Abstract
In this paper, phosphoric acid (H3PO4), hydrochloric acid (HCl), and hydrogen peroxide (H2O2) were employed for the modification of oil-based drill cutting ash (OBDCA) for the first time. The adsorption of rhodamine B (RhB) on modified oil-based drill cutting ash (MOBDCA) in an aqueous medium was investigated. H2O2-modified OBDCA had the optimal adsorption efficiency for RhB. The physical and chemical properties of MOBDCA were analyzed using X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), ζ-potential, N2 adsorption–desorption isotherm, and pore size distribution. The effect of the pH value (3–11), reaction time (10–720 min), and initial RhB concentration (10–200 mg/L) on RhB adsorption was discussed. The adsorption kinetics highly fitted with the pseudo-second-order model (R2 > 0.99), which indicated that the adsorption process was dominated by chemisorption. The adsorption isotherm fitted well with the Langmuir and Freundlich models (R2 > 0.97), which indicated the monolayer adsorption process and the heterogeneous adsorption process, respectively. The theoretic adsorption capacity (50 mg/g) for RhB was achieved by H2O2-modified OBDCA. This paper provides a promising method of resource utilization of OBDCA to treat organic pollutants.
Introduction
With the rapid development of industry, more and more industrial wastewater is discharged into the environment without sufficient treatment, resulting in increasingly serious pollution of water resources.1,2 Common pollutants in the water environment include organic dyes, heavy metals, pesticides, antibiotics, and hormone drugs.3−5 Among them, dyes are the main source of water pollution, which are widely used in papermaking, textile, leather, printing, and dyeing industries.6−10 Rhodamine B (RhB), a xanthene-type cationic dye, is commonly used in rubber, paper, and plastic manufacturing industries.11−13 Insufficient treatment for RhB in water bodies could result in environmental pollution and cause harm to human health.14 Therefore, the removal of RhB is of great significance.
The main methods for treating dyes in wastewater include adsorption, flocculation, chemical oxidation, reverse osmosis, photocatalytic degradation, and so on.15−19 Among them, adsorption technology has attracted much attention due to its advantages such as simplicity, eco-friendliness, and recyclability of adsorbents.20,21 Traditional materials used to adsorb dyes usually include zeolite, biological materials, nanomaterials, carbonaceous materials, ion exchange resins, and so on.22−24
Oil-based drill cutting ash (OBDCA) is a solid waste that is produced from the pyrolysis product of oil-based drill cuttings (OBDC). In China, the yield of OBDCA is up to 200 thousand tons, which cannot be fully utilized due to the huge quantity. The main utilization method of OBDCA is as a raw material for brick and cement.25−27 In our previous study, OBDCA was developed as a novel material of environmental remediation for metal polluted soil and water.28,29 However, OBDCA is currently not used in the removal of organic matter due to the poor adsorption efficiency for organic matter. To enhance adsorption for organic matter, appropriate modification of OBDCA should be studied. Acid modifications have been widely used to improve the adsorption capacity of the material for pollutants. The possible reasons are that acid modification could remove soluble salts, introduce new functional groups, increase the pore volume, and enhance the specific surface area for the material.30−32 In this study, three common acids, H3PO4, HCl, and H2O2 were selected to modify OBDCA for improving the adsorption capacity for organic pollutants.
The main aims of this study were (1) to modify OBDCA using three acids; (2) to compare the characterization of modified OBDCA (MOBDCA) and OBDCA; (3) to investigate the effect of the modification method and pH value on RhB removal; and (4) to study the adsorption kinetics and adsorption isotherm of RhB on MOBDCA and OBDCA.
Characterization of OBDCA and MOBDCA
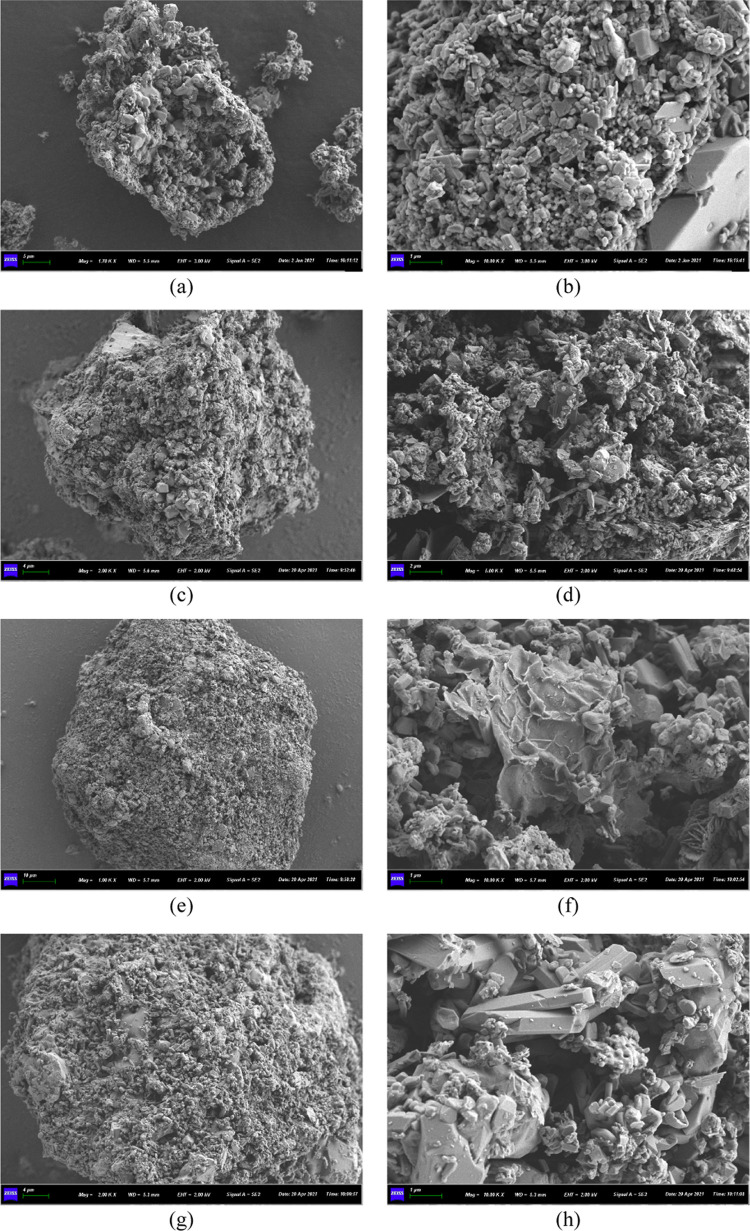
The morphology characterization of OBDCA and MOBDCA was analyzed using a scanning electron microscope (SEM) (Figure 1). OBDCA was an irregular particle with a relatively coarse surface structure, which indicated the presence of impurity on OBDCA. For M1-OBDCA, the morphology of M1-OBDCA had no obvious difference with OBDCA. For M2-OBDCA and M3-OBDCA, the morphology of the two samples was an irregular particle shape with a relatively smooth surface structure, which indicated less impurity on OBDCA. The change of morphology was due to the stronger oxidation and corrosion effect of HCl and H2O2 than H3PO4 for OBDCA modification.
Figure 1.
SEM photographs of the samples: (a, b) OBDCA, (c, d) M1-OBDCA, (e, f) M1-OBDCA, and (g, h) M1-OBDCA.
The surface functional group of OBDCA and MOBDCA was analyzed using Fourier transform infrared spectroscopy (FTIR; Figure 2). For OBDCA, the obvious band appeared at 3436 cm–1 was attributed to the O–H stretching vibration peak;33 the band appeared at 1625 cm–1 was attributed to O–H bending vibration peak.34 The band appeared at 1087 cm–1 was attributed to the C–N stretching vibration peak.35 The bands appeared at 601–468 cm–1 were ascribed to C–C=O stretching vibration peaks.36 The FTIR spectra of the three modified OBDCA samples had no obvious difference compared with those of OBDCA, which indicated that modification had not changed the functional group for OBDCA.
Figure 2.
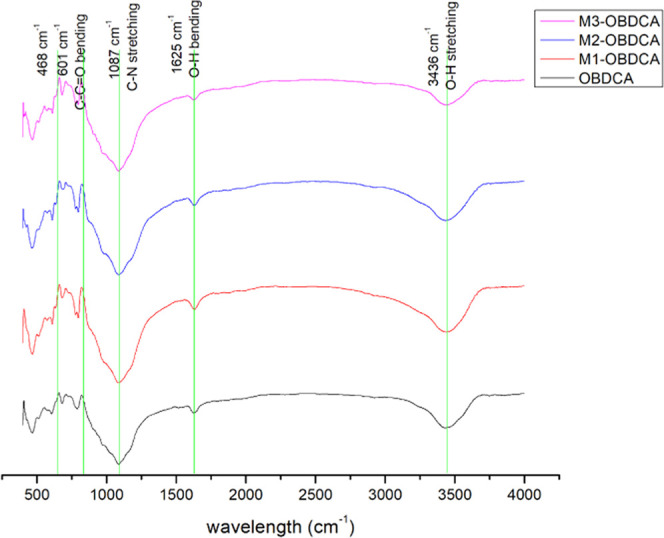
FTIR spectra of MOBDCA and OBDCA.
The composition of OBDCA and MOBDCA was analyzed using X-ray diffraction (XRD) (Figure 3). For OBDCA, many mess peaks appeared in the XRD pattern of OBDCA, which indicated the complex composition of OBDCA. According to the index result, the main diffraction planes observed in OBDCA included quartz (SiO2, PDF 79-1907), barite (BaSO4, PDF 76-0213), and vaterite (CaCO3, PDF 74-1816).37−39 For M1-OBDCA and M3-OBDCA, the XRD pattern had no obvious difference compared with that of OBDCA. For M2-OBDCA, the diffraction planes corresponding to barite and vaterite disappeared. This was due to the reaction of CaCO3 with HCl to form CaCl2, which resulted in the reduction of vaterite in M2-OBDCA. In addition, although BaSO4 could not react with HCl, the solubility of BaSO4 increased in the presence of HCl, which also resulted in the reduction of barite in M2-OBDCA.
Figure 3.
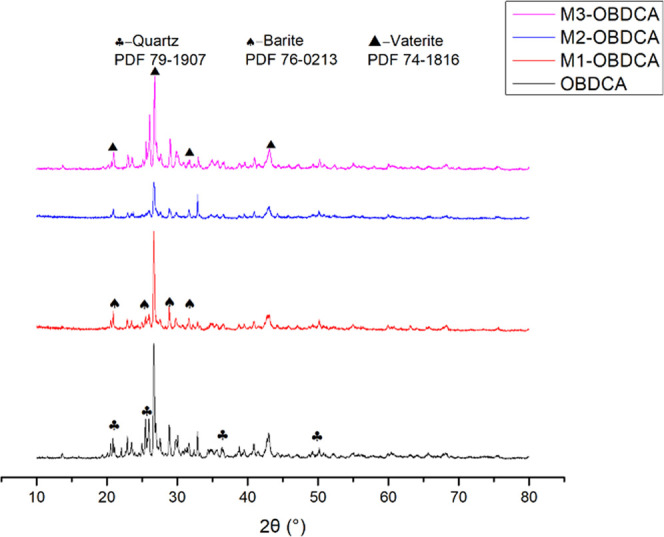
XRD patterns of MOBDCA and OBDCA.
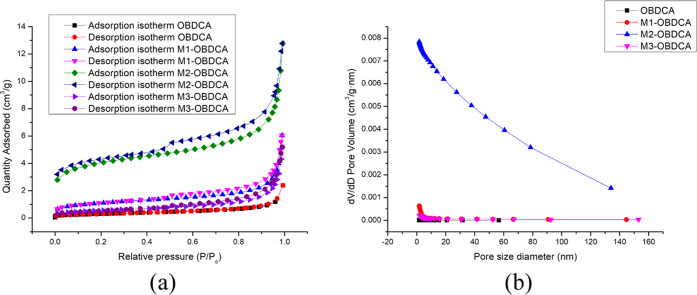
Figure 4 shows the N2 adsorption/desorption isotherms and pore size distributions of OBDCA and MOBDCA. For OBDCA, according to the Brunauer–Deming–Deming–Teller (BDDT) classification, the N2 adsorption/desorption isotherms of OBDCA were irreversible approximate type II with no obvious hysteresis loop; the data of pore size distribution indicated the non-porous structure of OBDCA with a very small pore volume. For M1-OBDCA and M3-OBDCA, N2 adsorption/desorption isotherms and pore size distributions were similar to those of OBDCA. For M2-OBDCA, the N2 adsorption/desorption isotherms of OBDCA were irreversible approximate type II with an obvious hysteresis loop; despite the relatively high pore volume, the data of pore size distribution still indicated the non-porous structure of M3-OBDCA due to the small total pore volume. According to the result of the BET parameter (Table 1), the order of the surface area and total pore volume was as follows: OBDCA < M3-OBDCA < M1-OBDCA < M2-OBDCA. The highest surface area was obtained for M2-OBDCA due to the reduction of barite, vaterite, and other impurities after HCl modification.
Figure 4.
BET analysis of MOBDCA and OBDCA: (a) N2 isotherm and (b) pore size distribution.
Table 1. Brunauer–Emmett–Teller (BET) Parameters of MOBDCA and OBDCA.
| BET surface area (m2/g) | total volume (cm3/g) | average pore diameter (nm) | |
|---|---|---|---|
| OBDCA | 1.0993 | 0.0037 | 13.4 |
| M1-OBDCA | 3.8590 | 0.0093 | 9.7 |
| M2-OBDCA | 14.5641 | 0.0197 | 5.2. |
| M3-OBDCA | 1.9371 | 0.0080 | 12.6 |
The colloidal dispersion stability and potential value under different pH values were analyzed using ζ-potential analysis (Figure 5). The obtained pHpzc values for OBDCA and MOBDCA were all less than 3.0, which indicated the negative charge on the surface of OBDCA and MOBDCA when pH > 3.0.
Figure 5.
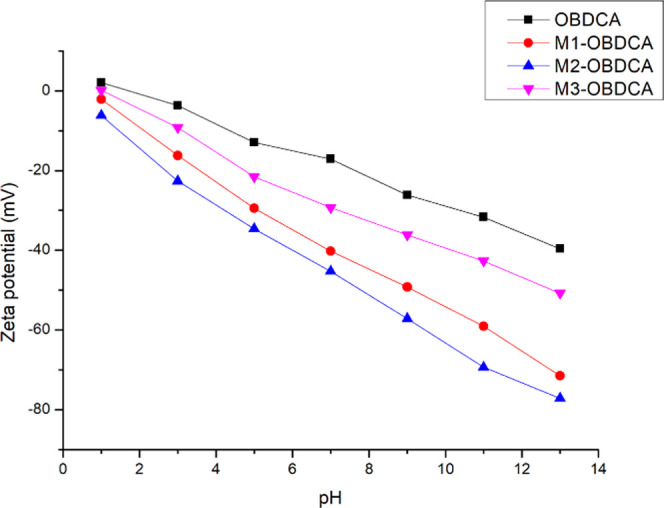
ζ-Potential measurement of OBDCA and MOBDCA under different pH values.
Results and Discussion
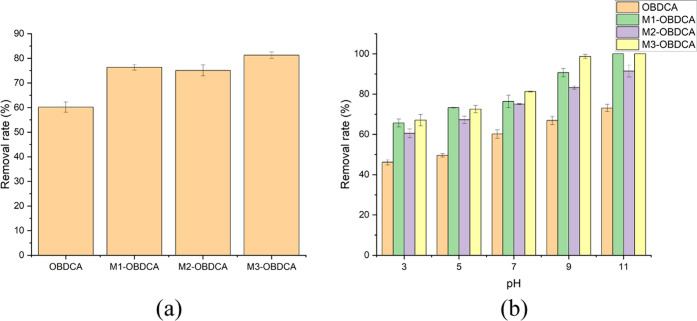
Figure 6a shows the effect of the modification method of OBDCA on RhB adsorption. Three modified OBDCA samples had higher adsorption capacity for RhB than OBDCA. The order of the removal rate for RhB was as follows: OBDCA (60.1%) < M2-OBDCA (75.0%) < M1-OBDCA (76.3%) < M3-OBDCA (81.3%). The conclusion was not consistent with the BET surface area of the adsorbent. Especially, M2-OBDCA with the highest BET surface area had little enhanced adsorption capacity compared with OBDCA. This was possible due to the discussion mentioned in XRD patterns. HCl modification could corrode the surface of OBDCA to generate more adsorption sites for RhB adsorption. However, barite, vaterite, and other impurities in OBDCA, which could also adsorb the dye at a certain degree, were also reduced after HCl modification.40−42 The H3PO4 and H2O2 modification could oxidize and corrode the surface of OBDCA with relatively intact retention of barite and vaterite. In addition, among the three MOBDCA, M3-OBDCA had the lowest BET surface area and the optimal adsorption efficiency for RhB, which could indicate that the H2O2 modification could enhance the hydrophilicity and affinity of OBDCA for RhB.43
Figure 6.
(a) Effect of the modification method of OBDCA on the removal rate of RhB. Experimental conditions: dosage of the adsorbent: 1 g/L, pH: 7, reaction time: 180 min, and initial RhB concentration: 10 mg/L. (b) Effect of the pH value on the removal rate of RhB. Experimental conditions: dosage of the adsorbent: 1 g/L, pH: 3–11, reaction time: 180 min, and initial RhB concentration: 10 mg/L.
Figure 6b shows the effect of pH on RhB adsorption. For the four adsorbents, the pH value had an obvious effect on the removal rate of RhB, and the removal rate increased with increasing pH value. This was because (i) RhB is a weakly basic dye, which has a positive surface charge.44 (ii) According to the result of the ζ-potential analysis of the adsorbent, the surface charge of the adsorbent was negative when pH > 3, while there was a strong electrostatic attraction between the adsorbent and the RhB solution when pH > 3. Therefore, a higher pH value was conducive for RhB adsorption due to the more obvious electrostatic attraction.
Adsorption Kinetics and Adsorption Isotherm
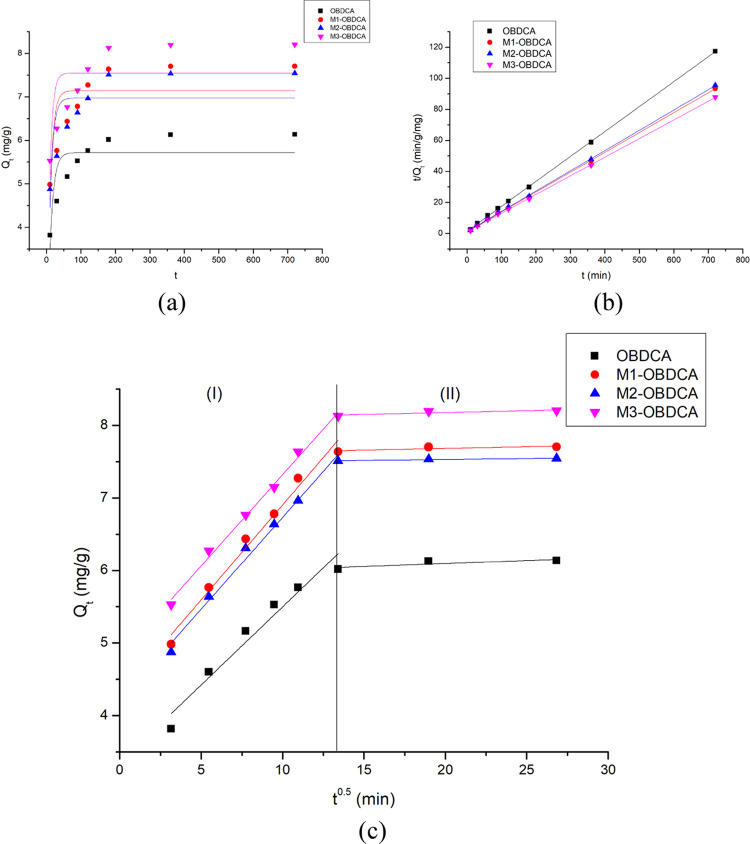
The pseudo-first-order model, pseudo-second-order model, and intraparticle diffusivity model were employed to determine the kinetic mechanism of the adsorption process.45,46 The kinetic plots and the corresponding parameters are shown in Figure 7 and Table 2, respectively. According to the calculated coefficients (R2), the pseudo-second-order kinetic model was more suitable to explain the adsorption process of RhB on the adsorbent, which indicated that the main adsorption process was controlled by chemisorption. The intraparticle diffusivity model was also suitable to fit the adsorption process. As shown in Figure 7c, the intraparticle diffusivity plot was divided into two steps ((I) and (II)). For the first (I) step, the experiment data had a high coefficient and the fitted curve was close to the origin, which indicated that this step was mainly controlled by intraparticle diffusivity.47 For the second (II) step, the experiment data had a low coefficient and the fitted curve was far from the origin, which indicated that this step was mainly controlled by extraparticle diffusivity.
Figure 7.
(a) Pseudo-first-order, (b) pseudo-second-order, and (c) intraparticle diffusivity model plots. Experimental conditions: dosage of the adsorbent: 1 g/L, pH: 7, reaction time: 10–720 min, and initial RhB concentration: 10 mg/L.
Table 2. Adsorption Kinetic Model Parameters.
| pseudo-first-order | |||
|---|---|---|---|
| k1 (min–1) | qm (mg/g) | R2 | |
| OBDCA | 0.09 ± 0.01 | 5.7 ± 0.1 | 0.6434 |
| M1-OBDCA | 0.10 ± 0.01 | 7.1 ± 0.3 | 0.5446 |
| M2-OBDCA | 0.10 ± 0.01 | 6.9 ± 0.4 | 0.5391 |
| M3-OBDCA | 0.11 ± 0.01 | 7.5 ± 0.6 | 0.4644 |
| pseudo-second-order | |||
| qe (mg/g) | k2 (g/mg/min) | R2 | |
| OBDCA | 6.0 ± 0.2 | 0.017 ± 0.001 | 0.9998 |
| M1-OBDCA | 7.8 ± 0.3 | 0.013 ± 0.001 | 0.9997 |
| M2-OBDCA | 7.6 ± 0.2 | 0.013 ± 0.001 | 0.9970 |
| M3-OBDCA | 8.3 ± 0.2 | 0.012 ± 0.001 | 0.9967 |
| intraparticle diffusivity | |||
| kw (min–1) | b | R2 | |
| OBDCA | 0.21 ± 0.01 (I)/0.0082 ± 0.0004(II) | 3.3 ± 0.3 (I)/5.9 ± 0.3 (II) | 0.9473 (I)/0.4541 (II) |
| M1-OBDCA | 0.26 ± 0.03(I)/0.0043 ± 0.0006(II) | 4.3 ± 0.2 (I)/7.6 + 0.5 (II) | 0.9805 (I)/0.3683 (II) |
| M2-OBDCA | 0.23 ± 0.03 (I)/0.0027 ± 0.0003(II) | 4.2 + 0.3 (I)/7.4 + 0.6 (II) | 0.9861 (I)/0.8207 (II) |
| M3-OBDCA | 0.25 ± 0.02(I)/0.0052 ± 0.0004(II) | 4.8 + 0.3 (I)/8.1 + 0.7(II) | 0.9931 (I)/0.4531 (II) |
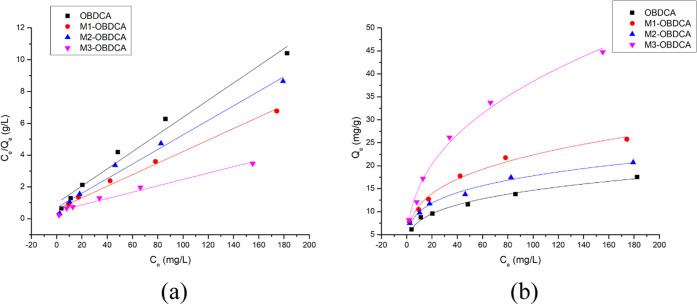
The Langmuir and Freundlich models were employed to fit the adsorption isotherm of RhB on the adsorbent.48,49 The isotherm plots and the corresponding parameters are shown in Figure 8 and Table 3, respectively. According to the calculated coefficients (R2), the Langmuir and Freundlich isotherm models were both suitable to explain the adsorption process of RhB on the adsorbent. The parameters kl (<1) and n (<1) indicated the homogeneous, monolayer, and favorable adsorption process. The theoretic adsorption capacity, Qm, was calculated from the Langmuir equation in the following order: OBDCA (18 mg/g) < M2-OBDCA (21 mg/g) < M1-OBDCA (30 mg/g) < M3-OBDCA (50 mg/g).
Figure 8.
(a) Langmuir and (b) Freundlich model plots. Experimental conditions: dosage of the adsorbent: 1 g/L, pH: 7, reaction time: 180 min, and initial RhB concentration: 10–200 mg/L.
Table 3. Adsorption Isotherm Parameters.
| Freundlich | |||
|---|---|---|---|
| kf | n | R2 | |
| OBDCA | 4.3 ± 0.3 | 0.27 ± 0.01 | 0.9880 |
| M1-OBDCA | 5.8 ± 0.4 | 0.29 ± 0.02 | 0.9869 |
| M2-OBDCA | 5.6 ± 0.3 | 0.25 ± 0.01 | 0.9858 |
| M3-OBDCA | 6.3 ± 0.5 | 0.39 ± 0.03 | 0.9902 |
| Langmuir | |||
|---|---|---|---|
| kl (L/mg) | Qm (mg/g) | R2 | |
| OBDCA | 0.055 ± 0.003 | 18 ± 1 | 0.9757 |
| M1-OBDCA | 0.061 ± 0.004 | 30 ± 2 | 0.9872 |
| M2-OBDCA | 0.066 ± 0.002 | 21 ± 1 | 0.9822 |
| M3-OBDCA | 0.043 ± 0.002 | 50 ± 3 | 0.9744 |
Comparison with Other Adsorbents for RhB
The adsorption capacity of M3-OBDCA for RhB was compared with other adsorbents (Table 4). The adsorption capacity of M3-OBDCA was not at a high level compared with some adsorbents. Different from other adsorbents, OBDCA is a solid waste and the modification of OBDCA was very low cost. Meanwhile, the use of M3-OBDCA for RhB adsorption achieved waste utilization, which had a certain environmental significance. However, the regeneration of used M3-OBDCA may result in secondary environmental pollution and disproportionate cost consumption. The used M3-OBDCA was considered to be utilized and developed to prepare other materials, such as bricks, cement-based materials, and proppants.25,26,50
Table 4. Comparison with Other Adsorbents for RhB Adsorption.
Experimental Section
Materials and Instruments
Materials: OBDC was provided by Agriculture and Forestry Department of Fuling Shale Gas Company. OBDCA was obtained by pyrolysis of OBDC. The pyrolysis conditions were as follows: pyrolysis temperature was 750 °C, pyrolysis time was 45 min, and heating rate was 6 °C/min. OBDCA was ground and sieved through a 200-mesh screen. Phosphoric acid (H3PO4), hydrochloric acid (HCl), rhodamine B (RhB), hydrogen peroxide (H2O2), and (NaOH) were bought from Aladdin Company.
Instruments: An automatic surface area and porosity analyzer (ASAP 2020M) was used to analyze the N2 adsorption isotherms and pore size distribution of the samples. A ζ-potential analyzer (NanoPlus) was used to analyze the ζ-potential of the samples. An X-ray diffractometer (D8 Advance) was used to analyze the X-ray diffraction (XRD) pattern of the samples. A field emission scanning electron microscope (FE-SEM; JSM-IT300) was used to measure the surface morphologies of the samples. A Fourier transform infrared spectrometer (Nexus) was used to analyze the Fourier transform infrared spectroscopy (FTIR) spectrum of the samples.
Modification of OBDCA
H3PO4, HCl, and H2O2 were used for OBDCA modification. In the modification process, 20 g of OBDCA was added into each 1000 mL conical flask with 800 mL of a H3PO4 (30%) solution, 800 mL of a HCl (10%) solution, and 800 mL of a H2O2 (30%) solution, separately. Then, the conical flasks were sealed and shaken in a shaker for 24 h at 25 °C. Modified OBDCA was collected and rinsed using ultrapure water until the pH was neutral. The modified OBDCA was dried using a dryer for 24 h at 120 °C. H3PO4-, HCl-, and H2O2-modified OBDCA were denoted as M1-OBDCA, M2-OBDCA, and M3-OBDCA, respectively.
Adsorption Experiment
Adsorption tests were carried out using batch experiments. OBDCA (0.1 g) was added into 150 mL conical flasks with 100 mL of the RhB solution. H2SO4 (0.1 mol/L) and NaOH (0.1 mol/L) were used to adjust the pH value. Then, the conical flasks were sealed and shaken in a shaker for 3 h at 25 °C. After adsorption, the RhB aqueous solution was filtered using a syringe filter to remove the OBDCA residue. The concentration of RhB in the filtrate was measured using an ultraviolet spectrometer at λmax wavelength of HA (wavelength: 554 nm). The removal rate (%) and unit adsorption quantity (mg/g) of RhB were calculated according to eqs 1 and 2, respectively. All experiments were carried out three times at least.
| 1 |
| 2 |
where C0 and Ct are the initial concentration (mg/L) and residue concentration (mg/L) after the adsorption of RhB, respectively; V is the volume of the solution (L); and W is the weight of the dosage of the adsorbent (g).
The adsorption kinetics of RhB on OBDCA was fitted with the pseudo-first-order, pseudo-second-order, and intraparticle diffusivity models (eqs 3–5, respectively).
| 3 |
| 4 |
| 5 |
where Qe and Qt are the adsorption capacities (mg/g) at equilibrium and at time t, respectively. k1 and k2 are the pseudo-first-order and pseudo-second-order constants, respectively.
The adsorption isotherm of RhB on OBDCA was fitted with the Langmuir and Freundlich models (eqs 6 and 7, respectively).
| 6 |
| 7 |
where Qm is the theoretic adsorption capacity (mg/g). kl and kf are the Langmuir and Freundlich constants, respectively. kw and b both are intraparticle diffusivity constants.
Conclusions
OBDCA was modified using HCl, H2O2, and H3PO4 to enhance the adsorption capacity for organic pollutants. H2O2-modified OBDCA showed good adsorption performance for RhB adsorption due to enhanced adsorption sites and the presence of barite and vaterite. The result of adsorption isotherms indicated that the adsorption process belonged to heterogeneous adsorption. The result of adsorption kinetics indicated that the adsorption process was dominated by chemisorption and the adsorption rate was controlled by both the intraparticle diffusion stage and the extraparticle diffusivity stage. Although the adsorption capacity of M3-OBDCA for RhB (50 mg/g) was not at a high level compared with other adsorbents, this study achieved the resource utilization of OBDCA for the treatment of organic pollutants at first.
Acknowledgments
The authors sincerely thank the grant funded by the Study on Comprehensive Control of Rocky Desertification and Ecological Service Function Improvement in Karst Peaks (No. 2016YFC0502402) and Fuling Shale Gas Environmental Exploration Technology of National Science and Technology Special Project (Grant No. 2016ZX05060). This work was also financially supported by the National Natural Science Foundation of China (No. 51709254) and Youth Innovation Promotion Association, Chinese Academy of Sciences (No. 2020335)
The authors declare no competing financial interest.
References
- Frag E. Y.; Mohamed N. M.; Elashery S. E. A. Exploitation of o-benzoyl benzoic acid as an efficient electroactive material for selective determination of Cr (III) ions in pharmaceutical samples and industrial waste water using carbon sensor. Anal. Chim. Acta 2021, 1154, 338322 10.1016/j.aca.2021.338322. [DOI] [PubMed] [Google Scholar]
- Senthil R. A.; Wu Y.; Liu X.; Pan J. A facile synthesis of nano AgBr attached potato-like Ag2MoO4 composite as highly visible-light active photocatalyst for purification of industrial waste-water. Environ. Pollut. 2021, 269, 116034 10.1016/j.envpol.2020.116034. [DOI] [PubMed] [Google Scholar]
- Cong X.; Zhang J.; Pu Y. A novel living environment exposure matrix of the common organic air pollutants for exposure assessment. Ecotoxicol. Environ. Saf. 2021, 215, 112118 10.1016/j.ecoenv.2021.112118. [DOI] [PubMed] [Google Scholar]
- Zhou K.; Wu J.; Liu H. Spatiotemporal variations and determinants of water pollutant discharge in the Yangtze River Economic Belt, China: A spatial econometric analysis. Environ. Pollut. 2021, 271, 116320 10.1016/j.envpol.2020.116320. [DOI] [PubMed] [Google Scholar]
- Saravanan A.; Senthil Kumar P.; Jeevanantham S.; Karishma S.; Tajsabreen B.; Yaashikaa P. R.; Reshma B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595 10.1016/j.chemosphere.2021.130595. [DOI] [PubMed] [Google Scholar]
- Thasneema K. K.; Dipin T.; Thayyil M. S.; Sahu P. K.; Messali M.; Rosalin T.; Elyas K. K.; Saharuba P. M.; Anjitha T.; Hadda T. B. Removal of toxic heavy metals, phenolic compounds and textile dyes from industrial waste water using phosphonium based ionic liquids. J. Mol. Liq. 2021, 323, 114645 10.1016/j.molliq.2020.114645. [DOI] [Google Scholar]
- Razak M. R.; Yusof N. A.; Aris A. Z.; Nasir H. M.; Haron M. J.; Ibrahim N. A.; Johari I. S.; Kamaruzaman S. Phosphoric acid modified kenaf fiber (K-PA) as green adsorbent for the removal of copper (II) ions towards industrial waste water effluents. React. Funct. Polym. 2020, 147, 104466 10.1016/j.reactfunctpolym.2019.104466. [DOI] [Google Scholar]
- Raza W.; Lee J.; Raza N.; Luo Y.; Kim K.-H.; Yang J. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. 10.1016/j.jiec.2018.11.024. [DOI] [Google Scholar]
- Geng J.; Chang J. Synthesis of magnetic Forsythia suspensa leaf powders for removal of metal ions and dyes from wastewater. J. Environ. Chem. Eng. 2020, 8, 104224 10.1016/j.jece.2020.104224. [DOI] [Google Scholar]
- Sun Z.; Yang J.; Qi Y.; Wang F.; Hong W.; Li H.; Jiang Y. Facile preparation of hydroxyl-rich mesoporous magnesium silicate with excellent adsorption performance. Surf. Interfaces 2020, 20, 100519 10.1016/j.surfin.2020.100519. [DOI] [Google Scholar]
- Liang Y.; Huang G.; Zhang Q.; Yang Y.; Zhou J.; Cai J. Hierarchical porous carbons from biowaste: Hydrothermal carbonization and high-performance for Rhodamine B adsorptive removal. J. Mol. Liq. 2021, 330, 115580 10.1016/j.molliq.2021.115580. [DOI] [Google Scholar]
- Vigneshwaran S.; Sirajudheen P.; Karthikeyan P.; Meenakshi S. Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of Malachite green and Rhodamine B dyes. Surf. Interfaces 2021, 23, 100920 10.1016/j.surfin.2020.100920. [DOI] [Google Scholar]
- Vigneshwaran S.; Park C. M.; Meenakshi S. Designed fabrication of sulfide-rich bi-metallic-assembled MXene layered sheets with dramatically enhanced photocatalytic performance for Rhodamine B removal. Sep. Purif. Technol. 2021, 258, 118003 10.1016/j.seppur.2020.118003. [DOI] [Google Scholar]
- Song C.; Wang L.-j.; Sun S.-m.; Wu Y.; Xu L.-j.; Gan L. Preparation of visible-light photocatalysts of Bi2O3/Bi embedded in porous carbon from Bi-based metal organic frameworks for highly efficient Rhodamine B removal from water. Carbon 2021, 174, 760. 10.1016/j.carbon.2020.10.018. [DOI] [Google Scholar]
- Gan L.; Geng A.; Song C.; Xu L.; Wang L.; Fang X.; Han S.; Cui J.; Mei C. Simultaneous removal of rhodamine B and Cr(VI) from water using cellulose carbon nanofiber incorporated with bismuth oxybromide: The effect of cellulose pyrolysis temperature on photocatalytic performance. Environ. Res. 2020, 185, 109414 10.1016/j.envres.2020.109414. [DOI] [PubMed] [Google Scholar]
- Abdel-Aziz R.; Ahmed M. A.; Abdel-Messih M. F. A novel UV and visible light driven photocatalyst AgIO4/ZnO nanoparticles with highly enhanced photocatalytic performance for removal of rhodamine B and indigo carmine dyes. J. Photochem. Photobiol., A 2020, 389, 112245 10.1016/j.jphotochem.2019.112245. [DOI] [Google Scholar]
- Yang S.; Feng Y.; Liu N.; Zhao Y.; Wang X.; Zhang Z.; Chen H.; Yu Y. Enhancement on the removal of Rhodamine B (RhB) by means of the Enlarged Anode Electric Biological (EAEB) reactor. Chemosphere 2020, 245, 125566 10.1016/j.chemosphere.2019.125566. [DOI] [PubMed] [Google Scholar]
- He Y.; Lv H.; Daili Y.; Yang Q.; Junior L. B.; Liu D.; Liu H.; Ma Z. Construction of a new cascade photogenerated charge transfer system for the efficient removal of bio-toxic levofloxacin and rhodamine B from aqueous solution: Mechanism, degradation pathways and intermediates study. Environ. Res. 2020, 187, 109647 10.1016/j.envres.2020.109647. [DOI] [PubMed] [Google Scholar]
- Shi P.; Hu X.; Wang Y.; Duan M.; Fang S.; Chen W. A PEG-tannic acid decorated microfiltration membrane for the fast removal of Rhodamine B from water. Sep. Purif. Technol. 2018, 207, 443–450. 10.1016/j.seppur.2018.06.075. [DOI] [Google Scholar]
- Hou Y.; Huang G.; Li J.; Yang Q.; Huang S.; Cai J. Hydrothermal conversion of bamboo shoot shell to biochar: Preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J. Anal. Appl. Pyrolysis 2019, 143, 104694 10.1016/j.jaap.2019.104694. [DOI] [Google Scholar]
- Bethi B.; Manasa V.; Srinija K.; Sonawane S. H. Intensification of Rhodamine-B dye removal using hydrodynamic cavitation coupled with hydrogel adsorption. Chem. Eng. Process. 2018, 134, 51–57. 10.1016/j.cep.2018.10.017. [DOI] [Google Scholar]
- Rachna K.; Agarwal A.; Singh N. B. Preparation and characterization of zinc ferrite—Polyaniline nanocomposite for removal of rhodamine B dye from aqueous solution. Environ. Nanotechnol. Monit. Manage. 2018, 9, 154–163. 10.1016/j.enmm.2018.03.001. [DOI] [Google Scholar]
- Chen X.; Li H.; Liu W.; Zhang X.; Wu Z.; Bi S.; Zhang W.; Zhan H. Effective removal of methyl orange and rhodamine B from aqueous solution using furfural industrial processing waste: Furfural residue as an eco-friendly biosorbent. Colloids Surf., A 2019, 583, 123976 10.1016/j.colsurfa.2019.123976. [DOI] [Google Scholar]
- Zhao Y.; Zhu L.; Li W.; Liu J.; Liu X.; Huang K. Insights into enhanced adsorptive removal of Rhodamine B by different chemically modified garlic peels: Comparison, kinetics, isotherms, thermodynamics and mechanism. J. Mol. Liq. 2019, 293, 111516 10.1016/j.molliq.2019.111516. [DOI] [Google Scholar]
- Wang C.-q.; Lin X.-y.; He M.; Wang D.; Zhang S.-l. Environmental performance, mechanical and microstructure analysis of concrete containing oil-based drilling cuttings pyrolysis residues of shale gas. J. Hazard. Mater. 2017, 338, 410–427. 10.1016/j.jhazmat.2017.05.051. [DOI] [PubMed] [Google Scholar]
- Ikotun J. O.; Okeniyi J. O.; Akinlabi E. T.; Akinlabi S. A.; Okeniyi E. T.; Olanrewaju D. O. Physicochemical and mineralogical characterization datasets from oil drill cuttings in comparison with other cement types for cement partial-replacement in concrete. Chem. Data Collect. 2019, 19, 100176 10.1016/j.cdc.2019.100176. [DOI] [Google Scholar]
- Wang C.-q.; Lin X.-y.; Mei X.-d.; Luo X.-g. Performance of non-fired bricks containing oil-based drilling cuttings pyrolysis residues of shale gas. J. Cleaner Prod. 2019, 206, 282–296. 10.1016/j.jclepro.2018.09.128. [DOI] [Google Scholar]
- Yang H.; Huang S.; Zhang Y.; Zhou B.; Manzoor Ahmed S.; Liu H.; Liu Y.; He Y.; Xia S. Remediation effect of Cr (VI)-contaminated soil by secondary pyrolysis oil-based drilling cuttings ash. Chem. Eng. J. 2020, 398, 125473 10.1016/j.cej.2020.125473. [DOI] [Google Scholar]
- Liuyang X.; Yang H.; Huang S.; Zhang Y.; Xia S. Resource utilization of secondary pyrolysis oil-based drilling cuttings ash for removing Cr (VI) contaminants: Adsorption properties, kinetics and mechanism. J. Environ. Chem. Eng. 2020, 8, 104474 10.1016/j.jece.2020.104474. [DOI] [Google Scholar]
- Mobarak M.; Selim A. Q.; Mohamed E. A.; Seliem M. K. Modification of organic matter-rich clay by a solution of cationic surfactant/H2O2: A new product for fluoride adsorption from solutions. J. Cleaner Prod. 2018, 192, 712–721. 10.1016/j.jclepro.2018.05.044. [DOI] [Google Scholar]
- Chen M.; Wang F.; Zhang D.-l.; Yi W.-m.; Liu Y. Effects of acid modification on the structure and adsorption NH4+-N properties of biochar. Renewable Energy 2021, 169, 1343–1350. 10.1016/j.renene.2021.01.098. [DOI] [Google Scholar]
- Wei F.; Guo X.; Liao J.; Bao W.; Chang L. Ultra-deep removal of thiophene in coke oven gas over Y zeolite: Effect of acid modification on adsorption desulfurization. Fuel Process. Technol. 2021, 213, 106632 10.1016/j.fuproc.2020.106632. [DOI] [Google Scholar]
- Ahmad M. A.; Eusoff M. A.; Oladoye P. O.; Adegoke K. A.; Bello O. S. Statistical optimization of Remazol Brilliant Blue R dye adsorption onto activated carbon prepared from pomegranate fruit peel. Chem. Data Collect. 2020, 28, 100426 10.1016/j.cdc.2020.100426. [DOI] [Google Scholar]
- Ghosh I.; Kar S.; Chatterjee T.; Bar N.; Das S. K. Adsorptive removal of Safranin-O dye from aqueous medium using coconut coir and its acid-treated forms: Adsorption study, scale-up design, MPR and GA-ANN modeling. Sustainable Chem. Pharm. 2021, 19, 100374 10.1016/j.scp.2021.100374. [DOI] [Google Scholar]
- Chanajaree R.; Sriuttha M.; Lee V. S.; Wittayanarakul K. Thermodynamics and kinetics of cationic/anionic dyes adsorption on cross-linked chitosan. J. Mol. Liq. 2020, 322, 114507 10.1016/j.molliq.2020.114507. [DOI] [Google Scholar]
- Xia J.; Gao Y.; Yu G. Tetracycline removal from aqueous solution using zirconium-based metal-organic frameworks (Zr-MOFs) with different pore size and topology: Adsorption isotherm, kinetic and mechanism studies. J. Colloid Interface Sci. 2021, 590, 495–505. 10.1016/j.jcis.2021.01.046. [DOI] [PubMed] [Google Scholar]
- Siyal A. A.; Shamsuddin M. R.; Rabat N. E.; Zulfiqar M.; Man Z.; Low A. Fly ash based geopolymer for the adsorption of anionic surfactant from aqueous solution. J. Cleaner Prod. 2019, 229, 232–243. 10.1016/j.jclepro.2019.04.384. [DOI] [Google Scholar]
- Wang Z.; Wu H.; Xu Y.; Shu K.; Yang J.; Luo L.; Xu L. Effect of dissolved fluorite and barite species on the flotation and adsorption behavior of bastnaesite. Sep. Purif. Technol. 2020, 237, 116387 10.1016/j.seppur.2019.116387. [DOI] [Google Scholar]
- Song X.; Cao Y.; Bu X.; Luo X. Porous vaterite and cubic calcite aggregated calcium carbonate obtained from steamed ammonia liquid waste for Cu2+ heavy metal ions removal by adsorption process. Appl. Surf. Sci. 2021, 536, 147958 10.1016/j.apsusc.2020.147958. [DOI] [Google Scholar]
- Nagpal M.; Kakkar R. Selective adsorption and separation of toxic cationic dyes using hierarchically porous SDBS modified vaterite microspheres (Hr-SMV). J. Phys. Chem. Solids 2020, 146, 109598 10.1016/j.jpcs.2020.109598. [DOI] [Google Scholar]
- Chen Y.-Y.; Yu S.-H.; Jiang H.-F.; Yao Q.-Z.; Fu S.-Q.; Zhou G.-T. Performance and mechanism of simultaneous removal of Cd(II) and Congo red from aqueous solution by hierarchical vaterite spherulites. Appl. Surf. Sci. 2018, 444, 224–234. 10.1016/j.apsusc.2018.03.081. [DOI] [Google Scholar]
- Khalilzadeh Shirazi E.; Metzger J. W.; Fischer K.; Hassani A. H. Removal of textile dyes from single and binary component systems by Persian bentonite and a mixed adsorbent of bentonite/charred dolomite. Colloids Surf., A 2020, 598, 124807 10.1016/j.colsurfa.2020.124807. [DOI] [Google Scholar]
- Sun Z.; Huang D.; Duan X.; Hong W.; Liang J. Functionalized nanoflower-like hydroxyl magnesium silicate for effective adsorption of aflatoxin B1. J. Hazard. Mater. 2020, 387, 121792 10.1016/j.jhazmat.2019.121792. [DOI] [PubMed] [Google Scholar]
- Wang X.; Chen S.; Sun J.; Zhang D.; Yan Z.; Xu X.; Song J. Synthesis of large pore sized mesoporous carbon using alumina-templated strategy for high-performance RhB removal. Microporous Mesoporous Mater. 2021, 318, 110993 10.1016/j.micromeso.2021.110993. [DOI] [Google Scholar]
- Mishra S.; Sahoo S. S.; Debnath A. K.; Muthe K. P.; Das N.; Parhi P. Cobalt ferrite nanoparticles prepared by microwave hydrothermal synthesis and adsorption efficiency for organic dyes: Isotherms, thermodynamics and kinetic studies. Adv. Powder Technol. 2020, 31, 4552–4562. 10.1016/j.apt.2020.10.001. [DOI] [Google Scholar]
- Zhao S.; Zhan Y.; Wan X.; He S.; Yang X.; Hu J.; Zhang G. Selective and efficient adsorption of anionic dyes by core/shell magnetic MWCNTs nano-hybrid constructed through facial polydopamine tailored graft polymerization: Insight of adsorption mechanism, kinetic, isotherm and thermodynamic study. J. Mol. Liq. 2020, 319, 114289 10.1016/j.molliq.2020.114289. [DOI] [Google Scholar]
- Chen J.; Liao Y.; Wan X.; Tie S.; Zhang B.; Lan S.; Gao X. A high performance MoO3@MoS2 porous nanorods for adsorption and photodegradation of dye. J. Solid State Chem. 2020, 291, 121652 10.1016/j.jssc.2020.121652. [DOI] [Google Scholar]
- Naushad M.; Alqadami A. A.; Al-Kahtani A. A.; Ahamad T.; Awual M. R.; Tatarchuk T. Adsorption of textile dye using para-aminobenzoic acid modified activated carbon: Kinetic and equilibrium studies. J. Mol. Liq. 2019, 296, 112075 10.1016/j.molliq.2019.112075. [DOI] [Google Scholar]
- Değermenci G. D.; Değermenci N.; Ayvaoğlu V.; Durmaz E.; Çakır D.; Akan E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Cleaner Prod. 2019, 225, 1220–1229. 10.1016/j.jclepro.2019.03.260. [DOI] [Google Scholar]
- Utilization of Cr-Contaminated Oil-Based Drilling-Cuttings Ash in Preparation of Bauxite-Based Proppants. Environ. Eng. Sci..
- Yang Z.; Wu G.; Gan C.; Cai G.; Zhang J.; Ji H. Effective adsorption of arsenate, dyes and eugenol from aqueous solutions by cationic supramolecular gel materials. Colloids Surf., A 2021, 616, 126238 10.1016/j.colsurfa.2021.126238. [DOI] [Google Scholar]
- Li Y.; Yan X.; Hu X.; Feng R.; Zhou M. Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water. Chem. Eng. J. 2019, 375, 122003 10.1016/j.cej.2019.122003. [DOI] [Google Scholar]
- Huang Z.; Lai Z.; Zhu D.; Wang H.; Zhao C.; Ruan G.; Du F. Electrospun graphene oxide/MIL-101(Fe)/poly(acrylonitrile-co-maleic acid) nanofiber: A high-efficient and reusable integrated photocatalytic adsorbents for removal of dye pollutant from water samples. J. Colloid Interface Sci. 2021, 597, 196–205. 10.1016/j.jcis.2021.04.020. [DOI] [PubMed] [Google Scholar]
- Rahdar S.; Rahdar A.; Zafar M. N.; Shafqat S. S.; Ahmadi S. Synthesis and characterization of MgO supported Fe–Co–Mn nanoparticles with exceptionally high adsorption capacity for Rhodamine B dye. J. Mater. Res. Technol. 2019, 8, 3800–3810. 10.1016/j.jmrt.2019.06.041. [DOI] [Google Scholar]
- Rubab R.; Ali S.; Rehman A. U.; Khan S. A.; Khan A. M. Templated synthesis of NiO/SiO2 nanocomposite for dye removal applications: Adsorption kinetics and thermodynamic properties. Colloids Surf., A 2021, 615, 126253 10.1016/j.colsurfa.2021.126253. [DOI] [Google Scholar]