Abstract
Objectives
The anti-Mullerian hormone (AMH) test has been promoted as a way to inform women about their future fertility. However, data consistently show the test is a poor predictor of natural fertility potential for an individual woman. As fertility centre websites are often a primary source of information for reproductive information, it is essential the information provided is accurate and reflects the available evidence. We aimed to systematically record and categorise information about the AMH test found on Australian and New Zealand fertility clinic websites.
Design
Content analysis of online written information about the AMH test on fertility clinic websites.
Setting
Accredited Australian and New Zealand fertility clinic websites.
Methods
Data were extracted between April and June 2020. Any webpage that mentioned the AMH test, including blogs specifically about the AMH test posted since 2015, was analysed and the content categorised.
Results
Of the 39 active accredited fertility clinics’ websites, 25 included information about the AMH test. The amount of information varied widely, and embodied four overarching categories; (1) the utility of the AMH test, (2) who the test is suitable for, (3) possible actions in response to the test and (4) caveats and limitations of the test. Eight specific statements about the utility of the test were identified, many of which are not evidence-based. While some websites were transparent regarding the test’s limitations, others mentioned no caveats or included persuasive statements actively promoting the test as empowering for a range of women in different circumstances.
Conclusions
Several websites had statements about the utility of the AMH test that are not supported by the evidence. This highlights the need for higher standards for information provided on fertility clinic websites to prevent women being misled to believe the test can reliably predict their fertility.
Keywords: reproductive medicine, general medicine (see internal medicine), quality in healthcare
Strengths and limitations of this study.
First study to robustly and systematically assess publicly available anti-Mullerian hormone (AMH) test information on fertility clinic websites.
Two researchers independently assessed all the extracted information about the AMH test, with any inconsistencies resolved with an additional member of the study team.
Only written content was assessed (eg, videos were excluded), potentially missing relevant information on the AMH test.
Website content can change over time, meaning that different information may be identified if the study is repeated.
Introduction
A woman’s fertility declines with age, due to the reduction in the quality and quantity of her eggs over time.1 In women, the anti-Mullerian hormone (AMH) is exclusively produced by granulosa cells of ovarian follicles during the early stages of their development.2 AMH levels can be measured by a blood test, giving an indication of ovarian reserve, or the number of eggs remaining in the ovaries. In theory, higher levels of AMH indicate the presence of more eggs and higher fertility potential and low levels indicate that there are few eggs left and the woman is approaching menopause. Menopause typically occurs at approximately 50 years of age.3 However, loss of ovarian reserve is accelerated in approximately 10% of women leading to premature menopause and loss of fertility potential before the age of 40 years.4 The AMH test has been promoted as a way for women to find out how much longer they have to achieve pregnancy or how likely it is that pregnancy could be achieved at all,5 potentially encouraging proactive family planning and preventing childlessness caused by age-related infertility.6 Public interest in AMH testing is also increasing with the rise of elective egg freezing in women concerned about age-related fertility decline.7 8
While the AMH test may be valuable in assisted reproductive technology treatment (ART) management through indicating potential ovarian response and enabling personalised dose selection in stimulation protocols,9 10 it has limited predictability of live birth rates in both ART11 12 and spontaneous conception settings.13–15 In addition, while a low AMH level may reflect a quantitative decline in ovarian reserve, there is currently no consensus on the level which defines a depleted ovarian reserve. Indeed, pregnancy can still occur even at undetectable AMH levels, especially in young women.2 6 16 The AMH test is therefore not a reliable measure of fertility potential.13 It can also give false readings for women with polycystic ovary syndrome (PCOS) or who use oral contraceptives.17 The American College of Obstetricians and Gynaecologists recently released a statement against the use of AMH in women without a diagnosis of infertility as it is not supported by the evidence.18 Despite this, some fertility specialists and researchers19 have suggested that women in their late 20s have the test at regular intervals to monitor their fertility potential. In addition, online companies in countries such as the USA, Australia and the UK are now selling the test direct-to-consumers outside of clinical settings,7 offering women estimates of their fertility potential based on the results of the test. In Australia, AMH testing can occur in several ways, although women are predominantly referred by their general practitioners (GPs) or fertility specialists to get the test from pathology laboratories or fertility clinics with in-house pathology. The test is not covered by Australia’s universal health scheme and has out-of-pocket costs.
Fertility clinic websites along with social media are primary sources for women seeking reproductive information,20 such as egg freezing.21 When ‘AMH test’ or ‘egg timer test’ is entered into the Google search engine, fertility clinic websites are among the first websites to appear. In Australia and New Zealand, fertility clinics must be accredited by the Reproductive Technology Accreditation Committee (RTAC).22 The RTAC Code of Practice states that clinics ‘…must provide patients with information that is accurate, timely, in formats and language appropriate to the patient…’.22 Considering the popular narrative that the AMH test can predict fertility, the aim of this study was to systematically record and categorise any written information about the AMH test found on Australian and New Zealand fertility clinic websites.
Method
Setting
Accredited fertility clinics in Australia and New Zealand were identified from the list of accredited practices on the Fertility Society of Australia’s website.23 The websites of those clinics were accessed between April and June 2020. All webpages that mentioned the AMH test, including posts or blogs specifically about the AMH test which had been posted since 2015 were scrutinised. Analysis was restricted to written context (ie, videos and non-text data were excluded). Any webpages described as being specifically for clinicians (eg, GPs) were also excluded. Websites that did not mention the AMH test were excluded from further analysis.
Study design
A content analysis of the information on fertility clinic websites about the AMH test was conducted. Content analysis is a widely used analysis method which combines qualitative and quantitative methods to analyse text data, allowing the content and frequency of categories to be reported.24 Given the uncertain evidence about the utility of the AMH test, we aimed to systematically identify and categorise the statements made about the utility of the AMH test and related information. This method has previously been used to assess claims made on fertility clinic websites about the effectiveness of different treatments.20 The study team included public health researchers (TC, BN, SL, KH and KM), a general practitioner and clinical epidemiologist (JD), a registered nurse (KH) and fertility specialists (BWM and DL).
Patient and public involvement
No patients or public were involved. The data were derived from publicly available information on Australian and New Zealand fertility clinic websites.
Analysis
The analysis involved an iterative process with five members of the study team. After the number of eligible fertility clinic websites were ascertained and the data were extracted by one researcher (TC), content analysis was used to map out the areas of content that emerged and record and categorise the statements made about the AMH test, as well as additional observations. First, two researchers (TC and BN) independently reviewed information about the AMH test on 20 websites each to become familiar with the content and develop a list of recurring codes and themes. These codes and themes were discussed with a third researcher (SL) and informed an initial coding framework. All contents were then coded independently by two researchers (TC and SL) into the framework to ensure rigour. Further revisions to the framework were discussed and made as required during coding. The level of agreement between the two coders was tested using Cohen’s kappa and indicated a strong level of agreement (κ=0.83). Any inconsistencies in coding were then discussed and resolved, with a third researcher (BN) involved to come to a final agreement. Descriptive statistical analysis was used to calculate the frequency of each code, and quotes were chosen to illustrate findings.
Results
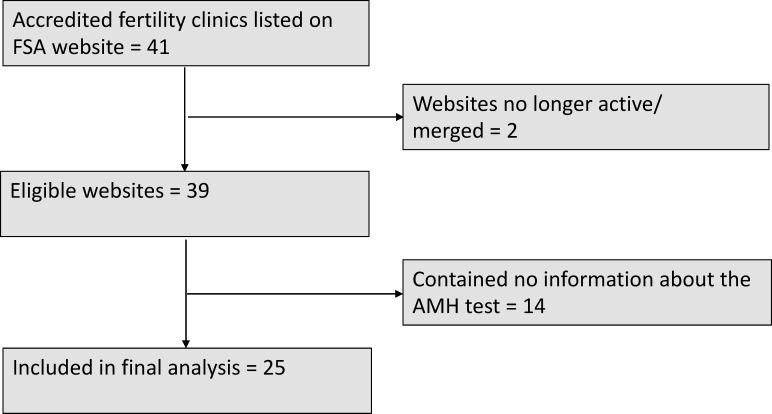
Of the 41 accredited fertility clinics listed on the Fertility Society of Australia’s website, two had merged with other fertility clinics, resulting in 39 eligible clinic websites. The number of web pages with content relating to the AMH test varied widely across websites from 0 up to 12 pages (mean: 3.4 webpages per clinic website). Of the 39 eligible websites, 14 (36%) did not mention the AMH test at all and 8 (21%) only listed the test or gave a very brief description of the test, which did not include any additional information such as potential benefits or limitations. The 14 websites that did not mention the AMH test were excluded from further analysis (see figure 1).
Figure 1.
Flow diagram of accredited fertility clinic websites included in the current study. AMH, anti-Mullerian hormone, FSA, Fertility Society Australia
Information about the AMH test on the remaining 25 clinic websites was organised into four overarching categories; statements about (1) the utility of the AMH test, (2) who the test is suitable for, (3) possible actions in response to the test and (4) statements reflecting caveats and limitations of the test. The overarching categories and their affiliated statements, quotes illustrating each statement and proportions of clinic websites containing each statement are shown in table 1. In addition, two patterns of observations arose when analysing the data. These included the use of persuasive language and contradictory information within and across websites.
Table 1.
Statements about the AMH test on fertility clinic websites (N=25 websites)
| Categories and codes | Example quote | n (%) |
| Statements about the utility of the AMH test | ||
| Indicator of ovarian reserve/number of eggs | ‘The amount of AMH gives an indication of the number of eggs being produced, or ovarian reserve’. | 19 (76%) |
| Indicates response to fertility treatment (ie, number of eggs collected, treatment/IVF success) |
‘An AMH is a measure of quantity and can infer how many eggs can be expected to develop in a fertility treatment cycle’. ‘It can also help a fertility specialist determine whether a woman is a good candidate to undergo certain fertility treatments and how successful those treatments may be’. |
9 (36%) |
| Assesses future fertility potential | ‘You might want to consider an Anti-Mullerian hormone (AMH) test to get some insight into the remaining quantity of eggs and number of fertile years you may have left’. | 9 (36%) |
| Assesses current fertility | ‘AMH levels decline at predictable rates hence the AMH test is a good snapshot of current fertility’. | 7 (28%) |
| Indicates polycystic ovary syndrome | ‘A low AMH may indicate low egg reserve and high levels of AMH can be indicative of Polycystic Ovary Syndrome (PCOS)’. | 6 (24%) |
| Predicts OHSS | ‘A high level may indicate an exaggerated response to the IVF medication’. | 4 (16%) |
| Indicates increased risk of miscarriage | ‘Women with diminished ovarian reserve have diminished fertility and increased risk of miscarriage’. | 4 (16%) |
| Predicts time to menopause and indicates risk of early menopause | ‘It may also identify women who may undergo early menopause, and therefore who may lose their fertility earlier than average’. | 2 (8%) |
| Statements about who the AMH test is suitable for | ||
| Women considering fertility treatment | ‘The AMH test is useful if: You are considering IVF or other fertility medications, as low levels of AMH may indicate a potentially poor response to IVF and conversely a high level may indicate an exaggerated response to the IVF medication’. | 12 (48%) |
| Women with risk factors for reduced fertility | ‘Women who have had chemotherapy or ovarian/endometrial surgery and want to find out what effect it has had on their future fertility’. | 9 (36%) |
| Women planning for pregnancy, now or in the future | ‘If you are planning on having children 1 day, it’s worth considering the egg timer test’. | 8 (32%) |
| Women who have been trying to conceive for 6 months and are seeking reassurance | ‘Women who have been trying to conceive for over 6 months, and are looking for reassurance that their ovarian reserve is appropriate for their age’. | 6 (24%) |
| Women who want to check their ovarian reserve/ are curious | ‘Women who would like to conceive in the future and are curious about their ovarian reserve’. | 6 (24%) |
| Women considering delaying pregnancy |
‘An AMH is often done to give reassurance to women who want to delay childbearing’. ‘Your doctor may recommend an AMH test if you are wanting to delay childbirth and are under 35 years old’. |
3 (12%) |
| Women undergoing IVF (to inform about dose change) | ‘A low ovarian reserve result may indicate: if already undergoing fertility treatment, may call for a larger dose of fertility medication’. | 1 (4%) |
| Women considering fertility preservation/egg freezing | ‘The expected success of the (egg freezing) procedure can be ascertained from an initial assessment of the ovarian reserve using a blood test for Anti-Mullerian Hormone (AMH) and an ultrasound scan of the ovaries and uterus’. | 1 (4%) |
| Women over 35 years trying to conceive | ‘If you are over 35 and haven’t fallen pregnant within 6 months of trying, we may begin our assessment by checking your ovarian reserve’. | 1 (4%) |
| Statements about possible actions in response to the result of the AMH test | ||
| Informs when to access fertility treatment | ‘This test provides a snapshot early on so a decision can be made on when to start trying for a baby and when to access fertility treatment’. | 10 (40%) |
| Assists with reproductive life planning (when to start trying/if need to bring forward plans) | ‘Once the ovaries run out of eggs, the body can’t produce more. The last remaining eggs may not be great quality – so it’s best to make an informed decision. The egg timer test can help with this’. | 9 (36%) |
| Informs when to undertake elective egg freezing | ‘It may however indicate the need for more proactive action such as beginning a family sooner or undertaking elective egg freezing’. | 7 (28%) |
| Enables tailored IVF drug dose | ‘When fertility treatments are required, the AMH serves as a guide to the dosage of medications used’. | 4 (16%) |
| Informs when to talk to a fertility specialist | ‘A low AMH level is indicative of poor egg reserve, and you should then consider discussing your situation further with a fertility specialist’. | 3 (12%) |
| Informs when to consider using donor eggs | ‘What if my ovarian reserve is low?: if you’ve experienced premature menopause, we can talk about options including using donor eggs’. | 3 (12%) |
| Stated caveats and limitations of the AMH test | ||
| Quantity not quality | ‘AMH indicates the quantity of eggs remaining in a woman’s ovary and does not indicate the quality of the eggs in the ovary’. | 9 (36%) |
| Cannot predict individual response/ does not predict chance of a live birth | ‘A woman with a low AMH level will have the same chance of conceiving naturally’. | 8 (32%) |
| Artificially lower or higher in certain women | ‘AMH measurement is not 100% reliable and can be artificially lower in women who are very young, who are taking the contraceptive pill or who are very lean exercisers or those with pituitary problems’. | 5 (20%) |
| Age is the most important factor of fertility | ‘Please remember that age is still a very important factor for fertility’. | 5 (20%) |
| Lacks sensitivity, specificity/imperfect test/ levels can fluctuate | ‘It is impossible to entirely predict a woman’s chances of conception, so a normal result should always be considered cautiously in relation to future fertility’. | 3 (12%) |
| Needs to be interpreted in conjunction with other factors/ needs specialist interpretation | ‘The interpretation of the AMH result will depend on your medical history, your family’s fertility history, lifestyle and other investigations into your fertility’. | 3 (12%) |
AMH, anti-Mullerian hormone; IVF, in vitro fertilisation; OHSS, ovarian hyperstimulation syndrome.
Statements about the utility of the AMH test
Eight specific statements about the utility of the test were identified, with 19 of the 25 websites listing at least one of these. The most common statement made about the usefulness of the test was that it is an indicator of ovarian reserve, or the number of eggs in the ovaries (76%; table 1). Other recurring statements included that the test indicates response to fertility treatment [eg, number of eggs collected (n=6) or vague treatment success statements (n=3) for example, ‘…a good predictor of IVF success’; 36%], assesses women’s future fertility potential (eg, how many fertile years ahead; 36%) or determines women’s current fertility status (28%).
Statements about who the test is suitable for
The test was recommended for a range of women in different circumstances and settings, including those undergoing assisted reproduction, women who were curious about their ovarian reserve and women who wanted to know their current and future fertility potential. The most common recommendations were for women considering fertility treatment (48%), women with risk factors for reduced fertility (eg, family history of premature ovarian failure, women who have had chemotherapy, ovarian tumour, endometriosis; 36%) and for women planning pregnancy, now or in the future (32%).
Statements about possible actions in response to the result of the test
Several websites included statements about possible actions in response to the result of the test, with the most common being that the test results can inform women when to access fertility treatment (40%), assist with reproductive life planning (36%) and inform when to undertake elective egg freezing (28%).
Stated caveats and limitations of the AMH test
Some websites had statements reflecting caveats or limitations of the test. The most commonly stated limitations were that the test is an indicator of egg quantity not quality (36%), it does not predict chance of conceiving or having a live birth (32%), that age is still the most important factor for fertility (20%) and that the results can be artificially lower or higher in certain women, such as women who are heavy exercisers, are on the contraceptive pill, have PCOS or are very young (20%).
Additional observations
Use of persuasive language
Some websites used persuasive language and assertions that actively promoted the test. The most common was adding a motivation or rationale for having the test (44%), such as stating ‘Information is power and lets you take charge of your fertility’. Some also communicated the growing popularity and demand for the test (eg, ‘more and more women are seeking reassurance about their ability to reproduce’; 8%) or emphasised the convenience of the test (eg, ‘a simple blood test’; 44%).
Confusing statements and contradictions
There were also a number of contradictions in the information provided across the websites. These included contradicting statements about whether the AMH test can (n=2) or cannot predict menopause (n=1), is an indicator (n=1) or is not an indicator of egg quality (n=9), whether the results need to be interpreted by a specialist (n=2) or by a GP (n=3), and whether the test is reliable (n=6) or can be artificially lower when using oral contraception (n=5). There was even conflicting, ambiguous and confusing statements within the same website on three of the websites (12%), with the most common being whether or not the blood sample can be taken while using oral contraception and whether the test assesses women’s fertility (eg, ‘…not a measure of fertility but an important tool in assessing potential fertility’ and then in the next paragraph ‘an AMH test can assess your current fertility’).
Discussion
This study systematically recorded and categorised information about the AMH test found on Australian and New Zealand fertility clinic websites. The information provided was highly variable across the websites, from providing none or minimal information on the AMH test to providing extensive information about the test. Some websites were found to be very transparent and upfront regarding the test’s limitations, while others did not mention limitations or included persuasive statements actively promoting the test (eg, promoting empowerment, proactive decision-making) for a wide range of women in different circumstances. In addition, despite some websites containing substantial information about the test, it was often disjointed and spread across several pages; therefore comprehensive information may be difficult for women to find in one place. There were also several confusing or unclear statements, as well as contradictions within and across websites.
Importantly, while a number of statements about the utility of the test were made across a number of websites, few are supported by high-level evidence. Statements for which there is some supporting evidence include the AMH test being an indicator of ovarian reserve10 25 in terms of egg quantity and it being associated with the number of eggs obtained in an in vitro fertilisation (IVF) cycle,9 26 27 although large variation in ovarian response remains unexplained.27 Statements with mixed evidence include low AMH levels indicating increased risk of miscarriage.28 29 There is preliminary evidence that high levels of AMH indicate PCOS,30 31 however more research is needed to confirm this and current PCOS guidelines recommend against using AMH as a diagnostic tool.32 Statements refuted by existing evidence include the test being able to predict a woman’s future fertility potential or current fertility status,13–15 33 or identifying a woman at risk of early menopause.6 Furthermore, it is important to note that although the AMH may be associated with outcomes at a population level, this does not mean it has predictive value for individuals. For example, while the AMH appears to be associated with age of menopause at a population level, the huge individual variation, imprecision in estimates and limited capacity in predicting the extreme ages of menopause (eg, it cannot identify those at risk of early menopause) means its clinical applicability in individual women is limited.34 Questions have also been raised about whether AMH adds substantive predictive value over and above readily available patient characteristics, such as age.9 35 Considering this, there were also several misleading corresponding statements about who the test is suitable for and possible actions to be taken in response to the test result. This was particularly the case for websites that recommended the test for women outside of fertility treatment settings18 (eg, women planning pregnancy now or in the future, women who are curious about their ovarian reserve) or websites that claimed the test assisted with reproductive life planning (when to start trying to conceive) or when to undertake elective egg freezing.
Consequently, many websites include incorrect, overstated or misleading statements about the ability of the AMH test to reliably predict fertility. This raises concerns that women who use the AMH test to plan timing of pregnancy may get a false sense of security about delaying pregnancy if their level is in the normal or high range, and give women with low readings unwarranted anxiety about their ability to conceive. This could in turn increase women’s perceived need to freeze their eggs,36 try to conceive earlier than they had planned or pursue fertility treatments when it may not be needed, increasing the risk of healthy individuals receiving unnecessary fertility care.37 While many clinics do not receive direct financial benefit from ordering the test, clinics would benefit from the outlined potential actions as a result of women getting the test result, such as seeing a fertility specialist, egg freezing or commencing fertility treatment. Although these findings may reflect the varied views held about the utility of the AMH test and mixed evidence supporting its use in practice, it likely increases confusion for women seeking information regarding the AMH test and perpetuates unrealistic expectations. Given fertility clinic websites have been found to be a primary source of information for people seeking fertility treatments,38 it is essential the information provided is accurate and reflects the highest level of available evidence.20
Our findings of misleading or inaccurate information on fertility clinic websites are similar to recent studies evaluating the quality of website information regarding oocyte cryopreservation and of various interventions used in addition to standard IVF procedures.20 39 For example, a recent analysis of the quality of information about elective oocyte cryopreservation on Australian and New Zealand fertility clinic websites found that more than half scored poor, indicating that women are not receiving the information they need to make well-informed choices.39 To make autonomous decisions, patients must be presented with accurate, balanced information regarding the risks, benefits and limitations. Websites that do not state limitations or include misleading statements are impeding consumer decision-making and placing a large burden on clinicians to dispel misconceptions.40 The decision to have an AMH test may appear to be empowering; however, this rests on the false assumption that the test is an accurate predictor of fertility status.40
To our knowledge, this is the first study to rigorously and systematically assess publicly available AMH-related information for women using the well-established content analysis method, which involved a number of members of a multidisciplinary study team. The current study only included blogs from 2015, so older posts were excluded. This decision was made as the quality of reporting on the test before this time was poor and we felt it was not a fair judgement of the clinics’ current information. A limitation of the study is that it is unclear how consumers would interpret the information. Future studies are needed to assess how women interpret and respond to the information captured. We also excluded non-text content, such as videos, which may have had more accurate information. Website content also changes over time, so a different set of reviewers at a later date might locate different information to what was captured. In addition, direct-to-consumer websites or fertility clinics in countries without accrediting bodies may have worse quality information, so replication in other settings is warranted.
In conclusion, some Australian and New Zealand fertility clinic websites contain a number of statements regarding the utility of the AMH test which are not supported by the evidence and are potentially misleading. Fertility clinics should provide information based on the best available evidence and be transparent about uncertainties and limitations. In particular, the lack of utility of the AMH test for women without a diagnosis of infertility needs to be much clearer to prevent women having this test believing that it can accurately gauge their current and future fertility. These are high-stake decisions for women, so high-quality, accurate information to enable informed decision-making is essential.
Supplementary Material
Footnotes
Twitter: @TessaCopp
Contributors: TC, KM and BN conceived the study. TC, BN, SL, KM and KH were involved in designing the study and the data analysis. TC extracted the data from the websites and drafted the manuscript. TC, BN, SL, KH, DL, JD, BWM and KM contributed to the interpretation of the analysis, and critically revised and approved the manuscript.
Funding: This project was supported by a National Health and Medical Research Council (NHMRC) Programme grant (APP1113532).
Competing interests: BWM reports consultancy for ObsEva, Merck, Merck KGaA and Guerbet.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available. The data were derived from publicly available information on Australian and New Zealand fertility clinic websites.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update 2013;19:67–83. 10.1093/humupd/dms043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewailly D, Laven J. Amh as the primary marker for fertility. Eur J Endocrinol 2019;181:D45–51. 10.1530/EJE-19-0373 [DOI] [PubMed] [Google Scholar]
- 3.Treloar AE. Menstrual cyclicity and the pre-menopause. Maturitas 1981;3:249–64. 10.1016/0378-5122(81)90032-3 [DOI] [PubMed] [Google Scholar]
- 4.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update 2002;8:141–54. 10.1093/humupd/8.2.141 [DOI] [PubMed] [Google Scholar]
- 5.Tremellen K, Savulescu J. Ovarian reserve screening: a scientific and ethical analysis. Hum Reprod 2014;29:2606–14. 10.1093/humrep/deu265 [DOI] [PubMed] [Google Scholar]
- 6.Depmann M, Broer SL, van der Schouw YT, et al. Can we predict age at natural menopause using ovarian reserve tests or mother's age at menopause? A systematic literature review. Menopause 2016;23:224–32. 10.1097/GME.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 7.Kyweluk MA. Quantifying fertility? Direct-to-consumer ovarian reserve testing and the new (in)fertility pipeline. Soc Sci Med 2020;245:112697. 10.1016/j.socscimed.2019.112697 [DOI] [PubMed] [Google Scholar]
- 8.Ethics Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org, Ethics Committee of the American Society for Reproductive Medicine . Planned oocyte cryopreservation for women seeking to preserve future reproductive potential: an ethics Committee opinion. Fertil Steril 2018;110:1022–8. 10.1016/j.fertnstert.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 9.Broer SL, van Disseldorp J, Broeze KA, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update 2013;19:26–36. 10.1093/humupd/dms041 [DOI] [PubMed] [Google Scholar]
- 10.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (art). Hum Reprod Update 2010;16:113–30. 10.1093/humupd/dmp036 [DOI] [PubMed] [Google Scholar]
- 11.Broekmans FJ, Kwee J, Hendriks DJ, et al. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006;12:685–718. 10.1093/humupd/dml034 [DOI] [PubMed] [Google Scholar]
- 12.Revelli A, Biasoni V, Gennarelli G, et al. Ivf results in patients with very low serum AMH are significantly affected by chronological age. J Assist Reprod Genet 2016;33:603–9. 10.1007/s10815-016-0675-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017;318:1367–76. 10.1001/jama.2017.14588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagen CP, Vestergaard S, Juul A, et al. Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril 2012;98:1602–8. 10.1016/j.fertnstert.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Casadei L, Manicuti C, Puca F, et al. Can anti-Müllerian hormone be predictive of spontaneous onset of pregnancy in women with unexplained infertility? J Obstet Gynaecol 2013;33:857–61. 10.3109/01443615.2013.831050 [DOI] [PubMed] [Google Scholar]
- 16.Gleicher N, Weghofer A, Barad DH. Anti-Müllerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril 2010;94:2824–7. 10.1016/j.fertnstert.2010.04.067 [DOI] [PubMed] [Google Scholar]
- 17.Birch Petersen K, Hvidman HW, Forman JL, et al. Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan. Hum Reprod 2015;30:2364–75. 10.1093/humrep/dev197 [DOI] [PubMed] [Google Scholar]
- 18.ACOG Committee opinion no. 773: the use of Antimüllerian hormone in women not seeking fertility care. Obstet Gynecol 2019;133:e274–8. 10.1097/AOG.0000000000003162 [DOI] [PubMed] [Google Scholar]
- 19.Evans A, de Lacey S, Tremellen K. Australians' understanding of the decline in fertility with increasing age and attitudes towards ovarian reserve screening. Aust J Prim Health 2018;24:428–33. 10.1071/PY18040 [DOI] [PubMed] [Google Scholar]
- 20.Spencer EA, Mahtani KR, Goldacre B, et al. Claims for fertility interventions: a systematic assessment of statements on UK fertility centre websites. BMJ Open 2016;6:e013940. 10.1136/bmjopen-2016-013940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson EL, Gispanski L, Fields K, et al. Knowledge and decision making about future fertility and oocyte cryopreservation among young women. Hum Fertil 2021;24:112–21. 10.1080/14647273.2018.1546411 [DOI] [PubMed] [Google Scholar]
- 22.Reproductive Technology Accreditation Committee . Code of practice for assisted reproductive technology units. Australia FSo, ed. Melbourne, 2017. [Google Scholar]
- 23.Fertility Society of Australia and New Zealand . RTAC scheme & code of practice: accredited units: fertility society of Australia and New Zealand, 2021. Available: https://www.fertilitysociety.com.au/code-of-practice/#copanz
- 24.Weber RP. Basic content analysis California. Thousand Oaks: Sage, 1990. [Google Scholar]
- 25.de Vet A, Laven JSE, de Jong FH, et al. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 2002;77:357–62. 10.1016/S0015-0282(01)02993-4 [DOI] [PubMed] [Google Scholar]
- 26.Karkanaki A, Vosnakis C, Panidis D. The clinical significance of anti-Müllerian hormone evaluation in gynecological endocrinology. Hormones 2011;10:95–103. 10.14310/horm.2002.1299 [DOI] [PubMed] [Google Scholar]
- 27.Rustamov O, Wilkinson J, La Marca A, et al. How much variation in oocyte yield after controlled ovarian stimulation can be explained? A multilevel modelling study. Hum Reprod Open 2017;2017:hox018. 10.1093/hropen/hox018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peuranpää P, Hautamäki H, Halttunen-Nieminen M, et al. Low anti-Müllerian hormone level is not a risk factor for early pregnancy loss in IVF/ICSI treatment. Hum Reprod 2020;35:504–15. 10.1093/humrep/deaa008 [DOI] [PubMed] [Google Scholar]
- 29.Lyttle Schumacher BM, Jukic AMZ, Steiner AZ. Antimüllerian hormone as a risk factor for miscarriage in naturally conceived pregnancies. Fertil Steril 2018;109:1065–71. 10.1016/j.fertnstert.2018.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook CL, Siow Y, Brenner AG, et al. Relationship between serum Müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril 2002;77:141–6. 10.1016/S0015-0282(01)02944-2 [DOI] [PubMed] [Google Scholar]
- 31.Eilertsen TB, Vanky E, Carlsen SM. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Hum Reprod 2012;27:2494–502. 10.1093/humrep/des213 [DOI] [PubMed] [Google Scholar]
- 32.Teede H, Misso M, Tassone EC, et al. Anti-Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metab 2019;30:467–78. 10.1016/j.tem.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 33.Lam MT, Li HWR, Wong CYG, et al. Women's age and total motile normal morphology sperm count predict fecundability: a prospective cohort study. BMJ Sex Reprod Health 2020;46:279–86. 10.1136/bmjsrh-2020-200639 [DOI] [PubMed] [Google Scholar]
- 34.Depmann M, Eijkemans MJC, Broer SL, et al. Does AMH relate to timing of menopause? results of an individual patient data meta- analysis. J Clin Endocrinol Metab 2018;103:3593–600. 10.1210/jc.2018-00724 [DOI] [PubMed] [Google Scholar]
- 35.Lambalk CB. Anti-Müllerian hormone, the Holy Grail for fertility counselling in the general population? Hum Reprod 2015;30:2257–8. 10.1093/humrep/dev199 [DOI] [PubMed] [Google Scholar]
- 36.Pritchard N, Kirkman M, Hammarberg K, et al. Characteristics and circumstances of women in Australia who cryopreserved their oocytes for non-medical indications. J Reprod Infant Psychol 2017;35:108–18. 10.1080/02646838.2016.1275533 [DOI] [PubMed] [Google Scholar]
- 37.Kamphuis EI, Bhattacharya S, van der Veen F, et al. Are we overusing IVF? BMJ 2014;348:g252. 10.1136/bmj.g252 [DOI] [PubMed] [Google Scholar]
- 38.Human Fertilisation & Embryology Authority . Pilot national fertility patient survey. Human Fertilisation & Embryology Authority: London, 2018. [Google Scholar]
- 39.Beilby K, Dudink I, Kablar D, et al. The quality of information about elective oocyte cryopreservation (EOC) on Australian fertility clinic websites. Aust N Z J Obstet Gynaecol 2020;60:605–9. 10.1111/ajo.13174 [DOI] [PubMed] [Google Scholar]
- 40.Bayefsky MJ, DeCherney AH, King LP. Respecting autonomy-a call for truth in commercial advertising for planned oocyte cryopreservation. Fertil Steril 2020;113:743–4. 10.1016/j.fertnstert.2019.12.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available. The data were derived from publicly available information on Australian and New Zealand fertility clinic websites.