Abstract
Background
Most cell therapy trials failed to show an improvement in global left ventricular (LV) function measures after myocardial infarction (MI). Myocardial segments are heterogeneously impacted by MI. Global LV function indices are not able to detect the small treatment effects on segmental myocardial function which may have prognostic implications for cardiac events. We aimed to test the efficacy of allogeneic cardiosphere-derived cells (CDCs) for improving regional myocardial function and contractility.
Methods
In this exploratory analysis of a randomised clinical trial, 142 patients with post-MI with LVEF <45% and 15% or greater LV scar size were randomised in 2:1 ratio to receive intracoronary infusion of allogenic CDCs or placebo, respectively. Change in segmental myocardial circumferential strain (Ecc) by MRI from baseline to 6 months was compared between CDCs and placebo groups.
Results
In total, 124 patients completed the 6-month follow-up (mean (SD) age 54.3 (10.8) and 108 (87.1%) men). Segmental Ecc improvement was significantly greater in patients receiving CDC (−0.5% (4.0)) compared with placebo (0.2% (3.7), p=0.05). The greatest benefit for improvement in segmental Ecc was observed in segments containing scar tissue (change in segmental Ecc of −0.7% (3.5) in patients receiving CDC vs 0.04% (3.7) in the placebo group, p=0.04).
Conclusions
In patients with post-MI LV dysfunction, CDC administration resulted in improved segmental myocardial function. Our findings highlight the importance of segmental myocardial function indices as an endpoint in future clinical trials of patients with post-MI.
Trial registration number
Keywords: MRI, coronary artery disease, epidemiology
Key questions.
What is already known about this subject?
Most cell therapy trials failed to show an improvement in global left ventricular function measures after myocardial infarction (MI).
What does this study add?
Administration of allogeneic cardiosphere-derived cells (CDCs) in patients with post-MI improves regional myocardial function.
How might this impact on clinical practice?
Improvement in regional myocardial function with administration of CDCs may provide survival benefit for these patients.
Introduction
Cell therapy has been proposed as a potential strategy to partly reverse myocardial damage and enhance myocardial function after myocardial infarction (MI).1–4 While preclinical and some clinical studies have suggested a benefit in enhancing left ventricular (LV) function,2 5 6 myocardial perfusion7 or reducing infarct size,2 significant controversy persists on the value of cell therapy, given that several clinical trials have failed to improve LV ejection fraction (LVEF).1 3 4 8
The ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) study was designed to test the hypothesis that administration of allogeneic cardiosphere-derived cells (CDC) to patients with large MI would produce a reduction in infarct size 12 months after cell administration. Based on an interim analysis performed after all enrolees had completed 6 months of follow-up, the study was interrupted on the premise that its continuation would be futile given failure to impact the predesignated primary endpoint (infarct size).9 Despite this disappointment regarding infarct size, primary analysis of ALLSTAR study revealed favourable changes in secondary measures including LV dilatation, and reductions in NT-pro-BNP in CDC-treated patients.9 Global LV function by LVEF did not improve by CDCs. However, LVEF do not consider the effect of CDCs on different segments of the myocardium, which may be heterogeneous across different myocardial regions (given the spatially localised nature of ischaemic injury in MI).
Detection of heterogeneous treatment effects at the segmental level requires highly sensitive and reproducible tools. LV strain is a sensitive and accurate index of myocardial deformation that directly reflects LV myocardial contractility at the segmental as well as global ventricular levels, and has prognostic value superior to LVEF in predicting myocardial functional deterioration in different types of disease,10 as well as in asymptomatic individuals.11 In patients with MI, LV strain is a strong predictor of mortality and heart failure admissions over and above LVEF and traditional risk factors.12 Multimodality feature tracking has enabled quantification of myocardial strain from cine images which also allow for quantification of LV volumes and LVEF routinely in clinical cardiac MRI protocols.13
In this exploratory study, circumferential LV strain in ALLSTAR study participants was quantified using MRI cine images at baseline and follow-up visit to: (1) test the efficacy of CDCs for improving segmental postinfarct myocardial contractility; (2) investigate the differential effect of CDCs in infarcted versus remote segments; (3) test whether the improvement in myocardial contractility relates to changes in infarct size; (4) identify the determinants of beneficial responses to cells.
Methods
The design of the ALLSTAR phase II trial has been described previously.9 14 The ALLSTAR phase II trial assessed safety and efficacy of intracoronary delivery of allogeneic CDCs (CAP-1002) in patients with recent or chronic MI aged 18 years or older enrolled across 30 US centres to receive either one dose of intracoronary CAP-1002 or placebo. Participants had MI within the prior 4 weeks to 12 months before enrolment, and had undergone successful percutaneous coronary intervention with resultant thrombolysis in MI flow of 3 in the infarct related artery, had postinfarct LVEF <45% by any clinically accepted imaging method, and infarct size (scar) of ≥15% of LV mass by MRI.9 14
Procedures
Intracoronary infusion of CAP-1002 or placebo was performed on day 0, at least 4 weeks after the index MI. CDCs were obtained from donor hearts at the time of cardiac transplantation and manufactured according to a comprehensive quality control programme as described.14 The CAP-1002 (25 million human allogeneic CDCs) and placebo were administered intracoronarily.14 Participants were hospitalised for 20–24 hours after infusion with continuous cardiac monitoring for potential adverse events.
Randomisation, masking, follow-up protocol and endpoints
The randomisation for phase II trial was performed with 2:1 ratio favouring the CAP-1002 group stratified by recent (4 weeks to 90 days) and chronic (90 days to 12 months) index MI as well as presence of donor specific antibodies against human leucocyte antigens. Investigators, participants, readers at the MRI Core Laboratory and all sponsor staff were blinded to treatment assignment until completion of the 6-month follow-up for the entire cohort. The randomisation and allocation concealment were monitored by Steering Committee and Data and Safety Monitoring Board. The safety follow-up protocol is described in online supplemental file. Following the prespecified interim analysis on the 6-month follow-up data, the sponsor decided to terminate the study given the low probability to achieve a statistically significant difference at the end of the 12-month follow-up for the primary endpoint of LV scar size reduction. At the time of study termination, 124 and 67 participants had completed 6-month and 12-month follow-up, respectively.
openhrt-2021-001614supp001.pdf (136.9KB, pdf)
Cardiac MRI: image acquisition and analysis
The details of the cardiac MRI protocol are described in the online supplemental file. MRI using 1.5 or 3T scanners was performed at baseline, and at 6-month and 12-month follow-ups for efficacy assessment. MRI technologists reviewed and matched the slice positioning and MRI parameters between baseline and follow-up MRIs in order to improve reproducibility of image acquisition. Cine images using steady-state free precession with retrospective gating were used for quantification of LV volumes, LVEF and myocardial strain. For assessment of myocardial scar, late gadolinium enhancement (LGE) images were acquired using breath-hold inversion recovery-prepared gated TurboFLASH sequence 15 min after intravenous administration of 0.2 mmol/kg gadolinium contrast. In participants with cardiac implantable electrical devices (CIED) (n=16), cine images were acquired using fast gradient echo pulse sequence and LGE images were acquired using a wideband sequence to avoid device induced artefacts.15 This resulted in adequate image quality for endpoint quantification in all participants with a CIED.
De-identified MRI images were analysed by a single experienced reader with over 10 years of experience reading such images in the MRI Core Laboratory at Johns Hopkins University. LV volumes and LVEF were quantified from contiguous short axis cine slices using Food and Drug Administration approved software (QMass 7.4, Medis Medical Imaging Systems, The Netherlands). Myocardial scar from LGE images was quantified using the full width at half maximum method (QMass 7.4) as described.16 The LV scar was presented as a percentage of the total myocardial mass. Multimodality Tissue Tracking software (MTT 6.1, Toshiba, Japan) was used to obtain circumferential strain (Ecc) from short axis cine images as described in online supplemental file and elsewhere.13 This method uses a pixel-to-pixel matching technique by defining angle-independent motion vectors from multiple tracking points to find identical pixels in successive frames to generate strain curves.13 The mid-wall peak systolic circumferential strain (Ecc) was determined from strain curves, with lower Ecc values representing greater systolic myocardial contraction. The segmental myocardial scar and circumferential strain were mapped according to the American Heart Association 16-segment model.17
Statistical analysis
The current analysis included all infused participants who completed the 6-month follow-up. Descriptive data are presented as mean (SD), or number (%). We used mixed-effects linear regression models with the treatment group and visit number as fixed effects and the patient as the random effect to compare the change in peak Ecc from baseline to 6 months between CAP-1002 versus placebo groups. The resultant regression coefficients represent the relative difference in the slope of baseline to 6-month changes between the CAP-1002 and placebo groups.
As exploratory analyses in order to identify the determinants of response to CAP-1002, we plotted the change in Ecc from baseline to 6 months against baseline LVEF, LV end diastolic volume index (LVEDVi) and LV scar percent. Subgroup efficacy analyses using mixed-effects linear regression models were performed, to study the treatment response across the baseline values of LVEF, LVEDV, or infarct size.
The significance level was set at p<0.05.
Results
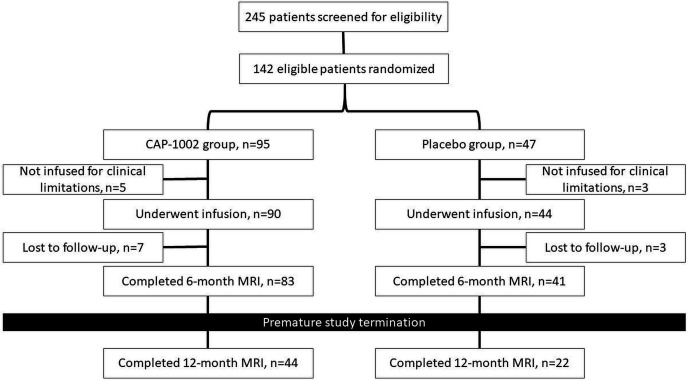
Between January 2014 and September 2016, 245 patients were screened for eligibility and 142 were randomised and enrolled in the study (95 in the CAP-1002 group vs 47 in the placebo group, figure 1). Five subjects in the CAP-1002 and three subjects in the placebo groups were not treated due to clinical limitations. Ten participants (seven in CAP-1002 and three in placebo) were lost to follow-up, leaving 83 and 41 participants at 6-month follow-up in the CAP-1002 and placebo groups, respectively. Baseline characteristics of participants are shown in table 1. Thirty-seven and 17 participants in CAP-1002 and placebo groups had recent MI, respectively. No primary safety endpoint events were observed in the study. Primary safety and efficacy analysis results were reported.9
Figure 1.
The trial flowchart.
Table 1.
Demographics and baseline clinical characteristics
| Placebo (n=41) | CAP-1002 group (n=83) | P value | |
| Age, mean (SD) | 53.5 (10.2) | 54.7 (11.1) | 0.56 |
| Male gender, n (%) | 36 (87.8) | 72 (86.7) | 0.99 |
| Chronicity of MI, n (%) | |||
| Recent | 17 (41.5) | 37 (44.6) | 0.85 |
| Chronic | 24 (58.5) | 46 (55.4) | |
| Race, n (%) | |||
| White | 34 (82.9) | 73 (87.9) | 0.17 |
| Black or African-American | 6 (14.6) | 7 (8.4) | |
| Asian | 0 | 3 (3.6) | |
| American-Indian or Alaska Native | 1 (2.4) | 0 | |
| Diabetes mellitus, n (%) | 11 (26.8) | 18 (21.7) | 0.65 |
| Hypertension, n (%) | 27 (65.8) | 48 (57.8) | 0.70 |
| ACE inhibitors or ARBs, n (%) | 35 (85.4) | 76 (91.6) | 0.35 |
| Beta blockers, n (%) | 40 (97.6) | 79 (95.2) | 0.99 |
| Diuretics, n (%) | 18 (43.9) | 34 (41.0) | 0.85 |
| Aldosterone antagonists, n (%) | 12 (29.3) | 28 (33.7) | 0.69 |
| Antithrombotic agents, n (%) | 41(100) | 81 (97.6) | 0.99 |
| Lipid lowering agents, n (%) | 39 (95.1) | 79 (95.2) | 0.99 |
| Aspirin, n (%) | 41(100) | 78 (93.4) | 0.17 |
ARB, angiotensin receptor blocker; MI, myocardial infarction.
As shown in table 2, LVEDVi remained stable from 102.7 mL/m2 (20.4) at baseline to 103.7 mL/m2 (23.4) at 6-month follow-up in the CAP-1002 group, while it increased from 105.2 mL/m2 (25.9) to 110.9 mL/m2 (26.5) in the placebo group (p=0.02). There was a similar trend for 3.1 mL/m2 (7.7) increase in LV end-systolic volume index (LVESVi) in the placebo group compared with only 0.3 mL/m2 (9.4) increase in the CAP-1002 group (p=0.08, table 2). Proportional to LVEDVi changes, the stroke volume index increased by 2.5 mL/m2 (5.7) in the placebo group compared with 0.7 mL/m2 (5.7) increase in the CAP-1002 group (p=0.04, table 2). There were no differences in 6-month change in LVEF, cardiac output and LV mass index between the CAP-1002 and placebo groups (table 2). LV scar per cent changes from baseline to 6 months in the CAP-1002 (−1.1% (1.7)) and placebo groups (−0.8% (2.1)) were similar (p=0.43).
Table 2.
Endpoint analysis of LV function and structure between baseline and 6 months in whole cohort
| Placebo (n=41) | CAP-1002 group (n=83) | Between-group p value | |
| LVEDVi at baseline, mL/m2 | 105.2 (25.9) | 102.7 (20.4) | 0.02 |
| LVEDVi at 6 months, mL/m2 | 110.9 (26.5) | 103.7 (23.4) | |
| Within-group p value | 0.001 | 0.41 | |
| Change in LVEDVi, mL/m2 | 5.7 (10.3) | 1.0 (11.3) | |
| LVESVi at baseline, mL/m2 | 64.8 (22.8) | 62.5 (18.0) | 0.08 |
| LVESVi at 6 months, mL/m2 | 68.0 (22.9) | 62.8 (20.2) | |
| Within-group p value | 0.01 | 0.78 | |
| Change in LVESVi, mL/m2 | 3.1 (7.7) | 0.3 (9.4) | |
| SVi at baseline, mL/m2 | 40.3 (6.6) | 40.2 (6.2) | 0.04 |
| SVi at 6 months, mL/m2 | 42.9 (7.2) | 40.9 (6.9) | |
| Within-group p value | 0.007 | 0.24 | |
| Change in SVi, mL/m2 | 2.5 (5.7) | 0.7 (5.7) | |
| LVEF at baseline, % | 39.7 (7.8) | 40.0 (6.7) | 0.93 |
| LVEF at 6 months, % | 39.3 (8.1) | 40.4 (7.1) | |
| Within-group p value | 0.58 | 0.38 | |
| Change in LVEF, % | 0.3 (3.4) | 0.4 (4.4) | |
| CO at baseline, L/min | 5.8 (1.2) | 5.3 (1.1) | 0.92 |
| CO at 6 months, L/min | 5.7 (1.2) | 5.3 (1.2) | |
| Within-group p value | 0.73 | 0.98 | |
| Change in CO, L/min | −0.05 (0.9) | −0.001 (0.8) | |
| LVMi at baseline, g/m2 | 77.1 (13.6) | 74.7 (13.5) | 0.72 |
| LVMi at 6 months, g/m2 | 76.5 (13.4) | 74.3 (12.6) | |
| Within-group p value | 0.21 | 0.27 | |
| Change in LVMi, g/m2 | 0.6 (3.3) | 0.4 (3.3) | |
| Scar mass at baseline, g/m2 | 37.7 (12.0) | 34.2 (11.7) | 0.7 |
| Scar mass at 6 months, g/m2 | 36.4 (12.6) | 32.3 (11.3) | |
| Within-group p value | <0.001 | <0.001 | |
| Change in scar mass, g/m2 | −1.7 (2.8) | −1.8 (2.9) | |
| Scar per cent at baseline, % | 22.9 (5.5) | 22.0 (5.5) | 0.43 |
| Scar per cent at 6 months, % | 22.3 (6.3) | 20.9 (5.4) | |
| Within-group p value | 0.01 | <0.001 | |
| Change in scar per cent, % | −0.8 (2.1) | −1.1 (1.7) |
Within-group p value refers to the changes from baseline to 6 months within CAP-1002 and placebo groups separately.
Between-group p value refers to the relative difference in the slope of change from baseline to 6 months between CAP-1002 and placebo groups (ie, treatment effect in CAP-1002 vs placebo groups).
Bold font indicates statistical significance.
CO, cardiac output; LV, left ventricle; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; LVMi, left ventricular mass index; SVi, stroke volume index.
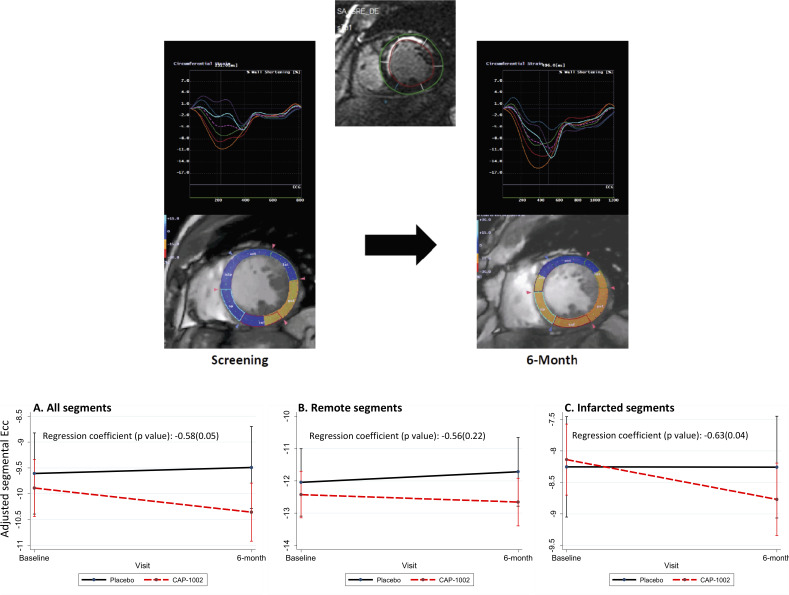
Segmental Ecc (from 16 LV segments) improved (became more negative) from −9.9% (5.2) at baseline to −10.4% (5.3) at 6 months in participants who received cell therapy compared with participants in the placebo group in whom it worsened from −9.7% (5.1) at baseline to −9.5% (5.0) at 6 months (p=0.05 between groups, table 3 and figure 2). When adjusted for LV scar size, the regression coefficient for observed benefit in segmental Ecc favouring the CAP-1002 group increased from −0.58 (p=0.05) to −0.62 (p=0.02). Similar signals for benefit in segmental Ecc favouring the CAP-1002 group were noted in participants with either recent or chronic MI, although the differences did not reach statistical significance (online supplemental tables 1 and 2). The greatest benefit for improvement in segmental Ecc was observed in infarcted segments with segmental scar >5% (change in segmental Ecc of −0.7% (3.5) in the CAP-1002 vs 0.04% (3.7) in the placebo group, p=0.04 between groups) compared with remote segments (change in segmental Ecc of −0.2% (4.5) in the CAP-1002 vs 0.4% (3.8) in the placebo group, p=0.22 between groups, table 3 and figure 2). Importantly, as shown in figure 3, improvement in segmental Ecc was proportional to LV scar size reduction at the segmental level (r=0.05, p=0.03).
Table 3.
Endpoint analysis of global and segmental circumferential strain between baseline and 6 months in whole cohort
|
|
Mean (SD) | Coefficient (between-group p value) | ||||
| Placebo | CAP-1002 group | Unadjusted | Adjusted for LV scar per cent | Adjusted for LVEDVi | Adjusted for LVEDVi and scar per cent | |
| All segments | ||||||
| Segmental Ecc at baseline, % | −9.7 (5.1) | −9.9 (5.2) | −0.58 (0.05) | −0.62 (0.02) | −0.49 (0.11) | −0.51 (0.06) |
| Segmental Ecc at 6 months, % | −9.5 (5.0) | −10.4 (5.3) | ||||
| Within-group p value | 0.25 | <0.001 | ||||
| Change in segmental Ecc, % | 0.2 (3.7) | −0.5 (4.0) | ||||
| Remote segments | ||||||
| Segmental Ecc at baseline, % | −12.2 (5.1) | 12.5 (5.0) | −0.56 (0.22) | – | −0.52 (0.27) | – |
| Segmental Ecc at 6 months, % | −11.8 (5.2) | −12.7 (5.5) | ||||
| Within-group p value | 0.11 | 0.22 | ||||
| Change in segmental Ecc, % | 0.4 (3.8) | −0.2 (4.5) | ||||
| Infarcted segments | ||||||
| Segmental Ecc at baseline, % | −8.3 (4.5) | −8.1 (4.6) | −0.63 (0.04) | – | −0.53 (0.09) | – |
| Segmental Ecc at 6 months, % | −8.2 (4.4) | −8.8 (4.5) | ||||
| Within-group p value | 0.82 | <0.001 | ||||
| Change in segmental Ecc, % | 0.04 (3.7) | −0.7 (3.5) | ||||
| Global Ecc at baseline, % | −8.4 (2.3) | −8.3 (2.9) | −0.63 (0.15) | −0.65 (0.14) | −0.53 (0.25) | −0.57 (0.20) |
| Global Ecc at 6 months, % | −8.3 (2.5) | −8.9 (3.0) | ||||
| Within-group p value | 0.65 | 0.03 | ||||
| Change in global Ecc, % | 0.1 (1.9) | −0.6 (2.6) | ||||
The regression coefficients represent the relative difference in the slope of change from baseline to 6 months between CAP-1002 and placebo groups.
Bold font indicates statistical significance.
Ecc, circumferential strain; LVEDVi, left ventricular end-diastolic volume index.
Figure 2.
Strain curves and colour-coded short axis images from circumferential strain analysis of baseline and 6-month MRI studies for a participant in CAP-1002 group with anteroseptal myocardial infarction. Segmental circumferential strain (Ecc) improved in several segments over 6-month period (top panel). Changes in segmental Ecc from baseline to 6-month in placebo versus CAP-1002 groups. The regression coefficients represent the relative difference in the slope of change from baseline to 6 months between CAP-1002 and placebo groups. (A) Segmental Ecc including all segments; (B) segmental Ecc in remote segments; (C) segmental Ecc in infarcted segments (bottom panel).
Figure 3.
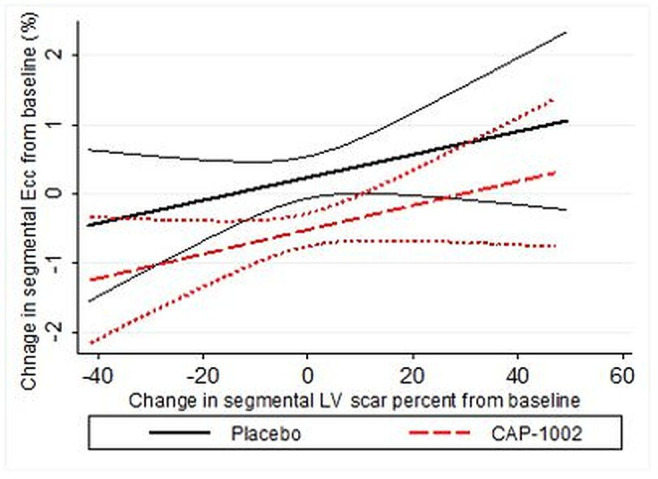
The association of baseline to 6-month changes in segmental circumferential strain (Ecc±95% CI) with baseline to 6-month changes in segmental left ventricular (LV) scar per cent.
Global Ecc changed from −8.3% (2.9) at baseline to −8.9% (3.0) at 6 months in the CAP-1002 group, while it slightly worsened in the placebo group from −8.4% (2.3) to −8.3% (2.5) in the placebo group, although the between group difference in trends did not reach statistical significance (p=0.15, table 3 and figure 2).
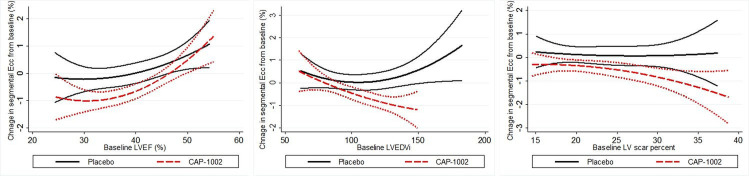
When changes in global Ecc were plotted against baseline LVEF and LV scar size, we observed that the Ecc improvement for CAP-1002 group relative to those in the placebo group were greater in participants with lower LVEF, larger LVEDVi and greater LV scar size (figure 4). Accordingly, we performed an efficacy analysis of Ecc improvement in the subgroups of participants with baseline LVEF <50% of the population (LVEF cut-off=41%) and in those with LV scar size and LVEDVi >50% of the entire cohort (scar size and LVEDV cut offs=18.8% and 100 mL/m2, respectively). As shown in table 4, the regression coefficients for improvement in segmental Ecc in these subgroups were 34%–110% greater than for the entire cohort.
Figure 4.
The changes in segmental circumferential strain (Ecc±95% CI) from baseline to 6 months versus baseline left ventricular ejection fraction (LVEF), left ventricular volume index (LVEDVi) and LV scar per cent.
Table 4.
Subgroup endpoint analysis of global and segmental circumferential strain between baseline and 6 months in whole cohort
| Mean (SD) | Coefficient (between-group p value) | |||||
| Placebo | CAP-1002 group | Unadjusted | Adjusted for LV scar per cent | Adjusted for LVEDVi | Adjusted for LVEDVi and scar per cent | |
| LVEF <41% | ||||||
| Segmental Ecc at baseline, % | −8.5 (4.4) | −8.3 (4.6) | −0.92 (0.02) | −0.96 (0.006) | −0.94 (0.02) | −0.89 (0.01) |
| Segmental Ecc at 6 months, % | −8.4 (4.7) | −9.3 (5.1) | ||||
| Within-group p value | 0.8 | <0.001 | ||||
| Change in segmental Ecc, % | 0.04 (3.4) | −1.03 (3.2) | ||||
| Global Ecc at baseline, % | −7.4 (1.6) | −6.7 (2.2) | −1.19 (0.02) | −1.23 (0.02) | −1.25 (0.02) | −1.18 (0.03) |
| Global Ecc at 6 months, % | −7.1 (1.8) | −7.9 (2.6) | ||||
| Within-group p value | 0.6 | <0.001 | ||||
| Change in global Ecc, % | 0.23 (2.1) | −1.2 (2.0) | ||||
| LVEDVi >100 | −1.16 (0.004) | −1.22 (0.001) | −1.10 (0.009) | −1.07 (0.003) | ||
| Segmental Ecc at baseline, % | −8.9 (4.6) | −9.0 (5.0) | ||||
| Segmental Ecc at 6 months, % | −8.6 (4.8) | −10.0 (5.1) | ||||
| Within-group p value | 0.06 | <0.001 | ||||
| Change in segmental Ecc, % | 0.3 (3.2) | −1.0 (3.6) | ||||
| Global Ecc at baseline, % | −7.9 (1.9) | −7.6 (2.6) | −1.45 (0.009) | −1.5 (0.007) | −1.36 (0.02) | −1.37 (0.02) |
| Global Ecc at 6 months, % | −7.4 (1.9) | −8.6 (2.8) | ||||
| Within-group p value | 0.23 | 0.005 | ||||
| Change in global Ecc, % | 0.5 (1.9) | −1.0 (2.3) | ||||
| LV scar per cent >18.8% | ||||||
| Segmental Ecc at baseline, % | −9.6 (5.1) | −9.5 (5.1) | −0.78 (0.03) | – | −0.63 (0.08) | – |
| Segmental Ecc at 6 months, % | −9.4 (5.1) | −10.1 (5.4) | ||||
| Within-group p value | 0.26 | <0.001 | ||||
| Change in segmental Ecc, % | ||||||
| Global Ecc at baseline, % | −8.3 (2.3) | −7.9 (2.9) | −0.97 (0.05) | – | −0.81 (0.1) | – |
| Global Ecc at 6 months, % | −8.1 (2.6) | −8.7 (3.1) | ||||
| Within-group p value | 0.4 | 0.02 | ||||
| Change in global Ecc, % | 0.29 (2.0) | −0.73 (2.3) | ||||
The regression coefficients represent the relative difference in the slope of change from baseline to 6 months between CAP-1002 and placebo groups.
Bold font indicates statistical significance.
Ecc, circumferential strain; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction.
Twelve-month follow-up was completed only in a minority of participants (see the Methods section) and demonstrated no significant difference in global and segmental Ecc between CAP-1002 and placebo groups (n=22 in placebo vs n=44 in CAP-1002 groups, online supplemental table 3).
Discussion
The ALLSTAR study is the largest clinical trial of allogeneic CDCs to date, and the only one to focus on post-MI segmental LV dysfunction. Although ALLSTAR trial was neutral for the primary endpoint of reduction in LV infarct size, the present exploratory analysis demonstrated that participants with MI who received CAP-1002 experienced improved regional myocardial function measured as myocardial circumferential strain by MRI, 6 months after cell therapy. Such improvement persisted after adjusting for LVEDVi or LV scar per cent, was greater in infarcted segments, and proportional to segmental scar per cent. Finally, functional improvement was greater in participants who had more advanced disease at baseline reflected by greater LV scar size, dilated LV and lower LVEF.
The data in the literature on efficacy of cell therapy for LV function are controversial. Despite some preclinical18 and clinical studies19–21 that documented improvement of LVEF secondary to cell therapy, most of the recent trials have failed to demonstrate improvement in LVEF.2 4 8 22–24 LVEF is very load dependent and tends to remain stable even in the presence of changes in LV volumes and remodelling.25–27 In our study, LVEF remained unchanged in the CAP-1002 treated group, due to parallel changes in LVEDVi and LVESVi.28 Furthermore, the extent of LV dysfunction after MI varies segment by segment depending on the involved culprit artery. Thus, global LV function indices such as LVEF often fail to identify patients that respond to treatment particularly in patients with ischaemic heart disease. Therefore, in these patients, markers of segmental LV function may be more likely to reflect the potential favourable outcomes of cell therapy earlier than LVEF changes or reverse remodeling.29 Earlier changes in strain compared with cardiac volumes and ejection fraction were similarly observed in other cardiac pathologies such as chemotherapy-induced cardiomyopathy and atrial fibrillation.30 31
The prognostic value of global strain for predicting major cardiovascular events (MACE) in patients with post-MI have been shown in multiple studies12 32 even in the absence of association between LVEF and MACE.33 Furthermore, at regional level, number of segments with abnormal strain has been associated with all-cause mortality after MI.34 Even though the mean difference between cell therapy and placebo groups in terms 6-month change in segmental Ecc in our study was small (0.7%), but, it was shown that favourable changes as small as 1% in strain are associated with at least 13%–23% reduction in MACE rate.33 35 ALLSTAR participants are being followed for occurrence of MACE and the prognostic value of improved segmental Ecc in this trial will be published after completion of follow-up.
Fewer previous studies have examined the effects of cell therapy on myocardial function at the segmental level and most of those used subjective techniques such as the wall motion by echocardiography.36 Conversely, myocardial circumferential strain is a quantitative parameter that has been used to study the infarcted heart for several decades, measured at first with mechanical gauges,37 then with sonomicrometers38 and more recently with MRI tagging,39 MRI feature tracking40 or speckle-tracking echocardiography.41 In the ASTAMI trial, bone marrow cell therapy in acute patients with MI, did not result in improvement of regional function by speckle-tracking echocardiography,41 but in the study by Herbots et al,42 it led to improved longitudinal strain rate by echocardiography. In the study by Williams et al,29 circumferential strain by MRI tagging revealed improved regional function by stem cell therapy, but the sample size was quite small (n=8). Our study is the largest cell therapy trial to measure myocardial circumferential strain in a comprehensive manner using MRI feature tracking, demonstrating improvement in regional LV function by CAP-1002 administration.
The ALLSTAR trial was not designed to address mechanism, which has been the subject of much speculation in previous studies of cell therapy in patients with MI. Although there was evidence for myocardial regeneration in experimental studies,43 it has become clear that transplanted stem cells have poor engraftment and direct differentiation to cardiomyocytes is not the underlying mechanism of action.44 Paracrine effects possibly mediated by exosomes leading to reduced inflammation and apoptosis could mediate the improved contractility, reverse remodelling and reduced scar size shown in this and previous studies.45–48 Moreover, our study demonstrates that reduced scar size at the segmental level was associated with improved myocardial strain, and similar to the REPAIR-AMI trial,5 partially infarcted segments derived the greatest benefit from stem cell administration. Previous studies have also documented the presence of biopsy-proven inflammation in the LGE-hyperenhanced myocardial tissue regions in non-ischaemic cardiomyopathies.49 50 Animal studies have also shown that inflammation is an important mechanism leading to myocardial dysfunction after MI and cell therapy improves myocardial function by reducing the inflammation.51 52 It is plausible that, in this trial, reduced inflammation and subsequent cell preservation may have represented an important mechanism of CAP-1002 benefit, but that conjecture remains untested clinically.
In this study, myocardial strain enhancement after cell therapy was more prominent in patients with lower LVEF, greater LVEDVi and larger areas of replacement fibrosis. These findings are in line with previous studies such as CHART-1, and REPAIR-AMI where sicker patients achieved greater benefit from stem cell therapy.3 5 This highlights the importance of patient-tailored approaches in the management of post-MI myocardial dysfunction based on disease severity markers and targeting well-defined patient populations for development of effective novel therapies in future clinical trials.53
Study limitations include the fact that the trial was interrupted before completion of the 12-month follow-up for all participants, use of a single administration of CDCs as opposed to repeated stem cell administrations and use of stop-flow delivery into a sole coronary artery. We now appreciate that multiple doses of cells can yield additive benefits in preclinical models of post-MI dysfunction.54 Single administration of CDCs in addition to lack of 12-month data in ~50% of participants may have contributed to the absence of a beneficial treatment effect between the CAP-1002 and placebo groups at 12-month follow-up. Stop-flow intracoronary delivery, while standard at the time of designing ALLSTAR, is now known to be inferior to multivessel non-occlusive intracoronary delivery.55 In addition, focus on infarct size as the primary endpoint may also have been limiting. However, the relationship between improved myocardial shortening with reduced scar at the segmental level does suggest that scar growth containment leading to reduced scar was at the core of the beneficial effects of CAP-1002. ALLSTAR included patients with post-MI with mild to moderate LV dysfunction (LVEF <45%). Previous studies have shown that patients with more severe MI benefit the most from cell therapy56 and inclusion of patients with more severe LV dysfunction in ALLSTAR could have yielded on more robust efficacy. Finally, this is a post-hoc exploratory analysis within ALLSTAR and this needs to be taken into consideration for interpretation of the results of the study. Study strengths included the randomised double-blinded design which allows for comparisons between changes in parameters of myocardial structure and function over time. The population was highly uniform and consisted exclusively of patients with large first MIs which, although less common today than in the pre-reperfusion era, remain the most feared consequence of coronary artery disease. The implementation of rigorous, state of the art MRI methods across a large number of recruiting centres in the USA and Canada underscore the solidity of findings reported in this study.
Conclusion
In this exploratory study, the administration of allogeneic CAP-1002 cells to patients with MI resulted in improved segmental function, particularly within infarcted segments. The segmental functional improvement induced by CAP-1002 was associated with reduced segmental LV scar. CAP-1002 induced myocardial functional improvement was greater in patients with reduced LVEF, dilated LV indexed as greater LVEDVi and greater infarct size. The utility of circumferential strain in this study may have implication for endpoint selection and design of future clinical trials in the field of cardiovascular medicine. The prognostic value of segmental Ecc improvement in occurrence of MACE in ALLSTAR is yet to be published after completion of MACE follow-up.
Footnotes
Contributors: MRO, RRM, BA-V, DA, LM, EM and JACL participated in conception, design and interpretation of the data. MRO drafted the initial manuscript and all other authors contributed to revising it and have given final approval of the version to be submitted. MRO and JP were responsible for statistical analysis of the manuscript. TC, TDH, GK, FVA, DJK, TJP, RS, JHT, RDS, LM, EM, JACL contributed to data collection and quality control of the study.
Funding: This work was supported by the California Institute of Regenerative Medicine and Capricor Therapeutics.
Competing interests: DA, RDS and LM are employed at Capricor therapeutics. RDS, LM and EM hold stocks at Capricor therapeutics. EM is the founder of Capricor therapeutics. TDH is advisory board member at Capricor therapeutics. The remaining authors have no disclosures.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available. The authors declare that the supporting data for this manuscript will not be available to other researchers.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Each clinical centre had independent approval and oversight committees, the investigation conforms with the principles outlined in the Declaration of Helsinki, and all participants provided written informed consent and release of medical information. The trial was monitored by a Data Safety Monitoring Board. The ALLSTAR phase II trial was supported by the California Institute of Regenerative Medicine and Capricor Therapeutics.
References
- 1.Wollert KC, Meyer GP, Müller-Ehmsen J, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J 2017;38:2936–43. 10.1093/eurheartj/ehx188 [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012;379:895–904. 10.1016/S0140-6736(12)60195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartunek J, Terzic A, Davison BA, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 2017;38:648–60. 10.1093/eurheartj/ehw543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gyöngyösi M, Wojakowski W, Lemarchand P, et al. Meta-analysis of cell-based cardiac studies (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res 2015;116:1346–60. 10.1161/CIRCRESAHA.116.304346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schächinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol 2004;44:1690–9. 10.1016/j.jacc.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 6.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 2014;311:62–73. 10.1001/jama.2013.282909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobi J, Solanes N, Fernández-Jiménez R, et al. Intracoronary administration of allogeneic adipose tissue-derived mesenchymal stem cells improves myocardial perfusion but not left ventricle function, in a translational model of acute myocardial infarction. J Am Heart Assoc 2017;6:e005771. 10.1161/JAHA.117.005771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hare JM, Sanina C. Bone marrow mononuclear cell therapy and granulocyte colony-stimulating factor for acute myocardial infarction: is it time to reconsider? J Am Coll Cardiol 2015;65:2383-7. 10.1016/j.jacc.2015.03.571 [DOI] [PubMed] [Google Scholar]
- 9.Makkar RR, Kereiakes DJ, Aguirre F, et al. Intracoronary allogeneic heart stem cells to achieve myocardial regeneration (ALLSTAR): a randomized, placebo-controlled, double-blinded trial. Eur Heart J 2020;41:3451-3458. 10.1093/eurheartj/ehaa541 [DOI] [PubMed] [Google Scholar]
- 10.Edvardsen T, Rosen BD, Pan L, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging--the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2006;151:109–14. 10.1016/j.ahj.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 11.Choi E-Y, Rosen BD, Fernandes VRS, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Eur Heart J 2013;34:2354–61. 10.1093/eurheartj/eht133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ersbøll M, Valeur N, Mogensen UM, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013;61:2365–73. 10.1016/j.jacc.2013.02.061 [DOI] [PubMed] [Google Scholar]
- 13.Ohyama Y, Ambale-Venkatesh B, Chamera E, et al. Comparison of strain measurement from multimodality tissue tracking with strain-encoding MRI and harmonic phase MRI in pulmonary hypertension. Int J Cardiol 2015;182:342–8. 10.1016/j.ijcard.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 14.Chakravarty T, Makkar RR, Ascheim DD, et al. Allogeneic heart stem cells to achieve myocardial regeneration (ALLSTAR) trial: rationale and design. Cell Transplant 2017;26:205–14. 10.3727/096368916X692933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashid S, Rapacchi S, Vaseghi M, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology 2014;270:269–74. 10.1148/radiol.13130942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 2004;44:2383–9. 10.1016/j.jacc.2004.09.020 [DOI] [PubMed] [Google Scholar]
- 17.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. 2002;4:203–10. 10.1081/JCMR-120003946 [DOI] [PubMed] [Google Scholar]
- 18.Zwetsloot PP, Végh AMD, Jansen of Lorkeers SJ, Goumans MR, Doevendans PA, Chamuleau SA, et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ Res 2016;118:1223–32. 10.1161/CIRCRESAHA.115.307676 [DOI] [PubMed] [Google Scholar]
- 19.Fisher SA, Doree C, Mathur A, et al. Meta-Analysis of cell therapy trials for patients with heart failure. Circ Res 2015;116:1361–77. 10.1161/CIRCRESAHA.116.304386 [DOI] [PubMed] [Google Scholar]
- 20.Florea V, Rieger AC, DiFede DL, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the trident study). Circ Res 2017;121:1279–90. 10.1161/CIRCRESAHA.117.311827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308:2369–79. 10.1001/jama.2012.25321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.San Roman JA, Sánchez PL, Villa A, et al. Comparison of different bone marrow-derived stem cell approaches in reperfused STEMI. A multicenter, prospective, randomized, open-labeled TECAM trial. J Am Coll Cardiol 2015;65:2372–82. 10.1016/j.jacc.2015.03.563 [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Avilés F, Sanz-Ruiz R, Bogaert J, et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST-segment elevation myocardial infarction and left ventricular dysfunction. Circ Res 2018;123:579–89. 10.1161/CIRCRESAHA.118.312823 [DOI] [PubMed] [Google Scholar]
- 24.Fisher SA, Zhang H, Doree C, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev 2015;158. 10.1002/14651858.CD006536.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng S, Fernandes VRS, Bluemke DA, et al. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2009;2:191-8. 10.1161/CIRCIMAGING.108.819938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mewton N, Opdahl A, Choi E-Y, et al. Left ventricular global function index by magnetic resonance imaging--a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the multi-ethnic study of atherosclerosis. Hypertension 2013;61:770–8. 10.1161/HYPERTENSIONAHA.111.198028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eng J, McClelland RL, Gomes AS, et al. Adverse left ventricular remodeling and age assessed with cardiac MR imaging: the multi-ethnic study of atherosclerosis. Radiology 2016;278:714–22. 10.1148/radiol.2015150982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Late-Breaking science Abstracts from the American Heart Association’s scientific sessions 2017 and Late-Breaking Abstracts in resuscitation science from the resuscitation science symposium 2017. Circulation 2017;136:e448–67. 10.1161/CIR.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 29.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 2011;108:792-6. 10.1161/CIRCRESAHA.111.242610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Yum B, Palumbo MC, et al. Left atrial strain impairment precedes geometric remodeling as a marker of post-myocardial infarction diastolic dysfunction. JACC Cardiovasc Imaging 2020;13:2099–113. 10.1016/j.jcmg.2020.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poterucha JT, Kutty S, Lindquist RK, et al. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr 2012;25:733–40. 10.1016/j.echo.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 32.Mangion K, Carrick D, Carberry J, et al. Circumferential strain predicts major adverse cardiovascular events following an acute ST-segment-elevation myocardial infarction. Radiology 2019;290:329–37. 10.1148/radiol.2018181253 [DOI] [PubMed] [Google Scholar]
- 33.Gavara J, Rodriguez-Palomares JF, Valente F, et al. Prognostic Value of Strain by Tissue Tracking Cardiac Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Imaging 2018;11:1448–57. 10.1016/j.jcmg.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 34.Wang N, Hung C-L, Shin S-H, et al. Regional cardiac dysfunction and outcome in patients with left ventricular dysfunction, heart failure, or both after myocardial infarction. Eur Heart J 2016;37:466–72. 10.1093/eurheartj/ehv558 [DOI] [PubMed] [Google Scholar]
- 35.Reindl M, Tiller C, Holzknecht M, et al. Prognostic implications of global longitudinal strain by Feature-Tracking cardiac magnetic resonance in ST-elevation myocardial infarction. Circ Cardiovasc Imaging 2019;12:e009404. 10.1161/CIRCIMAGING.119.009404 [DOI] [PubMed] [Google Scholar]
- 36.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 2012;307:1717–26. 10.1001/jama.2012.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tennant R, Wiggers CJ. The effect of coronary occlusion on myocardial contraction. Am J Physiol-Legacy Content 1935;112:351–61. 10.1152/ajplegacy.1935.112.2.351 [DOI] [Google Scholar]
- 38.Theroux P, Ross J, Franklin D, et al. Regional myocardial function and dimensions early and late after myocardial infarction in the unanesthetized dog. Circ Res 1977;40:158–65. 10.1161/01.res.40.2.158 [DOI] [PubMed] [Google Scholar]
- 39.Azevedo CF, Amado LC, Kraitchman DL, et al. Persistent diastolic dysfunction despite complete systolic functional recovery after reperfused acute myocardial infarction demonstrated by tagged magnetic resonance imaging. Eur Heart J 2004;25:1419–27. 10.1016/j.ehj.2004.06.024 [DOI] [PubMed] [Google Scholar]
- 40.Ashikaga H, Mickelsen SR, Ennis DB, et al. Electromechanical analysis of infarct border zone in chronic myocardial infarction. Am J Physiol Heart Circ Physiol 2005;289:H1099–105. 10.1152/ajpheart.00423.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beitnes JO, Gjesdal O, Lunde K, et al. Left ventricular systolic and diastolic function improve after acute myocardial infarction treated with acute percutaneous coronary intervention, but are not influenced by intracoronary injection of autologous mononuclear bone marrow cells: a 3 year serial echocardiographic sub-study of the randomized-controlled ASTAMI study. Eur J Echocardiogr 2011;12:98–106. 10.1093/ejechocard/jeq116 [DOI] [PubMed] [Google Scholar]
- 42.Herbots L, D'hooge J, Eroglu E, et al. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J 2009;30:662–70. 10.1093/eurheartj/ehn532 [DOI] [PubMed] [Google Scholar]
- 43.Segers VFM, Lee RT. Stem-Cell therapy for cardiac disease. Nature 2008;451:937. 10.1038/nature06800 [DOI] [PubMed] [Google Scholar]
- 44.Bolli R. Repeated cell therapy: a paradigm shift whose time has come. Circ Res 2017;120:1072–4. 10.1161/CIRCRESAHA.117.310710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T-S, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 2012;59:942–53. 10.1016/j.jacc.2011.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malliaras K, Li T-S, Luthringer D, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 2012;125:100–12. 10.1161/CIRCULATIONAHA.111.042598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chimenti I, Smith RR, Li T-S, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res 2010;106:971–80. 10.1161/CIRCRESAHA.109.210682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallet R, Dawkins J, Valle J. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2016;38:201–11. 10.1093/eurheartj/ehw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Cobelli F, Pieroni M, Esposito A, et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 2006;47:1649–54. 10.1016/j.jacc.2005.11.067 [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815–22. 10.1016/j.jacc.2004.11.069 [DOI] [PubMed] [Google Scholar]
- 51.Epstein SE, Lipinski MJ, Luger D. Persistent inflammation, stem Cell–Induced systemic Anti‐Inflammatory effects, and need for repeated stem cell injections: critical concepts influencing optimal stem cell strategies for treating acute myocardial infarction and heart failure. J Am Heart Assoc 2018;7. 10.1161/JAHA.118.008524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luger D, Lipinski MJ, Westman PC, et al. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res 2017;120:1598–613. 10.1161/CIRCRESAHA.117.310599 [DOI] [PubMed] [Google Scholar]
- 53.Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the cardiovascular round table of the European Society of cardiology. Eur Heart J 2016;37:747–54. 10.1093/eurheartj/ehv213 [DOI] [PubMed] [Google Scholar]
- 54.Reich H, Tseliou E, de Couto G, et al. Repeated transplantation of allogeneic cardiosphere-derived cells boosts therapeutic benefits without immune sensitization in a rat model of myocardial infarction. J Heart Lung Transplant 2016;35:1348–57. 10.1016/j.healun.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 55.Tseliou E, Kanazawa H, Dawkins J, et al. Widespread myocardial delivery of heart-derived stem cells by Nonocclusive Triple-Vessel intracoronary infusion in porcine ischemic cardiomyopathy: superior attenuation of adverse remodeling documented by magnetic resonance imaging and histology. PLoS One 2016;11:e0144523–e23. 10.1371/journal.pone.0144523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malliaras K, Marbán E, EJBmb M. Cardiac cell therapy: where we've been, where we are, and where we should be headed. Br Med Bull 2011;98:161–85. 10.1093/bmb/ldr018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001614supp001.pdf (136.9KB, pdf)
Data Availability Statement
No data are available. The authors declare that the supporting data for this manuscript will not be available to other researchers.