Abstract
Background
There is very limited evidence on the existence of cancer-related perceived stigma and self-blame among patients with advanced cancer in Asia, and how they are associated with psychosocial outcomes. This study aimed to address the gap in the current literature by (1) assessing perceived stigma, behavioural self-blame and characterological self-blame among Vietnamese patients with advanced cancer, and (2) investigating the associations of perceived stigma and self-blame (behavioural and characterological) with depression, emotional well-being and social well-being.
Methods
This cross-sectional study involved 200 Vietnamese patients with stage IV solid cancer. Depression was measured using the Center for Epidemiologic Studies Depression (CES-D) Scale. Emotional well-being and social well-being were measured with the relevant domains of the Functional Assessment of Cancer Therapy-General (FACT-G) scale. Perceived stigma was assessed using the sense of stigma subscale of Kissane’s Shame and Stigma Scale. Behavioural self-blame and characterological self-blame were measured by the patients’ answers to the questions on whether their cancer was due to patient’s behaviour or character. Multivariable linear regressions were used to investigate the associations while controlling for patient characteristics.
Results
Approximately three-fourths (79.0%, n = 158) of the participants reported perceived stigma with an average score of 20.5 ± 18.0 (out of 100). More than half of the participants reported behavioural self-blame (56.3%, n = 112) or characterological self-blame (62.3%, n = 124). Higher perceived stigma was associated with lower emotional well-being (ß = -0.0; p = 0.024). Behavioural self-blame was not significantly associated with depressive symptoms, emotional well-being or social well-being. Patients who reported characterological self-blame reported greater depressive symptoms (ß = 3.0; p = 0.020) and lower emotional well-being (ß = -1.6; p = 0.038).
Conclusion
Perceived stigma and self-blame were common amongst Vietnamese advanced cancer patients. Perceived stigma was associated with lower emotional well-being while characterological self-blame were associated with greater depressive symptoms and lower emotional well-being. Interventions should address perceived stigma and self-blame among this population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12904-021-00803-5.
Keywords: Perceived stigma, Behavioural self-blame, Characterological self-blame, Vietnam, Advanced cancer
Background
The perception of stigma and self-blame among cancer patients is well-documented in several countries [1–9]. Perceived stigma in relation to being a cancer patient has been observed across all cancer types [1, 6, 10–12], and refers to the patient’s perception that others feel prejudice against them because of their cancer diagnosis which results in less social acceptance [12, 13]. Self-blame refers to the attribution of self as the cause of a situation, and can be either behavioural or characterological depending on the focus of blame [14]. Behavioural self-blame in the context of cancer focuses on one’s own risky health behaviour which is modifiable, such as smoking and drinking. On the contrary, characterological self-blame focuses on an individual’s character that is relatively hard to modify, such as the type of person they are.
Understanding cancer patients’ perceptions of being stigmatized and self-blame is important as these are parts of a patient’s experience with the disease and may lead to additional burden on cancer patients, further reducing their quality of life [6, 7, 9]. Several studies have shown that perceived stigma is associated with higher depressive symptoms, and lower emotional and social well-being amongst cancer patients [1, 6, 10–12]. Evidence on the associations of self-blame with depression, emotional well-being and social well-being, however, are limited and not conclusive [6, 11, 15–18]. In addition, evidence on self-blame seems to suggest that characterological self-blame was more detrimental on psychological well-being than behavioural self-blame [19, 20]. The majority of the studies mentioned above, however, were conducted in Western countries. Culture might play a crucial role in influencing how individuals perceive and respond to life-threatening diseases [21]. Beliefs such as cancer being contagious or a punishment from God still exist amongst people of Asian origins [21–23]. In addition, avoidance of cancer-related communication is very common in Asian countries [24, 25]. Such differences in cultural belief and practice may affect aspects of the experience of Asian patients, such as cancer-related stigma and self-blame, differently than those of Western societies. However, very little is known about perceived stigma and self-blame among cancer patients in Asian countries like Vietnam and the extent to which they are associated with psychosocial well-being. In Vietnam, the burden of cancer has risen rapidly in recent decades. An estimation by the International Agency for Research on Cancer (IARC) reported a total of 164,671 new cases and 114,871 cancer deaths in Vietnam in 2018 [26, 27]. These figures are three times higher than in 1990 [28]. Despite the drastically increasing cancer burden in Vietnam over the past thirty years, studies focusing on the underlying factors for psychosocial well-being of cancer patients in Vietnam remain scarce.
The aims of this study were 1) to assess the prevalence of perceived stigma and self-blame among advanced cancer patients in Vietnam, and 2) to investigate the associations of perceived stigma and self-blame (behavioural and characterological) with psychosocial well-being. We hypothesized that patients who reported perceived stigma, behavioural or characterological self-blame will report higher depressive symptoms, lower emotional well-being, and lower social well-being. Our findings will serve as an initial situational analysis for policy makers and health care providers to understand the relationship between perceived stigma, self-blame and psychosocial well-being amongst advanced cancer patients in Vietnam.
Methods
Participants and study setting
This study analysed data collected from the Vietnam site of the Asian Patient Perspectives Regarding Oncology Awareness, Care and Health (APPROACH) study, a cross-sectional multi-country study [29]. Data was collected by trained interviewers using a structured questionnaire through face-to-face interviews with inpatient and outpatient participants recruited at the Hue Central Hospital in Vietnam. The inclusion criteria included (1) being at least 21 years of age; (2) having been diagnosed with stage 4 solid cancer; (3) being aware of cancer diagnosis; (4) being a citizen of Vietnam. Ethics approvals were obtained from the National University of Singapore-Institutional Review Board (NUS-IRB B-15–319), Singapore and Hue Central Hospital Ethics Committee, Vietnam (230/QD-BVH-HDDD).
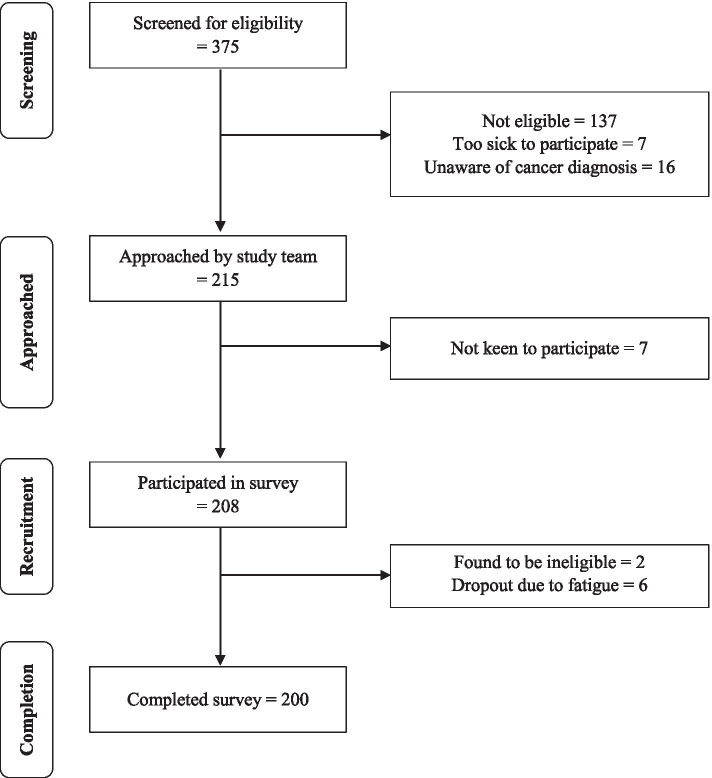
Recruitment was carried out between February 2018 to July 2018 (Fig. 1). The medical records of 375 patients were screened for eligibility. During the initial screening, a total of 238 patients were found to be eligible. Of these, 16 were not aware of their cancer diagnosis and therefore were not recruited. From the remaining participants, 7 were too ill and 7 were not interested in the study. Informed consent was then obtained from the remaining 208 participants. Of the 208 participants, 2 were subsequently found to be ineligible while 6 participants decided to withdraw from the study due to fatigue. One participant did not answer questions on self-blame and was excluded from the analyses related to self-blame. The final analytical sample consisted of 200 participants for perceived stigma and 199 participants for self-blame.
Fig. 1.
Recruitment flowchart
Survey development and outcomes
The questionnaire which was developed as part of the APPROACH study included questions developed by the study investigators in consultation with oncologists and questions taken from validated instruments. The questions were first developed in English (Additional file 1). For this study site, the questionnaire was then translated by professional translators into Vietnamese, and back-translated into English. The original and back-translated English versions were compared, and reconciliations made where necessary. Further revisions were made based on feedback gathered from the physicians and cognitive interviews with ten eligible patients from the study site.
Perceived stigma
Perceived stigma was measured by summing the 6 items on sense of stigma from Kissane’s Shame and Stigma Scale and transforming the total score on a scale of 0 to 100 [13]. A total score of 0 indicated no perceived stigma. A higher score indicated a higher level of perceived stigma. This scale displayed good internal consistency with a Cronbach’s alpha of 0.78.
Self-blame
Behavioural self-blame was identified by asking “How much do you blame yourself for any behaviour that may have led to your cancer?”. Characterological self-blame was assessed by asking “How much do you blame yourself for the kind of person you are (e.g., being the unlucky person who has things like cancer happen to them)?”. Four options were available: “1-Not at all”, “2-Somewhat”, “3-Very much” and “4-Completely”. The responses were dichotomized into presence (Somewhat/Very much/Completely) or absence (Not at all) of the respective self-blame. In addition, information on patient’s perceived reasons for cancer was collected. The patients were asked to choose from a list that contained the following options: smoking, chewing betel nut/tobacco, consumption of alcohol, being overweight, stress/anxiety, previous bad deeds, God’s will and old age. They were also allowed to specify any other reasons that were not in the list.
Depression
Depression was measured via a validated Vietnamese version of the 20-item scale developed by the Center for Epidemiologic Studies (CES-D). Possible score ranged between 0 and 60, with higher CES-D score indicating higher depressive symptoms. A CES-D score of 16 and above suggests the presence of depression [30]. The scale displayed strong reliability with a Cronbach’s alpha of 0.91.
Emotional well-being
Emotional well-being (EWB) was measured using the 6-item EWB component of the validated Vietnamese version of the Functional Assessment of Cancer Therapy-General (FACT-G) [31]. Participants were asked to rate their responses on a 5-point scale ranging from 0 to 4. The possible score for EWB ranged between 0 to 24. Higher EWB score indicated better emotional well-being. The EWB component demonstrated good internal consistency with a Cronbach's alpha of 0.84.
Social well-being
Social well-being (SWB) was assessed with the 7-item SWB component of FACT-G [31]. This component required participants to rate their responses on a 5-point scale which ranged between 0 and 4. The possible score for SWB ranged between 0 to 28. Higher SWB score indicated better social well-being. This SWB component had a good internal consistency with a Cronbach’s alpha of 0.74.
Patient characteristics
Socio-demographic characteristics such as age, sex, marital status, years of education, religion, and cancer type were captured through the survey. Financial distress was assessed by summing three questions [32]: (1) How well does the amount of money you have enable you to cover the cost of your treatment? (2) How well does the amount of money you have enable you to take care of your daily needs? (3) How well does the amount of money you have enable you to buy those little ‘extras’, that is, those small luxuries?. Three possible options were available for these three questions: “0-Very well”, “1-Fairly well”, “2-Poorly”. The possible score for financial distress ranged between 0 to 6, with higher scores reflecting higher financial distress. We also assessed participants’ awareness of disease severity by asking participants to report the current stage of their cancer. Patients who reported advanced stage was considered as having accurate awareness of disease severity. Type of cancer was identified from the medical records.
Utilization of and interest in using mental health services
Utilization of mental health services was assessed by asking if patients have seen any of the listed mental health care workers (e.g., psychiatrist, psychologist, medical social worker) as part of their cancer treatment. Interest in using mental health services was determined by asking patients to indicate if they would use mental health services if they were referred.
Statistical analysis and sample size
Demographic information was summarized with mean and standard deviation (SD) for continuous variables while categorical variables were presented with number, percentage and 95% confidence intervals (95 CI). To investigate the associations, we conducted separate multivariable linear regressions where dependent variables were depressive symptoms (CES-D), emotional well-being (EWB) or social well-being (SWB). The independent variables of interest were perceived stigma, behavioural self-blame or characterological self-blame. These analyses were controlled for patient demographics such as sex (male = 1, female = 0), age, marital status (married = 1, separated/widowed/divorced/never married = 0), years of education, religious affiliation (religious affiliation such as Christian, Buddhist or Taoist = 1, no religious affiliation = 0), financial distress, accurate awareness of disease severity (advanced stage = 1, early stage/don’t know = 0), and cancer type (lung cancer = 1, breast cancer = 1, colorectal cancer = 1, nasopharyngeal cancer = 1, other cancer types = 0).
The sample size of this study was found to be sufficient for the regressions used in this study, assuming an alpha error probability of 0.01 and power of 0.8. To control for Type 1 error due to multiple testing, critical significance level of 0.05 was adjusted according to the Holm’s method [33]. More information on the Holm’s method is available in Additional file 2. All analyses were conducted using Stata 15.
Results
Participant characteristics
Demographic information of study participants was tabulated in Table 1. Participants’ ages ranged from 22 to 87 years, with a mean age of 55.2 ± 11.1 years. There were slightly more male (53.5%, n = 107) than female participants. The two most common types of cancer among the participants were breast cancer (22.5%, n = 45) and lung cancer (22.0%, n = 44). Participants received 9.8 ± 3.4 years of education on average. Majority of the participants were married (85.5%, n = 171). Slightly less than half (47.0%, n = 94) of the participants reported having a religious affiliation. The average reported financial distress was 4.1 ± 2.0 on a scale of 0 to 6. Despite being aware of their cancer diagnosis, about half of the participants (48.5%, n = 97) were not aware that they were in the advanced stage of the cancer.
Table 1.
Demographics of study participants (N = 200)
| Characteristics | N (%) or mean ± SD |
|---|---|
| Age | 55.2 ± 11.1 |
| Sex | |
| Male | 107 (53.5%) |
| Female | 93 (46.5%) |
| Marital status | |
| Married | 171 (85.5%) |
| Separated, widowed, divorced, never married | 29 (14.5%) |
| Years of education received | 9.8 ± 3.4 |
| Having a religious affiliation | 94 (47.0%) |
| Cancer type | |
| Breast cancer | 45 (22.5%) |
| Lung cancer | 44 (22.0%) |
| Nasopharyngeal cancer | 21 (10.5%) |
| Colorectal cancer | 20 (10.0%) |
| Other cancers a | 70 (35.0%) |
| Financial distress | 4.1 ± 2.0 |
| Accurate awareness about disease severity (i.e. being at advanced stage) | |
| Aware | 103 (51.5%) |
| Not aware | 97 (48.5%) |
| Perceived reasons for cancer | |
| Old age | 158 (79.0%) |
| Smoking | 109 (54.5%) |
| Alcohol consumption | 102 (51.0%) |
| God’s will | 82 (41.0%) |
| Stress | 44 (22.0%) |
| Chewing betel nut/tobacco | 33 (16.5%) |
| Being overweight | 21 (10.5%) |
| Previous bad deeds | 13 (6.5%) |
| Other reasons b | 15 (7.5%) |
| Perceived stigma | |
| Prevalence | 158 (79.0%) |
| Score | 20.5 ± 18.0 |
| Prevalence of behavioural self-blame | 112 (56.3%) |
| Prevalence of characterological self-blame | 124 (62.3%) |
| Depression (CES-D) | |
| Patients with CES-D above 16 | 133 (66.5%) |
| Score | 20.2 ± 9.5 |
| Emotional well-being | 13.3 ± 5.6 |
| Social well-being | 21.6 ± 4.5 |
| Utilization of mental health services | |
| Ever used | 6 (3.0%) |
| Never used | 174 (87.0%) |
| Not sure | 20 (10.0%) |
| Interest in using mental health services should they be referred | |
| Interested | 47 (24.2%) |
| Not interested | 56 (28.9%) |
| Not sure | 91 (46.9%) |
aOther cancers included cancer at bladder, brain, cervical, gastric, kidney, liver, oesophageal, ovarian, oral, pancreas, prostate, soft palate, parotid gland, submandibular gland, melanoma, thymus, bile ducts or ampulla of Vater
bOther perceived reasons for cancer included genetic factors, environmental pollution, exposure to chemical substance including insecticide, type of food consumed, and consumption of contaminated food
More than three-fourths (79.0%, n = 158) of the participants reported perceived stigma. On a scale of 0 to 100 where higher score reflects higher perceived stigma, the average score for perceived stigma was 20.5 ± 18.0. More than half of the participants reported behavioural self-blame (56.3%, n = 112) or characterological self-blame (62.3%, n = 124). The top reasons cited as contributing factors of their disease included old age (79.0%, n = 158), smoking (54.5%, n = 109), alcohol consumption (51.0%, n = 1 02) and God’s will (41.0%, n = 82).
The average depressive symptoms score was 20.2 ± 9.5 (out of 60), and two-thirds of the participants (66.5%, n = 133) reported a score of 16 and above, indicative of potential presence of depression. The average emotional well-being score of the study participants were 13.3 ± 5.6 (out of 24). The average social well-being score was 21.6 ± 4.5 (out of 28).
Only six participants (3.0%) reported having received mental health services while 20 participants (10.0%) were not sure if they have received mental health services before. Among the 194 participants who have never used or are not sure if they have used mental health services, about one-quarter (24.2%, n = 47) indicated interest to use mental health services if they were referred.
Associations of perceived stigma and self-blame with depression, emotional well-being and social well-being
Consistent with our hypothesis, participants who reported higher perceived stigma had lower emotional well-being (ß = -0.1). However, our analyses showed no associations of perceived stigma with depressive symptoms and with social well-being (Table 2).
Table 2.
Associations of perceived stigma, behavioural self-blame, characterological self-blame with depression, emotional well-being, and social well-beinga
|
Coefficient (ß) |
95% confidence interval (95 CI) | p-value | |
|---|---|---|---|
| Perceived stigma | |||
| Model 1: Depressive symptoms | 0.1 | 0.1, 0.2 | 0.000 |
| Model 2: Emotional well-being | -0.0 * | -0.1, 0.0 | 0.024 |
| Model 3: Social well-being | 0.0 | -0.1, 0.0 | 0.098 |
| Presence of behavioral self-blame | |||
| Model 4: Depressive symptoms | 1.7 | -1.0, 4.4 | 0.225 |
| Model 5: Emotional well-being | -1.4 | -3.1, 0.2 | 0.088 |
| Model 6: Social well-being | -0.4 | -1.7, 0.9 | 0.538 |
| Presence of characterological self-blame | |||
| Model 7: Depressive symptoms | 3.0 * | 0.5, 5.5 | 0.020 |
| Model 8: Emotional well-being | -1.6 * | -3.1, -0.1 | 0.038 |
| Model 9: Social well-being | 0.8 | -0.4, 2.0 | 0.191 |
aMultivariable linear regressions controlled for gender, age, marital status, education, religion, financial distress, awareness of disease severity, and type of cancer
*denotes statistical significance after the Holm’s adjustment
Contrary to our hypotheses regarding behavioural self-blame, the findings showed no significant associations of behavioural self-blame with depressive symptoms, emotional well-being and social well-being. On the other hand, participants who experienced characterological self-blame reported greater depressive symptoms (ß = 2.7) and lower emotional well-being (ß = -1.8). However, no significant association was found between social well-being and characterological self-blame. Full list of estimates is available in Additional file 3.
Discussion
Our study showed that more than half of the Vietnamese advanced cancer patients in our sample reported perceived stigma, behavioural self-blame or characterological self-blame. Although the perceived stigma score reported by our study population may seem low, the figure was higher than those reported by cancer patients in other studies [13, 34]. Our findings also showed that perceived stigma was significantly associated with lower emotional well-being, consistent with other studies [12, 35].
Our findings showed that the relationship between self-blame and psychological outcomes depended on the type of blame, where only characterological self-blame was associated with greater depressive symptoms and lower emotional well-being. This finding is consistent with the Theory of Learned Helplessness which suggests that the perceived lack of control over an outcome such as cancer may affect an individual’s emotions and mental health negatively [36, 37]. The theory suggests that characterological self-blame is more detrimental because it is related to attributes that are relatively non-modifiable or non-controllable [37].
Based on these findings, intervention strategies to reduce self-blame may consider addressing characterological reasons. Although our findings on self-blame are consistent with some studies [19, 38], others reported that both types of self-blame were associated with poorer psychological outcomes [39–41]. The mixed findings in the literature suggest that cultural or other factors might be moderating the relationship between behavioural self-blame and psychological outcomes. This could be a topic for future studies.
Contrary to our hypothesis, social well-being was not significantly associated with perceived stigma, behavioural or characterological self-blame. This could be related to the relatively high social capital and social trust among Vietnamese as compared to countries with similar economic development [42]. In addition, as a society that is deeply rooted in Confucian philosophy, Vietnamese hold firm to the teaching that parents are to be respected regardless of their qualities or faults. Similarly, parents are expected to do their best for their children even at the expense of their own well-being [42]. These cultural norms might have prevented social well-being to decrease for cancer patients even when experiencing cancer stigma or self-blame.
Lastly, the fact that two-thirds of the participants reported a CES-D score of 16 and above, an indicator of possible depression, is worth noting. Equally alarming is the extremely low utilization rate of mental health services among this study population and the relatively low interest in using mental health services. Studies have reported that individuals experiencing a mental condition such as depression are not likely to seek help due to stigmatization and fear for discrimination [43–45]. Due to the lack of resources or lack of help-seeking behaviour, these individuals might not receive the necessary intervention to address their depression [46]. In view of this, it is important to ensure that the psychological needs of advanced cancer patients in Vietnam are adequately addressed through the provision of mental health services. This also echoes the calls of Lancet Commission on Palliative Care and Pain Relief Study Group to alleviate serious health-related suffering among terminally ill patients in low- and middle- income countries like Vietnam and reinforces the urgency of integrating an affordable Essential Package for palliative care into the national health system, which includes interventions to address the psychological needs of terminally ill patients [47].
Strengths and limitations
To the best of our knowledge, this study is the first to show presence of perceived stigma, behavioural self-blame and characterological self-blame among Vietnamese advanced cancer patients. There are several limitations to this study. First, self-blame was not assessed using an instrument that has been psychometrically validated. Second, we measured behavioural self-blame and characterological self-blame as dichotomous variables (presence versus absence of self-blame). However, the associations of self-blame with depression, and emotional and social well-being may vary based on the intensity of self-blame. Third, since 69 participants in this study reported both behavioural and characterological self-blame, it is possible that we were not able to separate the effects of behavioural self-blame and characterological self-blame on the psychosocial outcomes. Fourth, although the participants in this study were recruited from Hue Central Hospital which is one of the largest hospitals in the country, we acknowledge the possibility that the results may not be generalizable to patients who live in the rural areas of Vietnam. Lastly, as this was a cross-sectional study, our results only imply associations but not causations.
Implications
Our results demonstrated that patients who reported perceived stigma and characterological self-blame reported greater depressive symptoms and lower emotional well-being, reiterating the importance of assessing and addressing these issues. Although the coefficient for the association between CES-D score and characterological self-blame was not large, any additional increase in CES-D score should be given attention as the mean CES-D score of this population suggests presence of depression. It is also noteworthy that the effect sizes of the associations of characterological social blame with emotional well-being and social well-being were considered moderate based on a meta-analysis on the clinical relevance of changes in these scales [48]. Healthcare professionals involved in the direct care of advanced cancer patients are urged to include mental health assessment to screen for these issues as part of clinical routine. Our results suggest that patients who experience perceived stigma or characterological self-blame can benefit from interventions that address negative inner thoughts and teach coping mechanisms surrounding the issue of cancer stigma and characterological self-blame. Future studies should identify the predictors for individuals who are more susceptible to cancer-related perceived stigma and characterological self-blame amongst cancer patients in Vietnam. Knowing this allows not only health care providers to identify this vulnerable population, but also allows policy makers to design targeted approaches to reduce perceived stigma and self-blame among cancer patients in Vietnam.
Conclusion
This study serves as an initial situational analysis for policy makers and healthcare providers by providing evidence on the presence of perceived stigma, behavioural self-blame or characterological self-blame among Vietnamese advanced cancer patients. The findings show evidence that perceived stigma was associated with lower emotional well-being, and characterological self-blame was associated with higher depressive symptoms and lower emotional well-being. Early identification of patients who are affected by perceived stigma or self-blame is important to ensure that relevant interventions are offered to these patients in a timely manner.
Supplementary Information
Additional file 2. Holm’s correction for the p-values
Additional file 3. Full list of estimates
Acknowledgements
We thank patients who were interviewed as part of this study for their time and effort.
Abbreviations
- IARC
International Agency for Research on Cancer
- APPROACH
Asian Patient Perspectives Regarding Oncology Awareness, Care and Health
- NUS-IRB
National University Singapore-Institutional Review Board
- FACT-G
Functional Assessment of Cancer Therapy-General
- CES-D
Center for Epidemiologic Studies Depression scale
- EWB
Emotional well-being
- SWB
Social well-being
- SD
Standard deviation
- CI
95% Confidence intervals
Authors’ contributions
SO, NTP, JJL and IT conceptualized the paper. SO and JJL analysed the data. SO, NTP, JJL, NHP, TDQP, KT, HBD, IT, CM and EAF participated in the interpretation of the data and writing or critical review of the paper. All authors read and approved the final manuscript.
Funding
This study was funded by Lien Centre for Palliative Care. The study funder had no role in study design, data collection, analysis and interpretation, or writing of the manuscript. The publication of study results was not contingent on the sponsor's approval or censorship of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approvals were obtained from the National University of Singapore-Institutional Review Board (NUS-IRB B-15–319), Singapore and Hue Central Hospital Ethics Committee, Vietnam (230/QD-BVH-HDDD). Verbal informed consent, the default method of obtaining informed consent for similar studies in Vietnam, was obtained prior to the interview as approved by the ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nguyen Tuong Pham, Email: nguvien@yahoo.com.
Jia Jia Lee, Email: audrey.jiajia@duke-nus.edu.sg.
Nhu Hiep Pham, Email: phamnhuhiepdr@gmail.com.
Thi Do Quyen Phan, Email: doquyen_cl@yahoo.com.
Khoa Tran, Email: drkhoa666@gmail.com.
Hoai Bao Dang, Email: dr.baodang@gmail.com.
Irene Teo, Email: irene.teo@duke-nus.edu.sg.
Chetna Malhotra, Email: chetna.malhotra@duke-nus.edu.sg.
Eric A. Finkelstein, Email: eric.finkelstein@duke-nus.edu.sg
Semra Ozdemir, Email: semra.ozdemir@duke-nus.edu.sg.
References
- 1.Gonzalez BD, Jacobsen PB. Depression in lung cancer patients: the role of perceived stigma. Psychooncology. 2012;21(3):239–246. doi: 10.1002/pon.1882. [DOI] [PubMed] [Google Scholar]
- 2.Rose S, Kelly B, Boyes A, Cox M, Palazzi K, Paul C. Impact of perceived stigma in people newly diagnosed with lung cancer: a cross-sectional analysis. In: Oncology nursing forum: 2018. Oncology Nursing Society; 2018: 737–747. [DOI] [PubMed]
- 3.Tripathi L, Datta SS, Agrawal SK, Chatterjee S, Ahmed R. Stigma perceived by women following surgery for breast cancer. Indian J Med Paediatr Oncol. 2017;38(2):146. doi: 10.4103/ijmpo.ijmpo_74_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson TJ, Kwon DM, Riley KE, Shen MJ, Hamann HA, Ostroff JS. Lung cancer stigma: does smoking history matter? Ann Behav Med. 2020;54(7):535–540. doi: 10.1093/abm/kaz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettridge K, Bowden JA, Chambers SK, Smith DP, Murphy M, Evans SM, Roder D, Miller CL. “Prostate cancer is far more hidden…”: Perceptions of stigma, social isolation and help-seeking among men with prostate cancer. Eur J Cancer Care. 2018;27(2):e12790. doi: 10.1111/ecc.12790. [DOI] [PubMed] [Google Scholar]
- 6.Phelan SM, Griffin JM, Jackson GL, Zafar SY, Hellerstedt W, Stahre M, Nelson D, Zullig LL, Burgess DJ, Van Ryn M. Stigma, perceived blame, self-blame, and depressive symptoms in men with colorectal cancer. Psychooncology. 2013;22(1):65–73. doi: 10.1002/pon.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa D, Umezawa S, Fujimori M, Miyashita M. Prevalence and associated factors of perceived cancer-related stigma in Japanese cancer survivors. Jpn J Clin Oncol. 2020;50(11):1325–1329. doi: 10.1093/jjco/hyaa135. [DOI] [PubMed] [Google Scholar]
- 8.Doble B, Lau E, Malhotra C, Ozdemir S, Teo I, Finkelstein EA, Group AS. The association of self-blame with treatment preferences in a multi-country cohort of advanced cancer patients from the APPROACH study. J Psychosom Res. 2020;139:110284. doi: 10.1016/j.jpsychores.2020.110284. [DOI] [PubMed] [Google Scholar]
- 9.Weiss J, Yang H, Weiss S, Rigney M, Copeland A, King JC, Deal AM. Stigma, self-blame, and satisfaction with care among patients with lung cancer. J Psychosoc Oncol. 2017;35(2):166–179. doi: 10.1080/07347332.2016.1228095. [DOI] [PubMed] [Google Scholar]
- 10.Lee JL, Kim KS. The relationships between stigma, distress, and quality of life in patients with lung cancer. J Korean Oncol Nurs. 2011;11(3):237–246. doi: 10.5388/jkon.2011.11.3.237. [DOI] [Google Scholar]
- 11.Nipp RD, El-Jawahri A, Fishbein JN, Eusebio J, Stagl JM, Gallagher ER, Park ER, Jackson VA, Pirl WF, Greer JAJC. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer. 2016;122(13):2110–2116. doi: 10.1002/cncr.30025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst J, Mehnert A, Dietz A, Hornemann B, Esser P. Perceived stigmatization and its impact on quality of life-results from a large register-based study including breast, colon, prostate and lung cancer patients. BMC Cancer. 2017;17(1):741. doi: 10.1186/s12885-017-3742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissane DW, Patel SG, Baser RE, Bell R, Farberov M, Ostroff JS, Li Y, Singh B, Kraus DH, Shah JP. Preliminary evaluation of the reliability and validity of the Shame and Stigma Scale in head and neck cancer. Head Neck. 2013;35(2):172–183. doi: 10.1002/hed.22943. [DOI] [PubMed] [Google Scholar]
- 14.Janoff-Bulman R. Characterological versus behavioral self-blame: inquiries into depression and rape. J Pers Soc Psychol. 1979;37(10):1798. doi: 10.1037/0022-3514.37.10.1798. [DOI] [PubMed] [Google Scholar]
- 15.Brunault P, Champagne AL, Huguet G, Suzanne I, Senon JL, Body G, Rusch E, Magnin G, Voyer M, Réveillère C. Major depressive disorder, personality disorders, and coping strategies are independent risk factors for lower quality of life in non-metastatic breast cancer patients. Psychooncology. 2016;25(5):513–520. doi: 10.1002/pon.3947. [DOI] [PubMed] [Google Scholar]
- 16.AguadoLoi CX, Baldwin JA, McDermott RJ, McMillan S, Martinez Tyson D, Yampolskaya S, VandeWeerd C. Risk factors associated with increased depressive symptoms among Latinas diagnosed with breast cancer within 5 years of survivorship. Psychooncology. 2013;22(12):2779–2788. doi: 10.1002/pon.3357. [DOI] [PubMed] [Google Scholar]
- 17.Kitano A, Yamauchi H, Hosaka T, Yagata H, Hosokawa K, Ohde S, Nakamura S, Takimoto M, Tsunoda H. Psychological impact of breast cancer screening in Japan. Int J Clin Oncol. 2015;20(6):1110–1116. doi: 10.1007/s10147-015-0845-0. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Zhu X, Yang Y, He J, Yi J, Wang Y, Zhang J. Cognitive emotion regulation: characteristics and effect on quality of life in women with breast cancer. Health Qual Life Outcomes. 2015;13(1):51. doi: 10.1186/s12955-015-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plaufcan MR, Wamboldt FS, Holm KE. Behavioral and characterological self-blame in chronic obstructive pulmonary disease. J Psychosom Res. 2012;72(1):78–83. doi: 10.1016/j.jpsychores.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tangney JP, Dearing RL. Shame and guilt. New York: Guilford Press; 2003. [Google Scholar]
- 21.Dein S. Explanatory models of and attitudes towards cancer in different cultures. Lancet Oncol. 2004;5(2):119–124. doi: 10.1016/S1470-2045(04)01386-5. [DOI] [PubMed] [Google Scholar]
- 22.Lui C-W, Ip D, Chui WH. Ethnic experience of cancer: a qualitative study of Chinese-Australians in Brisbane Queensland. Soc Work Health Care. 2009;48(1):14–37. doi: 10.1080/00981380802440403. [DOI] [PubMed] [Google Scholar]
- 23.Wong-Kim E, Sun A, Merighi JR, Chow EA. Understanding quality-of-life issues in Chinese women with breast cancer: a qualitative investigation. Cancer Control. 2005;12(4_suppl):6–12. doi: 10.1177/1073274805012004S02. [DOI] [PubMed] [Google Scholar]
- 24.Pang A, Ho S, Lee S-C. Cancer physicians’ attitude towards treatment of the elderly cancer patient in a developed Asian country. BMC Geriatr. 2013;13(1):1–8. doi: 10.1186/1471-2318-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong A, Shin DW, Kim SY, Yang HK, Park JH. Avoidance of cancer communication, perceived social support, and anxiety and depression among patients with cancer. Psychooncology. 2016;25(11):1301–1307. doi: 10.1002/pon.4060. [DOI] [PubMed] [Google Scholar]
- 26.Global Cancer Observatory—Vietnam population fact sheets. [http://gco.iarc.fr/today/data/factsheets/populations/704-viet-nam-fact-sheets.pdf].
- 27.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 28.Anh PTH, Duc NB. The situation with cancer control in Vietnam. Jpn J Clin Oncol. 2002;32(suppl_1):S92–S97. doi: 10.1093/jjco/hye127. [DOI] [PubMed] [Google Scholar]
- 29.Jacob J, Palat G, Verghese N, Chandran P, Rapelli V, Kumari S, Malhotra C, Teo I, Finkelstein E, Ozdemir S. Health-related quality of life and its socio-economic and cultural predictors among advanced cancer patients: evidence from the APPROACH cross-sectional survey in Hyderabad-India. BMC Palliative Care. 2019;18(1):94. doi: 10.1186/s12904-019-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas JL, Jones GN, Scarinci IC, Mehan DJ, Brantley PJ. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. Int J Psychiatry Med. 2001;31(1):25–40. doi: 10.2190/FUFR-PK9F-6U10-JXRK. [DOI] [PubMed] [Google Scholar]
- 31.D C: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT Scales) (Version 4.1). Evanston: Center on Outcomes Research and Education (CORE) Evanston Northwestern Healthcare; 2004.
- 32.Malhotra C, Harding R, Teo I, Ozdemir S, Koh GC, Neo P, Lee LH, Kanesvaran R, Finkelstein E. Financial difficulties are associated with greater total pain and suffering among patients with advanced cancer: results from the COMPASS study. Support Care Cancer. 2019;28:1–9. doi: 10.1007/s00520-019-05208-y. [DOI] [PubMed] [Google Scholar]
- 33.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979:65–70.
- 34.Tseng W-T, Lee Y, Hung C-F, Lin P-Y, Chien C-Y, Chuang H-C, Fang F-M, Li S-H, Huang T-L, Chong M-Y. Validation of the Chinese version of the shame and stigma scale in patients with head and neck cancer. Cancer Manage Res. 2019;11:10297. doi: 10.2147/CMAR.S228843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graves KD, Jensen RE, Cañar J, Perret-Gentil M, Leventhal K-G, Gonzalez F, Caicedo L, Jandorf L, Kelly S, Mandelblatt J. Through the lens of culture: quality of life among Latina breast cancer survivors. Breast Cancer Res Treat. 2012;136(2):603–613. doi: 10.1007/s10549-012-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maier SF, Seligman ME. Learned helplessness: theory and evidence. J Exp Psychol Gen. 1976;105(1):3. doi: 10.1037/0096-3445.105.1.3. [DOI] [Google Scholar]
- 37.Else-Quest NM, LoConte NK, Schiller JH, Hyde JS. Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychol Health. 2009;24(8):949–964. doi: 10.1080/08870440802074664. [DOI] [PubMed] [Google Scholar]
- 38.Tilghman-Osborne C, Cole DA, Felton JW, Ciesla JA. Relation of guilt, shame, behavioral and characterological self-blame to depressive symptoms in adolescents over time. J Soc Clin Psychol. 2008;27(8):809–842. doi: 10.1521/jscp.2008.27.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett KK, Compas BE, Beckjord E, Glinder JG. Self-blame and distress among women with newly diagnosed breast cancer . J Behav Med. 2005;28(4):313–323. doi: 10.1007/s10865-005-9000-0. [DOI] [PubMed] [Google Scholar]
- 40.Glinder JG, Compas BE. Self-blame attributions in women with newly diagnosed breast cancer: a prospective study of psychological adjustment. Health Psychol. 1999;18(5):475. doi: 10.1037/0278-6133.18.5.475. [DOI] [PubMed] [Google Scholar]
- 41.Malcarne VL, Compas BE, Epping-Jordan JE, Howell DC. Cognitive factors in adjustment to cancer: attributions of self-blame and perceptions of control. J Behav Med. 1995;18(5):401–417. doi: 10.1007/BF01904771. [DOI] [PubMed] [Google Scholar]
- 42.Dalton R, Ong N-N, Hac PM, Nghi PT. Social relations and social capital in Vietnam: findings from the 2001 World Values Survey. Comp Sociol. 2002;1(3–4):369–386. doi: 10.1163/156913302100418646. [DOI] [Google Scholar]
- 43.Mahalik JR, Di Bianca M. Help-seeking for depression as a stigmatized threat to masculinity. Prof Psychol Res Pract. 2021;52:146–55. doi: 10.1037/pro0000365. [DOI] [Google Scholar]
- 44.Ibrahim N, Amit N, Shahar S, Wee L-H, Ismail R, Khairuddin R, Siau CS, Safien AM. Do depression literacy, mental illness beliefs and stigma influence mental health help-seeking attitude? A cross-sectional study of secondary school and university students from B40 households in Malaysia. BMC Public Health. 2019;19(4):1–8. doi: 10.1186/s12889-019-6862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnyder N, Panczak R, Groth N, Schultze-Lutter F. Association between mental health-related stigma and active help-seeking: systematic review and meta-analysis. Br J Psychiatry. 2017;210(4):261–268. doi: 10.1192/bjp.bp.116.189464. [DOI] [PubMed] [Google Scholar]
- 46.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5(8):466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 47.Knaul FM, Farmer PE, Krakauer EL, De Lima L, Bhadelia A, Kwete XJ, Arreola-Ornelas H, Gómez-Dantés O, Rodriguez NM, Alleyne GA. Alleviating the access abyss in palliative care and pain relief—an imperative of universal health coverage: the Lancet Commission report. Lancet. 2018;391(10128):1391–1454. doi: 10.1016/S0140-6736(17)32513-8. [DOI] [PubMed] [Google Scholar]
- 48.King MT, Cella D, Osoba D, Stockler M, Eton D, Thompson J, Eisenstein A. Meta-analysis provides evidence-based interpretation guidelines for the clinical significance of mean differences for the FACT-G, a cancer-specific quality of life questionnaire. Patient Relat Outcome Meas. 2010;1:119. doi: 10.2147/PROM.S10621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Holm’s correction for the p-values
Additional file 3. Full list of estimates
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.