Abstract
Introduction
Knee osteoarthritis (OA) is associated with chronic knee pain and functional disability that negatively affect the ability to carry out normal daily activities. Patients are offered a large variety of non-surgical treatments, often not in accordance with clinical guidelines. This observational study will provide a comprehensive overview of treatment pathways for knee OA during the first 2 years after consulting an orthopaedic surgeon, including timing and order of treatment modalities, predictors of treatment outcomes, cost-effectiveness of treatment pathways and patients’ views on different treatment pathways.
Methods and analysis
Patients with primary referrals to an orthopaedic surgeon due to knee OA are consecutively invited to participate and fill out a questionnaire prior to their consultation with an orthopaedic surgeon. Follow-up questionnaires will be obtained at 6 and 24 months after inclusion. Based on a prospective cohort study design, including questionnaires and register data, we will (1) describe treatment pathways for knee OA during the first 2 years after consulting an orthopaedic surgeon; (2) describe the characteristics of patients choosing different treatment pathways; (3) develop predictive models for patient-self-determined classifications of good and poor treatment outcomes; (4) evaluate the cost-effectiveness of treatment pathways that live up to clinical guidelines versus pathways that do not; based on a qualitative study design using semistructured individual interviews, we will (5) describe the patients’ perspectives on treatment pathways for knee OA.
Ethics and dissemination
The study is approved by the Danish regional ethical committee (journal number H-17017295) and the Danish Data Protection Agency (journal number AHH-2017–072). Data will be anonymised and handled in line with the General Data Protection Regulation and the Danish Data Protection Act. The study results will be submitted to international open-access peer-reviewed journals and disseminated at conferences.
Trial registration number
Keywords: knee, musculoskeletal disorders, rehabilitation medicine, quality in healthcare, orthopaedic & trauma surgery
Strengths and limitations of this study.
This study protocol outlines a multidisciplinary research approach using different methodologies to achieve a comprehensive overview of treatment pathways for knee osteoarthritis (OA) during the first 2 years after consulting an orthopaedic surgeon.
Patients are consecutively included in the cohort as they are referred to two outpatient clinics (representing both urban and more rural areas) due to knee OA to strengthen the representativeness of the study population.
The results may be more relevant for patients with more severe knee OA, rather than the whole disease spectrum, since the cohort is composed of patients consulting an orthopaedic surgeon due to their knee OA.
A pragmatic approach was used to estimate sample size as there were no specific guidelines on sample size estimation for prediction models.
By using self-reported questionnaires to detail previous treatment, there is a risk of missing data and recall bias.
Introduction
Knee osteoarthritis (OA) is associated with chronic knee pain and functional disability that negatively affects the ability to carry out regular daily activities.1 Knee OA is the most common form of arthritis with a prevalence increase of 27.5% worldwide from 2010 to 2019.2 In 2019, 528 million people were estimated to have OA, and the knee joint is the most common OA site that causes disability.2 Consequently, knee OA places a major economic burden to the society expected to increase in the future.3 In order to address the increasing burden, evidence-based and individualised treatment strategies are needed.
Total knee replacement (TKR) is considered cost-effective for end-stage knee OA,4 but timing is difficult.5 Skou et al’s recent randomised trial has shown that, in patients who are eligible for surgery, TKR, in addition to non-surgical treatment (patient education, neuromuscular exercise, dietary advice, use of insoles and pain medication), was more effective—and with clinically relevant effect sizes—in relieving pain and improving physical function compared with non-surgical treatment alone at 1 year. However, patients treated non-surgically also gained clinically important improvements and had a much lower risk of serious adverse events compared with those who had surgery. Skou et al’s findings imply that many patients who are deemed eligible for a TKR can gain substantial improvements with an optimised non-surgical treatment approach.6
National and international clinical guidelines on the management of knee OA recommend patient education, exercise and weight loss intervention, if overweight, as core non-surgical treatments for knee OA.1 7–9 However, several studies have highlighted that compliance with the recommendations is poor,10–12 and patients are offered a large variety of non-surgical treatments, some in accordance with clinical guidelines and some not.13 A systematic review showed that only 39% of the patients are offered referral or recommendation to exercise, and 35% are offered education and self-management.14
Healthcare practitioners are expected to adhere to clinical guidelines in the shared decision-making process with the patient. Patients have reported that insufficient information about possible treatment options, lack of information on individual consequences of having knee OA, and access to local care can influence the decision-making on different treatments.15 16 Further, previous research has indicated that patients’ decision to undergo TKR is influenced by the interaction between the orthopaedic surgeon and the patient.17 Challenges with the implementation of clinical guidelines are also a possible factor that may influence which treatments patients are offered.18 19 The poor compliance with clinical guidelines, including the patients’ and clinicians’ reported barriers with usage of different treatment modalities, stresses the need to clarify challenges and barriers related to different treatment modalities, and which treatment modalities are applied in which patients, at which stage in their knee OA disease course.
To our knowledge, no previous large-scale studies have provided a comprehensive overview of different treatment pathways or the timing and order, predictors of effect and cost-effectiveness of different treatment options for knee OA, as well as clarifying patients’ considerations on different treatments at the point in time when patients consult an orthopaedic surgeon. Such an overview would be helpful in order to design, evaluate and implement individualised treatment strategies.
Objectives
The objectives of this study were
To describe which treatment pathways patients pursue for knee OA during the first 2 years after consulting an orthopaedic surgeon.
To describe the characteristics of patients choosing different treatment pathways.
To develop prediction models for good and poor treatment outcomes of different treatment modalities and/or pathways used for knee OA.
To evaluate the cost-effectiveness of treatment pathways that adhere to clinical guidelines versus pathways that do not.
To describe the patients’ perspectives on their treatment pathways for knee OA.
Methods and analysis
The study will use a prospective cohort study design (objectives 1–4) in which patients are included when consulting an orthopaedic surgeon with follow-ups at 6 and 24 (primary) months after inclusion. A qualitative study design will be used for objective 5. The study was prospectively registered with ClinicalTrials.gov. Since registration, the most significant edits to the registration and protocol (current protocol V.2.0, 21 December 2020) include a specification of primary and secondary outcomes as reflected in the updated registration on clinicaltrials.gov on 8 January 2021. Reporting of the study will follow the The Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines for observational studies.20 Also the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guidelines21 for objective 3, the Consolidated Health Economic Evaluation Reporting Standards statement22 for objective 4, and the Consolidated criteria for Reporting Qualitative research23 for objective 5 will be followed to facilitate subsequent reporting.24
Prospective cohort study
Participants
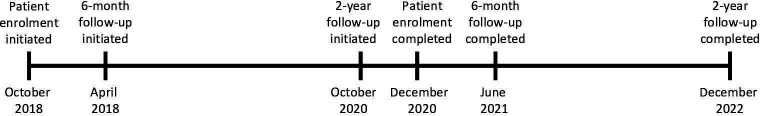
For the prospective cohort study (objectives 1–4), patients with primary referrals to an orthopaedic surgeon due to unilateral or bilateral knee OA are consecutively invited to participate. A pragmatic approach to inclusion based on the general practitioners’ diagnosis of knee OA is applied, irrespective of which diagnostic criteria the general practitioners use. Additionally, patients referred with unspecified diagnoses such as ‘knee pain’ or ‘knee problems’ will be invited if their age is ≥40 years, as this is the lowest age limit proposed by international recommendations for clinical OA criteria.25 Patients are included from the outpatient clinics at the departments of orthopaedic surgery at Copenhagen University Hospital Hvidovre and Næstved Hospital in Denmark (representing both urban and more rural areas). Patient enrolment was initiated in October 2018 and was completed ultimo December 2020. Data collection is ongoing with an expected completion of follow-up in December 2022 (figure 1). Exclusion criteria are previous total or unicompartmental knee replacement or osteotomy around the study knee, and inability to read and write Danish.
Figure 1.
Timeline for data collection for the prospective cohort study.
Procedure
At inclusion, patients with primary referrals to an orthopaedic surgeon due to knee OA are identified from lists of patients referred to the hospital and contacted by a member of the research team through a secure email, with information about the study, an invitation to participate and a link to a patient self-reported questionnaire within 3 days–2 weeks before the consultation with the orthopaedic surgeon. Patients who have not responded at the day of their consultation are asked by a member of the research team to fill out the questionnaire on an iPad or a paper-based questionnaire in the waiting room, prior to their consultation with the orthopaedic surgeon. Patient consent is collected electronically through their response to the questionnaire that also marks the enrolment in the study. At 6 months and 2 years after inclusion, links to the questionnaire will be sent electronically. For patients who do not have secure email (approximately 15% based on actual numbers from the cohort so far), paper versions of the questionnaire and a prepaid return envelope will be sent by post. Two reminders are sent after 1 week and additionally 2 weeks for electronic and 5 weeks for paper-based questionnaires.
In Denmark, patients with knee OA initially visit their general practitioner who may option to refer to an orthopaedic surgeon. This study follows a pure observational design, in which patients are invited to share their pursued treatment pathways through questionnaires, in the 2-year period after consulting the orthopaedic surgeon. The study does not interfere with the chosen treatment, and patients will be followed up for 2 years, whether they are offered surgical treatment or not.
Outcomes
Two primary outcomes will be assessed using the patient-self-determined classifications of achieving good or poor treatment outcomes from inclusion to 2-year follow-up defined by a patient acceptable symptom state (PASS) and a treatment failure (TF) anchor question.26 27 The PASS anchor question was developed for patients with OA and asks, ‘Taking into account all the activities you have during your daily life, your level of pain, and also your functional impairment, do you consider that your current knee state is satisfactory? (yes/no)’. Patients responding ‘yes’ to this question will be categorised as having a good treatment outcome.26 Patients responding ‘no’ will be asked the TF anchor question: ‘Would you consider your current state as being so unsatisfactory that you think the treatment has failed? (yes/no)’. Patients answering no to the PASS and subsequently answering yes to the TF anchor question will be defined as having a poor treatment outcome. The PASS and TF questions at the 6-month follow-up will act as secondary outcomes. Another secondary outcome will be based on a supplementary anchor question asking about the patients’ experienced degree and importance of change in their knee problems: ‘How are your knee problems now compared with for 2 years (6 months) ago, when you first consulted the orthopaedic surgeon?’ Patients will respond to this question on a seven-level Likert scale ranging from ‘better, an important improvement’ to ‘worse, an important deterioration’.28 29 Further, the Oxford Knee Score (OKS) at 6 months and 2 years, and the change in OKS over time will act as an additional secondary outcome. The OKS evaluates self-reported knee pain and function on a scale ranging from 0 (worst) to 48 (best), and has presented sufficient validity, reliability and responsiveness characteristics for use in this patient population.30 31
Collected outcomes and predictive variables
A number of variables will be collected at inclusion (prior to the consultation with the orthopaedic surgeon) and at 6 month and 2 year follow-ups after the consultation with the orthopaedic surgeon (table 1) to describe different treatment pathways and to identify possible predictive variables for treatment outcome following different treatments.
Table 1.
Collected outcomes and predictive variables at inclusion and at 6-month and 2-year follow-ups
| Collected outcomes and predictive variables (response categories) | Source of data | Inclusion | 6-month follow-up | 2-year follow-up |
| Primary outcomes | ||||
| PASS (yes/no) | Patient-reported | X | ||
| Self-reported TF (yes/no) | Patient-reported | X | ||
| Secondary outcomes | ||||
| PASS (yes/no) | Patient-reported | X | ||
| Self-reported TF (yes/no) | Patient-reported | X | ||
| Degree and importance of change in knee pain and function (ranging from ‘better, an important improvement’ to ‘worse, an important deterioration’) | Patient-reported | X | X | |
| Oxford Knee Score (12 items) | Patient-reported | X | X | X |
| Predictive variables | ||||
| Biological gender (female/male) | Extracted from personal identification number | X | ||
| Age (years) | Extracted from personal identification number | X | ||
| Height (cm) | Patient-reported | X | ||
| Weight (kg) | Patient-reported | X | ||
| Body Mass Index (kg/m2) | Calculated | X | ||
| Residential status (alone/cohabiting) | Patient-reported | X | ||
| Level of education (elementary school/high school/vocational education/short-cycle higher education/medium-cycle higher education/long-cycle higher education or more) | Patient-reported | X | ||
| Occupation (retired, early retiree or on early retirement/sick leave part time or full time/unemployed/on the labour market or student part time or full time) | Patient-reported | X | ||
| Smoking (yes/no, but I used to/no never; if yes: average number of daily cigarettes is recorded) | Patient-reported | X | ||
| Comorbidities (list of 15 diseases) | Patient-reported | X | ||
| Which knee to be examined by the orthopaedic surgeon (right/left/both) | Patient-reported | X | ||
| Duration of knee problems (ranging from 0 months to more than 10 years) | Patient-reported | X | ||
| Degree of knee pain (Visual Analogue Scale 0 (no pain)–100 (worst imaginable pain (100 mm scale)) | Patient-reported | X | X | X |
| Localisation of pain/discomfort elsewhere in the body (marked on a full body pain mannequin) | Patient-reported | X | X | X |
| Expectations to the following treatment (surgery/injection into the knee joint/training sessions or other treatment/weight loss (if overweight)/treatment for pain/no treatment/other) | Patient-reported | X | ||
| Type of healthcare provider who has examined/treated the knee OA (general practitioner/orthopaedic surgeon/rheumatologist/physiotherapist/occupational therapist/dietitian/osteopath/chiropractor/personal trainer in the gym/alternative therapist (such as massage therapist, healer, body self-development system therapist, reflexologist, acupuncturist or similar)/other/no examination or treatment) | Patient-reported | X | X | X |
| Number of consultations/treatments for knee OA for each healthcare provider | Patient-reported | X | X | |
| Treatment for knee OA (table 2) | Patient-reported | X | X | X |
| Osteoarthritis Quality Indicator Questionnaire (15 items) | Patient-reported | X | X | X |
| Previous knee injury that was examined by a healthcare provider (none/right knee/left knee/both knees) | Patient-reported | X | ||
| Previous joint surgery in lower limb (hip(right/left), knee(right/left) or ankle(right/left)) | Patient-reported | X | ||
| Type of previous joint surgery in lower limb (arthroscopic/open surgery/total or partial replacement) | Patient-reported | X | ||
| Short version of the Arthritis Self-efficacy Scale (11 items) | Patient-reported | X | ||
| Self-reported physical activity level (none/30 min/1 hour/2 hours/more than 2 hours) | Patient-reported | X | X | X |
| Self-reported health condition (3-level version of the European Quality of Life - 5 Dimensions (EQ-5D-3L)) (five items and European Quality of Life Visual Analogue Scale (EQ-VAS)) | Patient-reported | X | X | X |
| Radiographical knee OA severity (grade 0, none–4, severe) | Radiographical assessment | X | ||
| Knee alignment (anatomical tibiofemoral axis) | Radiographical assessment | X | ||
| Knee OA wear pattern (patellofemoral/lateral/medial/involving two to three compartments) | Radiographical assessment | X | ||
| Type of knee replacement surgery for patients who are surgically treated (total/unicompartmental knee replacement) | National Patient Register | X | X | |
| Information for the cost-effectiveness study | ||||
| Healthcare costs (hospitalisation, surgery, medication, primary and secondary healthcare provider visits and home-help services) | Statistics Denmark | X | X | |
| Healthcare costs (healthcare provider visits not covered by public healthcare system) | Patient-reported | X | X | |
| Short-term sick leave (<21 days) | Patient-reported | X | X | |
| Long-term sick leave (≥21 days) | Statistics Denmark | X | X | |
OA, osteoarthritis; PASS, patient acceptable symptom state; TF, treatment failure.
At inclusion
The patient self-reported questionnaire includes patient demographics, comorbidities, duration of knee problems and other affected joints, and surgical history in the knees, hips or ankles. Knee pain and function will be evaluated with the OKS. Average knee pain during the past week is measured with a Visual Analogue Scale (VAS) (ranging from 0 (no pain) to 100 (worst imaginable pain) (100 mm scale)),32 which has sufficient validity and reliability characteristics to measure knee pain.33 34 The patients mark areas where they currently have pain or discomfort on a pain manikin (19 areas), which has been shown to be reliable to assess musculoskeletal pain.35 Physical activity level can affect the treatment outcome and is reported as the average time spent on physical activity every week, with a non-validated single item question proposed by the International Consortium for Health Outcomes Measurement.36 The quality of previous treatment for knee OA is assessed with the revised version of the patient self-reported Osteoarthritis Quality Indicator Questionnaire (OA-QI) that had improved validity, reliability and responsiveness.37 The OA-QI consists of 16 items but only 15 for this study as one question concerning referral for assessment for operation has been excluded from the questionnaire. Further questions elaborate on the previous treatment used for knee OA, including type of healthcare provider consulted. Furthermore, the patients will be asked about their expectations to the treatment they are about to undergo, and their perceived self-efficacy will be evaluated using the 11-item version of the Arthritis Self-efficacy Scale (ASES) that subscales pain and other symptoms.38 The currently not validity tested Danish version of ASES was chosen for lack of better alternatives to capture self-efficacy.39 Finally, the 3-level version of the European Quality of Life - 5 Dimensions (EQ-5D-3L) measures the patients’ self-reported health status on the five domains mobility, self-care, usual activities, pain/discomfort, anxiety/depression and an additional European Quality of Life Visual Analogue Scale (EQ-VAS) of current self-reported overall health status (ranging from 0 (worst) to 100 (best)).40
Standing anteroposterior and lateral short filmed knee radiographs are routinely taken prior to the primary consultation with the orthopaedic surgeon. Radiographical evaluations include (1) the Kellgren-Lawrence classification of radiographical OA severity (five grades ranging from 0 (none) to 4 (severe))41; (2) knee alignment, measured as the anatomical tibiofemoral axis42; (3) the OA wear pattern, which will be recorded as patellofemoral, lateral or medial, or involving two to three compartments. Radiographical analyses will be performed by SMB, supervised by LHI (>5 years radiographical review experience) and AT (orthopaedic surgeon).
At follow-up
Additionally, at the 6-month and 2-year follow-ups after the consultation with the orthopaedic surgeon, the type of knee replacement will be extracted from the National Patient Register for patients who are surgically treated and will be registered as a total or unicompartmental knee replacement. Patients are asked about the type of healthcare provider who has examined/treated the knee OA since inclusion and the type of treatment for knee OA received since last questionnaire. Patients who are surgically treated since inclusion will be asked to answer which other treatment modalities they have undergone until surgery. In addition, the follow-up questionnaire includes information on the number of consultations/treatments and the cost per treatment for treatment modalities that are not covered by the national health security system. Finally, for patients who are active on the labour market, details about short-term sick leave are asked for.
Sample size
The sample size for the cohort is based on a pragmatic approach based on the number of primary referrals from patients with knee OA during a 2 year inclusion period. Taken together, Copenhagen University Hospital Hvidovre and Næstved Hospital receive primary referrals from approximately 3000 patients with knee OA per year. With a 2-year period and an expected inclusion rate of 65% (based on the first year of inclusion), we expect to include 3900 patients. With an expected 65% follow-up rate, full data will be obtained from approximately 2535 patients at 2-year follow-up.
Our sample size considerations are based on objective 3, to develop a prognostic prediction model. Although there are no specific guidelines on adequate sample size to develop a prognostic prediction model,43 for binary prediction models, at least 10 outcome events per variable (EPVs) has been suggested as a rule of thumb.44 45 It has also been suggested that at least 20 EPVs are required for models that include low-prevalence binary predictors; however, it is recommended that the rule of thumb of EPV should be data driven.46 An expected event rate of approximately 25% of patients responding no to the PASS will be used, which is the previously found proportion in patients undergoing primary TKR.27 With a minimum of 20 EPVs and approximately 30 predictor variables, a total number of 2400 patients would be required. Decreasing to a minimum of 10 EPV would require 1200 patients.
Data analysis plan
A flowchart of patients will be presented, including the number of patients excluded and unwilling to participate, stating the reasons for exclusion or missing data. Furthermore, a table of key patient characteristics will be outlined. Multiple and single imputation will be used to handle missing data.
Data analysis plan for the descriptive studies
The first descriptive study (objective 1) will present the treatment pathways that patients with primary referrals to an orthopaedic surgeon due to knee OA pursue during the 2-year follow-up period, or until surgery for those undergoing knee arthroplasty. Patients select which treatments they have received for knee OA from a predefined list of 18 treatments in the questionnaire at inclusion (prior to the consultation with the orthopaedic surgeon), and at 6-month and 2-year follow-ups after the consultation with the orthopaedic surgeon. These treatments will be grouped into 13 treatment categories (table 2). Based on national and international clinical guidelines, the treatments will be classified into (1) core treatment (education/self-management, exercise, weight loss, if needed (patients with Body Mass Index (BMI)≥2547), and participation in Good Life with osteoArthritis in Denmark48); (2) supplements to core treatment (pharmacological treatments, intra-articular injections, walking aids and devices, stretching and joint mobilisation); (3) end-stage treatment (total or unicompartmental knee arthroplasty); and (4) uncertain or not recommended treatment (arthroscopic surgery, passive treatment and complementary medicine) (table 2).1 7–9 49 As the questionnaires do not contain other questions about weight loss intervention than diet or dietary guidance, we will additionally also classify the combination of the treatment categories education/self-management and exercise as (1) core treatment. A sensitivity analysis will be performed to investigate subgroups of exercise (supervised, unsupervised and water-based). The order of undertaken treatment will be defined based on response to three questionnaires: (1) treatment up until inclusion (before consultation with the orthopaedic surgeon), (2) treatment from inclusion until 6 months of follow-up and (3) treatments from 6 months of follow-up until to 2 year follow-up. Results will be presented as the percentages of patients undertaking different treatment pathways. The total number of possible treatment pathways based on the 13 treatment categories and three questionnaire time points is too high to enable a description of all pathways. Therefore, we will present the most common pathways using a data-driven approach that best describes the distribution of data. Additionally, we will also describe the proportion of patients pursuing treatment pathways that live up to clinical guidelines, that is, (1) core treatment, (2) core treatment followed by or in combination with any supplemental treatment, (3) core treatment followed by knee replacement and (4) core treatment followed by or in combination with any supplemental or other treatment followed by knee replacement. We anticipate that some patients will receive treatment in line with clinical guidelines but occasionally seek non-guideline-adherent treatments in addition. We believe the most important aspect is whether or not the recommended core treatments have been used. We have therefore chosen to classify treatment pathways as adherent to clinical guidelines, as long as the patients have received the core treatments, disregarding any additional non-recommended treatments used.
Table 2.
Predefined list of 18 treatments in the questionnaires, grouped into 13 treatment categories and the overall classification of treatments
| Classification of treatments | Treatment categories | Predefined list of treatments in the questionnaire |
| Core treatment | 1. Education/self-management. | 1. Information and guidance on living with OA. |
| 2. Exercise. | 2. Exercise and gymnastics (strength training, fitness or other type of exercise) under the supervision of a physiotherapist or similar. | |
| 3. Water-based exercise in groups or under supervision. | ||
| 4. Exercise on your own (strength training, fitness or other type of exercise). | ||
| 3. Weight loss, if needed. | 5. Diet or dietary guidance. | |
| 4. GLA:D.* | 6. Participation in GLA:D.* | |
| Supplements to core treatment | 5. Pharmacological treatment. | 7. Pharmacological treatment (including painkillers). |
| 6. Intra-articular injections. | 8. Injection into the knee joint. | |
| 7. Walking aids and devices. | 9. Insoles. | |
| 10. Assessment of the need for walking aid (walking stick, crutches, etc). | ||
| 8. Stretching. | 11. Stretching. | |
| 9. Joint mobilisation. | 12. Other manual therapy. | |
| End-stage treatment | 10. Total or unicompartmental knee arthroplasty.† | 13. Knee arthroplasty. |
| Uncertain or not recommended treatment | 11. Arthroscopic surgery. | 14. Arthroscopic surgery. |
| 12. Passive treatment. | 15. Acupuncture. | |
| 16. Massage. | ||
| 17. Ultrasound, laser or other type of electrotherapy. | ||
| 13. Complementary medicine. | 18. Complementary medicine (such as healing, body self-development system, craniosacral therapy or similar). |
Patients select which treatments they have received for knee OA at inclusion and at 6-month and 2-year follow-ups.
*GLA:D is an evidence-based programme that includes education and supervised neuromuscular exercise delivered by certified physiotherapists.
†Type of knee arthroplasty will be extracted from the National Patient Register.
GLA:D, Good Life with osteoArthritis in Denmark; OA, osteoarthritis.
The second descriptive study (objective 2) will focus on the demographic, functional and radiological characteristics (table 1) in patients choosing the different treatment pathways. For each treatment pathway identified in the first descriptive study, patient characteristics will be presented descriptively. Depending on the identified treatment pathways it may also be relevant to explore and present patient characteristics for selected treatment modalities. Investigating the relationship between patient characteristics and the treatment pursued will help clarify whether patients with certain characteristics are more likely to pursue certain treatment pathways.
Data analysis plan for the prediction study
Through the prediction study (objective 3), we will investigate what predicts good and poor outcomes of available treatment modalities. Prognostic models will be developed using machine learning software packages for the statistical software program R.50 Two separate prognostic models will be developed—one model for the PASS and one for the TF outcome.
The patients’ self-determined classifications of good treatment outcomes, defined as answering yes or no to the PASS question will be the dichotomised dependent variable. Likewise, for the TF model, the dependent variable will be the patients’ self-determined classifications of poor treatment outcomes dichotomised into ‘TF’, if answering yes to the TF question, or ‘not TF’ if answering either yes to the PASS question or no to the subsequent TF question. Collected possible predictive variables (table 1), along with the 13 different categories of treatments that patients have received (table 2) at the three different time points, will be included as independent predictive variables. Variables such as BMI, preoperative OKS, physical function and prior knee arthroscopy have previously been shown to be clinically and statistically relevant predictive variables associated with treatment outcome after primary TKR.51 52 To identify which variables to include in the final model, models with and without specific variables will be compared with evaluate the possible loss in accuracy when excluding these variable.
To develop and determine which machine learning model to use, different models will be compared, for example, neural network and random forest. We intend to split collected data into two data sets so that approximately 70% of the total data can be used for developing/training the prediction models and approximately 30% of the total data can be used for validating/testing the models’ actual predictive performance.43 53 The decision on which statistical model to use depends on the distribution of data and which machine learning model showing the best predictive performance (mean accuracy) closest to 1.00 (100%). Validating the predictive performance of the best model is important21 and is done by using the model on the validating data set for the purpose of evaluating the actual predictive performance estimated based on the development data set.54–57 Different metrics will be used to evaluate the predictive performance and as a rule of thumb the accuracy of the model can be ranked from high (0.90 to 1.00) to moderate (0.70 to 0.89) to low (0.50 to 0.69),58 59 with 0.70 previously used as cut-off for a clinically relevant model.60
Qualitative study
We will conduct a qualitative study (objective 5) focusing on the patients’ perspectives on the choices and experiences of treatment for knee OA. The qualitative study will ensure a better understanding of current practices, needs and challenges in the daily management of patients with knee OA, seen from the patient’s point of view. We expect that patient characteristics in terms of for example, gender, age, BMI, function, OA severity, length of symptoms, received treatments, other comorbidities and connection to the labour market of patients included in the prospective cohort study will vary largely. Therefore, we will strive to include patients with a wide range of these patient characteristics for the qualitative study to better reflect the views of the general population with knee OA. Patients eligible for the qualitative study are selected by a screening of questionnaires from patients included in the TREATright study in both Copenhagen University Hospital Hvidovre and Næstved Hospital. Patients will be recruited from Copenhagen University Hospital Hvidovre and Næstved Hospital and individual interviews will be performed. Approximately 20 patients will be recruited, but the actual number of patients recruited for the qualitative study will depend on information power.61 The number of patients needed will be evaluated continuously and recruitment will end when sufficient information power is obtained61 to avoid recruiting too few or too many patients.62 63 Sufficient information power is influenced by the study aim, sample specificity, use of established theory, quality of dialogue and analysis strategy, and can be considered adequate when new knowledge has been developed with reference to the objectives of the study.61
The interviews with patients will be performed as semistructured interviews, and an interview guide will be prepared prior to the interviews.64 The interview guide will include themes such as patients’ experiences of knee OA, choices and treatment experiences, both in relation to the experience of the effect and when in the course they have received treatment, where and how, as well as experiences with the organisation around their treatment. In addition, the interview guide will be informed by experiences from the survey and descriptive studies. Furthermore, the TREATright patient representatives (see the Patient and public involvement section) will be invited to comment on the guide and to contribute with their perspectives on important and essential questions to be covered. Subsequently, the interview guide will be tested in a couple of pilot interviews on relevant patients after which the interview guide will be adjusted. The interviews will take approximately 1 hour and will take place at Copenhagen University Hospital Hvidovre and Næstved Hospital or, if possible, at the patients’ own residence.
All interviews will be recorded and subsequently transcribed for further analysis. A qualitative content analysis involving a systematic text condensation and thematisation will be performed by SMB, in collaboration with AM and SR.65 66 The analysis will follow the following stages: reading all the material to get an overall impression, identifying units of meaning, representing different aspects of the patients’ perspectives and coding for these, condensing and abstracting the meaning within each of the coded groups, and summarising the content of each code group to generalised descriptions reflecting the most important experiences of the patients.65 Principles for the coding and the choice of themes and categories will be discussed continually in the research team. Furthermore, the analysis will be presented for and discussed with patient representatives.
Cost-effectiveness study
A health economy analysis will be performed to evaluate the cost-effectiveness of treatment pathways that live up to clinical guidelines versus pathways that do not (as outlined under the descriptive studies). The outcome will be based on the EQ-5D-3L, which will be used to derive summary index values based on the Danish value set.40 Quality-adjusted life years (QALYs) for the cost utility analysis will be calculated using change in EQ-5D-3L summary index value from baseline to 2-year follow-up.
Information on healthcare costs and non-health care costs will be collected from Statistics Denmark, and additionally from the 6-month and 2-year follow-up questionnaires (table 1). Healthcare costs in the follow-up period include hospitalisation, surgery, medication, primary and secondary health provider visits, and home help services provided by the municipalities. Productivity costs estimated from weekly data on long-term sickness absence will be obtained from the National Register on Social Transfer Payments (the DREAM registry) and short-term sick leave (defined as sick leave less than 21 continuous days), will be asked for in the follow-up questionnaires, as well as cost for treatment that is not covered by the public health insurance system. For the purpose of collecting more accurate data and to reduce recall bias, the primary source of data is registry based. Only information on short-term sick leave and cost for treatment that is not covered by the public health insurance system are patient reported.
An incremental cost-effectiveness ratio (ICER) will be calculated by dividing the incremental cost by the QALYs gained. In Denmark, there is no officially accepted and recognised willingness-to-pay threshold. Therefore, the ICER will be compared with threshold applied by NICE (£20 000–£30 000).67
Patient and public involvement
To ensure study importance, relevancy and research usefulness from an end-user perspective, patients are involved in the research planning and continuous development of the project.68 69 The study aims and research questions were discussed with two patients with knee OA. Furthermore, initial pretesting of the questionnaire was performed on 11 patients. Further, six patients with knee OA were appointed as TREATright patient representatives and are invited to the study site at Copenhagen University Hospital Hvidovre two to three times a year to be involved in the process of developing the questionnaires and the interview guide and share their views on the research development and results, and contribute with their ideas on how to disseminate the results to people with knee OA.
Ethics and dissemination
We received a waiver (journal number: H-17017295) from the Danish regional ethical committee. Approval from the Danish Data Protection Agency to handle patient-sensitive information from both study sites was acquired (journal number: AHH-2017–072). All data will be handled in line with the General Data Protection Regulation and the Danish Data Protection Act. Data will be collected electronically through a REDCap database that ensures secure data storage. Paper-based questionnaires and other data will be securely stored in a locked cabinet. After data entry into the REDCap database, paper-based questionnaires will be shredded. Patients will be asked for permission to extract data from their medical records. For statistical data processing, only anonymised data will be extracted. After study completion, all data will be anonymised.
To ensure all relevant stakeholders are informed, we will strive for a wide distribution of the results through different news and social media, conferences and workshops. The results will be submitted to international open-access peer-reviewed journals.
Discussion
This study will provide an overview of treatment pathways used for knee OA in a 2-year time period after consulting an orthopaedic surgeon. The strength of this study is that patients are included consecutively from two large centres that represent both rural and urban areas of Denmark, increasing the representativeness of the study population. Although clinical care pathways for knee OA may vary largely between countries, we believe that our results will be of value also to other countries and healthcare systems.
This study will face a number of limitations. Although study invitation is based on referral from the general practitioners due to knee OA, we also include patients with unspecified diagnoses such as knee pain or knee problems if their age is ≥40 years, which introduces a risk of including a small number of patients that do not fit the diagnostic criteria for knee OA.
As part of this study is conducted as a prospective cohort study using self-reported questionnaires including retrospective information of previous treatment, there is a risk of recall bias.70 Although we risk patients not reporting accurate detail on treatments received, the self-report approach is the only possibility to collect this detailed information. Particularly for the cost-effectiveness analyses, considering that the primary source of data is collected through national registries with a high quality and completeness,71 we do not believe that the risk of bias is substantial. Only the information on short-term sick leave and cost for treatment that is not covered by the public health insurance system is self-reported.
Furthermore, when collecting possible predictive variables through self-reported questionnaires, we risk missing data. Therefore, for the predictive study, multiple and single imputation will be used.
Lastly, defining treatment pathways for the descriptive and cost-effectiveness studies that adhere to and do not adhere to clinical guidelines is challenging since clinical guideline recommendations are not always consistent.1 7–9 We have therefore used a pragmatic approach based on drawing similarities between the different clinical guidelines. In addition, the classification is made based on the self-reported treatments that the patients have received limited to a level of detail that the patients can understand and thus answer in a meaningful way.
Supplementary Material
Footnotes
Twitter: @LIngelsrud, @STSkou
Contributors: Study conception and design, critical revision for important intellectual content and final approval of the article: SMB, LHI, TB, STS, HMS, SR, AM, JK, TK and AT; acquisition of data and drafting of the manuscript: SMB and LHI.
Funding: This work was supported by the Region Zealand Health Science Research Foundation (grant number RSSF2017000636), the Copenhagen University Hospital Hvidovre local fund (grant numbers E-21210-03 and E-21210-08), the Copenhagen University Hospital Hvidovre strategic fund (grant numbers E-21210-11 and E-21210-15), the Næstved-Slagelse-Ringsted Hospitals’ local fund (grant numbers 111.1043 and 111.2113), Candys Foundation (grant number 2019-302), Helsefonden (grant number 20-B-0286) and the Danish Rheumatism Association (grant number R161-A5285).
Competing interests: STS is the associate editor of the Journal of Orthopaedic & Sports Physical Therapy, has received grants from The Lundbeck Foundation, personal fees from Munksgaard and TrustMe-ED, all of which are outside the submitted work. He is cofounder of Good Life with Osteoarthritis in Denmark, a not-for profit initiative hosted at University of Southern Denmark aimed at implementing clinical guidelines for osteoarthritis in clinical practice. STS is currently funded by a grant from Region Zealand (Exercise First) and a grant from the European Research Council under the European Union's Horizon 2020 research and innovation programme (grant agreement number 801790). Both are unrelated to the current project. TB has received speaker’s honoraria for talks or expert testimony on the efficacy of exercise therapy to enhance recovery after surgery at meetings or symposia held by biomedical companies (Zimmer Biomet and Novartis). He has received fees for writing textbook chapters (Munksgaard) and for organising postgraduate education, such as postgraduate courses in clinical exercise physiology or PhD courses on clinical research methodology. He is an exercise physiologist as well as a physical therapist; hence, a potential cognitive bias is exercise preference/love of exercise. HMS is the associate editor of The Knee. AT has received grants and personal fees from Zimmer Biomet outside the submitted work.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.National Clinical Guideline Centre (UK) . Osteoarthritis: care and management in adults. London: National Institute for Health and Care Excellence (UK), 2014. [PubMed] [Google Scholar]
- 2.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019 [published correction appears in Lancet 2020 Nov 14;396(10262):1562]. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmon JH, Rat AC, Sellam J, et al. Economic impact of lower-limb osteoarthritis worldwide: a systematic review of cost-of-illness studies. Osteoarthritis Cartilage 2016;24:1500–8. 10.1016/j.joca.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Kamaruzaman H, Kinghorn P, Oppong R. Cost-Effectiveness of surgical interventions for the management of osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2017;18:1–17. 10.1186/s12891-017-1540-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet 2018;392:1672–82. 10.1016/S0140-6736(18)32344-4 [DOI] [PubMed] [Google Scholar]
- 6.Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med 2015;373:1597–606. 10.1056/NEJMoa1505467 [DOI] [PubMed] [Google Scholar]
- 7.Sundhedsstyrelsen . Knæartrose - nationale kliniske retningslinjer og faglige visitationsretningslinjer [Danish Health Authority. Knee osteoarthritis - national clinical guidelines and visitation guidelines], 2012. [Google Scholar]
- 8.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–89. 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 9.Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 10.Spitaels D, Hermens R, Van Assche D, et al. Are physiotherapists adhering to quality indicators for the management of knee osteoarthritis? an observational study. Musculoskelet Sci Pract 2017;27:112–23. 10.1016/j.math.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 11.Basedow M, Esterman A. Assessing appropriateness of osteoarthritis care using quality indicators: a systematic review. J Eval Clin Pract 2015;21:782–9. 10.1111/jep.12402 [DOI] [PubMed] [Google Scholar]
- 12.Husted RS, Bandholm T, Rathleff MS, et al. Perceived facilitators and barriers among physical therapists and orthopedic surgeons to pre-operative home-based exercise with one exercise-only in patients eligible for knee replacement: a qualitative interview study nested in the QUADX-1 trial. PLoS One 2020;15:e0241175. 10.1371/journal.pone.0241175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingelsrud LH, Roos EM, Gromov K, et al. Patients report inferior quality of care for knee osteoarthritis prior to assessment for knee replacement surgery - a cross-sectional study of 517 patients in Denmark. Acta Orthop 2020;91:82–7. 10.1080/17453674.2019.1680180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagen KB, Smedslund G, Østerås N, et al. Quality of community-based osteoarthritis care: a systematic review and meta-analysis. Arthritis Care Res 2016;68:1443–52. 10.1002/acr.22891 [DOI] [PubMed] [Google Scholar]
- 15.Rosemann T, Wensing M, Joest K, et al. Problems and needs for improving primary care of osteoarthritis patients: the views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord 2006;7:48. 10.1186/1471-2474-7-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali SA, Walsh KE, Kloseck M. Patient perspectives on improving osteoarthritis management in urban and rural communities. J Pain Res 2018;11:417–25. 10.2147/JPR.S150578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traumer L, Sørensen EE, Kusk KH, et al. Investigating the motives of patients with knee oa undergoing a TKR: a qualitative interview study. Musculoskeletal Care 2018;16:380–7. 10.1002/msc.1244 [DOI] [PubMed] [Google Scholar]
- 18.Ferreira de Meneses S, Rannou F, Hunter DJ. Osteoarthritis guidelines: barriers to implementation and solutions. Ann Phys Rehabil Med 2016;59:170–3. 10.1016/j.rehab.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 19.Egerton T, Diamond LE, Buchbinder R, et al. A systematic review and evidence synthesis of qualitative studies to identify primary care clinicians' barriers and enablers to the management of osteoarthritis. Osteoarthritis Cartilage 2017;25:625–38. 10.1016/j.joca.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 21.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur Urol 2015;67:1142–51. 10.1016/j.eururo.2014.11.025 [DOI] [PubMed] [Google Scholar]
- 22.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (cheers) statement. Eur J Health Econ 2013;14:367–72. 10.1007/s10198-013-0471-6 [DOI] [PubMed] [Google Scholar]
- 23.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 24.Bandholm T, Christensen R, Thorborg K, et al. Preparing for what the reporting checklists will not tell you: the prepare trial guide for planning clinical research to avoid research waste. Br J Sports Med 2017;51:1494–501. 10.1136/bjsports-2017-097527 [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis 2010;69:483–9. 10.1136/ard.2009.113100 [DOI] [PubMed] [Google Scholar]
- 26.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 2005;64:34–7. 10.1136/ard.2004.023028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingelsrud LH, Terluin B, Gromov K, et al. Which Oxford knee score level represents a satisfactory symptom state after undergoing a total knee replacement? Acta Orthop 2021;92:85–90. 10.1080/17453674.2020.1832304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–15. 10.1016/0197-2456(89)90005-6 [DOI] [PubMed] [Google Scholar]
- 29.Ingelsrud LH, Roos EM, Terluin B, et al. Minimal important change values for the Oxford knee score and the forgotten joint score at 1 year after total knee replacement. Acta Orthop 2018;89:541–7. 10.1080/17453674.2018.1480739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br 2007;89:1010–4. 10.1302/0301-620X.89B8.19424 [DOI] [PubMed] [Google Scholar]
- 31.Dawson J, Fitzpatrick R, Murray D, et al. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63–9. 10.1302/0301-620x.80b1.7859 [DOI] [PubMed] [Google Scholar]
- 32.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (vas pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CpGs), short Form-36 bodily pain scale (SF-36 BPs), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63 Suppl 11:S240–52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 33.Alghadir AH, Anwer S, Iqbal A, et al. Test-Retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res 2018;11:851–6. 10.2147/JPR.S158847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crossley KM, Bennell KL, Cowan SM, et al. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Arch Phys Med Rehabil 2004;85:815–22. 10.1016/s0003-9993(03)00613-0 [DOI] [PubMed] [Google Scholar]
- 35.Southerst D, Côté P, Stupar M, et al. The reliability of body pain diagrams in the quantitative measurement of pain distribution and location in patients with musculoskeletal pain: a systematic review. J Manipulative Physiol Ther 2013;36:450–9. 10.1016/j.jmpt.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 36.Rolfson O, Wissig S, van Maasakkers L, et al. Defining an international standard set of outcome measures for patients with hip or knee osteoarthritis: consensus of the International Consortium for health outcomes measurement hip and knee osteoarthritis Working group. Arthritis Care Res 2016;68:1631–9. 10.1002/acr.22868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Østerås N, Tveter AT, Garratt AM, et al. Measurement properties for the revised patient-reported osteoarthritis quality indicator questionnaire. Osteoarthritis Cartilage 2018;26:1300–10. 10.1016/j.joca.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 38.Lorig K, Chastain RL, Ung E, et al. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum 1989;32:37–44. 10.1002/anr.1780320107 [DOI] [PubMed] [Google Scholar]
- 39.Garratt AM, Løchting I, Smedslund G, et al. Measurement properties of instruments assessing self-efficacy in patients with rheumatic diseases. Rheumatology 2014;53:1161–71. 10.1093/rheumatology/ket374 [DOI] [PubMed] [Google Scholar]
- 40.Wittrup-Jensen KU, Lauridsen J, Gudex C, et al. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health 2009;37:459–66. 10.1177/1403494809105287 [DOI] [PubMed] [Google Scholar]
- 41.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gromov K, Korchi M, Thomsen MG, et al. What is the optimal alignment of the tibial and femoral components in knee arthroplasty? Acta Orthop 2014;85:480–7. 10.3109/17453674.2014.940573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–73. 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 44.Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–10. 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 45.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. 10.1016/s0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 46.Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol 2016;76:175–82. 10.1016/j.jclinepi.2016.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization Regional Office for Europe . Body mass index - BMI, 2020. Available: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi
- 48.Skou ST, Roos EM. Good Life with osteoArthritis in Denmark (GLA:D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord 2017;18:72. 10.1186/s12891-017-1439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roos EM, Juhl CB. Osteoarthritis 2012 year in review: rehabilitation and outcomes. Osteoarthritis Cartilage 2012;20:1477–83. 10.1016/j.joca.2012.08.028 [DOI] [PubMed] [Google Scholar]
- 50.R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.R-project.org/ [Google Scholar]
- 51.Sanchez-Santos MT, Garriga C, Judge A, et al. Development and validation of a clinical prediction model for patient-reported pain and function after primary total knee replacement surgery. Sci Rep 2018;8:3381. 10.1038/s41598-018-21714-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Judge A, Arden NK, Cooper C, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology 2012;51:1804–13. 10.1093/rheumatology/kes075 [DOI] [PubMed] [Google Scholar]
- 53.Steyerberg EW, Harrell FE, Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001;54:774–81. 10.1016/s0895-4356(01)00341-9 [DOI] [PubMed] [Google Scholar]
- 54.Harrell FE. Regression modeling strategies. 2nd ed. New York City, NY: Springer Science + Business Media, 2015. [Google Scholar]
- 55.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–38. 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moons KGM, Kengne AP, Grobbee DE, et al. Risk prediction models: II. external validation, model updating, and impact assessment. Heart 2012;98:691–8. 10.1136/heartjnl-2011-301247 [DOI] [PubMed] [Google Scholar]
- 57.Altman DG, Vergouwe Y, Royston P, et al. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338:b605. 10.1136/bmj.b605 [DOI] [PubMed] [Google Scholar]
- 58.Swets JA. Measuring the accuracy of diagnostic systems. Science 1988;240:1285–93. 10.1126/science.3287615 [DOI] [PubMed] [Google Scholar]
- 59.Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med 2003;29:1043–51. 10.1007/s00134-003-1761-8 [DOI] [PubMed] [Google Scholar]
- 60.El-Galaly A, Grazal C, Kappel A, et al. Can Machine-learning algorithms predict early revision TKA in the Danish knee arthroplasty registry? Clin Orthop Relat Res 2020;478:2088–101. 10.1097/CORR.0000000000001343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753–60. 10.1177/1049732315617444 [DOI] [PubMed] [Google Scholar]
- 62.Sandelowski M. Sample size in qualitative research. Res Nurs Health 1995;18:179–83. 10.1002/nur.4770180211 [DOI] [PubMed] [Google Scholar]
- 63.Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks, CA: SAGE, 1996. [Google Scholar]
- 64.Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs 2016;72:2954–65. 10.1111/jan.13031 [DOI] [PubMed] [Google Scholar]
- 65.Malterud K. Systematic text condensation: a strategy for qualitative analysis. Scand J Public Health 2012;40:795–805. 10.1177/1403494812465030 [DOI] [PubMed] [Google Scholar]
- 66.Pope C, Ziebland S, Mays N. Analysing qualitative research in health care. 3rd ed. Blackwell Publishing Ltd, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Institute for Health and Care Excellence . Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence (NICE), 2013. [PubMed] [Google Scholar]
- 68.Bandholm T, Henriksen M, Treweek S, et al. Make it real: four simple points to increase clinical relevance in sport and exercise medicine research. Br J Sports Med 2018;52:1407–8. 10.1136/bjsports-2018-099714 [DOI] [PubMed] [Google Scholar]
- 69.Puggaard RS, Ingelsrud LH, Jacobsen S, et al. Establishing research priorities related to osteoarthritis care via stakeholder input from patients. Dan Med J 2021;68:A09200683. [PubMed] [Google Scholar]
- 70.Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc 2016;9:211–7. 10.2147/JMDH.S104807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.