Abstract
Vaccine-associated cerebral venous thrombosis has become an issue following the extensive vaccination program of the Coronavirus Disease of 2019 (COVID-19) Vaccine AstraZeneca (ChAdOx1 vaccine). The importance of early diagnosis should be emphasized due to the high mortality rate without appropriate treatment. Young female populations in western countries have been reported to be at a greater risk of this vaccine related thrombotic event, but cases in East Asia are lacking. Herein, we present the first case of cerebral venous sinus thrombosis 10 days after ChAdOx1 vaccination in a middle-age Asian male in Taiwan.
Keywords: COVID19, ChAdOx1 vaccine, Thrombocytopenia, Cerebral venous sinus thrombosis, Emergency department
1. Introduction
Cerebral venous sinus thrombosis (CVST), a rare form of stroke (0.5%–1%), has been recently found to be associated with COVID-19 vaccination with the ChAdOx1 vaccine. As of April 2021, 40 cases had been reported among 38.1 million recipients in the UK and EEA by the European Medicine Agency (EMA) and the Medicines and Healthcare Products Regulatory Agency (MHRA). The majority of such cases have been female, under the age of 55, and receiving anticoagulation and have occurred between 4 and 16 days after vaccination [1]. Vaccine-associated CVST (VACVST) carries considerable thrombotic risk, despite only mild to moderate thrombocytopenia, and the mortality of VACVST is 29–33%, significantly higher than the usual mortality of 4.4% [2,3]. Immediate and appropriate treatment with non-heparin anticoagulant is associated with better short-term survival and long-term prognosis. Therefore, early diagnosis of CVST is vital. Past studies have reported that Asians carried a lower risk of venous thromboembolism than other races [4]. Unlike reports from the US and Europe, a report of VACVST had not yet been published with regard to the Asian population. In this case report, we present the first East Asian case of VACVST involving the left internal jugular vein with thrombotic thrombocytopenia in a middle-age Asian male in an emergency department (ED) in Taiwan.
2. Case report
A 52-year-old man with no underlying disease presented to the ED with nausea and thunderclap headache for 5 days. He also claimed pain on the left side of his neck, but he had no other symptoms. He received the ChAdOx1 vaccine 10 days prior, and headache developed gradually 5 days after vaccination. The patient's vital sign were as follows: body temperature 35.4 °C, blood pressure 129/90 mmHg, heart rate 67/min, respiratory rate 18/min. Physical examination revealed no neurological deficit with normal extra-ocular movement and visual power without photophobia, phonophobia, and meningeal signs. Laboratory work up revealed thrombocytopenia (Platelet 99*109/L) and elevated d-dimer (>20.0 mg/L) but was otherwise normal. Hyperdensity of the sinus, including Cord sign and dense vein sign at the left transverse and sigmoid sinuses, was discovered via non-enhanced computer tomography (CT) (Fig. 1 ). CT venogram revealed CVST at the left transverse sinus and sigmoid sinuses and thrombosis of the left internal jugular vein (Fig. 2 ). A diagnosis of VACVST with thrombotic thrombocytopenia was made. The oral anticoagulant agent Apixaban was immediately administered. Platelet factor 4 (PF-4) enzyme-linked immunosorbent assay (ELLISA) was confirmed positive 2 days after admission.
Fig. 1.
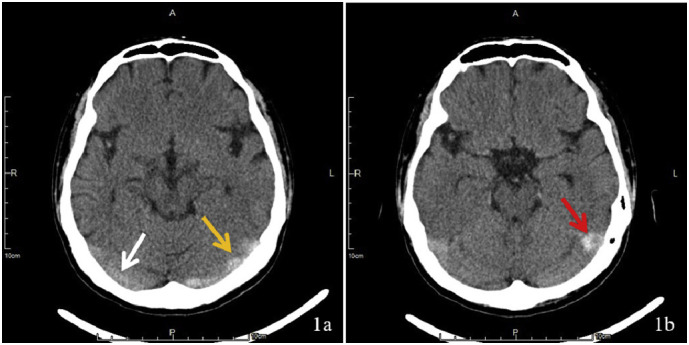
1a shows the hyperdensity of the left transverse sinus (yellow arrow) compared to the right transverse sinus (white arrow). 1b represents hyperdensity, with focal hypodensity in the distal left sigmoid sinus (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
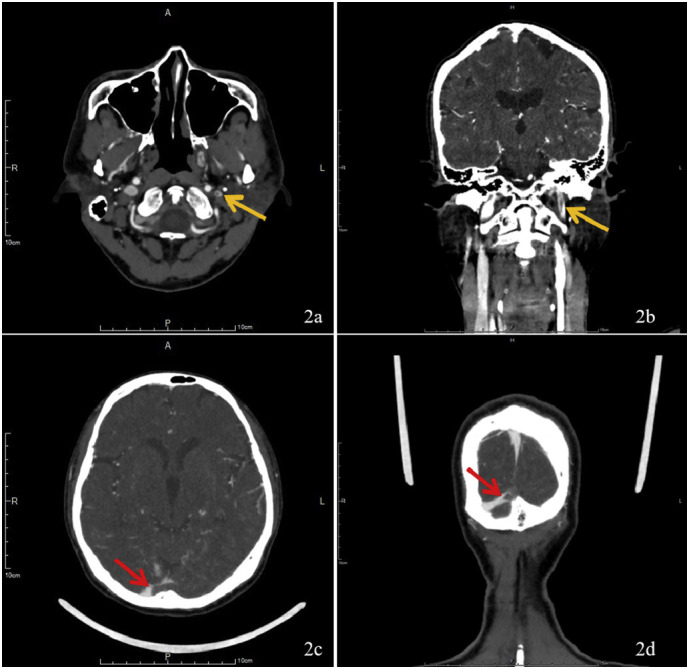
2a & 2b represent the filling defect (yellow arrow) in the left jugular vein compared to the right jugular vein. 2c & 2d show the filling defect (red arrow) in confluence of the sinus and represent the lack of contrast medium in the left transverse sinus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
The catastrophic COVID-19 pandemic has been ravaging the world since November 2019, infecting more than 171,000,000 people and causing over 3,570,000 deaths. The lack of valuable therapy for COVID-19 highlights the importance of an extensive vaccination program as the most powerful way to limit illness and death among this global crisis. Nevertheless, venous thromboembolism including CVST after vaccination has become a recent concern, and 40 cases among 38.1 million recipients of the ChAdOx1 vaccine have been reported in the UK and EEA by the EMA and MHRA. Based on the current information, the benefits of vaccination still outweigh the potential risks [1].
In the past, studies have discovered a lower risk of venous thromboembolism in Asians than in Caucasians [4]. Such a finding suggests the presence of acquired or genetic protective traits in East and Southeast Asian ethnic groups [5]. At the time of writing, no VACVST had been reported in most areas in Asia. This case report is the first of ChAdOx1 vaccine-related VACVST among more than 624,000 recipients in Taiwan.
Vaccine-associated cerebral venous sinus thrombosis is characterized by exposure to the ChAdOx1 vaccine 4–30 days prior to presentation and has a unique presentation compared to ordinary CVST. The EMA has indicated that the majority of sufferers are female gender, under the age of 55, receiving oral contraceptives, and receiving anticoagulation, and the event has typically occurred between 4 and 16 days after vaccination [1]. The pathophysiology of VACVST is believed to be associated with autoantibody, and, though still unclear, is thought to share a similar mechanism with Heparin-induced-thrombocytopenia (HIT), a process in which anti-PF-4 antibodies create a complex with the CXCL4 platelet factor and bind to the thrombocyte membrane's Fcγ-receptor IIa to cause thrombotic thrombocytopenia [6,7]. A current study of HIT prevalence in the Taiwan population also revealed a 13% carrier of anti-PF-4 antibody despite heparin exposure [8]. Like HIT, the clinical diagnosis of VACVST can be evaluated via adapted 4Ts score, which is determined by thrombocytopenia, timing after vaccination, thrombotic event or d-dimer level, and exclusion of other causes of thrombocytopenia. We used a positive finding of anti PF-4 antibody as confirmation. Our case both reached a maximum point of adapted 4Ts score and also carried the anti PF-4 antibody. Early treatment recommendations include such anticoagulants as danaparoid, argatroban, and possibly fondaparinux, but not heparin. Intravenous immunoglobulin (IVIG) should be initiated for ongoing thrombocytopenia, but only after confirmatory PF-4 ELLISA testing has been obtained since IVIG may cause false-negative test results. Fibrinogen should be corrected to >1.5 g/L with fibrinogen concentrate or cryoprecipitate. Antiplatelet agents are not recommended for treatment [6,9]. In our case, deterioration of thrombocytopenia developed after admission (decreased to 58*109/L), and IVIG 1 mg/kg was administered for 2 days (total dose 140 mg). The condition of thrombocytopenia subsequently improved, and the patient was discharged 10 days after admission, free of any symptoms.
In conclusion, although VACVST was announced by the EMA Pharmacovigilance Risk Assessment Committee, the overall risk of blood clots is not increased, and vaccination remains appropriate compared with the risks of the COVID-19 pandemic [1]. Nevertheless, clinicians should still be aware of fatal thrombotic events such as CVST with patients who have received the ChAdOx1 vaccine in all populations, even in the low-risk group of Asians.
References
- 1.Pinho A. COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low platelets. Eur. Med. Agency Publ. 2021;18 [Google Scholar]
- 2.European Medicines Agency Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant]) – COVID-19 Vaccine AstraZeneca (Other viral vaccines) 2021. https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-reportembolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant-covid_en.pdf;
- 3.Medicines and Healthcare products Regulatory Agency Coronavirus vaccine - weekly summary of Yellow Card reporting. 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting;
- 4.Klatsky A.L., Armstrong M.A., Poggi J. Risk of pulmonary embolism and/or deep venous thrombosis in Asian-Americans. Am J Cardiol. 2000;85(11):1334–1337. doi: 10.1016/s0002-9149(00)00766-9. [DOI] [PubMed] [Google Scholar]
- 5.Tran H.N., Klatsky A.L. Lower risk of venous thromboembolism in multiple Asian ethnic groups. Prev Med Rep. 2019;13:268–269. doi: 10.1016/j.pmedr.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan M., Berger J.S. Heparin-induced thrombocytopenia (HIT): review of incidence, diagnosis, and management. Vasc Med. 2020;25(2):160–173. doi: 10.1177/1358863X19898253. [DOI] [PubMed] [Google Scholar]
- 7.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y.-C., Lin C.-Y., Tsai C.-S. The frequency of heparin-induced thrombocytopenia in Taiwanese patients undergoing cardiopulmonary bypass surgery. J Formos Med Assoc. 2015;114(10):981–987. doi: 10.1016/j.jfma.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg J., Klamroth R., Langer F., Albisetti M., von Auer C., Ay C., et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41(3):184–189. doi: 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]


