ABSTRACT
Low magnesium intakes coupled with high calcium intakes and high calcium-to-magnesium (Ca:Mg) intake ratios have been associated with increased risk for multiple chronic conditions such as cardiovascular disease and metabolic syndrome, as well as some cancers (colorectal, prostate, esophageal), and total mortality. A high dietary Ca:Mg ratio (>2.60) may affect body magnesium status while, on the other hand, high intakes of magnesium could adversely impact individuals with an exceedingly low dietary Ca:Mg ratio (<1.70). Thus, a Ca:Mg ratio range of 1.70–2.60 (weight to weight) has been proposed as an optimum range. Data from NHANES surveys have shown the mean Ca:Mg intake ratio from foods alone for US adults has been >3.00 since 2000. One-third of Americans consume a magnesium supplement with a mean dose of 146 mg/d, and 35% of Americans consume a calcium supplement with a mean dose of 479 mg/d. Our review of Ca:Mg ratios in dietary supplements sold in the United States and listed in NIH's Dietary Supplement Label Database (DSLD) found a mean ratio of 2.90 across all calcium- and magnesium-containing products, with differences by product form. The ratios ranged from a low of 0.10 in liquid products to a high of 48.5 in powder products. Thirty-one percent of products fell below, 40.5% fell within, and 28.3% fell above the ratio range of 1.70–2.60. Our findings of calculated Ca:Mg ratios from dietary supplements coupled with food-intake data suggest that, in individuals with high calcium intakes from diet and/or supplements, magnesium supplementation may be warranted to establish a more favorable dietary Ca:Mg ratio in their total diet. Additional research may provide greater insight into whether the Ca:Mg ratio is a biomarker of interest for moderating chronic disease and which population groups may derive benefit from moderating that ratio.
Keywords: dietary supplement, calcium, magnesium, calcium-to-magnesium ratio, Ca:Mg, chronic disease, cancer
An evaluation of the Ca:Mg ratio in dietary supplements demonstrated 40.5% had a Ca:Mg ratio within the optimum range of 1.70–2.60 and over one-fourth showed ratios above this range.
Introduction
Low magnesium intakes coupled with high calcium intakes and high calcium-to-magnesium (Ca:Mg) intake ratios can increase the risk for cardiovascular disease (CVD) and metabolic syndrome (1, 2), colorectal cancer (3), prostate cancer (4), survival following breast cancer (5) and cancer mortality (6) as well as altered vitamin D status (7, 8). Dietary magnesium intakes have declined worldwide with the processing of foods, especially grains (9), while the ratio of Ca:Mg in the diet appears to be steadily increasing worldwide (10) as traditional diets give way to the modern processed-food diet. This trend has been quantified in the United States (Figure 1).
FIGURE 1.
NHANES data depicting rising Ca:Mg ratio over time (1977–2016) in US adults aged ≥20 y from food alone and food plus supplements for both women (A) and men (B). Ca:Mg, calcium-to-magnesium.
The 2-to-1 calcium-to-magnesium dietary intake ratio was first suggested in 1989 by the French magnesium researcher Jean Durlach as a high level not to be exceeded. He warned against excessive calcium relative to magnesium intakes—that is, one's calcium intake from all sources including food, water, and supplements should not exceed one's similarly total magnesium intake by >2 parts calcium to 1 part magnesium on a weight basis (11). Research since 1989 suggests an optimal range surrounding Durlach's recommendation of 2.00 and that either a high dietary Ca:Mg intake ratio (>2.60) or a low ratio (<1.70) can modify the effects of calcium and magnesium on disease risk (12).
Evidence for this range of dietary Ca:Mg intake ratio for optimal health is small but growing
A number of studies, 1 from China (12) and 9 from the United States (3–5, 13–18), have a priori evaluated the dietary Ca:Mg ratio in relation to breast, prostate, gastric, and colorectal cancer; CVD; and all-cause mortality. Described here and in Table 1 are the case-control and cohort studies. The earliest study, a case-control study (14), demonstrated that a high intake of magnesium was significantly related to a reduced risk of colorectal adenoma, but only among those who consumed dietary Ca:Mg ratios ≤2.78, not those with ratios >2.78. In the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial (13), participants consuming a higher calcium intake and Ca:Mg intake ratios between 1.70 and 2.50 demonstrated reduced risk of new cancers compared with controls without active disease on entry into the study (13). Data from the North Carolina–Louisiana Prostate Cancer Project (4) revealed that both African-American and European-American men diagnosed with prostate cancer with a total Ca:Mg intake ratio >2.50 had increased odds of having high-aggressive prostate cancer. However, in women diagnosed with breast cancer, participants, particularly postmenopausal women with the highest Ca:Mg ratio (>2.59), had a significantly lower risk of all-cause mortality than those with a Ca:Mg ratio <2.59 (5). In the NIH-AARP Diet and Health Study (19), increasing magnesium was associated with a reduced risk of noncardiac gastric carcinoma independent of the Ca:Mg ratio; however, in those with a Ca:Mg intake ratio <1.70 there was an increased risk of esophageal adenocarcinoma (16). Utilizing data from the NHANES 1999–2006, higher physical activity coupled with a dietary Ca:Mg ratio between 1.70 and 2.60 showed a reduced risk of death due to cancer (15). In 2 large cohort studies conducted in Chinese populations, one in men and one in women, with low Ca:Mg intake ratios (median ratio = 1.70), the dietary Ca:Mg intake ratio significantly modified mortality risk (12).
TABLE 1.
Ca:Mg Ratio Hypothesis Tested in Diet Studies1
| Study | Study design | Background diet or intervention | Average Ca, mg/d | Average Mg, mg/d | Median Ca:Mg ratio | Health outcome | Risk ratio |
|---|---|---|---|---|---|---|---|
| North Carolina–Louisiana Prostate Cancer Project (PCaP) (4) | Case-only study | Usual diet2 (with focus on dairy products) | AA, 980; EA, 11652 | AA, 421; EA, 4292 | AA, 2333; EA, 2803 | Ratio >2.50 increased odds of high-aggressive prostate cancer | OR, 1.65 [1.19–2.28] |
| Tennessee Colorectal Polyp Study (14) | Case-control | Usual diet2 | 981 | 321 | 2.78 | Ratio ≤2.78; increasing Mg associated with decreased risk of colorectal adenoma | OR, 0.38 [0.20–0.71; P < 0.01] |
| Western New York Exposures and Breast Cancer Study (WEB) (5) | Case-control | Usual diet2 | 1138 | 294 | 3.873 | Ratio >2.59; Mg was associated with lower risk of all cause-mortality | HR, 0.36 [0.17–0.77; P-trend = 0.01] |
| Vanderbilt, VA, Duke (18) | Case-control | Usual diet | Black, 544; White, 681 (median) | Black, 261; White, 322 (median) | Black, 2.00; White, 2.20 | Higher Ca:Mg ratio was related to a reduced odds of prostate cancer in Blacks, but not in Whites, for both low-grade (P-interaction, 0.04) and high-grade prostate cancer | OR, 0.66 [0.45–0.96; P = 0.03] |
| NHANES (15) | 1999–2006 Cohort | Usual diet2 and level of physical activity | 987–1221 | 302–375 | 3.25 | Higher physical activity associated with reduced risk of mortality due to cancer when Ca:Mg ratios were between 1.70 and 2.60 (interaction not significant) | HR, 0.48 [0.26–0.87; P < 0.001] |
| Shanghai Women's Study (12) | Prospective cohort | Usual diet | 493 | 279 | 1.704 | Ratio ≤1.70; Mg associated with increased risk of total mortality in women | Mg HR, 1.24 [1.02–1.51; P-trend = 0.02] |
| Shanghai Men's Health Study (12) | Prospective cohort | Usual diet | 513 | 298 | 1.704 | Ratio >1.70; Mg and Ca associated with reduced total mortality in men | Mg HR, 0.66 [0.50–0.88, P-trend = 0.01]; Ca HR, 0.59 [0.44–80, P-trend = 0.00] |
| Prostate, Lung, Colorectal, and Ovarian (PLCO) (13) | Prospective cohort | Screening tests vs. usual care (usual diet)2 | 1057–1161 | 395–431 | 2.50 | Ratio 1.70–2.50; Ca was associated with reduced risks of incident advanced adenoma and distal colorectal cancer | OR, 0.70 [0.49–1.01] |
| NIH-AARP Diet and Health Study (19) | Prospective cohort | Usual diet2 | 881 (median) | 358 (median) | 2.463 | Increased Mg was associated with a reduced risk of noncardiac gastric carcinoma; the associations did not significantly differ by ratio | |
| NIH-AARP Diet and Health Study (16) | Prospective cohort | Usual diet2 | 881 (median) | 358 (median) | 2.463 | Higher Mg intake was associated with an increased risk of esophageal adenocarcinoma when Ca:Mg ratios <1.70 | HR, 1.96 [1.01–3.77] |
| Calcium Polyp Prevention Study (3) | RCT | Calcium 1200 mg/d × 4 y | Ratio ≤2.60, 656; ratio >2.60, 1110 | Ratio ≤2.60, 321; ratio >2.60, 319 | 2.60 | Ratio ≤2.60; Ca was associated with a reduced risk of colorectal adenoma recurrence | RR, 0.68 [0.52–0.92; P- trend = 0.075 for interaction] |
| Personalized Prevention of Colorectal Cancer Trial (PPCCT) (17) | RCT | Personalized Mg supplementation | 1299 | 350 | 3.80 | Decreasing ratio to ∼2.30 improved cognitive function by 9.1% (P = 0.03) and modified APOE cytosine among those aged >65 y |
For studies that used control groups outcomes are compared with controls or reference tertile or quartile of intakes. Risk ratios are reported as HRs, ORs, or RRs and expressed with 95% CIs. AA, African Americans; Ca:Mg, calcium-to-magnesium; EA, European Americans; RCT, randomized controlled trial; RR, relative risk.
Total intakes include intake from supplements where noted.
Calculated average or median value.
Median ratio for the Shanghai study, men and women combined.
Few studies exist examining dietary Ca:Mg ratios <1.70 as well as >2.60, limiting the evidence base for establishing an optimum Ca:Mg reference range. Nonetheless, current evidence suggests that reduction in disease risk can occur with a dietary Ca:Mg ratio between 1.70 to 2.60, and that these benefits may be dependent on gender and the specific health outcome.
In US adults, the Ca:Mg intake ratio from foods alone has been >3.00 since 2000 (20). There are many dietary supplements available to this population that contain both calcium and magnesium, but a survey of their Ca:Mg ratio is lacking.
The objectives of this paper are to quantify the nutrient values of calcium and magnesium and the Ca:Mg ratio in dietary supplement products and to relate these findings to current literature on the health effects of varying Ca:Mg ratios.
Methods
The NIH's Dietary Supplement Label Database (DSLD) was used to identify dietary supplements containing both calcium and magnesium. The DSLD includes label-derived information from dietary supplement products marketed in the United States. As of February 2020, there were 61,054 labels categorized as On Market, of which 7370 were identified as containing calcium and magnesium. The Advanced Search feature was used to customize the search by ingredients (i.e., calcium and magnesium ≥10.0 mg), supplement form (i.e., tablet, liquids, powders), intended target group (>4 y), and by product type [i.e., containing only minerals or containing only vitamins and minerals (MVM)]. In the DSLD, an MVM is defined as containing only vitamins and minerals (MVM) and no other ingredients. Products in the database containing a combination of vitamins, minerals, and botanicals are coded separately. Only unique products and labels that included the amounts of calcium and magnesium were included in the final analyses (i.e., labels in multiple package sizes and flavors were deleted). The Excel 2010 statistical package (Microsoft Corporation) was used to calculate Ca:Mg ratios and mean, median, variance, and range of Ca:Mg values for each supplement form. Frequency distributions were also calculated and plotted for Ca:Mg ratio by supplement form. The Ca:Mg ratio by supplement form for the percentage of supplements below, within, and above the ratio range of 1.70–2.60 was determined. One-factor ANOVA was used to compare differences in Ca:Mg between products with significance set at P < 0.05.
Results
Approximately 12% of the labels in the DSLD were from products containing both magnesium and calcium. As a comparison, 463 labels in the DSLD were calcium-only containing products with amounts ranging from 19 to 3000 mg/serving, and 26 labels were from magnesium-only containing products with amounts ranging from 25 to 500 mg/serving. Of the 7 separate data searches performed in DSLD, one for each supplement form, conducted between 20 January 2020 and 10 February 2020, 4308 unique products were entered into data analysis for review after meeting the inclusion criteria (Table 2). One-factor ANOVA of ratio means demonstrated significant Ca:Mg differences between supplement categories (F value: 21.77; F critical factor: 2.1) with a P value <0.05.
TABLE 2.
Summary table of Ca:Mg ratio by product formulation in the DSLD1
| Formulation | Count, n | Median Ca:Mg | Mean ± SD Ca:Mg | Range of Ca:Mg2 |
|---|---|---|---|---|
| Soft gel cap | 83 | 2.00 | 2.49 ± 1.77 | 0.16–10.00 |
| Liquid | 172 | 2.00 | 2.39 ± 2.74 | 0.10–24.39 |
| Other | 390 | 2.00 | 3.14 ± 3.49 | 0.05–30.00 |
| MVM | 393 | 2.08 | 3.52 ± 4.00 | 0.24–30.00 |
| Capsule | 871 | 2.00 | 2.12 ± 2.19 | 0.05–27.78 |
| Powder | 889 | 2.31 | 3.70 ± 4.55 | 0.03–48.46 |
| Tablet | 1510 | 2.00 | 2.71 ± 2.85 | 0.04–30.00 |
Search parameters: must have calcium, must have magnesium, ≥10 mg, age ≥4, on market. Reported per serving size, 1 representative packaging size, duplicate flavors removed. Ca:Mg, calcium-to-magnesium; DSLD, Dietary Supplement Label Database; MVM, multivitamin-mineral.
P ≤ 0.05 by 1-factor ANOVA of means between product formulations.
We found a mean ratio of 2.90 across all calcium- and magnesium-containing products, with differences by product form. Powder products displayed the largest ratio spread from a low of 0.30 to a high of 48.5 (Table 2). Figure 2 displays the frequency distribution of Ca:Mg ratio by product form. Soft gels were the only supplement form that did not include products with a Ca:Mg ratio >10.0. Powders as consumed showed a significant number of products with a ratio of ≥20.0 and displayed the highest frequency (43%) of products exceeding the targeted ratio range. Overall, 31% of products fell below, 40.5% fell within, and 28.3% fell above the ratio range of 1.70–2.60 (Figure 3).
FIGURE 2.
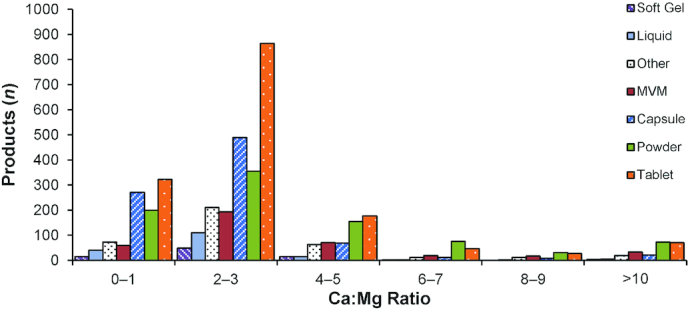
Calculated Ca:Mg ratio frequency distributions of products in the DSLD by product form: soft gel, liquid, other forms, MVM, capsule, powder, and tablet. Ca:Mg, calcium-to-magnesium; DSLD, Dietary Supplement Label Database; MVM, multivitamin-multimineral.
FIGURE 3.

Percentage of products in the DSLD below, within, and above the calculated Ca:Mg ratio range of 1.70–2.60 by formulation category: soft gel, liquid, other forms, MVM, capsule, powder, and tablet. Ca:Mg, calcium-to-magnesium; DSLD, Dietary Supplement Label Database; MVM, multivitamin-multimineral.
As some antacids carry a dietary supplement label, we searched the DSLD for products with antacid in the product name. Fourteen products were identified, only five contained magnesium along with calcium. The Ca:Mg ratios for these 5 products were all >2.6, and some substantially so, as calcium is the main ingredient in antacids.
Current status of knowledge
Understanding the physiology of calcium and magnesium helps to understand the potential impact of the Ca:Mg ratio. Within cells, the magnesium ion (Mg2+) is a physiological antagonist of the calcium ion (Ca2+) (21). Thus, the cellular Ca:Mg ratio is of major importance for Ca2+-dependent signaling events including the uncoupling mitochondrial electron transfer from ATP synthesis, activation, and overstimulation of enzymes including proteases, protein kinases, and NO synthase and Ca2+ transporting proteins. It is possible that cytosolic calcium activation results from a magnesium deficit. Even small changes in Mg2+ concentrations within the cell may cause disturbed Ca2+ signaling or Ca2+ toxicity (1, 21). Small increases in dietary calcium above normal requirements have been shown to exacerbate magnesium deficiency in rats fed a low-magnesium diet (22).
One theory to explain how excess calcium might antagonize magnesium is that magnesium and calcium share a homeostatic regulating system that involves the calcium-sensing receptor. Also, magnesium and calcium may compete during absorption in the gastrointestinal tract (12).
In relation to diseases, such as cancer, magnesium plays a key role in cell growth and mediates cancer pathology through maintaining genomic stability; regulation of cell differentiation, proliferation, and apoptosis; and prevention of angiogenesis. Cellular DNA studies have indicated that >30 genes are affected by up-and-down changes in Mg2+ content within the cell (23).
Several population studies report mean magnesium and calcium intakes, usually with a value for SD and often a range. Very few, if any, calculate Ca:Mg intake ratios for each subject. Thus, we have some medians but very few means or variance measures of this important intake ratio.
Impact on the Ca:Mg ratio by supplementation
In the United States, dietary supplement use is widespread, with over half of adults reporting use and older adults reporting the highest use (6, 24, 25). Pooled data from 6 cycles of NHANES (1999–2000 to 2009–2010) in 30,899 adults aged ≥20 y provided information on dietary supplement use (6). Of the 24,763 dietary supplement users, 33.3% used magnesium supplements, consuming a mean dose of 146 mg/d. Blumberg and colleagues (26) provided an NHANES analysis for survey years 2009–2012 in adults showing a 10–19% decrease in the prevalence of magnesium inadequacy (below the Estimated Average Requirement) with supplement use, except in adults >71 y of age, who retained a high prevalence of inadequacy of 55.2%.
Examining results from the NHANES surveys of the Ca:Mg ratio of dietary supplement users, it is most likely that supplements would tend to raise the Ca:Mg intake ratio in the population of supplement consumers as shown in Figure 1. This was also seen in our search of the DSLD: 12% of supplements in the marketplace contain both calcium and magnesium, with levels of calcium up to 3000 mg/serving. In Figure 1, the ratio calculated from supplement use versus from foods from What We Eat in America clearly shows a generalized Ca:Mg ratio higher for those using supplements.
In the North Carolina–Louisiana Prostate Cancer Project, the Ca:Mg ratio in supplement users was higher than in non–supplement users (average Ca:Mg of 2.55 compared with 2.36, respectively) (Table 1) (4), still within the suggested optimal range but indicating that calcium supplementation (and/or a diet of whole milk) was contributing to altering the ratios in a less favorable direction, as seen in the NHANES data presented earlier.
Last, in a randomized controlled trial (RCT), Dai and colleagues (3) found that the baseline dietary Ca:Mg ratio modified the effect of calcium supplementation on adenoma recurrence. Among subjects with a baseline ratio above the median (>2.6), calcium supplementation (1200 mg/d) had no effect on the risk of ≥1 recurrent adenomas. In contrast, among those with a baseline ratio less than or equal to the median (i.e., 2.6), 1200 mg/d calcium treatment was associated with reduced risk. Very recently, in the same RCT, reducing the Ca:Mg ratio from 3.8 to ∼2.3 using magnesium supplements significantly improved cognitive function among those aged >65 y, and led to significant demethylation in the APOE gene, a gene found to be an important genetic factor in Alzheimer disease.
Table 3 summarizes the studies in this review by Ca:Mg reference interval. As shown, the majority of studies show positive or improved outcomes when the Ca:Mg intake ratio falls within the range of 1.70–2.60. Both RCTs reviewed fell within this interval ratio. It is interesting to note that, within the NIH-AARP prospective cohort study, a Ca:Mg ratio <1.7 increased the risk of esophageal adenocarcinoma (16), whereas in noncardiac gastric carcinoma Ca:Mg intake ratio intervals showed no effect on risk (19), suggesting that cancer pathologies may act differently under different dietary conditions. Gender differences in total mortality were evident in the Shanghai Men's and Women's Health Study (12), with reduced risk of total mortality in men with a ratio >1.70, but in women an increased risk was seen with a ratio ≤1.70.
TABLE 3.
Summary of Ca:Mg ratio in diet studies1
| Higher magnesium/lower calcium intake | Optimum Ratio Range | Higher calcium/lower magnesium intake |
|---|---|---|
| Ratio <1.70 | Ratio, 1.70–2.60 | Ratio >2.60 |
| ↑ Total mortality in women (12) | ↓ Risk of colorectal adenoma recurrence (3), RCT | ↑ High-aggressive prostate cancer (4) |
| 1↑ Esophageal adenocarcinoma (16) | ↓ Total mortality in men (12) | ↓ All-cause mortality, breast cancer patients (5) |
| ↓ Incident adenoma and distal colorectal cancer (13) | ||
| ↓ Odds of prostate cancer (18) | ||
| ↓ Cancer mortality with exercise (15) | ||
| Improved cognitive function (17), RCT | ||
| ↓ Noncardiac gastric carcinoma2 (19) | ↓ Noncardiac gastric carcinoma2 (19) | ↓ Noncardiac gastric carcinoma2 (19) |
| 1↓ Colorectal adenoma (14) using a cutoff Ca:Mg ratio of 3.00 as a higher median ratio was observed |
Ca:Mg, calcium-to-magnesium; RCT, randomized controlled trial; ↓, decrease; ↑, increase.
Association did not differ by ratio category.
Not only has improving the Ca:Mg ratio reduced the risk of several chronic diseases it has also improved the status of serum vitamin D. Both in vitro and in vivo studies indicate that magnesium deficiency affects enzymes that synthesize and metabolize 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)2D] (27). In a precision-based randomized trial (7) in participants at risk for the development of adenomas or hyperplastic polyps, the investigators found that reducing Ca:Mg ratios by supplementing participants with magnesium glycinate to a ratio of ∼2.30 from 3.8 optimized vitamin D concentrations (i.e., increased vitamin D concentrations when baseline vitamin D concentrations were low, but decreased vitamin D concentrations when baseline vitamin D concentrations were high). In another study (8), 2 mo of magnesium supplementation (500 mg/d) in healthy postmenopausal women, many of whom were deemed to be vitamin D and magnesium deficient on entry into study, showed that serum vitamin D [25(OH)D] concentrations increased from a baseline of 23.6 ± 5.70 ng/mL to 27.8 ± 7.56 ng/mL. Although there was an increase in vitamin D concentrations, it was not statistically significant in this small sample. In the intervention group, the Ca:Mg intake ratio on entry into the study was 3.98 and on completion of study was 1.14. The authors concluded that a high intake of either dietary or supplemented magnesium could lessen the risk of vitamin D deficiency. This study shows how moderating the Ca:Mg intake ratio with supplementation can affect vitamin D status.
An exact estimate of cumulative exposure due to supplement use and the composition of the supplement coupled with current food patterns remains poorly defined. Magnesium supplements in the marketplace today contain elemental magnesium as an inorganic or organic salt. Inorganic salts (e.g., magnesium oxide) contain high levels of elemental magnesium but may exhibit limited bioavailability as a result of their poor solubility. On the other hand, organic sources of magnesium are highly soluble but provide a lesser amount of elemental magnesium (e.g., magnesium citrate) (28). Of interest is the increase in magnesium supplement sales by 10.2% in 2018 and is on track to pass calcium as the top-selling ingredient in mineral supplements, a trend that could possibly moderate favorably the Ca:Mg ratio in the population (29).
This review of dietary supplements in the marketplace showed that many products had a Ca:Mg ratio above an optimum range. It is not yet known how continual supplement use or how the selection of inorganic versus organic forms of elemental magnesium may impact an individual's overall diet. A limitation of this review is that the information on the amounts of magnesium and calcium in the products was derived from values printed on the label and were not analytically verified. In addition, the dietary supplements containing both calcium and magnesium in the DSLD are a representative sample of the supplements currently available to the American public but not exhaustive. As the dietary supplement marketplace is dynamic and ever changing, some off-market products may have been captured in the data searches while new products not yet registered in the DSLD may have been missed. In addition, data on current total dietary intakes, including supplements, are needed to estimate status in the populations and association with risk of disease.
Conclusions
This review is the first to calculate and examine the Ca:Mg ratio in dietary supplement products by product form in the US marketplace. All forms of dietary supplements contained products with a Ca:Mg ratio within the optimum range of 1.70–2.60, but in varying proportions, and over one-fourth of all products showed Ca:Mg ratios above this optimum range. Supplements as powders, typically marketed to the physically active population group and people who have difficulty swallowing large pills, showed a mean ratio of 3.70, and MVMs showed a mean ratio of 3.52. All supplement formulations warrant close scrutiny by consumers when considering long-term consumption in conjunction with their typical dietary pattern. Since Ca:Mg ratios from US diets are high, without including supplement intake, a supplement with a lower dietary Ca:Mg ratio could help to reduce the imbalance of these nutrients but would need to be based on an individual's overall usual diet. In the case of individuals with high calcium intakes for prevention of osteoporosis, magnesium supplementation may also be warranted to establish a more favorable Ca:Mg ratio in their overall diet. Additional research may provide greater insight into whether the Ca:Mg ratio is a biomarker of interest for moderating chronic disease and which population groups may derive benefit from moderating that ratio. At this time no conclusive recommendations can be put forth, but a suggested optimum ratio range is between 1.70 and 2.60.
ACKNOWLEDGEMENTS
We thank Joyce Merkel for her editorial assistance with the manuscript. The authors’ responsibilities were as follows—RBC: compiled and analyzed the data, drafted the manuscript, and had responsibility for the final content; AR, LGS, QD, and NAP: revised and edited the manuscript and contributed to the content; and all authors: read and approved the final manuscript.
Notes
Supported by the office of dietary supplements, nih.
Author disclosures: RBC is a senior research fellow with the CMER Center for Magnesium Education and Research. LGS holds stock in food and drug companies. AR serves as the director of CMER Center for Magnesium Education and Research and holds a patent on a magnesium cream product, QD Vanderbilt University Medical Center submitted a patent application (pending) for methylation biomarkers for measuring magnesium status. The other authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: Ca:Mg, calcium-to-magnesium (ratio); CVD, cardiovascular disease; DSLD, Dietary Supplement Label Database; MVM, multivitamin-mineral; RCT, randomized controlled trial; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Rebecca B Costello, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD, USA.
Andrea Rosanoff, CMER Center for Magnesium Education and Research, Pahoa, HI, USA.
Qi Dai, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Vanderbilt Ingram Cancer Center, Nashville, TN, USA.
Leila G Saldanha, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD, USA.
Nancy A Potischman, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated?. Nutr Rev. 2012;70(3):153–64. [DOI] [PubMed] [Google Scholar]
- 2.Moore-Schiltz L, Albert JM, Singer ME, Swain J, Nock NL. Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001–2010 data. Br J Nutr. 2015;114(6):924–35. [DOI] [PubMed] [Google Scholar]
- 3.Dai Q, Sandler R, Barry E, Summers R, Grau M, Baron J. Calcium, magnesium, and colorectal cancer. Epidemiology. 2012;23(3):504–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steck SE, Omofuma OO, Su LJ, Maise AA, Woloszynska-Read A, Johnson CS, Zhang H, Bensen JT, Fontham ETH, Mohler JLet al. Calcium, magnesium, and whole-milk intakes and high-aggressive prostate cancer in the North Carolina-Louisiana Prostate Cancer Project (PCaP). Am J Clin Nutr. 2018;107(5):799–807. [DOI] [PubMed] [Google Scholar]
- 5.Tao MH, Dai Q, Millen AE, Nie J, Edge SB, Trevisan M, Shields PG, Freudenheim JL. Associations of intakes of magnesium and calcium and survival among women with breast cancer: results from Western New York Exposures and Breast Cancer (WEB) Study. Am J Cancer Res. 2016;6(1):105–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen F, Du M, Blumberg JB, Ho Chui KK, Ruan M, Rogers G, Shan Z, Zeng L, Zhang FF. Association among dietary supplement use, nutrient intake, and mortality among U.S. adults: a cohort study. Ann Intern Med. 2019;170:(9):604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Q, Zhu X, Manson JE, Song Y, Li X, Franke AA, Costello RB, Rosanoff A, Nian H, Fan Let al. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr. 2018;108(6):1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vázquez-Lorente H, Herrera-Quintana L, Molina-López J, Gamarra-Morales Y, López-González B, Miralles-Adell C, Planells E. Response of vitamin D after magnesium intervention in a postmenopausal population from the province of Granada, Spain. Nutrients. 2020;12(8):2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosanoff A, Kumssa DB. Impact of rising body weight and cereal grain food processing on human magnesium nutrition. Plant Soil. 2020;457:5–23. [Google Scholar]
- 10.Rosanoff A, Capron E, Barak P, Mathews B, Nielsen F. Edible plant tissue and soil calcium:magnesium ratios: data too sparse to assess implications for human health. Crop Pasture Sci. 2015;66:1265–77. [Google Scholar]
- 11.Durlach J. Recommended dietary amounts of magnesium: Mg RDA. Magnes Res. 1989;2(3):195–203. [PubMed] [Google Scholar]
- 12.Dai Q, Shu XO, Deng X, Xiang YB, Li H, Yang G, Shrubsole MJ, Ji B, Cai H, Chow WHet al. Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study. BMJ Open. 2013;3(2):e002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Giri A, Zhu X, Shrubsole MJ, Jiang Y, Guo X, Ness R, Seidner DL, Giovannucci E, Edwards TLet al. Calcium:magnesium intake ratio and colorectal carcinogenesis, results from the Prostate, Lung, Colorectal, and Ovarian cancer screening trial. Br J Cancer. 2019;121(9):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, Smalley WE, Li M, Shyr Y, Zheng W. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr. 2007;86(3):743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibler EA, Zhu X, Shrubsole MJ, Hou L, Dai Q. Physical activity, dietary calcium to magnesium intake and mortality in the National Health and Examination Survey 1999–2006 cohort. Int J Cancer. 2020;146(11):2979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah SC, Dai Q, Zhu X, Peek RM Jr, Roumie C, Shrubsole MJ. Associations between calcium and magnesium intake and the risk of incident oesophageal cancer: an analysis of the NIH-AARP Diet and Health Study prospective cohort. Br J Cancer. 2020;122(12):1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Borenstein AR, Zheng Y, Zhang W, Seidner DL, Ness R, Murff HJ, Li B, Shrubsole MJ, Yu Cet al. Ca:Mg ratio, APOE cytosine modifications, and cognitive function: results from a randomized trial. J Alzheimers Dis. 2020;75(1):85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowke JH, Koyama T, Dai Q, Zheng SL, Xu J, Howard LE, Freedland SJ. Blood and dietary magnesium levels are not linked with lower prostate cancer risk in black or white men. Cancer Lett. 2019;449:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah SC, Dai Q, Zhu X, Peek RM Jr, Smalley W, Roumie C, Shrubsole MJ. Associations between calcium and magnesium intake and the risk of incident gastric cancer: a prospective cohort analysis of the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study. Int J Cancer. 2020;146(11):2999–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosanoff A. Rising Ca:Mg intake ratio from food in USA adults: a concern?. Magnes Res. 2010;23(4):S181–93. [DOI] [PubMed] [Google Scholar]
- 21.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46. [DOI] [PubMed] [Google Scholar]
- 22.Bertinato J, Lavergne C, Plouffe LJ, El Niaj HA. Small increases in dietary calcium above normal requirements exacerbate magnesium deficiency in rats fed a low magnesium diet. Magnes Res. 2014;27(1):35–47. [DOI] [PubMed] [Google Scholar]
- 23.Romani A. Magnesium in health and disease. In: Sigel A, Sigel H, Sigel RKOeditors. Interrelations between essential metal ions and human diseases. Dordrecht (Netherlands): Springer Netherlands; 2013. pp. 49–79. [Google Scholar]
- 24.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316(14):1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumberg JB, Frei B, Fulgoni VL, Weaver CM, Zeisel SH. Contribution of dietary supplements to nutritional adequacy in various adult age groups. Nutrients. 2017;9(12):1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, Ness RM, Seidner DL, Dai Q. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013;11:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blancquaert L, Vervaet C, Derave W. Predicting and testing bioavailability of magnesium supplements. Nutrients. 2019;11(7):1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nutrition Business Journal . NBJ's supplement business report: an analysis of markets, trends, competition and strategy in the U.S. dietary supplement industry. New York: New Hope Network; 2019. [Google Scholar]



