Abstract
Early recognition of Alzheimer’s disease (AD) in the prodromal period has not been robust yet will be necessary if effective disease-modifying drugs are to be useful in preventing or delaying the condition. The objective of this narrative review was to describe the current, evidenced based understanding of alterations in sensory data as potential biomarkers for AD. Review of empirical studies that tested senses as biomarkers for AD and were published in English within the past 50 years was completed. Eighteen empirical studies were identified that met the strict criteria for inclusion, with 12 of these studies being related to the olfactory system. Two studies examined auditory, two examined vision, one examined proprioception, and one examined taste. Thus, only olfaction has been studied to any extent, leaving a clear gap in the literature for the use of other senses. A promising area of research has begun to be reported concerning differences in responses to pain stimuli in AD relative to cognitively normal subjects. Pain is not a single sense like the others but integrates several senses and may allow for use as an early biomarker for AD, as it integrates several brain areas and pathways. Unlike the other senses, simple devices can be used to measure changes in pain perception in cognitively normal adults with genetic predispositions for possible AD, making this potentially useful for clinicians in the future.
Keywords: Alzheimer’s disease, biomarkers, ApoE4, smell, vision, taste proprioception, pain
In the most recent publication of the “Alzheimer’s Disease Facts and Figures” the Alzheimer’s Association presented evidence suggesting a potential of savings of $7 trillion dollars resulting from the early diagnosis of Alzheimer’s disease (AD), a point at which 70% of those with AD are diagnosed in the prodromal phase of the illness or prior to the development of observable signs or symptoms of AD (Alzheimer’s Association, 2019). One current challenge is the lack of robustness in methods that detect AD, particularly in the very early stages of the disease. Early subtle changes in memory and cognition occur over the course of months to years making it difficult to recognize symptoms in patients, and often health care providers wrongly misattribute small changes in cognitive function as a process of normal aging (Knopman et al., 2000). Evidence of a complex pathophysiological process of AD is accumulating that indicates pathological change is detectable years before, rather than at the beginning of, the prodromal phase as neuropathologic examinations of adults clinically diagnosed without cognitive impairment reveal similar pathological signatures to those with AD (Dubois et al., 2007).
Recently, The National Institute on Aging (NIA) published a framework for the diagnosis of AD that shifted the understanding of AD as a syndrome or the clinical consequence of one or more diseases toward a biological process that incorporates measurable physiological parameters or biomarkers (Jack et al., 2018). Current biomarkers of AD are grouped into those of β amyloid deposition (A), pathologic τ (T), and neurodegeneration (N) and are typically gathered by advanced imaging and lumbar puncture (Jack et al., 2018). In the primary care setting it is difficult to detect pre-clinical AD because detection methods using imaging, invasive CSF test, and biomarker testing are rarely available outside of specialty clinics (Mortamais et al., 2017). Health care providers are advised to focus on the core criteria for diagnosis of clinical AD, which do not require biomarker evidence, but rely on clinical evaluation and neuropsychological testing (Tolea & Galvin, 2013). Since there is variation in the diagnostic abilities of primary care providers, research has focused on finding non-invasive biomarkers that may be helpful to health care providers in identifying AD (Romano et al., 2020).
Alterations in sensations may be a useful biomarker. Senses are multisystem physiological mechanisms of perception traditionally categorized as smell, taste, sight, hearing, and touch, but also include the ability of humans to sense temperature (thermoception), body position (proprioception), pain (nociception), balance (equilibrioception), and touch, thirst and hunger (mechanoception; Matlin & Foley, 1992). Stimuli interact with sensory receptors that initiate action potentials processed in the central nervous system to elicit an action (Matlin & Foley, 1992). In early AD, pathological changes in the brain may result in alteration in sensory perception and provide insight that may be helpful to identify those at risk for AD. This paper aims to explore what is known about current biomarkers using a focused and limited narrative review of the scientific literature pertaining to sensory changes as potential biomarkers for AD. Whittemore and Knafl (2005) contend that a narrative review is the preferred method of organizing a body of literature, as this type of review allows for a combination of diverse methods to provide an in-depth understanding of a phenomena of interest and deepen understanding. The question posed for this review was “What is the current understanding of alterations in senses as potential biomarkers for AD?”
Methods
An analysis of the existing literature was undertaken to meet the study aim. Empirical studies published in English were included in this analysis. These included studies that examined traditional senses (sight, hearing, taste, smell, and touch); the detection of other stimuli (thermoception, proprioception, nociception, equilibrioception, and mechanoreception); and response to internal stimuli (hunger and thirst) that used multiple senses. Further, to be considered eligible, the sense had to be investigated in the study as a biomarker defined as a measurable and quantifiable biological parameter that can be used as an index for dementia. Data items extracted from the literature included method of sensation-testing, measure of clinical disease, and association with diagnosis.
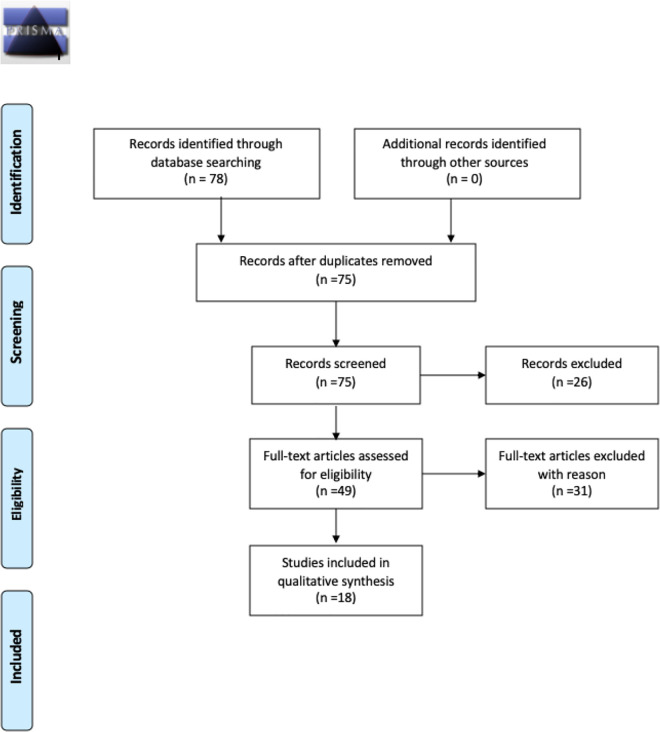
A search of English-language studies reporting associations between alterations in sensory modalities and diagnosis of dementia was conducted using three databases: PubMed (May 2020), CINAHL (May 2020), and PsycINFO (May 2020). Search terms included “sensation,” “dementia,” “Alzheimer’s disease,” and “biomarker” as well as each sense individually (i.e., sight, hearing, taste, smell, touch, thermoception, proprioception, nociception, equilibrioception, and mechanoreception). All potential studies identified by this search (N = 78) were exported directly to EndNote and reviewed for eligibility after duplicated studies were removed.
Articles were excluded if not topically related based on title and subsequent reading of the abstract to assure that a sense was investigated as a biomarker, defined as a measurable and quantifiable biological parameter used as an index for Alzheimer’s disease. Further, articles identified as literature reviews, editorials, or commentaries were excluded, and manuscripts without readily available full texts were removed from the sample (see Figure 1). Studies were organized according to sensory systems. Data were extracted independently by the first author (RR) using a piloted data collection form, and any ambiguous information was settled by consensus among all authors. The quality of the studies was assessed using the study design-specific (e.g., observational cohort or cross-sectional studies quality assessment tool) tool developed by the National Heart, Lung, and Blood Institute (NHLBI) and Research Triangle Institute International (Study Quality Assessment Tools, 2020). We awarded point values (e.g., No = 0, Yes = 1, Good = 2, Fair = 1, Poor = 0) to each assessment of quality and totaled them for the final score quality score so that the higher the total score the better the quality.
Figure 1.
PRISMA diagram.
Results
A total of 78 manuscripts were identified in the initial sample. The final literature review sample consisted of 18 studies after removing three duplicate manuscripts, 28 topically unrelated (e.g., ligand correlations to disease state, behavioral paradigms, depression related studies) or methodologically unacceptable manuscripts (e.g., reviews, commentaries, and editorials), 26 non-empirical manuscripts, and three non-human studies. The olfactory system was investigated in 12 manuscripts and was the most represented sense in the final sample. The other sensory systems in the final sample included auditory, vision, proprioception, and taste. No other sensory system studies were identified by this search.
Olfactory
Olfaction has been proposed as a biomarker for AD since Esiri and Wilcock (1984) compared the olfactory bulbs in AD patients and normal controls and found that patients with AD had collections of neurofibrillary tangles in the anterior olfactory nucleus. There were 12 manuscripts reviewed concerning the olfactory sense as a biomarker for AD. All manuscripts were cross-sectional analyses, with 1 manuscript published in 1996 by Serby et al. having a 5-year follow-up (see Table 1). The sample size of studies ranged from n = 30 to n = 728 and compared participants with AD to those with mild cognitive impairment, frontotemporal dementia, or healthy controls. Five studies were conducted in the United States, while the remaining studies were conducted in Japan (n = 2), Canada (n = 1), Sweden (n = 1), Italy (n = 1), Germany (n = 1), and China (n = 1). The quality of the manuscripts reviewed ranged from a score of 8–11, suggesting high-quality design and analysis, as shown in Tables 1 and 2.
Table 1.
Description of Sample of 12 Studies on Olfaction as a Biomarker.
| Author (Year) | Sample Size | Study Design | Assessment of Sensation | Assessment of Cognition | Quality Score |
|---|---|---|---|---|---|
| Serby (1996) | N = 28 FDR* N = 28 controls |
Cross-sectional, two-group comparison | UPSIT** | MMSE***, ADAS+ | 8 |
| Wang (2002) | N = 28 MCI N = 30 controls |
Cross-sectional, two-group, age-matched comparison | CC-SIT++ | CAMCOG-C +++, MMSE |
10 |
| Peters (2003) | N = 14 AD N = 8 MCI N = 8 controls |
Cross-sectional, three group, age-matched comparison | Butanol odor, odor discrimination, and odor identification | MMSE, CDR | 10 |
| Olofsson et al. (2010) | N = 1,236 | 5-year longitudinal cohort study | SOIT^ | MMSE, Vocabulary^^ | 11 |
| Jimbo (2011) | N = 109 AD N = 40 controls |
Cross-sectional, two-group, age-matched comparison | OSIT-J^^^ | MMSE, ADAS-cog$ | 9 |
| Masaoka (2013) | N = 16 AD N = PD N = DM1 N = 67 controls |
Cross-sectional, multi group comparison | UPSIT | — | 10 |
| Growdon (2015) | N = 215 CN | Cross-sectional one-group analysis | UPSIT | MMSE, BNT-30$$, AmNART$$$ | 10 |
| Albers (2016) | N = 10 AD N = 29 MCI N = 74 SCC N = 70 controls |
Four-group, cross-sectional analysis | OPIDoo, POEMooo, OASa | CDR, MMSE, BNT, TrailsB@ | 9 |
| Lafaille-Magnan (2017) | N = 274 | Cross-sectional | UPSIT | RBANS@@, MoCA | 11 |
| Quarmley (2017) | N = 262 AD N = 174 MCI N = 292 controls |
Cross-sectional three-group comparison | SS-OIT | MoCA | 11 |
| Tonacci (2017) | N = 85 MCI N = 41 controls |
Cross-sectional age- and gender-matched | OTTaa, ODTaaa, OIT | Free DCT@@@, RAVLT#, ROCF##, BSS###, MMSE, CDR, ADL, ADAS-cog | 9 |
| Kouzuki (2018) | N = 40 AD N = 34 MCI N = 40 controls |
Cross-sectional analysis | OSIT-J | MMSE, ADAS-J, TDASo | 10 |
Note. Mild cognitive impairment, Alzheimer’s disease, cognitively normal, Parkinson’s disease, diabetes mellites type 1, *First-degree relatives, **University of Pennsylvania Smell Identification Test, ***Mini Mental State Exam, +Alzheimer’s Disease Assessment Scale, ++Cross-Cultural Smell Identification Test, +++Cambridge Cognitive Examination Chinese version, ^Scandinavian Odor Identification Test, ^^Cureman and Salde, 1959, ^^^Odor Stick Identification Test for the Japanese, $Alzheimer’s Disease Assessment Scale-cognitive subscale, $$ Boston Naming Test, $$$ American National Adult Reading Test, @Trails Making Test B, @@ Repeatable Battery for the Assessment of Neuropsychological Status, @@@ Dot Counting Test, #Rey Auditory Verbal Learning Test, ##Rey–Osterrieth complex figure test, ###Bhatia Battery of Performance tests of Intelligence, oTouch Panel-type Dementia Assessment Scale, ooUniversity of Pennsylvania Smell Identification Test, oooPerception of Odor Episodic Memory, aOdor Awareness Scale, aaOdor Threshold Testing, aaaOdor Detection Threshold.
Table 2.
Quality Scores of Auditory, Vision, Proprioception, and Taste Studies.
| Author (Year) | Quality Score |
|---|---|
| Laptinskaya et al. (2018) | 10 |
| Tuwaig et al. (2017) | 10 |
| Balachandar et al. (2017) | 9 |
| Risacher et al. (2013) | 9 |
| Tu et al. (2015) | 9 |
| Naudin et al. (2015) | 10 |
Note. Study Quality Assessment Tools. (2020). National Heart, Lung, and Blood Institute. Retrieved July 13, 2020 from https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
Serby and colleagues (1996) found that first-degree relatives performed worse on a measure of odor identification when compared to a control group of community-dwelling older adults; further, the authors argued that impaired olfaction may be present before clinical symptoms. Similarly, two studies of participants with mild cognitive impairment (MCI), a known risk factor for AD, showed odor identification was impaired in people with MCI when compared to age- and gender-matched, cognitively normal controls, further adding to the evidence that suggests changes in the olfactory system may be useful in identifying those at risk for AD (Peters et al., 2003; Tonacci et al., 2017). These subsequent studies used similar methods to Serby et al. (1996). The manuscripts in the sample all found significant associations with measures of olfaction and AD diagnosis (Quarmley et al., 2017). Two studies that specifically evaluated APOE4 status found significant associations with deficits in odor identification (Lafaille-Magnan et al., 2017; Wang et al., 2002). Most studies found olfactory function decreased in AD and MCI patients and was associated with either cerebral spinal fluid biomarker levels (Kouzuki et al., 2018; Lafaille-Magnan et al., 2017), genetic status (Lafaille-Magnan et al., 2017; Wang et al., 2002), worse performance on measures of cognitive function (Albers et al., 2016; Jimbo et al., 2011; Lafaille-Magnan et al., 2017; Masaoka et al., 2013; Peters et al., 2003), or changes in brain structure as observed through imaging modalities (Growdon et al., 2015).
Though olfaction has been the most common sense evaluated as a potential biomarker for AD, challenges may occur when using olfaction as a screening process in primary care because assuring reliability and specificity can be problematic since conditions such as seasonal allergies can yield confusing results. Cultural exposure to certain scents may vary and make it difficult to find a generalizable test. Offensive scents may also stimulate the trigeminal nerve rather than the olfactory nerve, further complicating the reliability of screening.
Taste
Taste and smell are closely related, as both senses converge in the caudal orbitofrontal cortex, yet research has mostly focused on the association between smell and AD. Neuroimaging techniques are able to visualize taste perception in cerebral representations and suggest that gustatory processing may be impaired in people with brain disease (Camicioli et al., 1998; Lang et al., 2006). Naudin et al. (2015) conducted a cross-sectional analysis and found that people with AD gave significantly more incorrect responses on salty, bitter, and sweet taste identification tasks when compared to healthy controls.
Auditory
The next most common sensory system reported as a potential biomarker has been hearing. The quality of the 2 studies included in this review can be considered strong, with scores of 10 on the design-specific quality assessment tool. Waldton (1974) first showed functional impairment of the cranial nerves in 66 women with senile dementia. Further hearing loss becomes more prevalent as people age, making it a notable sense to study in the AD population. Autopsy studies have confirmed hallmarks of AD pathology, Aβ plagues, and τ were identified in the cochlear nucleus and throughout the auditory system in patients with AD. Of the auditory manuscripts reviewed, found positive associations with changes in auditory response and either markers of AD (total-τ and p-τ) or measures of cognition (Laptinskaya et al., 2018; Tuwaig et al., 2017).
The first study, published by Tuwaig et al. (2017), was a 2018 cross-sectional analysis of 187 cognitively normal, high-risk members of the PREVENT-AD cohort that found two tests of auditory abilities were associated with measures of cognition, concentration of total-τ and p-τ, measures of hippocampal and entorhinal cortical volumes, and cortical thickness. However, the authors did not find an association between the measures of central auditory processing and APOE4 carrier status.
The second auditory study reported significant changes in the event-related response in an EEG signal caused by alteration of auditory stimulation after a long interstimulus interval, and this change in EEG signal was associated with worse episodic memory performance at 5-year follow-up (Laptinskaya et al., 2018). Although limited, there is growing evidence of hearing loss as a potential indicator of AD; however, it is unclear if these changes are associated with AD pathology or with temporal lobe abnormalities related to aging (Murphy, 2019).
Vision
Changes in vision has been identified as another possible marker of AD, given that amyloid-β plagues have been identified in the aqueous humorous of AD patients undergoing cataract surgery. In 2013, Risacher and colleagues accurately identified people with MCI relative to healthy controls using vision testing (Risacher et al., 2013). The authors also determined that people with AD and MCI demonstrated significant contrast sensitivity visual deficits, which also is associated with worse performance of cognitive measures.
In a later cross-sectional analysis, Balachandar and colleagues (2017) found that people with mild AD exhibited significant associations between deficits on visuospatial measures and changes in resting-state functional connectivity. Vision changes may prove to be an inadequate biomarker, as there remains discussion on the timing of vision pathology and AD clinical disease as well as the fact that alteration in vision is a part of healthy aging in addition to part of other confounding disease processes (Murphy, 2019).
Proprioception
Proprioception, or the awareness and perception of one’s body in space, has the potential to be an early marker of AD pathology, as motor decline is observed in all stages of AD (Boyle et al., 2007). A slowing of walking speed that proceeds cognitive decline was first observed 2 decades ago and is explained by the overlap in the brain structures (i.e., the cerebellum, basal ganglia, and motor cortex) responsible for walking and involved in attentional, executive, and visuospatial functions (Camicioli et al., 1998). Tu and colleagues (2015) used a virtual supermarket task to discriminate between participants with AD and frontotemporal dementia (Tu et al., 2015). The authors found that people with AD had more impairment in orientation compared to people with FTD and concluded that orientation had the same level of diagnostic sensitivity as other measures of episodic memory (Tu et al., 2015). This evidence supports the use of tests of proprioception as a tool to identify persons with AD.
Discussion
The current state of the literature shows a paucity of research in how non-traditional senses may be used as biomarkers for AD. Yet, the results do suggest further research is warranted as the majority of studies reviewed demonstrate that sense perception changes at different diagnosis categories (e.g., AD, MCI, normal). Results from this review are promising as they suggest that future research in this area could shed light on other methods of assessment that may be more feasible and less invasive than current practice recommendations such as lumbar puncture, MRI, and PET scans to identify those with Alzheimer’s disease. Overall, olfaction was the most widely studied sense. Researchers of the 12 manuscripts reviewed showed measures of olfaction were associated with changes in cognition, imaging, and APOE status. Though limited in scope, this review demonstrates how a lack of evidence supporting how other senses could be used as a biomarker for AD serves as a clear gap in the literature. Evidence from all manuscripts included in the current review indicates deficits or changes in senses can potentially be used to differentiate between people with AD, MCI, and cognitively normal adults.
One possible, yet under studied area for AD biomarkers is pain. AD is well-known to cause changes in brain structures and metabolism (Monroe et al., 2017; Monroe et al., 2018). The hippocampus and prefrontal cortex experience the most brain volume loss with healthy aging; however, in individuals with AD, the entorhinal cortex and hippocampus and the lateral temporal lobe and neocortex have significantly more volume loss compared to healthy individuals (Monroe et al., 2012). As AD progresses, damage typically occurs in both the lateral and medial pain network. Studies have shown that neurofibrillary tangles and β amyloid plaques accumulate in the amygdala (Kromer Vogt et al., 1990), orbitofrontal cortex (Tekin et al., 2001), insula, PAG (Parvizi et al., 2000), and striatum (Selden et al., 1994), all of which comprise the rostral pain system (Monroe et al., 2012). People with AD have alterations in pain perceptions, and this has been shown to be different when compared to cognitively normal adults (Monroe et al., 2015, 2016; Romano et al., 2019). Measuring pain perception in individuals who are very early in the process of AD is not complex and could be adapted to most primary care practices. Also important is that the association between changes in pain perception in AD is theoretically sound (Monroe et al., 2012). Pain is not a single sense per se, as multiple areas of the brain are used in pain perception, and this complexity may allow alterations in pain perception to be used as an early biomarker for AD.
Implications for Research
New therapeutic agents are being explored that hold promise for clinicians to be able to offer treatment that alters the progression of AD (Cummings et al., 2019). However, the state of the science in identifying non-invasive biomarkers for AD that can be identified prior to the onset of cognitive impairment is still in the early stages. The results of this review suggest that additional focus should be directed on alternation of senses as a potentially useful biomarker. Further the results of this review identify a clear gap in the literature as only one nontraditional sense manuscript was identified through this review. If senses prove to be useful noninvasive biomarkers then clinicians might have feasible, simple, and non-invasive methods available to identify people who have early or asymptomatic AD for early treatment.
Limitations
Narrative reviews have a number of potential limitations. Defined parameters and boundaries can pose a challenge. In this study, the selection of the search terms was believed to be appropriate, but there is a possibility that some work may have been missed, as this is a rarely explored field of scientific work. Every effort was made to assure that the widest search of the actual research literature was conducted, but the language and/or MeSH terms may still be ambiguous. Further, the majority of data collection was conducted by the primary author; however, the final sample of manuscripts were reviewed by all authors and any disagreement on interpretation of the results were discussed and interpreted by group consensus.
Acknowledgment
The authors would like to acknowledge Curtis Roby for his editorial assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Raymond R. Romano III  https://orcid.org/0000-0003-1951-2412
https://orcid.org/0000-0003-1951-2412
Todd B. Monroe  https://orcid.org/0000-0003-3156-5808
https://orcid.org/0000-0003-3156-5808
References
- Albers A. D., Asafu-Adjei J., Delaney M. K., Kelly K. E., Gomez-Isla T., Blacker D., Johnson K. A., Sperling R. A., Hyman B. T., Betensky R. A., Hastings L., Albers M. W. (2016). Episodic memory of odors stratifies Alzheimer biomarkers in normal elderly. Annals of Neurology, 80(6), 846–857. 10.1002/ana.24792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 15(3), 321–387. 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- Balachandar R., Bharath S., John J. P., Joshi H., Sadanand S., Saini J., Kumar K. J., Varghese M. (2017). Resting-state functional connectivity changes associated with visuospatial cognitive deficits in patients with mild Alzheimer disease. Dementia and Geriatric Cognitive Disorders, 43(5–6), 229–236. 10.1159/000457118 [DOI] [PubMed] [Google Scholar]
- Boyle P. A., Wilson R. S., Buchman A. S., Aggarwal N. T., Tang Y., Arvanitakis Z., Kelly J., Bennett D. A. (2007). Lower extremity motor function and disability in mild cognitive impairment. Experimental Aging Research, 33(3), 355–371. 10.1080/03610730701319210 [DOI] [PubMed] [Google Scholar]
- Camicioli R., Howieson D., Oken B., Sexton G., Kaye J. (1998). Motor slowing precedes cognitive impairment in the oldest old. Neurology, 50(5), 1496–1498. 10.1212/wnl.50.5.1496 [DOI] [PubMed] [Google Scholar]
- Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. (2019). Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s & Dementia (NY), 5, 272–293. 10.1016/j.trci.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’Brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P. J., Scheltens P. (2007). Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. The Lancet Neurology, 6(8), 734–746. 10.1016/s1474-4422(07)70178-3 [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Wilcock G. K. (1984). The olfactory bulbs in Alzheimer’s disease. J Neurol Neurosurg Psychiatry, 47(1), 56–60. 10.1136/jnnp.47.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Growdon M. E., Schultz A. P., Dagley A. S., Amariglio R. E., Hedden T., Rentz D. M., Johnson K. A., Sperling R. A., Albers M. W., Marshall G. A. (2015). Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology, 84(21), 2153–2160. 10.1212/WNL.0000000000001614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R., Jr, Bennett D. A., Blennow K., Carrillo M. C., Dunn B., Haeberlein S. B., Holtzman D. M., Jagust W., Jessen F., Karlawish J., Liu E., Molinuevo J. L., Montine T., Phelps C., Rankin K. P., Rowe C. C., Scheltens P., Siemers E., Snyder H. M., Sperling R., & Contributors. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia, 14(4), 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo D., Inoue M., Taniguchi M., Urakami K. (2011). Specific feature of olfactory dysfunction with Alzheimer’s disease inspected by the Odor Stick Identification Test. Psychogeriatrics, 11(4), 196–204. 10.1111/j.1479-8301.2011.00387.x [DOI] [PubMed] [Google Scholar]
- Knopman D., Donohue J. A., Gutterman E. M. (2000). Patterns of care in the early stages of Alzheimer’s disease: Impediments to timely diagnosis. Journal of the American Geriatrics Society, 48(3), 300–304. 10.1111/j.1532-5415.2000.tb02650.x [DOI] [PubMed] [Google Scholar]
- Kouzuki M., Suzuki T., Nagano M., Nakamura S., Katsumata Y., Takamura A., Urakami K. (2018). Comparison of olfactory and gustatory disorders in Alzheimer’s disease. Neurological Sciences, 39(2), 321–328. 10.1007/s10072-017-3187-z [DOI] [PubMed] [Google Scholar]
- Kromer Vogt L. J., Hyman B. T., Van Hoesen G. W., Damasio A. R. (1990). Pathological alterations in the amygdala in Alzheimer’s disease. Neuroscience, 37(2), 377–385. 10.1016/0306-4522(90)90408-v [DOI] [PubMed] [Google Scholar]
- Lafaille-Magnan M.-E., Poirier J., Etienne P., Tremblay-Mercier J., Frenette J., Rosa-Neto P., Breitner J. C. S. (2017). Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology, 89(4), 327–335. 10.1212/WNL.0000000000004159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C. J., Leuschner T., Ulrich K., Stossel C., Heckmann J. G., Hummel T. (2006). Taste in dementing diseases and parkinsonism. Journal of the Neurological Sciences, 248(1–2), 177–184. 10.1016/j.jns.2006.05.020 [DOI] [PubMed] [Google Scholar]
- Laptinskaya D., Thurm F., Küster O. C., Fissler P., Schlee W., Kolassa S., von Arnim C. A. F., Kolassa I.-T. (2018). Auditory memory decay as reflected by a new mismatch negativity score is associated with episodic memory in older adults at risk of dementia. Frontiers in Aging Neuroscience, 10, 13. 10.3389/fnagi.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka Y., Pantelis C., Phillips A., Kawamura M., Mimura M., Minegishi G., Homma I. (2013). Markers of brain illness may be hidden in your olfactory ability: A Japanese perspective. Neuroscience Letters, 549, 182–185. 10.1016/j.neulet.2013.05.077 [DOI] [PubMed] [Google Scholar]
- Matlin M. W., Foley H. J. (1992). Sensation and perception (3rd ed.) Allyn & Bacon. [Google Scholar]
- Monroe T. B., Beach P. A., Bruehl S. P., Dietrich M. S., Rogers B. P., Gore J. C., Atalla S. W., Cowan R. L. (2017). The impact of Alzheimer’s disease on the resting state functional connectivity of brain regions modulating pain: A cross sectional study. Journal of Alzheimer’s Disease, 57(1), 71–83. 10.3233/JAD-161187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe T. B., Fillingim R. B., Bruehl S. P., Rogers B. P., Dietrich M. S., Gore J. C., Atalla S. W., Cowan R. L. (2018). Sex differences in brain regions modulating pain among older adults: A cross-sectional resting state functional connectivity study. Pain Medicine, 19(9), 1737–1747. 10.1093/pm/pnx084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe T. B., Gibson S. J., Bruehl S. P., Gore J. C., Dietrich M. S., Newhouse P., Atalla S., Cowan R. L. (2016). Contact heat sensitivity and reports of unpleasantness in communicative people with mild to moderate cognitive impairment in Alzheimer’s disease: A cross-sectional study. BMC Medicine, 14, 74. 10.1186/s12916-016-0619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe T. B., Gore J. C., Bruehl S. P., Benningfield M. M., Dietrich M. S., Chen L. M., Newhouse P., Fillingim R., Chodkowski B., Atalla S., Arrieta J., Damon S. M., Blackford J. U., Cowan R. L. (2015). Sex differences in psychophysical and neurophysiological responses to pain in older adults: A cross-sectional study. Biology of Sex Differences, 6, 25. 10.1186/s13293-015-0041-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe T. B., Gore J. C., Chen L. M., Mion L. C., Cowan R. L. (2012). Pain in people with Alzheimer disease: Potential applications for psychophysical and neurophysiological research. Journal of Geriatric Psychiatry and Neurology, 25(4), 240–255. 10.1177/0891988712466457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M., Ash J. A., Harrison J., Kaye J., Kramer J., Randolph C., Pose C., Albala B., Ropacki M., Ritchie C. W., Ritchie K. (2017). Detecting cognitive changes in preclinical Alzheimer’s disease: A review of its feasibility. Alzheimer’s & Dementia, 13(4), 468–492. 10.1016/j.jalz.2016.06.2365 [DOI] [PubMed] [Google Scholar]
- Murphy C. (2019). Olfactory and other sensory impairments in Alzheimer disease. Nature Reviews Neurology, 15(1), 11–24. 10.1038/s41582-018-0097-5 [DOI] [PubMed] [Google Scholar]
- Naudin M., Mondon K., El-Hage W., Perriot E., Boudjarane M., Desmidt T., Lorette A., Belzung C., Hommet C., Atanasova B. (2015). Taste identification used as a potential discriminative test among depression and Alzheimers disease in elderly: A pilot study. Psychiatry Research, 228(2), 228–232. 10.1016/j.psychres.2015.03.021 [DOI] [PubMed] [Google Scholar]
- Olofsson J. K., Nordin S., Wiens S., Hedner M., Nilsson L. G., Larsson M. (2010). Odor identification impairment in carriers of ApoE-∊4 is independent of clinical dementia. Neurobiol Aging, 31(4), 567–577. 10.1016/j.neurobiolaging.2008.05.019 [DOI] [PubMed] [Google Scholar]
- Parvizi J., Van Hoesen G. W., Damasio A. (2000). Selective pathological changes of the periaqueductal gray matter in Alzheimer’s disease. Annals of Neurology, 48(3), 344–353. https://www.ncbi.nlm.nih.gov/pubmed/10976641 [PubMed] [Google Scholar]
- Peters J. M., Hummel T., Kratzsch T., Lotsch J., Skarke C., Frolich L. (2003). Olfactory function in mild cognitive impairment and Alzheimer’s disease: An investigation using psychophysical and electrophysiological techniques. The American Journal of Psychiatry, 160(11), 1995–2002. 10.1176/appi.ajp.160.11.1995 [DOI] [PubMed] [Google Scholar]
- Quarmley M., Moberg P. J., Mechanic-Hamilton D., Kabadi S., Arnold S. E., Wolk D. A., Roalf D. R. (2017). Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. Journal of Alzheimer’s Disease, 55(4), 1497–1507. 10.3233/JAD-160842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher S. L., WuDunn D., Pepin S. M., MaGee T. R., McDonald B. C., Flashman L. A., Wishart H. A., Pixley H. S., Rabin L. A., Paré N., Englert J. J., Schwartz E., Curtain J. R., West J. D., O’Neill D. P., Santulli R. B., Newman R. W., Saykin A. J. (2013). Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiology of Aging, 34(4), 1133–1144. 10.1016/j.neurobiolaging.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano R. R., 3rd, Carter M. A., Anderson A. R., Monroe T. B. (2020). An integrative review of system-level factors influencing dementia detection in primary care. Journal of the American Association of Nurse Practitioners, 32(4), 299–305. 10.1097/jxx.0000000000000230 [DOI] [PubMed] [Google Scholar]
- Romano R. R., Anderson A. R., Failla M. D., Dietrich M. S., Atalla S., Carter M. A., Monroe T. B. (2019). Sex differences in associations of cognitive function with perceptions of pain in older adults. Journal of Alzheimer’s Disease, 70(3), 715–722. 10.3233/JAD-190142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden N., Geula C., Hersh L., Mesulam M. M. (1994). Human striatum: Chemoarchitecture of the caudate nucleus, putamen and ventral striatum in health and Alzheimer’s disease. Neuroscience, 60(3), 621–636. 10.1016/0306-4522(94)90491-x [DOI] [PubMed] [Google Scholar]
- Serby M., Mohan C., Aryan M., Williams L., Mohs R. C., Davis K. L. (1996). Olfactory identification deficits in relatives of Alzheimer’s disease patients. Biological Psychiatry, 39(5), 375–377. 10.1016/0006-3223(95)00472-6 [DOI] [PubMed] [Google Scholar]
- Tekin S., Mega M. S., Masterman D. M., Chow T., Garakian J., Vinters H. V., Cummings J. L. (2001). Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Annals of Neurology, 49(3), 355–361. https://www.ncbi.nlm.nih.gov/pubmed/11261510 [PubMed] [Google Scholar]
- Tolea M. I., Galvin J. E. (2013). Current guidelines for dementia screening: Shortcomings and recommended changes. Neurodegenerative Disease Management, 3(6), 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonacci A., Bruno R. M., Ghiadoni L., Pratali L., Berardi N., Tognoni G., Cintoli S., Volpi L., Bonuccelli U., Sicari R., Taddei S., Maffei L., Picano E. (2017). Olfactory evaluation in mild cognitive impairment: Correlation with neurocognitive performance and endothelial function. European Journal of Neuroscience, 45(10), 1279–1288. 10.1111/ejn.13565 [DOI] [PubMed] [Google Scholar]
- Tu S., Wong S., Hodges J. R., Irish M., Piguet O., Hornberger M. (2015). Lost in spatial translation—A novel tool to objectively assess spatial disorientation in Alzheimer’s disease and frontotemporal dementia. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 67, 83–94. 10.1016/j.cortex.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Tuwaig M., Savard M., Jutras B., Poirier J., Collins D. L., Rosa-Neto P., Fontaine D., Breitner J. C. S. (2017). Deficit in central auditory processing as a biomarker of pre-clinical Alzheimer’s disease. Journal of Alzheimer’s Disease, 60(4), 1589–1600. 10.3233/JAD-170545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldton S. (1974). Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiatrica Scandinavica, 50(5), 539–547. 10.1111/j.1600-0447.1974.tb09714.x [DOI] [PubMed] [Google Scholar]
- Wang Q. S., Tian L., Huang Y. L., Qin S., He L. Q., Zhou J. N. (2002). Olfactory identification and apolipoprotein E epsilon 4 allele in mild cognitive impairment. Brain Research, 951(1), 77–81. https://ac.els-cdn.com/S0006899302031372/1-s2.0-S0006899302031372-main.pdf?tid=5bd752a0-3761-48da-86f5-c8b8e2410412&acdnat=1536767275_6aa1e63279dede9ecd84fa3e4e7e7a2f [DOI] [PubMed] [Google Scholar]
- Whittemore R., Knafl K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52(5), 546–553. 10.1111/j.1365-2648.2005.03621.x [DOI] [PubMed] [Google Scholar]