Abstract
The intracellular bacterial pathogen Chlamydia trachomatis undergoes a developmental cycle consisting of two morphologically discrete developmental forms. The non-replicative elementary body (EB) initiates infection of the host. Once inside, the EB differentiates into the reticulate body (RB). The RB then undergoes multiple rounds of replication, before differentiating back to the infectious EB form. This cycle is essential for chlamydial survival as failure to switch between cell types prevents either host invasion or replication.
Limitations in genetic techniques due to the obligate intracellular nature of Chlamydia have hampered identification of the molecular mechanisms involved in the cell-type development. We designed a novel dual promoter-reporter plasmid system that, in conjunction with live-cell microscopy, allows for the visualization of cell type switching in real time. To identify genes involved in the regulation of cell-type development, the live-cell promoter-reporter system was leveraged for the development of a forward genetic approach by combining chemical mutagenesis of the dual reporter strain, imaging and tracking of Chlamydia with altered developmental kinetics, followed by clonal isolation of mutants. This forward genetic workflow is a flexible tool that can be modified for directed interrogation into a wide range of genetic pathways.
Introduction
Chlamydia trachomatis (Ctr) is an obligate intracellular pathogen that progresses through a biphasic developmental cycle that is essential for its survival and proliferation1 . This cycle consists of two developmental forms, the elementary body (EB) and the reticulate body (RB). The EB is replication incompetent but mediates cell invasion through effector induced endocytosis2 . Once in the host, the EB matures to the replicative RB. The RB carries out multiple rounds of replication prior to converting back to the EB in order to initiate subsequent rounds of infection.
The limited array of genetic tools has restricted most of the chlamydial research to biochemical studies or the use of surrogate systems. As a consequence, elucidation of gene regulation and control of the developmental cycle has been difficult3 , 4 . One of the more important challenges in the chlamydial field is the high resolution temporal tracking of the chlamydial developmental cycle and the identification of the proteins involved in its regulation. Gene expression during the chlamydial developmental cycle has traditionally been performed by destructive “end point” methods including RNAseq, qPCR, and fixed cell microscopy5 , 6 . Although these methods have provided invaluable information, the techniques employed are laborious and have low temporal resolution5 , 6 .
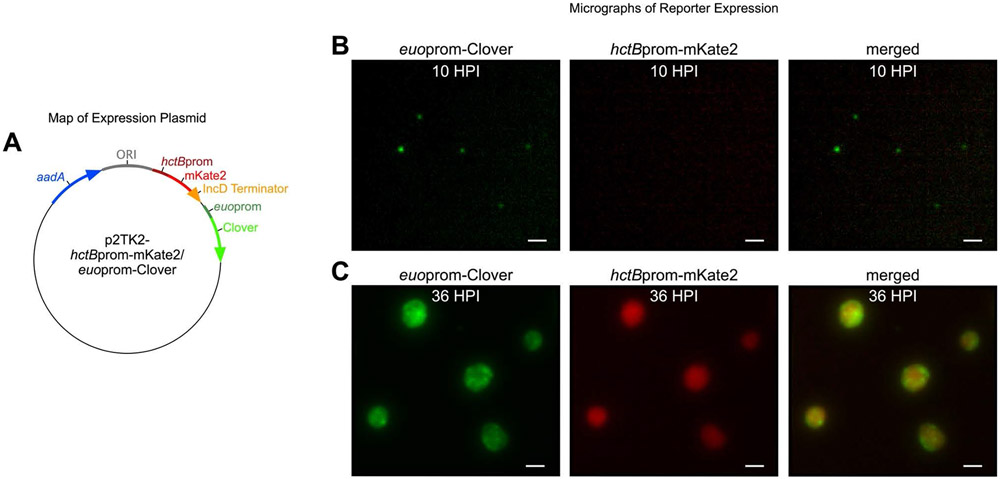
Within the last decade, genetic manipulation of Ctr has progressed with the introduction of plasmid transformation and methods for mutagenesis7 , 8 , 9 . For this study, a plasmid-based system was developed to monitor chlamydial development in individual inclusions in real time over the course of an infection. A chlamydial transformant was created that expressed both an RB and EB cell-type specific promoter-reporter. The RB specific reporter was constructed by fusing the promoter of the early RB gene euo upstream of the fluorescent protein Clover. EUO is a transcriptional regulator that represses a subset of late EB associated genes10 . The promoter of hctB, which encodes a histone-like protein involved in EB nucleoid condensation, was cloned directly upstream of mKate2 (RFP) to create the EB specific reporter11 . The backbone for hctBprom-mKate2/euoprom-Clover was p2TK2SW27 . The hctB and euo promoters were amplified from Ctr-L2 genomic DNA. Each promoter sequence consisted of ~100 base pairs upstream of the predicted transcription start site for the specified chlamydial gene plus the first 30 nucleotide (10 amino acids) of the respective ORF. The fluorescent FP variants were commercially obtained as Ctr codon optimized gene blocks and cloned in frame with the first 30 nucleotide of each chlamydial gene and promoter. The incD terminator was cloned directly downstream of mKate2. The second promoter-reporter was inserted downstream of the incD terminator. The ampicillin resistance gene (bla) in p2TK2SW2 was replaced with the aadA gene (Spectinomycin resistance) from pBam4. This resulted in the final construct p2TK2-hctBprom-mKate2/euoprom-Clover (Figure 1A) that was transformed into Ctr-L27 . This RB/EB reporter strain allowed for the observation of the developmental cycle within single inclusions using live-cell microscopy (Figure 1B,C).
Figure 1: Monitoring cell-type development with Ctr promoter-reporters.
(A) Schematic of the promoter-reporter construct, p2TK2-hctBprom-mKate2/euoprom-Clover. (B) Live-cell micrograph of euoprom-Clover and hctBprom-mKate2 expression in Ctr at 10 HPI (C) Live-cell micrograph of Ctr expressing euoprom-Clover and hctBprom-mKate2 at 36 HPI. Scale bar: 20 μm.
Employing our promoter-reporter construct in combination with chemical mutagenesis, a protocol was devised to track and isolate individual clones that exhibited developmental abnormalities from mutagenized populations of Ctr serovar L2. This protocol allows for the direct monitoring of individual chlamydial inclusions, tracking of the gene expression profiles over time, identifying chlamydial clones that express an altered developmental gene expression pattern, and clonal isolation of Chlamydia from individual inclusions.
Although this protocol has been created specifically for the identification of genes involved in chlamydial development, it could be easily adapted to interrogate any number of chlamydial genetic pathways.
Protocol
All Python scripts used in this protocol are available on Github https://github.com/SGrasshopper/Live-cell-data-processing
1. Mutagenize Reporter Chlamydia
NOTE: Ctr-L2-hctBprom-mKate2/euoprom-Clover EBs were directly mutagenized using ethyl methanesulfonate (EMS) in the axenic media CIP-1 as this media supports EB metabolism and maintenance of EB infectivity12 .
-
Thaw a chlamydial stock on ice containing ~3 x 107 EBs transformed with the p2TK2-hctBprom-mKate2/euoprom-Clover reporter plasmid and pellet at >14,000 x g for 30 min at 4 °C.
NOTE: Chlamydia organisms used for these experiments were 30% renografin density purified and frozen at −80 °C in 1x sucrose-phosphate-glutamate buffer (SPG).
Discard the supernatant and resuspend the EB pellet in 100 μL of CIP-1 buffer with sonication on ice at 10% power for 10 s. Divide the 100 μL of EB suspension into two 50 μL aliquots for mutagenized and mock treated samples.
Prepare 20 mg/mL of EMS-CIP-1 solution in a separate 1.5 mL microcentrifuge tube. To do so, add 6.8 μL of EMS in 375 μL total volume.
-
Add 50 μL of the EMS-CIP-1 solution into one of the chlamydial aliquots for mutagenesis and 50 μL of CIP-1 only to the other chlamydial aliquot for mock mutagenesis.
NOTE: Final EMS concentration is 10 mg/mL. The chlamydial titer, EMS concentration, and the time of exposure used in this protocol lead to approximately a 60-80% reduction in infectious progeny. This level of reduction corresponds to ~5-20 DNA lesions per chlamydial genome8 .
-
Incubate for 20 min at room temperature. The mutagenized EBs will be used directly to infect monolayers in section 2.
CAUTION: EMS is a known carcinogen. All equipment and materials that come in contact with EMS must be soaked in 1 M NaOH for 24 h before disposal, gloves should be used at all times during the protocol and cleanup of EMS materials.
2. Imaging of mutant Ctr
1. Host cell culture for imaging and isolation of mutagenized Ctr
Seed a 6 well glass bottom plate with 6 x 105 Cos-7 cells (ATCC) per well in 2 mL of complete media (RPMI-1640 supplemented with 10% fetal bovine serum and 10 mg/mL gentamicin). Use this glass bottom plate for imaging of mutagenized Ctr.
Seed a 24 well polystyrene plate with 1 x 105 Cos-7 cells (ATCC) per well in 1 mL complete media. Use this polystyrene plate for reinfection of isolated Chlamydia of interest.
Incubate both the plates at 5% CO2, 37 °C for approximately 18 h. Once cells reach confluency, replace media with complete media supplemented with 1 μg/mL of cycloheximide and incubate overnight.
2. Infecting the host cell culture with mutagenized Ctr
Infect 5 wells of the glass bottom plate with ~6 x 105 of mutagenized EBs in 1.5 mL/well ice cold HBSS. This will result in the MOI of ~ 0.3 as ~70% mortality rate is expected due to mutagenesis.
-
Infect the remaining well with ~2 x 105 mock mutagenized EBs in 1.5 mL/well ice cold HBSS. Without mutagenesis, expect less mortality, thus one-third of the inoculum is used to achieve the MOI of ~0.3.
NOTE: MOI of ~0.3 ensures that host cells are infected by a single EB and allows for the enough separation between infected cells for clonal isolation.
Incubate the plate for 15 min, with rocking, at 37 °C.
-
Wash the infected host cells with prewarmed (37 °C) HBSS containing 1 mg/mL heparin followed immediately by an HBSS rinse. Repeat heparin wash, immediately rinsing 2x with HBSS to ensure the heparin is removed.
NOTE: Heparin inhibits and can reverse the early electrostatic interactions between the host cell and EBs13 . The heparin washes remove EBs that have yet to enter the host cells, synchronizing the infection. When washing cells do so gently to prevent dislodging the cells from the surface of the wells. HBSS and heparin solutions contain residual EMS and should be placed in a beaker containing 1 M NaOH for 24 h before disposal.
Replace HBSS with 4 mL/well of prewarmed (37 °C) imaging media (complete media, 1 μg/mL cycloheximide, 20 mM HEPES, and no phenol red).
Fill the interwell spaces with prewarmed (37 °C) deionized H2O to aid in the temperature control and reduce evaporation. Incubate the plate at 37 °C incubator with 5% CO2 for 10 h.
3. Microscope set up and imaging
NOTE: Multicolor multiposition automated live-cell fluorescent imaging is used to collect time-lapse images to identify chlamydial mutants that differ in the developmental gene expression dynamics. This protocol utilizes the open source μManager software package for automated microscope control14 .
Begin the microscope setup 10 h post infection. Set the microscope stage incubator to 5% CO2, 37 °C. Place the infected 6 well glass bottom plate into the stage incubator and insert the sample thermistor into the interwell H2O.
Calibrate the XY stage using the High Content Screening (HCS) plugin. Click Plugins ∣ Acquisition Tools ∣ HCS Site Generator in the μManager microscope control software (JoVE61365_screenfile1, JoVE61365_screenfile2).
-
Select the 6-well plate template and generate an imaging position list consisting of 12 fields of view (FOV) per well within the HCS plugin (JoVE61365_screenfile3, JoVE61365_screenfile4). Open the Stage Position List and manually focus and set the initial Z position for each FOV using the Stage Control plugin (JoVE61365_screenfile5, JoVE61365_screenfile6). Adjust the XY coordinates of any FOV that has missing cells or does not contain a uniform monolayer.
NOTE: Due to the time it takes for each image to be captured, a maximum number of 72 FOV can be taken per 30 min interval.
Use a 20x objective lens for imaging. This magnification allows ~8 inclusions to be imaged per FOV while still providing the desired resolution.
Save the positions list as this will be used to locate the inclusions of interest after data analysis (JoVE61365_screenfile7).
Use the Auto Focus option in the imaging software, to set the focus for automated imaging (JoVE61365_screenfile8).
-
Use the following selections and values to produce the most consistent focus results using image based autofocus in μManager. In the Autofocus properties window select OughtaFocus from the drop-down menu and use the following settings. OughtaFocus-SearchRange_μm: 350, OughtaFocus-Tolerance_μm: 0.5, OughtaFocusCropFactor: 0.3, OughtaFocus-Exposure: 20, OughtaFocus-FFTLowerCutoff(%): 2.5, OughtaFocus-FFTUpperCutoff(%): 14, OughtaFocus-ShowImages: Yes, OughtaFocus-Maximize: SharpEdges, OughtaFocus-Channel: DIC.
NOTE: Reducing the autofocus imaging window by decreasing the crop factor allows for more consistent auto focusing. Selecting Yes for OughtaFocus-ShowImages allows the user to view the autofocus image.
Capture the kinetics of the developmental cycle by imaging for 24 h with 30 min time intervals (JoVE61365_screenfile9). Imaging between 12-36 HPI ensures that Chlamydia completes the developmental cycle but does not lyse the host cell.
-
Image the cell monolayers with a 250 ms exposure at 4% and 18% intensity in the GFP and RFP channels, respectively (JoVE61365_screenfile10). Detect the Clover (GFP) signal by excitation at 470 nm with a 514/30 nm bandpass emissions filter. Detect the mKate2 (RFP) signal by excitation at 595 nm and with a 641/75 nm bandpass emissions filter.
NOTE: Minimizing the excitation intensity is critical for minimizing fluorophore photobleaching and phototoxicity to Chlamydia. The minimum excitation intensity to generate a resolved image with a 200-300 ms exposure should be determined empirically in pilot studies.
Capture multiple Z-slices with a range of focus that ends on either side of the in-focus slice. In this experiment, at 20x magnification, this was achieved with 4 slices at 10 μm steps (JoVE61365_screenfile11).
-
Select Relative Z for imaging multiple slices in the acquisition window. The relative Z option uses the Z plane location saved in the imaging position list as the starting point for the next time interval. Input the appropriate Z-offset values for fluorescence imaging channels (JoVE61365_screenfile12).
NOTE: The image based focusing system is imperfect and over a 24 h imaging period this leads to focus drift. It was found that by capturing 3-4 Z focal planes for each time point an in-focus image was maintained. Z-offset is needed to correct for the differences in focal planes between the fluorescent image channels and the DIC channel. Empiric determination of this Z-offset will be needed.
Save images by selecting the root directory and naming the experiment. Use the μManager image stack file option to save images as tiff stack files (JoVE61365_screenfile13).
-
Record the experimental details in the Acquisitions Comments box in the Multi-D Acquisition window (JoVE61365_screenfile13). i.e., Well A1: Untreated control. Well A2-3 and B1-3: EMS Mutants. Imaging: 12-36HPI. Start the image acquisition at 12 HPI.
NOTE: If using μManager, leave the program, experimental setup, and microscope hardware running after the experiment is complete. The imaging sites will be revisited for inclusion isolation once analysis of the mutagenized population is completed.
3. Identify and isolate mutagenized Chlamydia with altered developmental phenotypes
1. Creating an in-focus image stack
Extract the most in-focus image from the Z-stacks using the saved image data which contains 4 Z slices per time point. Use the kurtosis measurement option (Analyze ∣ Measure) in ImageJ/FIJI to automatically identify the most in focus Z slice (highest kurtosis score) and create a new image stack with just these in-focus images. A Python script is included as a supplementary file (Reduce_Z_kertosis_2ch_JOVE.py) to automate this process.
2. Quantify fluorescence expression in individual inclusions
NOTE: To quantify the expression kinetics of the two reporters use the open source image analysis application ImageJ/FIJI and the plugin Trackmate15. Trackmate identifies ‘spots’ (corresponding to inclusions in this case) and follows them through a time-lapse image stack recording the X,Y location and signal intensity for each inclusion over time (JoVE61365_screenfile14). This information is saved as a CSV file and will be imported into a custom Python notebook for analysis.
Open the in-focus Z-reduced image stack in ImageJ/FIJI using Bio-formats Importer by clicking Plugins ∣ Bio-Formats ∣ Bio-formats Importer. Select Hyperstack and Composite (JoVE61365_screenfile15, JoVE61365_screenfile16).
Subtract the image background by clicking on Process ∣ Subtract Background using a Rolling ball radius of 50.0 pixels and enhance the image contrast to 0.3% for Saturated pixels (Process ∣ Enhance Contrast). Multiply the image values by 10.0 (Process ∣ Math ∣ Multiply) (JoVE61365_screenfile17 - JoVE61365_screenfile22).
-
In Trackmate (Plugins ∣ Tracking ∣ Trackmate), select an estimated blob diameter of 48 pixels (empirically determined based on the size of the inclusion at the end of imaging). Produce non-fragmented inclusion tracks by selecting a Linking max distance and Gap-closing max distance of 8.0 pixels and Gap-closing max frame gap of 1 (JoVE61365_screenfile23 - JoVE61365_screenfile25).
NOTE: To improve Trackmate’s ability to identify and track inclusions over the entire cycle a separate image channel is created by adding the euoprom and hctBprom channels together using the image math function in ImageJ/FIJI. This channel is then used by Trackmate to identify and follow inclusions over time. The fluorescent values of the euoprom and hctBprom channels are then recorded in channels 2 and 3.
-
Record tracks that meet a minimum continuous duration: Duration of track: 20 (JoVE61365_screenfile26). Analyze the tracks and save the Spots in track statistics as a CSV file (JoVE61365_screenfile27).
NOTE: This process has been automated using a custom Python script that is provided in the supplemental data (TrackMate_Zreduced_JOVE.py).
3. Identify inclusion tracks with altered developmental profiles
NOTE: To identify inclusions containing Chlamydia with altered developmental profiles, each inclusion track was visualized using Python notebook. These visualizations allow for the identification of inclusions with kinetic gene expression profiles that differed from the mock-treated population. The Python notebook used for identification of inclusions with altered developmental programming is provided in the supplemental data (EMS_Screen-Markdown).
Import the inclusion track data from the Spots in tracks statistics CSV files into Pandas data frame using the Import cell in the EMS_Screen-Markdown Python Notebook.
-
Baseline correct each track by subtracting the minimum value of each track from the rest of the track values using the Baseline Subtract cells. Save the resulting values as a pickle file using the Save as Pickle cell. This will permanently save the channel values after baseline subtraction for later retrieval.
NOTE: Baseline subtraction sets the starting fluorescent intensity of every inclusion to zero.
Eliminate traces from inclusions near the edges of the FOV using the Filter Edges cell as their fluorescent profiles may not be fully captured; these traces may produce false positive developmental profiles.
Calibrate the frame (totalFrames) values from the image slices to time (startTime, interval) values with the Time-lapse Calibration cell using the experimental start time and imaging time interval. Exclude partial inclusion reads by filtering out traces that do not extend over the last 20 h of the experiment (16-36 HPI) using the Track Duration Filter cell.
-
Separate tracks into individual data frames by experimental condition with the Assign Treatment cell. Filter for inclusions that exhibit sufficient growth using the Filter for Growth cell. In the Filter for Growth cell, empirically set the fluorescence intensity threshold for the euoprom channel by changing the values within the last two lines of code. This will filter out Chlamydia that did not grow.
NOTE: Be cautious when setting threshold filters, if the filter is too low the resulting scatter plots will be noisy, yet if filtering is set too high important mutants may be eliminated.
Calculate the percent mortality caused by EMS by dividing the number of mutagenized tracks/well by the number of mock-treated tracks/well. Multiply the number of mock-treated tracks by the initial dilution factor of 3 to calculate the number of mock-treated tracks/well. Use the Count Inclusion Tracks and Calculate Percent Mortality cells to perform this task.
Calculate the time to half-maximal expression for both early and late reporters for each track using the Calculate cell. These values will be used to compare mutagenized and mock populations to identify developmental mutants.
-
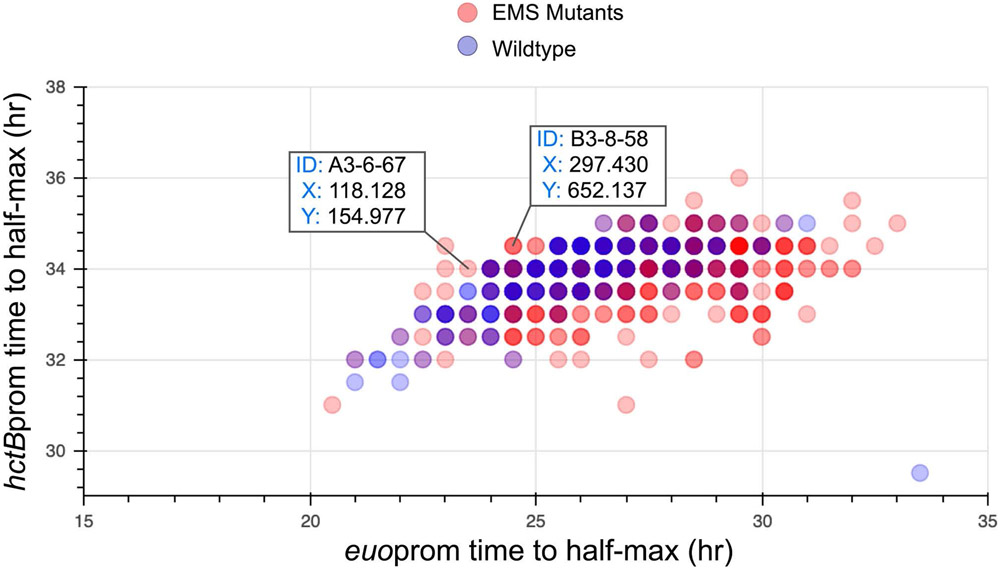
Within the Half-Max Plot cell use the bokeh plotting package to visualize the time to half-max expression of each promoter, graphing the euoprom time to half-maximal expression against that of hctBprom. Identify inclusions from the mutant population that fall outside the mock-treated scatter cloud using the bokeh interactive track ID explorer. Make note of the FOV and XY coordinates of the inclusions of interest (Figure 2).
NOTE: Pick candidate inclusions that visually fall outside of the control cloud as verification and statistical evaluation of each clone will be performed subsequently.
Within the Animated Plot cell visualize changes in promoter expression kinetics dynamically through time by graphing the expression intensities of euoprom against hctBprom using the plotting tool Plotly16. The scatter function of Plotly is used to animate the gene expression over time (Video 1).
-
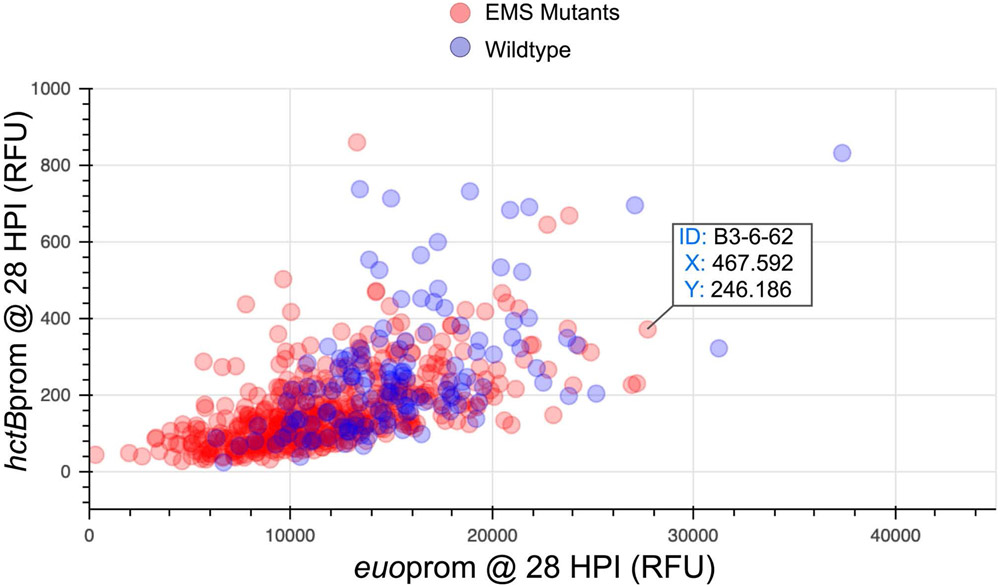
Visualize a snapshot from the Plotly animated graph in the Inclusion Locator cell, plotting euoprom and hctBprom expression at a specific time point (i.e. 28 HPI) using the bokeh package (Figure 3). Identify inclusions from the mutant population as described in step 3.3.8.
NOTE: This analysis needs to be performed quickly (<4 h) as the inclusions are still expanding and will start to lyse host cells ~48 h after infection.
Figure 2: Identification of representative isolates A3-6-67 and B3-8-58 by visualization of the time to half-maximal expression for each promoter.
The interactive graph is used to identify mutagenized Chlamydia exhibiting expression profiles that differ from the mock-treated control scatter cloud. Each spot on the graph represents a single inclusion. Inclusion spots A3-6-67 and B3-8-58 are highlighted as they fall outside of the mock-treated cloud, both exhibiting shorter time to half-maximal expression of the euo promoter in combination with longer time to half-maximal expression of hctB. euoprom: x-axis, hctBprom: y-axis.
Figure 3: Interactive snapshot for identification of inclusion location.
The graph presented is a snapshot at 28 HPI from the animated scatter plot (Video 1) and was used to identify the FOV and XY coordinate location of inclusions of interest. B3-6-62 is shown as it was chosen for isolation from the animated scatter plot.
4. Isolate developmental mutants from inclusions of interest
NOTE: To isolate Chlamydia from the inclusions that were determined to display altered gene regulation a micromanipulator with capillary needles was employed. The Well ID, FOV and X,Y coordinates of inclusions of interest were determined using the data visualization in section 3.3.
-
Prepare capillary needles by holding the center of the capillary tube in a flame and pulling both ends of the capillary tube until it has separated. Create an opening in the pulled capillary needle by breaking the pulled tip on a microscope slide. To break the needle, place the closed tip on the frosted portion of the microscope slide at an angle and apply pressure.
NOTE: Capillary tube specifications were 1.0 mm O.D., 0.5 mm I.D.
Check that the needle opening is approximately the size of an inclusion under a microscope, 20x objective.
Prepull ~25-30 capillary needles for isolation of candidate inclusions (one needle per inclusion).
Fill the microinjector with mineral oil ensuring that no air bubbles are present.
Attach a glass capillary needle to the microinjector and expel oil to the tip of the needle, expunging any air bubbles. Place the capillary needle in complete media and draw media up halfway. Filling the capillary needle with media prevents oil contamination in the well.
Using the saved position list in μManager from step 2.3.5, migrate to the well and FOV of an inclusion of interest identified in the Python visualization notebook.
Use the joystick of the micromanipulator to localize the capillary needle to the XY coordinates of the inclusion of interest.
Use the 595 nm excitation channel to visualize EBs for extraction and the phase/DIC white light channel for needle visualization. Maneuver the capillary needle to the inclusion, rupture the inclusion and then draw the EBs into the capillary needle using the microinjector.
-
Expel the EBs from the capillary needle into a single well of the prepared 24 well polystyrene plate prepared in step 2.1.2. Remove the capillary needle and replace with a fresh capillary needle for next inclusion extraction. Repeat section 3.4 for all candidate inclusions.
NOTE: For expansion and to ensure a high enough titer for re-imaging, incubate mutant isolates in a 5% CO2, 37 °C incubator until the majority of the host cells are infected (~1 week). Wells should be monitored closely as different isolates may exhibit different growth rates.
5. Harvest mutant isolates
On ice, disrupt the infected monolayer by scraping with a 1 mL micropipette tip. Transfer the media, cell debris, and released Chlamydia into a 1.5 mL microcentrifuge tube.
Pellet Chlamydia by centrifugation for 30 min at 4 °C, >14,000 x g. Remove the supernatant and resuspend pellet in 75 μL of ice cold 1x SPG. Aliquot into three 1.5 mL screw-cap microcentrifuge tubes. Store at −80 °C.
4. Verification of mutant isolate phenotypes
1. Host cell culture for imaging mutagenized isolates
Seed a 96 well glass bottom plate with 1.6 x 104 Cos-7 cells (ATCC) per well in 100 μL of complete media. Incubate at 5% CO2, 37 °C. Cells should reach confluency in approximately 24 h. After cells are confluent, replace media with complete media supplemented with 1 μg/mL cycloheximide, incubate overnight.
2. Infect cells with candidate isolates for phenotypic verification
Thaw mutant clones and wildtype Chlamydia on ice.
-
In the prepared 96 well plate, perform a two-fold serial dilution of mutant isolates, using one column per isolate (11 columns). Start with an initial dilution of 1:20 in 100 μl HBSS.
NOTE: Serial dilution of Chlamydia is performed to ensure mutant samples are imaged at an MOI < 1.
-
Infect the remaining (12th) column with wildtype Chlamydia at MOI ~0.5.
NOTE: Wildtype Chlamydia are used as a control for comparison against mutagenized isolates.
Incubate for 15 min rocking at 37 °C.
Wash infected host cells with prewarmed (37 °C) HBSS with 1 mg/mL of heparin and HBSS as specified in section 2.2.
Replace with 200 μL per well of prewarmed (37 °C) imaging media.
Fill the interwell spaces with prewarmed (37 °C) deionized H2O.
Incubate at 5% CO2, 37 °C for 10 h.
3. Microscope setup
NOTE: Refer to section 2.3 for microscope setup, this section will only contain the required setup modifications.
Select the 96 well plate template from the HCS plugin.
Empirically determine wells corresponding to an MOI < 1 for each mutant isolate. Clover expression under control of the euo promoter is observable at ~10 HPI making early visualization of inclusions possible (Figure 1B).
-
Select three wells per mutant isolate that correspond to an MOI < 1 and generate an imaging position list consisting of two FOV per well.
NOTE: Only 72 images can be taken per time interval due to hardware constraints, this equates to three dilutions (wells) per strain using two imaging sites per well if 12 samples are imaged.
Record the developmental cycle of each mutant isolate for 36 h at 30 min time intervals starting at 12 HPI.
Record the experimental details in the Acquisitions Comments box. i. e., Well ABC1: wildtype control. Well ABC2: Mutant strain 1, ABC3: Mutant strain 2, etc… Imaging: 12-48 HPI.
Start the image acquisition at 12 HPI.
5. Data analysis for isolate verification
1. Create in-focus image stacks and quantify fluorescence expression in individual inclusions
Generate fluorescent intensity traces for each inclusion as specified in section 3.1 - 3.2.
2. Verify Ctr mutagenized isolates
NOTE: To verify the altered developmental profiles of mutant isolates, their expression profiles are compared to the wildtype expression profile using Python notebook. The Python notebook used for verification of mutant clones with altered developmental programming is provided in the supplemental data (clone_check-Markdown).
Import and filter the inclusion trace data in the clone_check-Markdown Python notebook as done in section 3.3.
Calculate the mean and standard deviation (STD) from the traces of each isolate and wildtype control population using the Calculate Mean & STD cell.
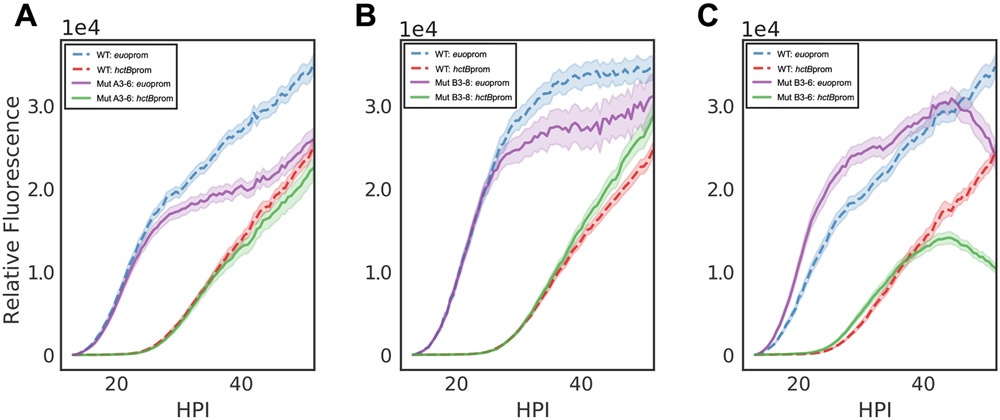
With the Graph Iso vs WT cell plot the mean and standard error of the mean (SEM) of each mutant clone against the wildtype control to determine if the mutant expression kinetics are divergent from the wildtype sample (Figure 4).
-
Determine if the isolated mutant population is clonal by plotting the mutant traces and comparing them to wildtype inclusion traces using a scatter plot as done in section 3.3 (steps 3.3.7-3.3.10) (Figure 2, Figure 3). If the isolate is a mixed population the plot will show one population overlaying with wildtype and a second distinct population outside of the wildtype scatter cloud. If the population looks mixed the mutant can be re-isolated using the original procedure described in section 3.4.
NOTE: To determine if the developmental profile of an isolate is statistically different from wildtype the curves for each isolate should be compared to wildtype using ANOVA.
Figure 4: Verification of representative mutant isolates.
Developmental profiles of mutagenized isolates A3-6, B3-8, and B3-6. (A) The A3-6 mutant exhibits a decrease euoprom expression at ~24 HPI. (B) The B3-8 mutant isolate exhibits a decrease euoprom expression at ~24 HPI, but an overall increase in hctBprom expression. (C) The B3-6 isolate exhibits increased levels of euoprom expression followed by a sudden loss of expression in both promoters at ~40 HPI. Each sample is the average of the specified population, n > 25. Cloud represents SEM.
Representative Results
Direct EMS mutagenesis of our promoter-reporter chlamydial strain resulted in an ~75% reduction in infectivity. Using the described live-cell imaging protocol, ~600 inclusions were imaged and tracked over a 24 h period. The fluorescent expression kinetics of both reporters in each inclusion was visualized using custom Python notebook scripts.
Two visualization approaches were implemented to identify candidate mutagenized Chlamydia for isolation. The first methodology (step 3.3.8) visualizes the time to half-maximal expression of euo and hctB promoters from individual chlamydial isolates in an interactive scatter plot (Figure 2). Inclusions were identified for isolation if they fell outside the mock-treated scatter cloud. Candidate clones were picked that visually fell outside of the control cloud. Verification of each clone was performed subsequently. Clones A3-6-67 and B3-8-58 were selected for isolation as they produced shorter times to half-maximal expression from the euo promoter and longer times for hctB (Figure 2).
The second visualization method for identifying inclusions with altered kinetics (steps 3.3.9-10) identifies individual inclusions based on visualization of dynamic gene expression from the two promoters (Video 1). Again, candidate clones with dynamic inclusion expression patterns that were noticeably distinct from control inclusions were picked. B3-6-62 was chosen due to increased fluorescent accumulation from the euo promoter between 23 and 29 HPI (Video 1). A snapshot of the animated graph was taken to identify the location of the inclusions of interest (Figure 3).
Using the two visualization methods, a total of 24 inclusions were identified for isolation. Of the 24 total isolates, 10 showed differential kinetics upon retesting. These isolates fell into three phenotypic categories; 8 isolates exhibited decreased euoprom expression at ~24 HPI, corresponding to the time of RB-EB conversion, as demonstrated by the clone A3-6-67 (Figure 4A). The remaining two clones displayed unique phenotypic profiles, the B3-8-58 isolate also exhibited decreased euoprom expression at ~24 HPI, yet an overall increase in hctBprom expression (Figure 4B), whereas B3-6-62 expressed increased levels of fluorescence from the euo promoter followed by a sudden loss of expression in both promoters (Figure 4C). Analysis of the live-cell micrographs for mutant B3-6-62 revealed that host cell lysis occurred in cells infected with this mutant much earlier than in wildtype infected cells (Video 2).
Discussion
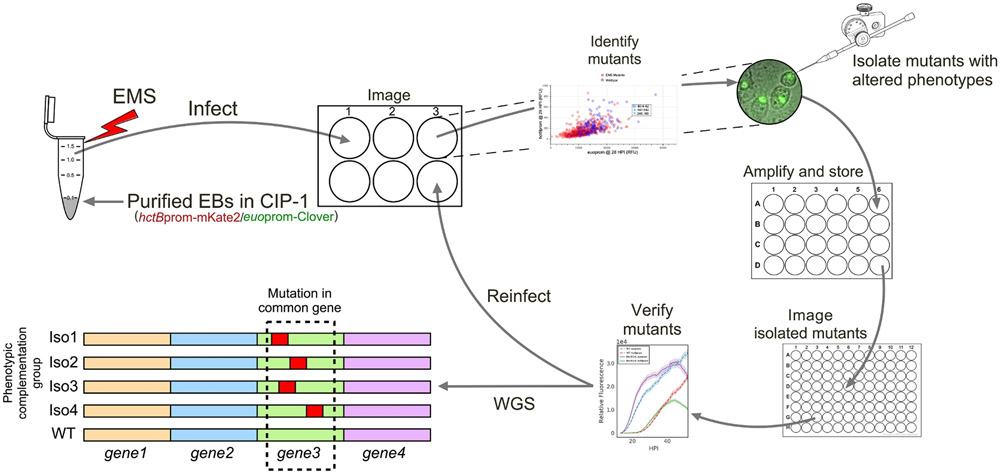
Dissecting the mechanisms that control the chlamydial developmental cycle has been hindered by the limitations of the currently available genetic tools. Employing our promoter-reporter Chlamydia in conjunction with live-cell automated microscopy, a system was built which enables monitoring of cell-type development in individual inclusions over a 24 h period. This system, in combination with chemical mutagenesis and direct inclusion isolation has established a method to rapidly and clonally select Chlamydia expressing altered developmental profiles (Figure 5).
Figure 5: Workflow for directed forward genetic analysis of promoter-reporter Ctr:
Ctr-L2-p2TK2-hctBprom-mKate2/euoprom-Clover EBs were directly mutagenized with EMS in axenic media, CIP-1. Mutagenized EBs were used to infect Cos-7 cell monolayers for imaging and fluorescent expression analysis. Chlamydia expressing altered developmental dynamics were identified by visualization in interactive graphs. Inclusions with altered developmental profiles were isolated using a micromanipulator. The phenotypes of the isolates were verified upon reinfection. Mutant isolates are subjected to WGS to identify DNA lesions associated with phenotypes.
Chlamydial EBs are metabolically active outside the host when provided with intracellular ionic conditions and an energy source5 , 12 . This EB axenic metabolism was leveraged to mutagenize purified EBs outside of host cells. In this protocol, metabolizing EBs were directly mutagenized with EMS. It was observed that EMS treatment effectively reduced EB viability and generated EBs that produced variable developmental kinetics as expected.
It is estimated that the described EMS mutagenesis protocol generates ~5-20 DNA changes/EB. The live-cell microscopy workflow described is capable of imaging ~8 inclusions per field of view (FOV) and 72 FOVs every in a 30 min interval. Therefore, it is estimated that the effects of ~3000-10,000 mutations can be visualized per run. Multiple runs (3-5) will result in visualization of the effects of 9,000-50,000 mutations. The Ctr-L2 genome encodes ~850 genes, suggesting this protocol will result in the visualization of >10 mutations per gene. These estimates indicate that genome coverage, while not complete, should be sufficient.
The strength of this protocol is the ability to track and record the expression kinetics of multiple promoter-reporters at the single inclusion resolution in near real-time. Forward genetics relies on observable phenotypes and clonal isolation. Past methods for forward genetics in Chlamydia relied on static observations and plaquing with agar overlays8. With our methodology, dynamic promoter activity is recorded throughout the developmental cycle and then visualized to identify inclusions that contain Chlamydia with altered gene expression kinetics. Identifying candidate inclusions using multiple parameters (i.e., the time to half-maximal expression and total fluorescent intensity at a given time point) results in distinct mutant pools that display different developmental kinetics. These Chlamydia are likely to have unique mutations that affect the regulation of separate genetic pathways. The fact that these profiles can be recorded live and visualized after a few hours allows time to locate and isolate the inclusions of interest from the infected monolayer. Although we focused on the gene expression dynamics during development, alternative gene reporters can be used to probe other regulatory pathways.
Depending on the genetic pathways being interrogated, caution should be taken with the addition of cycloheximide to host cells. Although incubation with cycloheximide improves the imaging characteristics of the monolayer by blocking replication of the host cells; this effect is achieved through inhibiting host protein synthesis. Inhibition of de novo host protein synthesis could influence the results of the genetic screen depending on the question asked.
Phototoxicity and photobleaching are major hurdles in longterm time-lapse microscopy. To overcome these issues, the specific characteristics of each fluorescent protein should be considered prior to experimentation. Clover and mKate2 have short maturation times (20-30 m) are photostable, and exhibit relatively large quantum yields17 , 18 . These qualities allow for the reduction of excitation intensity and exposure time, thus reducing the amount of phototoxicity and photobleaching incurred. The phase/DIC white light channel was employed for autofocusing as this spectrum of light was less phototoxic to Chlamydia.
For this protocol, EMS was used as a chemical mutagen. EMS causes G:C to A:T transitions via guanine alkylation19 . However, this protocol can be expanded to include alternative mutagens that can induce other kinds of genomic mutations. For instance, acridines are a class of DNA intercalating compounds which induce indels, increasing the chance of frame shifts and therefore null mutations20 .
With advances in chlamydial transformation techniques, mutated genes that are associated with phenotypic complementation groups can be knocked out via insertional gene disruption and genetic complementation for verification of genotype-phenotype linkage9. Recovering mutants that block RB to EB development could be problematic as mutations of interest may produce Chlamydia that cannot reinfect host cells. This technique can be modified to identify developmental genes by statistical associations (GWAS). The genomes of Chlamydia from isolated inclusions can be directly sequenced without expansion and verification. The high throughput nature of this technique would make statistical associations possible. Again, verification of these associations can be tested through gene disruption and complementation9 .
Supplementary Material
Identification of mutagenized Chlamydia exhibiting divergent expression kinetics using a dynamic gene expression plot. Promoter expression of euo and hctB for individual inclusions was plotted and visualized through time to identify mutagenized Chlamydia with altered expression dynamics. B3-6-62 was chosen for isolation as it exhibits higher euoprom expression in comparison to the wildtype cloud, euoprom: x-axis, hctBprom: y-axis.
Representative mutant B3-6-62 causes premature host-cell lysis. Time-lapse live-cell micrograph of B3-6-62 infected host-cells undergo premature lysis (~40 HPI).
Acknowledgments
We thank Dr. Anders Omsland at Washington State University for supplying the CIP-1 axenic media. This work was supported by NIH grant R01AI130072, R21AI135691 and R21AI113617. Additional support was provided by startup funds from the University of Idaho and the Center for Modeling Complex Interactions through their NIH grant P20GM104420.
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.AbdelRahman Y, Belland R The chlamydial developmental cycle. FEMS Microbiology Reviews. 29 (5), 949–959 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Clifton D et al. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proceedings of the National Academy of Sciences U. S. A 101 (27), 10166–10171 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu HHY, Tan M σ28 RNA polymerase regulates hctB, a late developmental gene in Chlamydia. Molecular Microbiology. 50 (2), 577–584 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koo I, Stephens R A Developmentally Regulated Two-component Signal Transduction System in Chlamydia. Journal of Biological Chemistry. 278 (19), 17314–17319 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Grieshaber S et al. Impact of Active Metabolism on Elementary Body Transcript Profile and Infectivity. Journal of Bacteriology. 200 (14), e00065–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belland R, et al. Genomic transcriptional profiling of the developmental cycle of. Proceedings of the National Academy of Sciences U. S. A 100 (14), 8478–8483 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, et al. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathogens. 7 (9), e1002258. (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen B, Valdivia R Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proceedings of the National Academy of Sciences U. S. A 109 (4), 1263–1268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller K, Wolf K, Fields K, Maurelli A Gene Deletion by Fluorescence-Reported Allelic Exchange Mutagenesis in Chlamydia trachomatis. MBio. 7 (1), e01817–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosario CJ, Tan M The early gene product EUO is a transcriptional repressor that selectively regulates promoters of Chlamydia late genes. Mol Microbiol. 84 (6), 1097–1107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brickman T, Barry C, Hackstadt T Molecular cloning and expression of hctB encoding a strain-variant chlamydial histone-like protein with DNA-binding activity. Journal of Bacteriology. 175 (14), 4274–4281 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omsland A, Sager J, Nair V, Sturdevant D, Hackstadt T Developmental stage-specific metabolic and transcriptional activity of Chlamydia trachomatis in an axenic medium. Proceedings of the National Academy of Sciences U. S. A 109 (48), 19781–19785 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H et al. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proceedings of the National Academy of Sciences U.S.A 93 (20), 11143–11148 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein A et al. Computer control of microscopes using μManager. Current Protocols in Molecular Biology. Chapter 14–20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinevez JY, et al. TrackMate: An open and extensible platform for single-particle tracking. Methods. 115, 80–90 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Plotly Technologies Inc. Collaborative data science. Montréal, QC, https://plot.ly. (2015). [Google Scholar]
- 17.Lam AJ, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nature Methods. 9 (10), 1005–1012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shcherbo D, et al. Far-red fluorescent tags for protein imaging in living tissues. Biochemical Journal. 418 (3), 567–574 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sega G A review of the genetic effects of ethyl methanesulfonate. Mutation Research. 134 (2-3), 113–142 (1984). [DOI] [PubMed] [Google Scholar]
- 20.Ferguson L, Denny W Frameshift mutagenesis by acridines and other reversibly binding DNA ligands. Mutagenesis. 5 (6), 529–540 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of mutagenized Chlamydia exhibiting divergent expression kinetics using a dynamic gene expression plot. Promoter expression of euo and hctB for individual inclusions was plotted and visualized through time to identify mutagenized Chlamydia with altered expression dynamics. B3-6-62 was chosen for isolation as it exhibits higher euoprom expression in comparison to the wildtype cloud, euoprom: x-axis, hctBprom: y-axis.
Representative mutant B3-6-62 causes premature host-cell lysis. Time-lapse live-cell micrograph of B3-6-62 infected host-cells undergo premature lysis (~40 HPI).