Abstract
The rapid growth of inhalable cannabis concentrates raises questions about the safety of acute and chronic exposure to these aerosol mixtures. Due to the nonpolar nature of the aerosol mixture created from cannabis vapor cartridges, traditional aqueous-based capture methods used in e-cigarette or tobacco cigarette studies for analysis of metals are insufficient. Moreover, hydrophobic cannabis concentrates are not miscible with dilute aqueous acids and therefore not ideal for metal spiking unlike electronic nicotine delivery systems. This study describes a method of spiking nonaqueous matrices with aqueous metals standards to investigate aerosolization and recovery of the metals. It also compares various methods for nonpolar aerosol capture and subsequent analysis of 10 metals (As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, and Sn) in two model cannabis matrices, flower and concentrate. Spiked cannabis concentrates were vaped in commercially available cartridges, and their aerosol mixtures were investigated for recovery of heavy metals via ICP-MS. Spiked flower samples were also combusted to compare collection rates of the 10 metals. Results show that not all metals that are present in the concentrate or flower can be fully recovered in the aerosol capture processes at standard voltage settings or combustion temperatures. These studies also demonstrate the importance of a nonpolar solvent as part of the aerosol collection to increase the recovery of some metals. The high concentration of some metals seen in the concentrate suggests that the devices themselves are potential routes of exposure. The ICP-MS analysis method was further validated by evaluating different parameters including linearity, matrix effect, limit of detection, limit of quantitation, and repeatability.
Introduction
The recreational and medicinal use of cannabis is growing rapidly across the United States. At the time of publication, 17 states have legalized adult recreational use1 and 36 states and four territories have some sort of medicinal use regulations.2 Historically, a common way of ingesting cannabis has been through a combustion process where the “flower/bud” of the plant is ignited and burned in a smoking apparatus and inhaled. More recently, a method of consuming cannabis referred to as “vaping” has been popularized.3 Vaping is a noncombustion process of heating the cannabis flower or extracted concentrates in a device that contains a resistance-heated coil, so the molecules are aerosolized and inhaled directly. Vaping cannabis has been shown to be an efficient and consistent method of administration for patients who use marijuana for medical reasons.4
The heavy metals content in vapor and/or aerosol mixtures of cigarettes and e-cigarettes has been investigated to varying degrees.5−10 Previous studies of electronic cigarette vapor have shown that there could be potential hazards concerning the levels of aerosolized metals resulting from the vaporization of e-cigarette liquid.7−10Cannabis sativa plants have the ability to remediate metals from soil,11,12 and there are products that have been recalled from legal markets for high levels of heavy metals.13−17 There have been limited studies on the presence of carcinogenic compounds in vaped cannabis aerosols18,19 and pesticides in the cannabis flower migrating into the smoke.20 Moir et al. determined heavy metals levels in mainstream smoke from tobacco and cannabis under two smoking conditions.21 Recently, Pappas et al. have proposed a methodology for aerosol collection and measurement of elemental constituents of cannabis vaping liquids and aerosols by ICP-MS based on e-cigarette studies.22 However, to our knowledge, there have been no publications that investigate the prevalence of heavy metals in a cannabis aerosol generated from a vaporizer device.
The nationwide outbreak of lung injuries and deaths by consumers using e-cigarettes and/or cannabis vaping products in 2019 raised many concerns about the safety of these products. The Centers for Disease Control (CDC) and the Food and Drug Administration (FDA) concluded that the likely source of the most serious health problems was mainly illegally manufactured cannabis concentrates containing vitamin E acetate.23−25 However, not all patients displayed the same symptoms, and their reported use of both nicotine and cannabis vaporizers leaves many unanswered questions.
Cannabis concentrates used in vaporizer cartridges are highly viscous mixtures that contain 40–90% by weight cannabinoid molecules with the remainder of the solution containing a combination of other plant-derived hydrocarbons such as terpenoids and phospholipids.26 They are often mixed with diluents to adjust viscosity or cannabis-derived or other botanically derived terpene mixtures for flavoring.4,27 In legal retail markets, cannabis concentrates can be created from a variety of solvent extraction and cleanup processes,28 sometimes followed by distillation. Commercially available cartridges may contain a variety of high-cannabinoid mixtures: “full spectrum” extracts, purified extracts from butane, propane, ethanol, or supercritical CO2 extractions, or distillate. This variety in available products creates some difficulty in creating a model matrix that will behave similarly to all retail products. For this study, we created a model matrix from a variety of cannabis concentrate oils that visibly mimic the viscosity and has similar cannabinoid content of commercially available products.
Cannabis vaporizer cartridges are generally sold pre-filled with concentrate oil and require a power source for heating the internal coils. The cartridges can take a variety of physical shapes and designs, but many of the components are metal including the heating coil, mouthpiece, and battery terminals, which are usually made from materials such as stainless steel (Fe, Cr, and Ni), brass (Cu and Zn), chromel (Cr and Ni), inconel (Ni, Cr, and Fe), or nichrome (Ni and Cr) as well as soldered battery connectors (Pb, Sb, and Sn).29 Temperatures of 135–334 °C have been measured for electronic devices containing liquids,30 and the dry heating coils were measured as high as 1000 °C. This means that at voltage and temperature settings of standard devices, dissolved metals or even fine metallic particles could have the potential to be inhaled into the consumer’s lungs.31 However, most state-based regulations only specify screening for Pb, As, Cd, and Hg.32,33 As a result, many standard screens would not identify the other metals that might be present from the cartridge device itself. Further, mainly due to the federal illegality of cannabis and the relatively recent availability of concentrate oils in cartridges, the metals exposure from nonpolar cannabis aerosols is poorly understood. Additionally, due to the nonpolar nature of the aerosol mixture created from cannabis vapor cartridges, traditional aqueous-based capture methods used in e-cigarette or tobacco cigarette studies for analysis of metals are insufficient.34,35 Moreover, the recent Colorado Marijuana Enforcement Division’s proposed rules, released in October 2020, will require “emissions testing” for marijuana concentrates in vaporized delivery devices,32 yet no validated method for the collection of cannabis vapor exists. Therefore, the goal of this work is to establish a method for the collection of the highly nonpolar cannabis aerosol and to investigate the recovery of metals spiked into a cannabis matrix by analyzing the aerosol for the presence of As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, and Sn. This timely study provides a new level of knowledge in an area lacking good quality data.
Results and Discussion
Model Cannabis Matrices
Hydrophobic cannabis oil samples are not miscible with the aqueous and acidic metals standards and are therefore not ideal for metal spiking unlike other electronic nicotine delivery systems (ENDSs).5,6 In order to incorporate aqueous metal standards into the nonpolar cannabis concentrate, we first dissolved the cannabis concentrate in a water-miscible solvent (2-propanol), then added a small volume of the metal standards, and homogenized the sample well. Finally, the excess organic solvent and water were removed with rotary evaporation to create a homogeneous solution of the cannabis concentrate spiked with metals similar in cannabinoid content to commercially available cartridges. The flower material was also spiked with aqueous metal standards and allowed to air-dry to create a comparison model matrix. Results before and after spiking for the concentrate and flower matrices are found in Table S1 in the Supporting Information. The percent recoveries of the spiked metals were generally high and ranged from 34.4% for Hg to 98.5% for Sn, with a target concentration of 10 μg/g. The low recovery of Hg in the concentrate may be due to its volatility and use of vacuum during the solvent removal procedure. As seen in the flower spiking without the use of vacuum, a higher Hg recovery (82.8%) is seen, indicating that the spiking procedure itself affects recovery in the final matrix. The high levels of Ni, Cu, and Mn in the unspiked flower are due to these being plant nutrients and naturally occurring.
Collection Method Optimization
Puff Parameters
The smoke machine can be programmed for many different puff profiles, and ideally, a standardized method might employ a CORESTA or ISO method34,35 puff profile. These experiments utilized a square profile with a 3 s puff and a 42 s resting period between puffs and 25 mL/s flow rate. These settings are similar to the CORESTA method, but with a slightly longer rest period to ensure that all visible aerosol had been dissolved in the impingers and the battery had consistent voltage before drawing another puff. We utilized 50 puffs for each experiment to ensure that sufficient concentrate had been consumed to obtain a signal within the instrument detection range after digestion and dilutions.
Impinger Solvent Investigations
Early experiments with only aqueous solvents resulted in condensing aerosol liquids and oil droplets clogging the glass frits of the impingers (see Figure S1 in the Supporting Information). Therefore, various organic solvents were incorporated into the first impinger to ensure that oil droplets were dissolved. Open-ended impingers were also investigated rather than fritted (data is not shown), but the bubbles were large and the vapor was not dissolving in the solvent, so the fritted impingers that create very small bubbles are preferred. In order to optimize the collection of the nonpolar aerosol mixtures, various impinger solvent combinations were investigated, as outlined in Table 1. The collection method outlined in Figure 1A was used for these experiments.
Table 1. Various Impinger Solvent Combinations Investigateda.
| impinger 1 | impinger 2 | abbreviation |
|---|---|---|
| acidic aqueous | acidic aqueous | 2AA |
| aqueous + 10% H2O2 | aqueous + 10% H2O2 | 2HAA |
| methanol | acidic aqueous | MeOH + AA |
| acetone | acidic aqueous | Ac + AA |
| hexanes | acidic aqueous | Hex + AA |
Acidic aqueous = 8% nitric acid, 2% hydrochloric acid, and 90% water (v/v %).
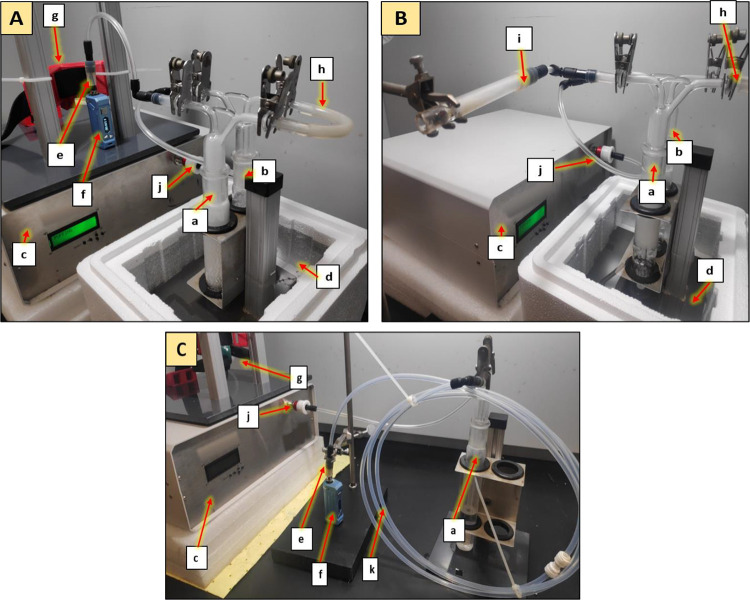
Figure 1.
Experimental setup for vaporized aerosol collection from the cannabis concentrate cartridge with impingers only (A), combusted aerosol collection from the cannabis flower (B), and tubing condensation and impinger (C). Components of the setup include (a) first impinger, (b) second impinger, (c) smoke machine, (d) ice bath, (e) cartridge with concentrate, (f) battery, (g) button pusher, (h) tubing between impingers, (i) combustion sample holder, (j) vacuum inlet, and (k) condensation tubing.
The spiked cannabis concentrate was vaped in triplicate using each one of these solvent combinations in Table 1, and the results are graphed in Figure 2. In the experiments that used organic solvents, after the aerosols were collected, the organic solvent was removed by evaporation in an open container and the residue was digested. It is useful to note here that the overall length of impinger connection tubing was minimized, and after aerosol collection, all tubing and connectors inside are rinsed with the organic impinger liquid to minimize condensate loss to surfaces.
Figure 2.
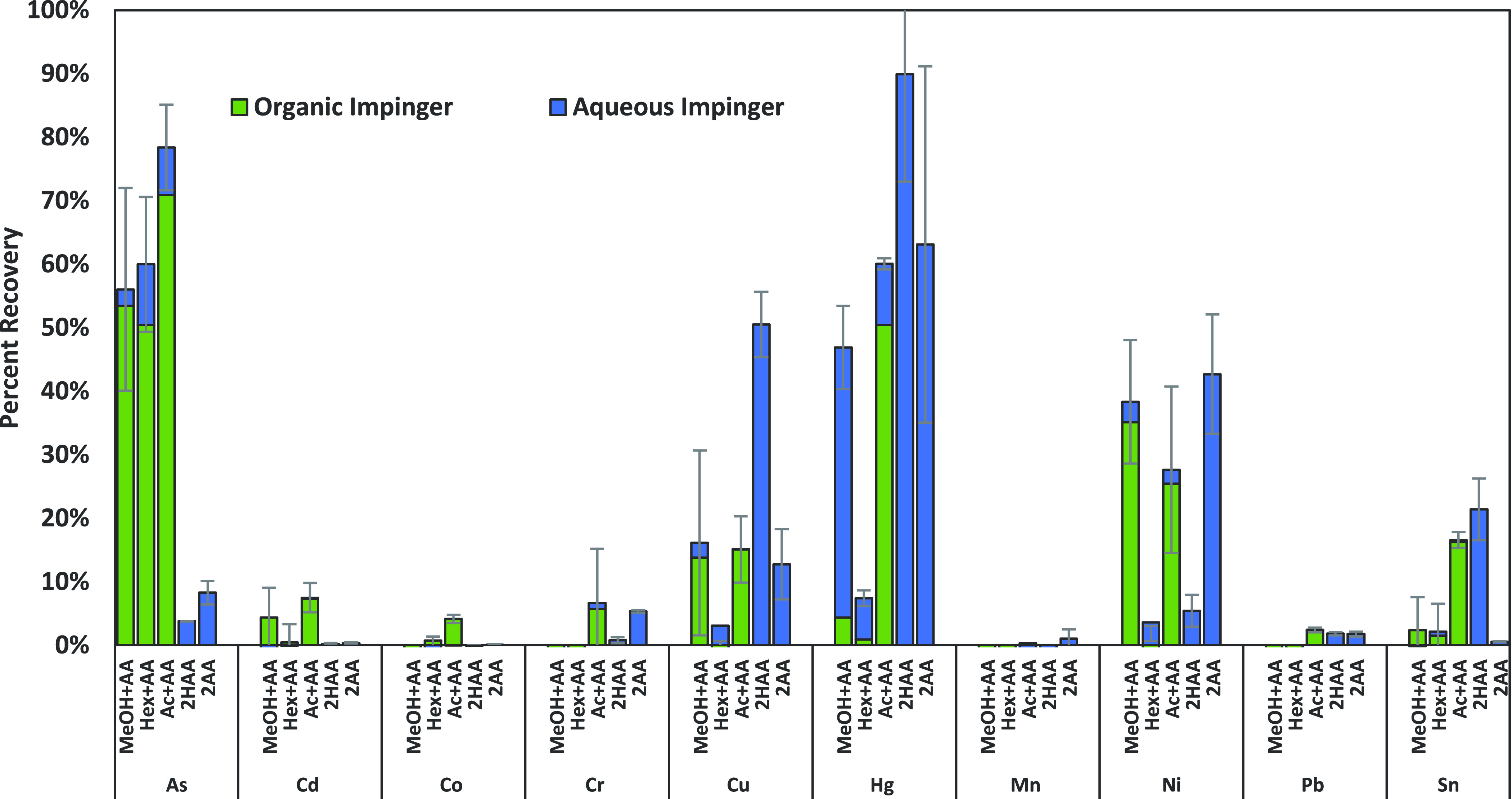
Amount of metals recovered from spiked cannabis concentrate aerosol in various combinations of impinger solvents, as depicted in Figure 1A. Values are reported as percent recovery of metals detected in spiked oil and are the average of three trials. Eight of the 10 metals had several values that were between the LOD and LOQ. All collection methods were able to recover arsenic and nickel above the LOQ. Impingers were analyzed separately and added together to determine total recovery. The green portion of the bar indicates the organic impinger signal, and the blue part of the bar indicates the aqueous impinger signal.
Figure 2 presents the concentration of metals recovered in vapor from a spiked oil sample with various solvent combinations in the two impingers. These recoveries are calculated based on the amount of metals spiked into the concentrate oil and the mass of oil consumed in the 50-puff experiment. The MeOH-AA combination captured five elements, and the percent recoveries of those were 56% for As, 16% for Cu, 47% for Hg, 38% for Ni, and 2.2% for Sn. The Hex-AA combination had similar recoveries to MeOH + AA and could also capture five elements but had very low recovery for nickel, which is an element of interest in cartridge studies. The Ac + AA combination demonstrated measurable recovery of nine elements: As 78%, Cd 7.5%, Co 4.1%, Cr 6.7%, Cu 15.1%, Hg 60.1%, Ni 28%, Pb 2.1%, and Sn 16% (Figure 2). When both impingers contained aqueous acidic solvents, four metals were able to be quantitatively captured (Figure 2) but also resulted in the aerosol condensing and clogging the frits in the impinger Figure 2 inserts (see Figure S1); therefore, it is important to incorporate an organic solvent to ensure that condensed oil droplets from the aerosol mixture are captured. Of the three organic solvents investigated, acetone could quantitatively capture more of the 10 metals and was the only one that could reliably capture Co. Methanol could capture five metals and had slightly higher recovery for two elements (Cu and Sn), but both of these were within the margins of error for similarity with other solvent systems. The hydrogen peroxide system could capture eight of the metals, with similar recoveries to the organic solvents, but the frit clogging in the aqueous media was problematic. Overall, average recoveries were quite low for many of the metals while also having large standard deviations, which has also been demonstrated in e-cigarette studies8,10 suggesting metals do not aerosolize efficiently. The highest recovery metals are the most volatile (As and Hg). The large standard deviations are indicative of the wide range of metal aerosolization that is seen in any single experiment. This points to the importance of requiring multiple experimental collections when considering regulatory requirements for aerosol testing of cannabis products. Further, the data in Figure 2 shows that without using an organic solvent, the aqueous-only impinger combinations would not capture as many of the aerosolized metals. However, the aqueous impinger was important to capture mercury, so both should be incorporated if a full suite of metals is to be reliably collected.
Tubing Condensation Capture Method
In order to investigate extractable, trace metals leaching from glassware, we compared a glass-free tubing condensation method similar to that described by Halstead et al.,10 where the vapor passes through a long length of chilled polyfluorinated tubing, then subsequently rinsed to collect the aerosol condensate. Two of the experiments also had aqueous impingers placed after the tubing to investigate the ability of the tubing alone to efficiently capture the aerosol mixture. The different tube rinsing and impinger combinations investigated are as follows: IMP refers to the impinger-only system with acetone in the first impinger followed by an aqueous impinger, no tubing. OR-AR-NI refers to the organic rinse-aqueous-rinse-no impinger setup where the tubing was rinsed with acetone first and then acidic aqueous solution but no impinger was used. This combination was glass-free. OR-IMP refers to the organic rinse-aqueous impinger setup that consisted of the tubing followed by an aqueous impinger (Figure 1C), and the tubing was rinsed with acetone for sample collection. AR-OR-IMP refers to the aqueous rinse-organic rinse-aqueous impinger, which had the tubing and an impinger (Figure 1C), and the tubing was rinsed with acidic aqueous solution first then acetone. This final combination allowed us to investigate the necessity for organic solvent use in sample collection. In each case, the individual rinse or impinger samples were analyzed separately to investigate its contribution to the total signal for the sample. The results for these four trials are graphed in Figure 3. These trials used a spiked distillate matrix, which contained 10% terpenes and was prepared similarly to the model concentrate matrix, as described in the Experimental Methods Section. The initial concentrations for the spiked distillate matrix and the background signal for the unspiked source material can be found in the Supporting information, Table S1.
Figure 3.
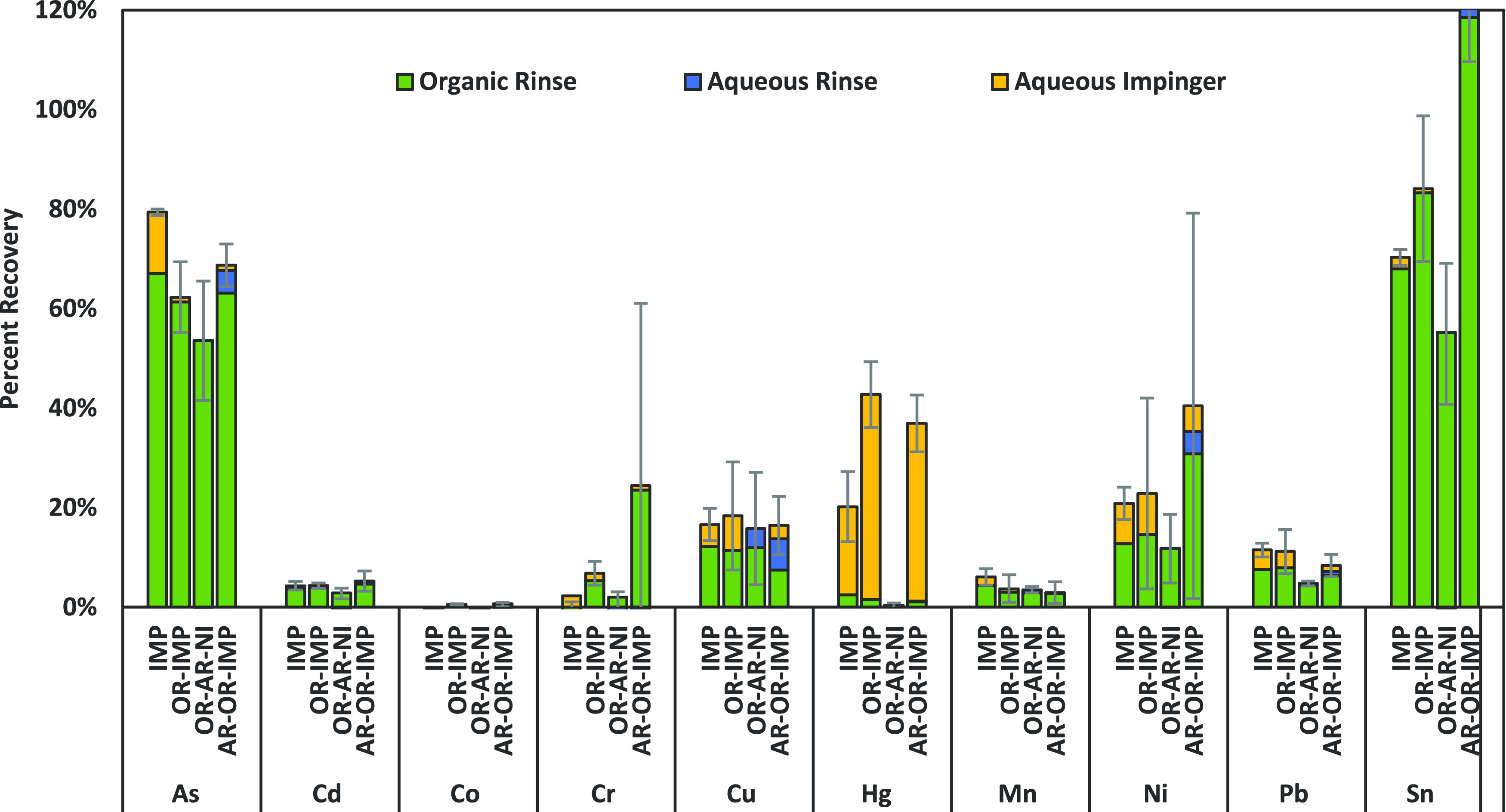
Percent recovery based on spiked amounts measured of each metal in the distillate matrix. Samples were collected from the aerosol using four different tubing condensation and impinger combinations, as depicted in Figure 1C. IMP = impingers only, OR-IMP = acetone rinse of tubing + acidic aqueous impinger, OR-AR-NI = acetone rinse of tubing + aqueous rinse of tubing + no impinger, and AR-OR-IMP = aqueous rinse + acetone rinse 2nd + acidic aqueous impinger. Colors indicate the contribution of individual solvent or impinger components to the total metal signal for each sample: green = organic (acetone) rinse, blue = aqueous rinse, and yellow = aqueous impinger. All data has a matched procedural blank subtracted.
Figure 3 shows the contribution of the various solvent rinse or impinger samples on the total signal for each experiment. For all elements except mercury, the organic impinger or organic rinse was the majority of the total signal, indicating the importance of including a nonpolar solvent in the cannabis aerosol collection. These data further show that the impinger collection methods described above and these tubing methods are directly comparable. However, for optimal mercury capture, an aqueous impinger is necessary. This is further shown in the impinger and glass-free OR-AR-NI method. Without the impinger, the capture of mercury was negligible and it also shows comparable recovery for other metals, indicating that leaching from glassware is insignificant.
Are the Metals Being Vaporized from the Spiked Oil?
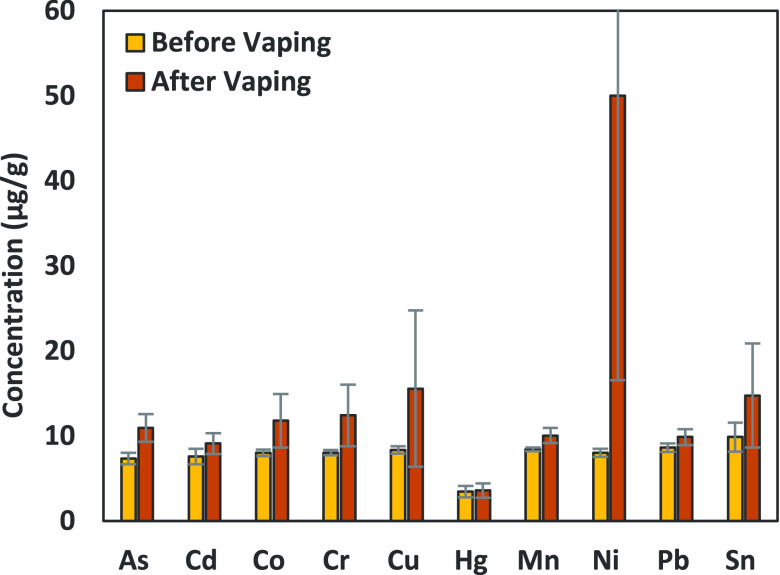
Given the relatively low recovery from spiked concentrate aerosol mixtures, we attempted to determine if they were being left behind and increasing in concentration within the remaining concentrate liquid. Full 1 g cartridges containing the spiked concentrate were vaped until they were approximately one-third full. The remaining concentrate was removed and analyzed to see if metals remained unvaporized in the concentrate. The concentrations of metals in the cannabis oil before and after vaping are shown in Figure 4.
Figure 4.
Concentrations of metals in spiked oil before vaping and after being vaped and then removed from the cartridge for analysis. Values are in μg/g of the concentrate and represent triplicate data points with error bars showing standard deviation.
Figure 4 shows slightly higher levels of all metals in the remaining oil but not enough to account for all the metals if they were not vaporized. Therefore, we hypothesize that some metals must be experiencing vaporization or aerosolization with the concentrate droplets, but some are remaining behind in the unvaporized oil. This figure also shows a high concentration of nickel above the spiking level and elevated levels of copper. The presence of nickel and copper could be due to leaching into the oil from the cartridge parts or being vaporized from the heating coils directly. The components of the cartridge were analyzed via ICP-MS, and the heating coil contains more than 46% nickel, while the metal body part is 64% copper (manuscript in preparation).
Spiked Flower Combustion vs Concentrate Vaporization
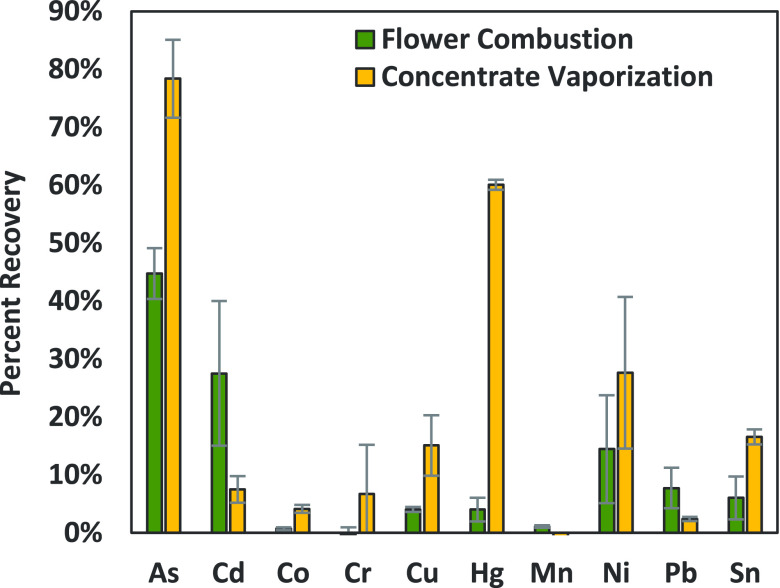
In order to investigate another matrix and heating source on the vaporization of metals and the feasibility of our collection system, cannabis flower and concentrate matrices were compared, utilizing the acetone-aqueous impinger combination (Ac + AA) (Figure 1B). The flower was combusted using one long continuous puff profile to avoid losing vapor between puffs while the coal still smoldered. The amounts of metals recovered using these two different matrices and heating methods can be found in Figure 5.
Figure 5.
Amounts of metals collected from the aerosol/vapor mixture from spiked flower combustion and spiked concentrate vaporization. Samples were collected with the acetone/aqueous impinger combination depicted in Figure 1A for the concentrate and Figure 1B for the flower. Values expressed as the % recovery of the original amount of metals spiked into the matrix. Data is the average of three trials, and error bars represent standard deviation.
Figure 5 shows higher recovery of spiked metals from concentrate vaporization for As, Cr, Cu, Hg, Ni, and Sn. Flower combustion had higher recoveries for Cd, Mn, and Pb after subtracting the naturally occurring background signal of some micronutrients in the flower. This demonstrates that even though Cd, Mn, and Pb are seen at low levels in spiked concentrate aerosol, they can be collected and analyzed using this aerosol collection method. Given the difficulty of measuring the actual temperatures of the heating coils or the combustion of flower during experiments, we cannot directly compare these two methods but suggest future work on the effect of temperature and voltage on metal vaporization rates.
Instrument Method Validation
Calibration Curves, Limit of Detection, and Limit of Quantification
Table 2 shows the results of the calibration curve parameters constructed for the studied samples, as well as the correlation coefficients, the limits of detection (LOD), and limits of quantification (LOQ) of the heavy metals analyzed. The analytical curves presented good linearity with correlation coefficients (R) higher than 0.999 for all the heavy metals studied. From Table 2, the limit of detection (LOD) values for all the metals analyzed ranged from 0.33 μg/g for Sn to 0.70 μg/g for Cr, and the limit of quantification (LOQ) values for all the metals analyzed ranged from 1.1 μg/g for Sn to 2.3 μg/g for Cr. The LOD and LOQ values obtained were low enough to detect the presence of metals of interest at trace levels in all samples.
Table 2. Correlation Coefficient (R) of the Calibration Curves, Recovery, RSD of Initial Calibration Verification (ICV), Laboratory Control Samples (LCS), Matrix Spike (MS), Continuing Calibration Verification (CCV), Limit of Detection (LOD), and Limit of Quantification (LOQ) Obtained for Each Element.
| element | R | ICV recovery (%) | % RSD (ICV) | LCS recovery (%) | % RSD (LCS) | CCV recovery (%) | % RSD (CCV) | MS recovery (%) | % RSD (MS) | LOD (μg /g) | LOQ (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 1.000 | 86 | 6.8 | 85 | 14 | 89 | 12 | 90 | 12 | 0.046 | 0.15 |
| Cd | 0.999 | 89 | 5.5 | 88 | 13 | 91 | 11 | 88 | 11 | 0.022 | 0.075 |
| Co | 0.999 | 85 | 9.1 | 90 | 8.3 | 87 | 15 | 88 | 15 | 0.090 | 0.30 |
| Cr | 1.000 | 90 | 6.4 | 86 | 12 | 99 | 14 | 101 | 14 | 0.13 | 0.42 |
| Cu | 0.999 | 91 | 7.0 | 100 | 14 | 89 | 11 | 116 | 11 | 0.035 | 0.12 |
| Hg | 1.000 | 94 | 6.3 | 99 | 15 | 96 | 9.3 | 97 | 9.3 | 0.084 | 0.28 |
| Mn | 1.000 | 88 | 6.3 | 92 | 17 | 90 | 14 | 149 | 14 | 0.046 | 0.16 |
| Ni | 1.000 | 90 | 5.2 | 92 | 14 | 92 | 12 | 100 | 12 | 0.024 | 0.080 |
| Pb | 1.000 | 92 | 6.4 | 94 | 13 | 93 | 12 | 96 | 12 | 0.089 | 0.30 |
| Sn | 1.000 | 94 | 5.5 | 87 | 14 | 90 | 12 | 98 | 12 | 0.066 | 0.22 |
Laboratory Control Sample
Laboratory control sample (LCS) recoveries and relative standard deviations (RSD) were also calculated from nine separate analyses during routine batch runs using eqs 1 and 2, respectively. The average values were taken, and corresponding results are summarized in Table 2. The percent recovery values of LCS results are in the range of 85% for As to 100% for Cu, and the RSD values ranged from 8.3% for Co to 17% for Mn. The LCS recovery obtained in this study falls within the normal acceptable range of 80–120%. As presented in Table 2, other quality control parameters like initial calibration verification (ICV) and continuing calibration verification (CCV) recoveries also fall within the normal acceptable range of 80–120%. The high percentage recovery and considerable RSD values obtained from all QC parameters validate the accuracy, reliability, and precision of our sample preparation and instrument readings of the metals investigated in this study.
Accuracy and Precision
The results of accuracy and precision of the digestion and analysis were evaluated through recovery tests. The accuracy of the method was determined by matrix spike (MS) recovery studies of the digested samples, and precision was expressed as relative standard deviation (RSD) of replicate results. The recovery values of the nine analyses of MS samples were calculated using eq 3, and RSD values were calculated using eq 2, and the results are also shown in Table 2. As can be seen in Table 2, the percent recovery of the metal analysis in the spike sample ranged between 88% of Co and 116% of Cu. The RSD values ranged between 9.3% of Hg and 15% of Co. The matrix spike recovery obtained in this study falls within the normal acceptable range of 75–125% for a good recovery study. However, the Mn showed very high recovery about 149%. This may be due to the sample matrix effect.
Conclusions
This study demonstrates improved methods to collect nonaqueous cannabis concentrate aerosols for metals analysis during vaporization. A fortification method to spike known concentrations is shown as well as optimization of aerosol collection using various solvents and collection methods. ICP-MS was used for metals analysis, and demonstration of the sample digestion and instrument validity for measuring these metals is shown. This demonstrates that organic solvents should be incorporated into the aerosol collection due to the nonpolar nature of these aerosol mixtures. Additionally, an aqueous impinger is necessary to capture mercury. Rinsing glassware and tubing and including the rinsate in the analysis is also important to reduce nonaqueous condensation loss. Further studies are currently in progress to further optimize this method in order to create a robust method for testing aerosol mixtures from cannabis products.
Experimental Methods
Chemicals and Reagents
All acids and chemicals were trace metal/ultrapure grade and used in sample digestion; HNO3 (65–68%), HCl (35–38%), and H2O2 (30%) were obtained from Fisher Chemicals (Ontario, Canada). The three different organic solvents methanol (MeOH) (HPLC grade), acetone (ACS grade), and hexane (HPLC grade) obtained from Fisher Scientific (Fair Lawn, NJ, USA) were used in liquid impinger solvents. Isopropyl alcohol/2 propanol (IPA) (99.8% purity, Techspray (Amarillo, TX, USA)), acetonitrile (HCN) (HPLC grade) (Alfa Aesar (Ward Hill, MA, USA)), and MeOH were used for sample spiking experiments. Analytical-grade metals stock standard solutions (1000 μg/mL) were purchased from Inorganic Ventures (Christiansburg, VA, USA). These stock standards were used to prepare calibration standard solutions and for spiking cannabis matrices. Reverse osmosis (RO) water was used for dilution and preparation of reagents and standard solutions as well as for rinsing glassware and sample bottles.
Model Matrices
Concentrate Stock/Blank
The unspiked stock cannabis concentrate sample was created in the laboratory by mixing a total of 100 g of cannabis concentrate and distillate samples sourced from different client lab samples that would have otherwise been destroyed. The mixture was heated to 120 °C for 1 h with stirring to ensure homogeneous mixing and complete decarboxylation of acidic cannabinoids to their neutral form. It was then transferred to two 50 mL tubes for storage at room temperature. This stock concentrate oil was used directly for experiments with unspiked (stock) matrices. Final cannabinoid, terpene, and residual solvent levels were determined, and values can be found in Tables S2–S4, respectively, in the Supporting Information.
Concentrate Spiking
In order to spike the hydrophobic cannabis concentrate oil with the aqueous metals standards, 10 g of cannabis concentrate stock was added to 15 mL of 2-propanol and warmed to 45 °C until the oil had been fully dissolved. Then, 100 μL of 1000 μg/mL of each metal (As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, and Sn) stock standard was directly added to the cannabis concentrate and isopropyl solution. The excess alcohol and water were evaporated under vacuum in a rotary evaporator, and the spiked oil was stored in glass jars at room temperature. Three samples were digested, and the metal concentrations were determined and can be found in Table S1.
Flower Spiking
The cannabis flower stock matrix was created by grinding and homogenizing the excess dried cannabis flower from different client lab samples that would have otherwise been destroyed. A 5 g portion of homogenized flower stock was placed in a weighing dish. Then, 50 μL of 1000 μg/mL metal stock standard of the following metals (As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, and Sn) was added for a final target concentration of 10 μg/g of each metal. Ten milliliters of DI water was added and a wooden applicator was used to stir the contents into a slurry. The dish was left in a fume hood overnight to dry. The spiked flower was then stored in a 50 mL conical plastic tube at room temperature. The three digested flower samples’ average metal concentrations can also be observed in Table S1.
Vapor Collection Design/Setup
Smoke Machine Parameters
In all experiments, vaporized/combusted aerosol was drawn using a CSM-STEP smoking machine (CH Technologies, USA) placed at the end of the collection apparatus (see Figure 1 for details). The smoking machine has an automatic button-pusher attachment to activate the battery power accurately during “inhalation” times. The following parameters were used for cartridge experiments: puff depth = 1.30 (25 mL/s as measured using a flow meter [Alicat Scientific WHISPER]), puff duration = 3 s, and time between puffs = 42 s. This puff interval was repeated for 25 cycles, then a 10 min “rest” period with no puffing, and then another 25 puffs (total of 50 puffs or 3750 mL per experiment). For flower combustion experiments, the puff depth was 12 mL/s and puff duration was a continuous pull until all sample was consumed and for 5 min after to ensure that all aerosol was dissolved in the impingers. All vapor capture experiments detailed in this work were completed in triplicate. Error bars are the standard deviation of the trial data points. The spiked and unspiked starting materials were also analyzed in triplicate and had their standard deviation calculated in the same way.
Vaporizer Cartridges and Battery
Commercially available “510 thread” 1 g glass tank cartridges (MG210, Mr. Green Supply) were filled with either the stock cannabis concentrate or spiked concentrate, depending on the purpose of the trial. The heat source was a Yocan Uni Pro Box Mod (Chino Hills, California) set to 3.5 V that was kept plugged into the wall power for consistent voltage delivery. Figure 1A shows the experimental setup for the vaporized aerosol collection from the cannabis concentrate cartridge. Air blanks of empty cartridges were obtained by setting up the same experimental collection system with an empty cartridge attached to the heat source, but the button was not activated during inhalation periods. This results in a sample that passes over all solid components of the collection system but is not heated and does not contain any cannabis matrix.
Flower Combustion
Around 200 mg of ground flower (unspiked blank stock or spiked, depending on the experiment) was loaded into a glass combustion apparatus (OG Chillum One-Hitter) and ignited with a standard butane lighter to begin combustion. Figure 1B shows the experimental setup for the combusted aerosol collection from the cannabis flower.
Impinger-only Collection Process
All experiments utilized two impingers containing 25 mL of liquid submerged in an ice bath to capture the metals from the aerosol mixture. Aqueous solutions contained either 8% nitric acid, 2% hydrochloric acid, and 90% deionized water (v/v %) or 10% volume H2O2. In some experiments, methanol, acetone, or hexane solvents were used in the first impinger. In these cases of organic solvents, after vapor collection, the solution was transferred to a digestion tube, and the solvent completely evaporated at 40 °C under gentle nitrogen stream overnight, leaving the residual sample to be digested for further metals analysis. In the case of acidic or hydrogen peroxide containing solutions, they were diluted to 2% final acid concentration (4× dilution factor), appropriate for direct injection into the ICP-MS.
Tubing Condensation Collection Process
In experiments utilizing a condensation collection method, 3 m of Savillex fluorinated ethylene propylene (FEP) tubing (1/4″ i.d., 96 mL of total volume) was connected directly to the cartridge device, and aerosol was passed through the length while it was submerged in an ice bath. The tubing was subsequently rinsed with approximately 20 mL of acetone three times, and the acetone rinsate that contains the condensate was dried down under gentle nitrogen stream in a 55 °C water bath for 2–4 h until only the oil residue remained. The sample was then prepared via microwave digestion as described below. In trials with an aqueous rinse, the tubing was rinsed with five 5 mL aliquots of impinger solution and diluted to 2% final acid concentration before direct injection into the instrument as a separate sample.
Connector Tubing and Impinger Glassware Rinse Process
After vapor was collected for impinger trials, all pieces of connector tubing and glassware were washed three times with a 10 mL aliquot of the impinger solvent being used in that trial. The rinsate and collected sample were both added to the appropriate storage vessel for further processing and analysis. This step is important to capture any condensate on tubing and glassware surfaces.
Filter Paper Usage
While it is common to incorporate the use of a glass fiber paper for aerosol collection and analysis, we encountered many issues that are discussed with data in the Supporting Information, Figures S2 and S3.
Microwave Digestion of Solid Samples and Organic Solvent Samples
Before metals analysis, cannabis concentrate, flower, cartridge pieces, filter paper, and dried organic solvent residues were digested as follows: 0.2 to 0.5 g of sample was added to a vial with 4.0 mL of concentrated nitric acid, 1.0 mL of hydrochloric acid, and 5.0 mL of water. The organic solvent impinger or tubing rinse samples that consisted of the residue remaining after solvent removal were digested with the same amount of acid and water but not weighed. All digestions are based on the EPA method 305236 and were performed on an Anton Paar Multiwave GO with the following parameters: a 12 min ramp to 80 °C, 12 min ramp to 130 °C, 12 min ramp to 185 °C, and maintained at 185 °C for 20 min. After digestion, samples were diluted with RO water to a final acid concentration of 2% HNO3 and 0.5% HCl, appropriate for ICP-MS analysis.
ICP-MS Method
EPA method 6020B37 was used to perform multielement measurements, with a Shimadzu inductively coupled plasma mass spectrometer 2030 (Shimadzu Scientific Instruments, Inc., Columbia, MD). The instrument has a micro-mist low-flow nebulizer (Glass Expansion, Melbourne, Australia), a quartz twister spray chamber, a quartz mini torch, and a sampler and skimmer cones made from nickel. The instrument was tuned for suitable sensitivity with cerium (Ce) oxide ratios <1:0% (156CeO+ = 140Ce+) and <2:0% doubly charged ions (70Ce+ = 140Ce++). The instrument was operated using collision cell gas in helium (He) mode (6:0 mL/min He). The optimized collision cell achieves superior ICP-MS sensitivity through efficient molecular ion interferences removal and high elemental ion transmission using helium gas. An internal standard of thallium and yttrium was added to all analyzed solutions (calibration standards, unknown samples, LCS, and method blanks) at 5 μg/L final concentration. Isotopes of thallium (Ti 203 and 205) and yttrium (Y 89) were monitored to compensate for instrument instability and possible matrix effects. The “matrix effects” were observed by the internal standard intensity recovery. (i.e., Cal blank, not digested sample (internal standard intensity) vs LCS/MS digested sample (internal standard intensity, 1 ppm gold (Au) solution spike was used to stabilize mercury (Hg) in all samples, QCs, and in calibration standards).
ICP-MS Method Validation
Instrument Calibration
The instrument was calibrated using a calibration blank and six working standard solutions of each metal to be analyzed: 0.5, 1.0, 2.5, 5.0, 10.0, and 25.0 μg/L of As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, and Sn, respectively. The response curves were recorded, and linearity and regression results are presented in Table 2.
Method Blank and Laboratory Control Samples Preparation
The method blank consists of all water and acids used for digestion, without any sample. It is prepared with each batch of samples and is digested, diluted, and analyzed the same as unknown samples.
The laboratory control sample (LCS) is a method blank with a known amount of analytes added. In this case, 250 μL of an intermediate stock solution with 1.0 μg/L each of As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, and Sn was added to the digestion tube containing the same acid and water as all other samples. After digestion and dilution, the final concentration of each metal was 5.0 μg/L. The percent LCS recoveries for each metal of interest were calculated using the following eq 1. LCS results are used to verify that the laboratory can acceptably perform the analysis in a clean matrix. The target recovery range for LCS samples was 80 to 120%.37
| 1 |
where LCS is the laboratory control sample results (μg/L), MB is the result of the method blank (μg/L), and S is the concentration of the element used to spike the LCS (μg/L).
The relative standard deviation for replicate analyses of the different samples was obtained by dividing the standard deviation by the mean value of the analytical data according to eq 2.
| 2 |
where S is the standard deviation of the nine replicate analyses and x̅ is the mean of the nine replicate analyses.
A series of “cold air blank” or procedural blanks were prepared on different days by drawing air through the entire system, without any cartridge or heat, and processed the same way as experimental samples. Their ICP-MS concentrations are found in Table S5 of the Supporting Information. The average value is subtracted from experimental samples to account for any background signal from glassware or solvents.
Accuracy and Precision
Accuracy and precision of the instrument method were assessed by the analysis of laboratory control samples (LCS) and matrix spike (MS) samples. Accuracy was evaluated through recovery studies of sample spikes. Precision was evaluated regarding repeatability by estimating the relative standard deviation (RSD) of the recovery percentage for each spiked level. In this study, the recovery test was done by creating a matrix spike as follows: 250 μL of an intermediate stock solution with 1.0 μg/L of nine metals standards was added to 0.2 g of cannabis concentrate stock in a digestion tube and digested using the same parameters as all other samples. After digestion and dilution, the final target concentration of the metals was 5.0 μg/L each. The percentage recoveries of the analyte were calculated using eq 3. MS analysis indicates a potential problem due to the sample matrix itself. The target recovery range for spiked samples was considered to be 75 to 125%.37
| 3 |
where Cs is the concentration of the spiked sample, Cu is the concentration of unspiked sample and Ct is the target concentration in μg/L.
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The LOD and LOQ were determined experimentally and defined as 3 and 10 times the standard deviation of the low-concentration spiked samples, respectively. For this study, both the LOD and LOQ were calculated using the six replicates of 0.5 μg/L matrix spike samples. LOD and LOQ sample spike recoveries were calculated using eq 3. The limit of detection (LOD) and limit of quantitation (LOQ) were calculated using eqs 4 and 5, respectively. After factoring in the sample weight (0.2 g) and a final volume (50 mL), the LOD and LOQ concentrations are expressed in μg/g. The percent recovery values for limit of detection were determined by eq 6 where Cs is the concentration of the spiked sample, Cu is the concentration of unspiked sample metal of interest in μg/L.
| 4 |
| 5 |
where SDss is the standard deviation of the six replicate spike samples.
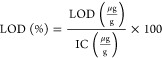 |
6 |
where LOD
(%) is the limit
of detection in percent recovery units, 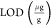 is the limit of detection in μg/g
units, and
is the limit of detection in μg/g
units, and 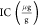 is the initial spiked oil
concentration
in μg/g units.
is the initial spiked oil
concentration
in μg/g units.
Determination of Heavy Metals in Samples
The digested samples were analyzed by ICP-MS for As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, and Sn, and the concentration in the original sample was calculated using eq 7.
| 7 |
where C is the concentration in μg/L from the instrument software’s evaluation of the calibration standards, V is the final volume of the digested solution (50 mL), df is the dilution factor (4), and m is the mass of the sample (∼0.2 g).
Sample Blank Adjustments
Each of the organic solvent trials (Table 1; MeOH + AA, Ac + AA, and Hex + AA) had an air blank subtracted for both the organic and aqueous impinger by using data from a blank trial with the Ac + AA solvent system. The aqueous samples had the metal concentrations of unused impinger liquid subtracted from them as a blank. In all experiments, both the impinger signals were added together for the total metal recovery. All spiked and unspiked materials had a method blank subtracted from them. When subtracting values, the raw instrument values were subtracted first, and then any unit conversions or calculations to the concentration in the matrix were conducted.
Acknowledgments
The authors would like to thank the Puyallup Tribe of Indians for its willingness to financially support this work through Medicine Creek Analytics. We would also like to thank the staff of MCA, Chris Johnson, Kyle Shelton, Lauren Christiansen, and Jen Juarez for assistance with sample and data analysis. Additionally, Dr. Julie Kowalski and Rachael Keener provided helpful comments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02740.
Tables of values related to model matrix profiles; cannabinoid percent, residual solvents, terpene concentrations, and metals concentrations, including procedural blank instrument values; images of impingers with and without organic solvents; and discussion and graphs related to filter paper use (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hartman M.Cannabis Overview. National Conference of State Legislatures. 2021.https://www.ncsl.org/research/civil-and-criminal-justice/marijuana-overview.aspx (accessed 2021/04/08).
- National Conference of State Legislatures .State Medical Marijuana Laws. 2021.https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (accessed 2021/05/18).
- Giroud C.; Cesare M. D.; Berthet A.; Varlet V.; Concha-Lozano N.; Favrat B. E-Cigarettes: A Review of New Trends in Cannabis Use. Int. J. Environ. Res. Public Health 2015, 12, 9988–10008. 10.3390/ijerph120809988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlet V.; Concha-Lozano N.; Berthet A.; Plateel G.; Favrat B.; De Cesare M.; Lauer E.; Augsburger M.; Thomas A.; Giroud C. Drug vaping applied to cannabis: Is “cannavaping” a therapeutic alternative to marijuana. Sci. Rep. 2016, 6, 25599. 10.1038/srep25599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas R. S.; Fresquez M. R.; Martone N.; Watson C. H. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J. Anal. Toxicol. 2014, 38, 204–211. 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresquez M. R.; Gonzalez-Jimenez N.; Gray N.; Watson C. H.; Pappas R. S. High-Throughput Determination of Mercury in Tobacco and Mainstream Smoke from Little Cigars. J. Anal. Toxicol. 2015, 39, 545–550. 10.1093/jat/bkv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . Public health consequences of e-cigarettes; The National Academies Press: 2018. [PubMed] [Google Scholar]
- Olmedo P.; Goessler W.; Tanda S.; Grau-Perez M.; Jarmul S.; Aherrera A.; Chen R.; Hilpert M.; Cohen J. E.; Navas-Acien A.; Rule A. M. Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ. Health Perspect. 2018, 126, 027010 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margham J.; McAdam K.; Forster M.; Liu C.; Wright C.; Mariner D.; Proctor C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- Halstead M.; Gray N.; Gonzalez-Jimenez N.; Fresquez M.; Valentin-Blasini L.; Watson C.; Pappas R. S. Analysis of Toxic Metals in Electronic Cigarette Aerosols Using a Novel Trap Design. J. Anal. Toxicol. 2020, 44, 149–155. 10.1093/jat/bkz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citterio S.; Santagostino A.; Fumagalli P.; Prato N.; Ranalli P.; Sgorbati S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. 10.1023/A:1026113905129. [DOI] [Google Scholar]
- Husain R.; Weeden H.; Bogush D.; Deguchi M.; Soliman M.; Potlakayala S.; Rudrabhatla S. Enhanced tolerance of industrial hemp (Cannabis sativa L.) plants on abandoned mine land soil leads to overexpression of cannabinoids. PLoS One 2019, 14, e0221570 10.1371/journal.pone.0221570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan Marijuana Regulatory Agency MRA Recalls Vape Cartridges With High Levels of Vitamin E Acetate. 2019. https://www.michigan.gov/som/0,4669,7-192-47796-517871--,00.html (accessed Nov 20, 2020).
- McGuinness L. B.CBD Oil Product Recalled Due to High Lead Levels, FDA Announces. Analytical Cannabis, May 20, 2020. https://www.analyticalcannabis.com/news/cbd-oil-product-recalled-due-to-high-lead-levels-fda-announces-312418 (accessed 2020/11/18).
- Biolchini A.Marijuana contaminated with heavy metals, pesticide recalled in testing lab investigation. Michigan Live, Aug 30, 2019. https://www.mlive.com/news/2019/08/marijuana-contaminated-with-heavy-metals-pesticide-recalled-in-testing-lab-investigation.html (accessed 2020/12/05).
- Donovan D.Maryland cannabis regulators warn of lead contamination risk as they expand tests for heavy metals. The Baltimore Sun, June 4, 2019. https://www.baltimoresun.com/health/marijuana/bs-md-cannabis-heavy-metals-20190605-story.html (accessed 2020/11/20).
- McGuinness L. B.Hawaiian Cannabis Vape Cartridges are Contaminated with Ethanol and Lead, Tests Find. Analytical Cannabis, June 19, 2020. https://www.analyticalcannabis.com/news/hawaiian-cannabis-vape-cartridges-are-contaminated-with-ethanol-and-lead-tests-find-312447 (accessed 2020/12/08).
- Meehan-Atrash J.; Luo W.; McWhirter K. J.; Strongin R. M. Aerosol Gas-Phase Components from Cannabis E-Cigarettes and Dabbing: Mechanistic Insight and Quantitative Risk Analysis. ACS Omega 2019, 4, 16111–16120. 10.1021/acsomega.9b02301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan-Atrash J.; Luo W.; Strongin R. M. Toxicant Formation in Dabbing: The Terpene Story. ACS Omega 2017, 2, 6112–6117. 10.1021/acsomega.7b01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.; Elzinga S.; Raber J. C. Determination of Pesticide Residues in Cannabis Smoke. J. Toxicol. 2013, 2013, 1–6. 10.1155/2013/378168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir D.; Rickert W. S.; Levasseur G.; Larose Y.; Maertens R.; White P.; Desjardins S. A Comparison of Mainstream and Sidestream Marijuana and Tobacco Cigarette Smoke Produced under Two Machine Smoking Conditions. Chem. Res. Toxicol. 2008, 21, 494–502. 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- Pappas R. S.; González-Jiménez N.; Gray N.; Halstead M.. Measurement of Elemental Constituents of Cannabis Vaping Liquids and Aerosols by ICP-MS. In Measuring heavy metal contaminants in cannabis and hemp; CRC Press: Boca Raton, 2020; Chapter 24. [Google Scholar]
- Centers for Disease Control and Prevention Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. February 25, 2020, https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed 2020/11/05).
- US Food and Drug Administration Respiratory Illnesses Associated with Use of Vaping Products. 2019, https://www.fda.gov/news-events/public-health-focus/lung-injuries-associated-use-vaping-products (accessed 2020/11/06).
- Layden J. E.; Ghinai I.; Pray I.; Kimball A.; Layer M.; Tenforde M. W.; Navon L.; Hoots B.; Salvatore P. P.; Elderbrook M.; Haupt T.; Kanne J.; Patel M. T.; Saathoff-Huber L.; King B. A.; Schier J. G.; Mikosz C. A.; Meiman J. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Final Report. N. Engl. J. Med. 2020, 382, 903–916. 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- Atakan Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan-Atrash J.; Strongin R. M. Pine rosin identified as a toxic cannabis extract adulterant. Forensic Sci. Int. 2020, 312, 110301. 10.1016/j.forsciint.2020.110301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T.; Montanes F.; Tallon S. J.; Fenton T.; King J. W. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. J. Supercrit. Fluids 2020, 161, 104850. 10.1016/j.supflu.2020.104850. [DOI] [Google Scholar]
- Gray N.; Halstead M.; Gonzalez-Jimenez N.; Valentin-Blasini L.; Watson C.; Pappas R. S. Analysis of Toxic Metals in Liquid from Electronic Cigarettes. Int. J. Environ. Res. Public Health. 2019, 16, 4450. 10.3390/ijerph16224450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Wang P.; Ito K.; Fowles J.; Shusterman D.; Jaques P. A.; Kumagai K. Measurement of heating coil temperature for e-cigarettes with a “top-coil” clearomizer. PLoS One 2018, 13, e0195925 10.1371/journal.pone.0195925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherrera A.; Olmedo P.; Grau-Perez M.; Tanda S.; Goessler W.; Jarmul S.; Navas-Acien A. The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Environ. Res. 2017, 159, 313–320. 10.1016/j.envres.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Regulated Marijuana Testing Program: Sampling and Testing Program. Colorado Code Regulations §212–3–4-115, 44(9), May 20, 2021.
- Heavy Metals Testing. Bureau of Cannabis Control, California Code of Regulations Title 16, Division 42, § 5723, 2018, 1–120.https://bcc.ca.gov/law_regs/readopt_text_final.pdf (accessed 2020/11/15).
- ISO ISO Method, 20768:2018, Vapor Products - Routine Analytical Vaping Machine - Definitions and Standard Conditions. https://www.iso.org/standard/69019.html (accessed 2020/09/15).
- CORESTA Recommended Method Number 81, Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection – Definitions and Standard Conditions. June, 2015. https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf (accessed 2020/09/15).
- United States Environmental Protection Agency Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices . Method 3052 (SW-846) 1996.
- United States Environmental Protection Agency Inductively Coupled Plasma-Mass Spectrometry. Method 6020B (SW-846), Rev. 2, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.