | (−42.15, 63.59) | 0.9162 |
| Time | −0.85 | (−1.88, 0.19) | 0.1084 | Time | −0.28 | (−1.23, 0.68) | 0.5631 |
| Testosterone × Time | 0.01 | (−0.02, 0.04) | 0.4258 | Cortisol × Time | −0.34 | (−4.52, 4.01) | 0.8728 |
| Motor development | |||||||
| Testosterone | −0.07 | (−0.51, 0.37) | 0.7397 | Cortisol | −21.99 | (−55.67, 37.29) | 0.3842 |
| Time | 0.32 | (−0.79, 1.44) | 0.5735 | Time | 0.12 | (−0.95, 1.19) | 0.8286 |
| Testosterone × Time | 0.00 | (−0.04, 0.03) | 0.8549 | Cortisol × Time | 1.50 | (−3.17, 6.40) | 0.5300 |
| Socioemotional development | |||||||
| Testosterone | −1.40 | (−2.85, 0.08) | 0.0636 | Cortisol | 10.26 | (−78.32, 460.68) | 0.9050 |
| Time | 0.50 | (−3.08, 4.20) | 0.7865 | Time | 3.38 | (0.35, 6.50) | 0.0288 |
| Testosterone × Time | 0.11 | (0.002, 0.22) | 0.0452 | Cortisol × Time | 3.71 | (−8.07, 17.00) | 0.5488 |
| Technology-dependence | |||||||
| Testosterone | −0.40 | (−2.66, 1.91) | 0.7266 | Cortisol | −78.33 | (−98.53, 219.40) | 0.2594 |
| Time | −2.37 | (−8.01, 3.62) | 0.4221 | Time | −3.31 | (−8.07, 1.70) | 0.1869 |
| Testosterone × Time | 0.05 | (−0.13, 0.22) | 0.5976 | Cortisol × Time | 9.42 | (−10.25, 33.41) | 0.3663 |
| Common health problems | |||||||
| Testosterone | −0.13 | (−1.08, 0.83) | 0.7884 | Cortisol | −35.72 | (−78.51, 92.23) | 0.4221 |
| Time | 0.51 | (−1.78, 2.85) | 0.6612 | Time | 0.14 | (−1.86, 2.19) | 0.8872 |
| Testosterone × Time | 0.01 | (−0.06, 0.08) | 0.7930 | Cortisol × Time | 2.47 | (−5.25, 10.81) | 0.5349 |
Note. Change % = (exp[estimated coefficient]−1)×100%; CI = confidence interval.
Figure 1.
The interactions between testosterone levels and time on (1) birth weight, (2) length, (3) head circumference, and (4) socioemotional development.
Using the Bayesian model, as shown in Table 5 in the Supplementary Material, we confirmed that testosterone was negatively associated with infant’s physical growth, health, and development. In particular, the data showed a strong evidence to support the alternative hypothesis in head circumference because the BF in testosterone is greater than 10, resulting in an overall average decrease in infant’s head circumference by −0.045 cm (95% CI = −0.067, −0.022) when testosterone level increased 1 ng/dL. Testosterone levels also had a positive interaction with time in infant’s growth and development, showing that these measures increased along with a higher testosterone level over time, particularly in infant’s length ( = 0.004; 95% CI = 0.000, 0.007; BF = 4.212) and head circumstance ( = 0.003; 95% CI = 0.001, 0.005; BF > 10). As the BF in cortisol ranged from 0.595 to 1.037, we did not find any evidence that our data could support either the alternative hypothesis or the null hypothesis.
Discussion
This study’s results supported our hypothesis that testosterone would a better predictive hormonal biomarker for physical growth and socioemotional development among VLBW infants than cortisol. Elevated testosterone levels, which were higher than those in other studies (Contreras et al., 2017; Fang et al., 2017), were longitudinally associated with physical growth delays over the 2 years of life. Such findings might have occurred because elevated testosterone levels were found to be related to preterm and low birthweight births in both human and animal studies (Cho et al., 2012; Quinn et al., 2014). The levels of testosterone and cortisol did not differ longitudinally between genders possibly because all saliva samples were collected after the testosterone surge between 1 and 3 months (Achermann, 2005). The mean testosterone levels decreased significantly from 6 months (48.8 pg/ml) to 24 months (12.9 pg/ml). However, the lower testosterone level at 24 months compared to 6 and 12 months is still considered relatively high since the levels were reported to be 2–3 times higher in preterm infants than in full-term infants from month 1 to month 6 (Kuiri-Hanninen et al., 2011). Testosterone levels are known to be highest immediately after birth followed by the second trimester and puberty (Achermann, 2005; Greaves et al., 2014). However, the relationship of elevated testosterone levels during early infancy to poor physical growth appeared to persist for 24 months. As we had expected, all physical growth variables (body weight, length, and head circumference) significantly increased as the infants became older and testosterone decreased per month (1 pg/ml).
We had expected that testosterone levels might be positively correlated with socioemotional developmental problems because boys have shown more of these developmental problems, such as a lack of self-regulation and lower communication skills, than girls (Saenz & Alexander, 2013). However, we found that elevated testosterone levels appeared to be related to fewer socioemotional developmental problems. This finding possibly occurred because testosterone levels were negatively correlated with all physical growth (bodyweight, length, and head circumference) and all physical growth were positively correlated with socioemotional developmental problems. Another possible explanation for this finding is that the infants with physical growth delays might not have enough physical strength for crying for long periods of time or stiffened and arched his back when picked up. We also found a significant positive interaction between testosterone and time on socioemotional developmental problems. That is, socioemotional developmental problems increased only when testosterone increased with time. As testosterone effects on socioemotional developmental problems became insignificant after adding time, time might be a stronger predictor than testosterone. More questions were thus included on ASQ: SE questionnaire at 24 months compared to 6 and 12 months (Squires et al., 2002) because more socioemotional developmental problems might have been expected to be seen with age. This is the first study to show the relationships among testosterone, physical growth, and socioemotional development longitudinally among the VLBW infants.
We also had expected that elevated testosterone levels might be a biological risk factor for infant health problems because boys are more likely to be born prematurely, and thus, they have greater neonatal mortality and morbidity than girls (Hintz et al., 2006). However, we did not find any associations between testosterone levels and infant health as measured by the amount of technology-dependence, such as use of oxygen and having a gastrostomy tube, or the number of common health problems such as gastroesophageal reflux and asthma. Elevated testosterone levels were also expected to be a contributing factor for problematic infant development as high testosterone levels in amniotic fluid and cord blood were negatively associated with language development in the first 3 years such that language problems were associated with higher testosterone levels (Hollier et al., 2013; Whitehouse, Mattes, Maybery, Sawyer, et al., 2012). However, we did not find any associations between testosterone and cognitive, motor, and language development over the 2 years after birth. It is possible that 24 months are too short a period to accurately identify subtle developmental problems. Also, we measured testosterone and cortisol levels first at 6 months. This might be too late to see the associations found by others who measured testosterone levels shortly after birth or at 3–4 months (Hollier et al., 2013; Saenz & Alexander, 2013).
We measured cortisol levels concurrently with testosterone levels at 6, 12, and 24 months CA because both levels are found to be positively correlated in amniotic fluid and neonates’ saliva (Cho et al., 2012; Sarkar et al., 2008). Although elevated prenatal cortisol levels are known to be a biological risk factor for preterm and low birthweight birth as well as problematic health and development (Bolten et al., 2011), cortisol levels in this study were not associated with any physical growth, health, or developmental variables in VLBW infants. A possible explanation is that elevated prenatal cortisol levels might be a more predictive of infant outcomes than postnatal levels as maternal ante- and postpartum cortisol levels compared to neonatal cortisol levels were more predictive of infant neurodevelopment at 6 months (Caparros-Gonzalez et al., 2019). Another explanation is that the group means of the cortisol levels at the three time points were identical (0.2 µg/dL). Having no differences in mean cortisol levels over time might make it more difficult to identify differences in infant’s outcomes. The other explanation is that cortisol levels were higher among neonates with smaller gestational age that altered HPA axis (D’Agata et al., 2019) and those levels were highest during the first week, then gradually decreased by the first month (Greaves et al., 2014). Altered HPA axis due to elevated cortisol levels might change with time as a negative association between cortisol levels and motor development was reported at 6 months in VLBW female infants (Cho et al., 2017) while no association was reported under 24 months in full-term infants (Macari et al., 2019).
All physical growth variables were positively correlated with one another and with health variables and with each other. However, developmental variables were not correlated, except that cognitive development was positively correlated with language and motor development and socioemotional development problems were negatively correlated with language development. The findings are understandable because many questions on the ASQ: SE questionnaire focused on communication skills such as “Does your baby let you know when she is hungry or sick?” at 6 months, “Does your baby make babbling sounds?” at 12 months, and “Does your child look at you when you talk to him?” at 24 months. Obviously, infants with better language skills could response to and communicate with mothers more efficiently.
Overall, this study had several limitations. First, we had an attrition rate of 20% during the 2 years period including three deaths before 40 weeks PMA. Maintaining a higher retention rate would have increased the study validity and power (Nicks et al., 2017). We reanalyzed the data using the Bayesian method for small sample size and found that the results were similar to those using mixed models. Second, the findings need to be confirmed before applying them to other groups of infants because other infants may have different baseline data including demographic characteristics, birth variables, and neonatal history. Third, recruitment from more than one medical center might be desirable for increasing power and generalizability.
There are numerous implications for future study. First, we recommend longitudinal and repeated measurements of the associations between hormonal biomarkers and infant outcomes during early childhood by using different methods (e.g., radioimmunoassay and liquid chromatography-mass spectrometry) and materials (e.g., hair and urine). As hormonal levels change with time, the associations may also change. Second, we recommend longitudinal and repeated measurements of the associations between maternal testosterone and cortisol levels during pregnancy and infant outcomes as maternal hormonal biomarkers were found to affect birth and infant outcomes. Finally, a lack of associations between cortisol levels and infant health and development outcomes during the 2 years after birth should be confirmed by using a larger sample size at multi sites.
There are also implications for nursing practice. First the fundamental factors of testosterone and cortisol, especially testosterone, could be used to understand why VLBW preterm infants have shown more health and developmental problems compared to NBW full-term infants. Second, in clinical findings, infant morbidity differs by gender in that males had a greater incidence of respiratory problems, neurological insults, severe sepsis and septic shock, developmental disorders, and immune disorders than females. As testosterone is a representative androgen, nurses could understand male vulnerability in infant health and development and develop a long-erm discharge plan for VLBW preterm infants and families.
Conclusion
With the present study, we were able to support our hypothesis that testosterone was a better predictor than cortisol of infant outcome variables including physical growth and socioemotional development in VLBW infants during the 2 years after birth. Our data showed that elevated cortisol levels were not always a risk factor. General gender-differences theories and the extreme male brain theory of autism have been recommended for use with caution because long-term effects of elevated pre- and postnatal testosterone on infant outcomes such as autistic-like traits at 2 years and early adulthood are uncertain (Kung et al., 2016; Whitehouse, Mattes, Maybery, Dissanayake, et al., 2012). Since we have found significant correlations and associations between testosterone and infant physical growth and socioemotional development, those theories might be applicable for predicting health and development among VLBW infants. Because VLBW infants will develop more socioemotional and behavioral problems over time, examining the associations beyond 24 months is necessary to establish an empirical basis for understanding the functions of these hormonal biomarkers and eventually to develop screening tools and interventions.
Supplemental Material
Supplemental Material, CHO_Supplements_ready for Associations Between Hormonal Biomarkers and Preterm Infant Health and Development During the First 2 Years After Birth by June Cho, Lung-Chang Chien and Diane Holditch-Davis in Biological Research For Nursing
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH; R01HD076871) to the first author.
ORCID iD: June Cho  https://orcid.org/0000-0003-1749-6527
https://orcid.org/0000-0003-1749-6527
Supplemental Material: Supplemental material for this article is available online.
References
- Achermann J. C. (2005). Development of the reproductive systems. In Brook C. G. D., Clayton P. E., Brown R. S. (Eds.), Clinical pediatric endocrinology (pp. 153–170). Blackwell. [Google Scholar]
- Bakdash J. Z., Marusich L. R. (2017). Repeated measures correlation. Frontiers in Psychology, 8(456), 1–13. 10.3389/fpsyg.2017.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. (2002). The extreme male brain theory of autism. Trends in Cognitive Sciences, 6(6), 248–254. [DOI] [PubMed] [Google Scholar]
- Bayley N. (2006). Bayley scales of infant development-III. Psychological Corporation. [Google Scholar]
- Bolten M. I., Wurmser H., Buske-Kirschbaum A., Papousek M., Pirke K. M., Hellhammer D. (2011). Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Archives of Women’s Mental Health, 14(1), 33–41. 10.1007/s00737-010-0183-1 [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M., Sideridis G., Bollati V., Hoxha M., Hacker M. R., Wright R. J. (2019). Maternal cortisol output in pregnancy and newborn telomere length: Evidence for sex-specific effects. Psychoneuroendocrinology, 102, 225–235. 10.1016/j.psyneuen.2018.12.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttler R. M., Bagci E., Brand H. S., den Heijer M., Blankenstein M. A., Heijboer A. C. (2018). Testosterone, androstenedione, cortisol and cortisone levels in human unstimulated, stimulated and parotid saliva. Steroids, 138, 26–34. 10.1016/j.steroids.2018.05.013 [DOI] [PubMed] [Google Scholar]
- Caparros-Gonzalez R. A., Romero-Gonzalez B., Gonzalez-Perez R., Lucena-Prieto L., Perez-Garcia M., Cruz-Quintana F., Peralta-Ramirez M. I. (2019). Maternal and neonatal hair cortisol levels are associated with infant neurodevelopment at six months of age. Journal of Clinical Medicine, 8(11), 2015. 10.3390/jcm8112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casavant S. G., Cong X., Fitch R. H., Moore J., Rosenkrantz T., Starkweather A. (2019). Allostatic load and biomarkers of stress in the preterm infant: An integrative review. Biological Research for Nursing, 21(2), 210–223. 10.1177/1099800418824415 [DOI] [PubMed] [Google Scholar]
- Cho J., Biasini F., Su X., Holditch-Davis D. (2017). Associations of testosterone and cortisol levels with cognitive, motor, and language development in very low birthweight infants. Nursing Research, 66(5), 350–358. 10.1097/NNR.0000000000000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Carlo W. A., Su X., McCormick K. L. (2012). Associations between salivary testosterone and cortisol levels and neonatal health and growth outcomes. Early Human Development, 88(10), 789–795. 10.1016/j.earlhumdev.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M., Raisingani M., Chandler D. W., Curtin W. D., Barillas J., Brar P. C., Prasad K., Shah B., David R. (2017). Salivary testosterone during the minipuberty of infancy. Hormone Research in Paediatrics, 87, 111–115. 10.1159/000454862 [DOI] [PubMed] [Google Scholar]
- D’Agata A. L., Roberts M. B., Ashmeade T., Dutra S. V. O., Kane B., Groer M. W. (2019). Novel method of measuring chronic stress for preterm infants: Skin cortisol. Psychoneuroendocrinology, 102, 204–211. 10.1016/j.psyneuen.2018.12.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Schoot R. V., Broere J. J., Perryck K. H., Zondervan-Zwijnenburg M., van Loey N. E. (2015). Analyzing small data sets using Bayesian estimation: The case of posttraumatic stress symptoms following mechanical ventilation in burn survivors. European Journal of Psychotraumatology, 6, 25216. 10.3402/ejpt.v6.25216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes Z. (2014). Using Bayes to get the most out of non-significant results. Frontiers in Psychology, 29, 10.3389/fpsyg.2014.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Wang L., Wu C., Shi H., Zhou Z., Montgomery S., Cao Y. (2017). Sex hormones, gonadotropins, and sex hormone-binding globulin in infants fed breast milk, cow milk formula, or soy formula. Scientific Reports, Open 7, 4332. 10.1038/s41598-017-04610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom J. D., Chiu Y. M., Hsu H. L., Devick K. L., Brunst K. J., Campbell R., Enlow M. B., Coull B. A., Wright R. J. (2018). Maternal lifetime trauma and birthweight: Effect modification by in utero cortisol and child sex. Journal of Pediatrics, 203, 301–308. 10.1016/j.jpeds.2018.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. G. A. M. (1987). Cerebral lateralization: Biological mechanisms, associations, and pathology. MIT Press. [DOI] [PubMed] [Google Scholar]
- Greaves R. F., Zacharin M. R., Donath S. M., Inder T. E., Doyle L. W., Hunt R. W.(2014). Establishment of hormone reference intervals for infants born <30 weeks’ gestation. Clinical Biochemistry, 47(15), 101–108. 10.1016/j.clinbiochem.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Gunnala V., Guo R., Minutti C., Durazo-Arvizu R., Laporte C., Mathews H., Kliethermis S., Bhatia R. (2015). Measurement of salivary cortisol level for the diagnosis of critical illness-related corticosteroid insufficiency in children. Pediatric Critical Care Medicine, 16(4), e101–106. 10.1097/PCC.0000000000000361 [DOI] [PubMed] [Google Scholar]
- Hamilton B. E., Martin J. A., Oysterman M. J. K., Rossen L. M. (May 2019). Birth: Provisional data for 2018. National vital statistics reports. National Center for Health Statistics. https://www.cdc.gov/nchs/data/vsrr/vsrr-007-508.pdf [Google Scholar]
- Hill C. A., Threlkeld S. W., Fitch R. H. (2011). Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. International Journal of Developmental Neuroscience, 29(4), 381–388. 10.1016/j.ijdevneu.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz S. R., Kendrick D. E., Vohr B. R., Kenneth Poole W., Higgins R. D., & NICHD Neonatal Research Network. (2006). Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatrica, 95(10), 1239–1248. 10.1080/08035250600599727 [DOI] [PubMed] [Google Scholar]
- Hollier L. P., Mattes E., Maybery M. T., Keelan J. A., Hickey M., Whitehouse A. J. (2013). The Association between perinatal testosterone concentration and early vocabulary development: A prospective cohort study. Biological Psychology, 92(2), 212–215. 10.1016/j.biopsycho.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Iwata S., Kinoshita M., Okamura H., Tsuda K., Saikusa M., Harada E., Saitoh S., Iwata O. (2019). Intrauterine growth and the maturation process of adrenal function. Peer-Reviewed Journal, 7, e6368, 1–11. 10.7717/peerj.6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury J. E., Gonzalez A., Levitan R., Masellis M., Basile V., Atkinson L. (2016). Maternal self-reported depressive symptoms and maternal cortisol levels interact to predict infant cortisol levels. Infant Mental Health Journal, 37(2), 125–139. 10.1002/imhj.21554 [DOI] [PubMed] [Google Scholar]
- Kuiri-Hanninen T., Seuri R., Tyrvainen E., Turpeinen U., Hamalainen E., Stenman U. H., Dunkel L., Sankilampi U. (2011). Increased activity of the hypothalamic-pituitary-testicular axis in infancy results in increased androgen action in premature boys. Journal of Clinical Endocrinology & Metabolism, 96(1), 98–105. 10.1210/jc.2010-1359 [DOI] [PubMed] [Google Scholar]
- Kung K. T., Constantinescu M., Browne W. V., Noorderhaven R. M., Hines M. (2016). No relationship between early postnatal testosterone concentrations and autistic traits in 18 to 30-months-old children. Molecular Autism, 7(15), 1–5. 10.1186/s13229-016-0078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macari G. H., Tapia A. G., Iniguez G., Weisstaub G. (2019). Psychomotor development and cortisol salivary levels in infants that live with their inmate mothers. Revista Chilena de Pediatría, 90(3), 275–282. 10.32641/rchped.v90i3.690 [DOI] [PubMed] [Google Scholar]
- Martin J. A., Oysterman M. J. K. (June 2018). Describing the increase in preterm births in the United States, 2014-2016 (National Center for Health Statistics Data Brief; No. 312). https://www.cdc.gov/nchs/data/databriefs/db312.pdf [PubMed] [Google Scholar]
- McEwen B. (2005). Stressed or stressed out: What is the difference? Journal of Psychiatry and Neuroscience, 30(5), 315–318. [PMC free article] [PubMed] [Google Scholar]
- Mezzullo M., Fazzini A., Gambineri A., Di Dalmazi G., Mazza R., Pelusi C., Vicennati V., Pasquali R., Pagotto U., Fanelli F. (2017). Parallel diurnal fluctuation of testosterone, androstenedione, dehydro-epiandrosterone and 17OHprogesterone as assessed in serum and saliva: Validation of a novel liquid chromatography-tandem mass spectrometry method for salivary steroid profiling. Clinical Chemistry and Laboratory Medicine, 55(9), 1315–1323. 10.1515/cclm-2016-0805 [DOI] [PubMed] [Google Scholar]
- Moore T. A., Berger A. M., Wilson M. E. (2014). A new way of thinking about complications of prematurity. Biological Research for Nursing, 16(1), 72–82. 10.1177/1099800412461563 [DOI] [PubMed] [Google Scholar]
- Nicks B. A., Shah M. N., Adler D. H., Bastani A., Baugh C. W., Caterino J. M., Clark C. L., Diercks D. B., Hollander J. E., Malveau S. E., Nishijima D. K., Stiffler K. A., Storrow A. B., Wilber S. T., Yagapen A. N., Sun B. C. (2017). Minimizing attrition for multisite emergency care research. Academic Emergency Medicine, 24(4), 458–466. 10.1111/acem.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan D., Kenyon C. J., Seckl J. R., Holmess M. C. (2004). Glucocorticoid exposure in late gestatin in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. American Journal of Physiology, Endocrinology, and Metabolism, 287(5), E863–870. 10.1152/ajpendo.00137.2004 [DOI] [PubMed] [Google Scholar]
- Quinn T. A., Ratnayake U., Castillo-Melendez M., Moritz K. M., Dickinson H., Walker D. W. (2014). Adrenal steroidogenesis following prenatal dexamethasone exposure in the spiny mouse. Journal of Endocrinology, 221(2), 347–362. 10.1530/JOE-13-0514 [DOI] [PubMed] [Google Scholar]
- Saenz J., Alexander G. M. (2013). Postnatal testosterone levels and disorder relevant behavior in the second year of life. Biological Psychology, 94(1), 152–159. 10.1016/j.biopsycho.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Sarkar P., Bergman K., O’Connor T. G., Glover V. (2008). Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: Possible implications for foetal programming. Journal of Neuroendocrinology, 20(4), 489–496. 10.1111/j.1365-2826.2008.01659.x [DOI] [PubMed] [Google Scholar]
- Schaadt G., Hesse V., Friederici A. D. (2015). Sex hormones in early infancy seem to predict aspects of later language development. Brain and Language, 141, 70–76. 10.1016/j.bandl.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Squires J., Bricker D., Twombly L. (2002). The ages and stages questionnaires: Social-emotional. A parent-completed, child-monitoring system for social-emotional behaviors. Brookes. [Google Scholar]
- Whitehouse A. J., Mattes E., Maybery M. T., Dissanayake C., Sawyer M., Jones R. M., Pennell C. E., Keelan J. A., Hickey M. (2012). Perinatal testosterone exposure and autistic-like traits in the general population: A longitudinal pregnancy-cohort study. Journal of Neurodevelopmental Disorders, 4(25), 1–12. 10.1186/1866-1955-4-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse A. J., Mattes E., Maybery M. T., Sawyer M. G., Jacoby P., Keelan J. A., Hickey M. (2012). Sex-specific associations between umbilical cord blood testosterone levels and language delay in early childhood. Journal of Child Psychology and Psychiatry, 53(7), 726–734. 10.1111/j.1469-7610.2011.02523.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, CHO_Supplements_ready for Associations Between Hormonal Biomarkers and Preterm Infant Health and Development During the First 2 Years After Birth by June Cho, Lung-Chang Chien and Diane Holditch-Davis in Biological Research For Nursing