Abstract
Kit is a growth factor receptor that regulates proliferation and/or survival of many embryonic and postnatal stem cell types. When mutated, it can induce malignant transformation of the host cells. To dissect the Kit role in the control of ESC pluripotency, we studied its expression during early mouse embryogenesis and during the process of ESC derivation from inner cell mass (ICM) cells. We followed the in vitro development of early mouse embryos obtained from transgenic mice carrying Kit promoter regions fused to EGFP (Kit-EGFP) and found that they initiate EGFP expression at morula stage. EGFP expression is then maintained in the blastocyst, within the ICM, and its levels increase when cultured in the presence of MAPK and GSK3β inhibitors (2i) plus LIF compared with the LIF-only condition. Kit-EGFP ESCs showed nonhomogeneous EGFP expression pattern when cultured in LIF condition, but they upregulated EGFP expression, as well as that of Sox2, Nanog, Prdm14, when shifted to 2i-LIF culture. Similarly, primordial germ cells (PGCs) in the process of embryonic germ cell (EGC) conversion showed enhanced EGFP expression in 2i-LIF. Kit expression was affected by manipulating Sox2 levels in ESCs. Chromatin immunoprecipitation experiments confirmed that Sox2 binds Kit regulatory regions containing Sox2 consensus sequences. Finally, Kit constitutive activation induced by the D814Y mutation increased ESC proliferation and cloning efficiency in vitro and in teratoma assays in vivo. Our results identify Kit as a pluripotency-responsive gene and suggest a role for Kit in the regulation of ESC proliferation.
Keywords: C-Kit, Embryo, Embryonic stem cells, LIF, Transgenic mouse, Xenogeneic stem cell transplantation, Proliferation
Introduction
Kit, the transmembrane receptor of the cytokine stem cell factor (SCF), is an important regulator of proliferation/survival of several embryonic and postnatal stem cell types such as melanoblasts, hemopoietic stem cells and germ cells [1, 2]. Kit maps to the W locus (white spotting) and its deletions or loss of function mutations affect these stem cell lineages. On the contrary, gain of function mutations or gene duplications have been identified in several neoplasias and have been hypothesized to be involved in the neoplastic transformation of hemopoietic and germ cells [3]. In the fetal germline, Kit is expressed both in primordial germ cells (PGCs) and in oocytes. After birth its expression is resumed in spermatogonia, to regulate the expansion of the male germ cell pool, whereas in postnatal oocytes, Kit mRNA is continuously expressed up to metaphase II stage and after fertilization it is completely degraded at 2-cell stage. Embryonic Kit expression can be detected at the blastocyst stage (within the pluripotent inner cell mass cells [ICMs]) [4] and in ESCs, the pluripotent stem cells derived from ICM of preimplantation blastocysts [5]. Although Kit null ESCs can be generated, they show growth and differentiation defects [6]. Similarly to some pluripotency genes such as Nanog or Prdm14, Kit is downregulated at implantation and then it is re-expressed at gastrulation only in PGCs [7, 8]. By using transgenic mice carrying different Kit promoter regions fused to EGFP (Kit-EGFP), we previously showed that 6.9 kb of the promoter are sufficient to drive EGFP expression only in germ cells (PGCs and oocytes, p13 line) [8]. Inclusion of 3 kb or 6 kb of the first Kit intron sequences (p18 and p70 transgenic line, respectively) extends EGFP expression to hemopoietic stem cells and to other stem cell lineages, including spermatogonia [8–11]. The regulation of Kit expression in germ cells depends on transcription factors that are developmentally regulated. We found that Sox2 promotes Kit expression in migratory PGCs [11], whereas the bHLH factors Sohlh1 and Sohlh2 are important for its expression in postnatal spermatogonia and oocytes [9]. The evidence that Kit expression pattern parallels that of pluripotency factors in early embryos, in PGCs and in ESCs, suggests that the regulation of its expression might be potentially associated to the ground state pluripotency. To test this hypothesis, we first studied Kit expression during early embryonic development and during ICM-ESC transition by following the activation of Kit-EGFP transgene expression in in vitro cultured 2-cell embryos obtained from the 3 transgenic lines [8]. We found that the first 6.9 kb of Kit-promoter specifically drive Kit expression at morula stage and that ground state culture conditions (2i-LIF) enhance both Kit and EGPF expression in blastocysts, in ESCs and in PGCs during embryonic germ cell (EGC) conversion. We found that when transfected into ESCs, Sox2 induced Kit expression whereas Sox2 deletion was accompanied by Kit downregulation. Of the 4 Sox2 consensus sequences contained within the promoter and first Kit intron, 3 of them were immunoprecipitated by Sox2 antibodies in chromatin immunoprecipitation (ChIP) assays, suggesting a direct effect of Sox2 on Kit transcription. Finally, by introducing a gain of function mutation of the Kit gene, we found that it stimulated ESCs proliferation both in vitro and in vivo.
Our results identify the Kit regulatory regions that drive Kit expression also during early embryogenesis, and respond to pluripotent ground state culture conditions, similarly to what has been shown for some pluripotency genes such as Nanog, Prdm14, Rex1, in ESCs. They also suggest a role for Kit in ESCs proliferation and tumor growth in mouse teratoma.
Materials and METHODS
Animal Models
Generation of Kit-KO, Kit-EGFP, and Sox2loxP/loxP mice have been previously described [8, 37, 41]. Sox2loxP/loxP were intercrossed with Rosa26Cre-Ert2 mice (Jackson Laboratories, Ann Harbor, MI) to generate Sox2loxP/loxP, Rosa26Cre-Ert2 mice. Sox2loxP/loxP Rosa26Cre-ERT mice were crossed to p18 mice to generate Sox2loxP/loxP, Rosa26Cre-Ert2 Kit-EGFP (p18) mice. Kit-KO mice were intercrossed with p18 to generate Kit-KO, Kit-EGFP. For embryo staging, 0.5 dpc corresponded to the day of vaginal plug. Knockin mice carrying the KitD814Y mutation (see below) were generated by injecting mutant ESCs into C57/B6 carrier blastocysts and transplanted the same day into foster CD1 mothers. All experiments were performed in compliance with the Tor Vergata University Institutional Animal Care and National Institutes of Health Intramural Animal Care and Use program. All procedures adhered to the standards published in Guide for the Care and Use of Laboratory Animals. Experimental procedures involving mice were approved by the Italian Ministry of Health. The structure of the targeting construct used to generate KitD814Y knockin ESCs is depicted below.
Construction of KitD814Y Targeting Vector
BAC (bacterial artificial chromosome) RP23–309C11 (from UCSC Genome Bioinformatic software), which consists of a 205 kb insert, including 68 kb of sequence upstream and 57 kb of sequence downstream of the Kit initiation and stop codons, respectively, was used to generate the Kit targeting vector. Bacteria containing BAC RP23–309C11 have been electroporated with mini-λ prophage DNA containing the essential components for recombination [42]. The BAC mutation has been generated using a 2-step recombination strategy for the missense mutations [43]. The correct insertion of the point mutation was checked by sequencing of mutated BAC using 2 opportune primers (Kit For240 = TAGTCATTAGAGCCCCGATC and Kit Rev500 = GTTACAGAAGGCTGGCACTT). KitD814Y knockin targeting vector was generated by homologous recombination inserting the neomycin cassette obtained from the pLTM260 vector flanked by loxP and frt sites (a generous gift of SK Sharan, NCI-FCRF, Frederick, MD) and 2 homology regions (ARM1 and ARM2) of the Kit gene at intron 17, into the previously D814Y mutated BAC. Kanamycin resistant positive recombinants were used for getting the BAC sequence (from intron 14 to intron 20) with the substitution D814Y (in exon 17) and the Neo cassette into the retrieval pDTa8 vector (a generous gift of SK Sharan, NCI) that contains ampicillin resistance and the DT (diphtheria toxin) gene for negative selection of electroporated ESCs. Correctly recombined colonies were checked for antibiotic resistance to both ampicillin and kanamycine.
Generation of Targeted ESCs
Eighty micrograms of the final targeting vector were linearized by cutting with NotI, purified and electroporated into 129iTL1 embryonic stem cells by standard methods at NCI. After selection with G418 antibiotic, 300 resistant colonies were expanded. Targeted ES cells were identified by southern blotting analysis by genomic DNA digestion with Bgl II and using an external probe mapping on exon 14 of c-Kit gene (Supporting Information Fig. S3). Three positive clones were identified. The targeted ESCs were injected into C57/B6 blastocysts to generate chimeric mice. Since no chimeras were obtained from the 3 positive targeted ESCs clones further experiments were performed on ESCs.
Derivation and Maintenance of ESCs
Two cells embryos at 1.5 dpc obtained from Kit-EGFP, Oct4-DE and Sox2loxp/loxp Rosa26Cre-Ert2 transgenic mice were flushed out from the oviduct and cultured in a 33 mm dish in KSOM medium (Embryomax Millipore) under mineral oil until the stage of blastocysts 3.5 dpc. To derive ESCs, individual transgenic blastocyst was placed and cultured for an additional day according to a modification of Ying et al. [13] in a 96-well plate in Knockout DMEM (Gibco, Grand Island, NY) supplemented with B27 (Gibco), insulin-transferrin-sodium selenite supplement (Gibco), 2% FBS (Gibco), 100 UI Penicilline (Gibco), 100 μg/ml Streptomycin (Gibco), 20 mM Glutamine (Gibco), Nonessential Amino Acids (Gibco), 100 nM β-Mercaptoethanol (Gibco) with 2i (PD325901, 1 μM: Axon Medchem; CHIR99021, 3 μM: Axon Medchem, London, U.K.) and LIF (1,000 μg/ml, Immunological Sci, Rome, Italy) on mouse embryonic fibroblasts (MEFs) feeders. The expanded ESC colonies were passaged by dissociating with TRypLE (Invitrogen, Milan, Italy). Until passage 4, ESCs were maintained on MEFs. From passage 4, ESCs, preplated to reduce MEF contamination, were stocked and subsequent experiments were performed using stocked ESC samples. ESCs were cultured in LIF condition medium (Knockout DMEM [Gibco] supplemented with 15% FBS [Gibco], 100 UI Penicilline [Gibco], 100 μg/ml Streptomycin [Gibco], 20 mM Glutamine [Gibco], Nonessential Amino Acids [Gibco], 100 nM β-Mercaptoethanol [Gibco] with LIF [1,000 U/ml]) or in 2i-LIF condition medium (Knockout DMEM [Gibco] supplemented with B27 [Gibco], insulin-transferrin-sodium selenite supplement [Gibco], 2% FBS [Gibco], 100 UI Penicilline [Gibco], 100 μg/ml Streptomycin [Gibco], 20 mM Glutamine [Gibco], Nonessential Amino Acids [Gibco], 100 nM β-Mercaptoethanol [Gibco] with 2i [PD325901, 1 μM: Axon Medchem; CHIR99021, 3 μM: Axon Medchem] and LIF [1,000 U/ml]) in feeder free on a dish coated with gelatin (0.25%; Sigma–Aldrich, Milan, Italy).
FACS Analysis
Kit-EGFP ESCs were dissociated with trypsin EDTA and quenched with DMEM containing 10% FBS. Fluorescence identification and quantitation were conducted on FACS Calibur (BD Biosciences, Milan, Italy).
PGC Culture
E9.5 and E13.5 gonads from CD-1 or Kit-EGFP (p18) mice were separated from mesonephros and dissociated with trypsin–EDTA working solution. Trypsin–EDTA was quenched and pipetted several times to obtain a single suspension. Cells were resuspended in 300 μl of MACS buffer (phosphate-buffered saline [PBS]-bovine serum album [BSA] 0.1% [wt/vol] and 0.2 mM EDTA). PGCs were isolated by MINI-MACS immunomagnetic cell sorter (Miltenyi Biotec, Milan, Italy) using anti-SSEA1 antibodies (GeneTex, Milan, Italy), according with the manufacturer’s instructions. Cells were then cultured 24 hours into transwell chambers (Falcon, Milan, Italy) [17] in Knockout DMEM medium in LIF or 2i-LIF condition supplemented with 25 ng/ml bFGF, BMP4 100 ng/ml, 10 ng/ml SCF and 10 μM Forskolin. PGCs were also cultured in the presence of LIF, PD and LIF, CH, and LIF or 2i+LIF to assay the impact of each inhibitor on Kit expression. Total RNA was extracted in Trizol (Invitrogen, Carlsbad, CA) for qRT-PCR.
EGC Derivation
Approximately 50 9.5 dpc PGCs, isolated by MINI-MACS procedure, were seeded onto mitomycin-treated Sl4-m220 cells [44] in 96 multiwell plates and cultured in 15% FCS Knockout DMEM medium containing SCF, bFGF, BMP4, and LIF (as above). After 2 days of culture, medium was replaced with LIF or 2i-LIF medium. PGC colonies and EGFP intensity were assessed by epifluorescence.
Transfection of ESCs
Sox2-pcDNA-plasmid was transfected into ESCs by lipofectamine 3,000 DNA transfection reagent (Invitrogen) as previously described [9]. After 24 hours cells were extracted in lysis buffer solution and Western blot analysis was performed on pools of different transfection experiments.
Cell Proliferation and Colony Assays
The 3H-thymidine incorporation assay was used to measure cell proliferation as previously described [45]. Briefly, 1,000 ES cells were seeded into 96-well plates in ES medium for 24 hours and then were pulsed with 1 μCi of [methyl-3H]-thymidine for 2 hours at 37°C. Cells and media were collected onto fiberglass filters and radioactivity retained on the dried filters was counted by liquid scintillation spectrometry. Colony assays were performed by seeding 20 or 200 single wt or mutant ESCs suspensions onto gelatin-treated wells that were cultured under appropriate conditions for 4 days. Cultures were then fixed with 4% paraformaldehyde (PFA) and stained for alkaline phosphatase (Fast red/Naphthol Kit, Sigma Aldrich) for 10 minutes for colony visualization. Number of AP stained (red/purple) pluripotent colonies were counted using a light microscope with 10X objective.
Semiquantitative RT-PCR and qRT-PCR
Total RNA from ES cells and PGCs was treated with DNase I to avoid potential contamination by genomic DNA. DNA-free RNA was reverse transcribed using Bioline (BIO-65043, London, U.K.) tetro cDNA Synthesis Kit according to the manufacturer’s instructions. For semiquantitative RT-PCR in ESCs and PGCs, 25 cycles were performed for Actin amplification and 38 cycles for all the other genes.
One step plus (Applied Biosystems, Monza, Italy) Real-time PCR System and SsoAdvanced Universal SYBR Green Supermix (BIO-RAD 172–5,271, Bio-Rad Laboratories, Hercules, CA; S.R.L., Segrate Milan, Italy) were used for quantitative RT-PCR (RT-qPCR). The comparative 2(-Delta Delta C[T]) method was used to determine the relative quantities of mRNA, using Gapdh mRNA as the endogenous normalizer. Sequences of oligonucleotides used for RT-PCR and qRT-PCR are indicated in Table 1.
Table 1.
List of primers used for gene expression and ChIP analyses.
| Table of primers | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|
| Oct4/Pou5F1 | CGAGTTGGGATAGGGCCTCTCTTGC | TTGGGCTAGAGAAGGATGTGGTT |
| Sox2 | GGAAAAGGGACTGAGTAGAGTGTGG | GCACATGAACGGCTGGAGCAACG |
| Nanog | TGAGACGGAGTCTTGCTCTG | GGCTTCTATTCAATGTTGTCCTTA |
| Prdm14 | TCTGTATGGAGCCATCGCTA | AATGAGGTCTGCATGAGGCA |
| Kit | GAGACGTGACTCCTGCCATC | TCATTCCTGATGTCTCTGGC |
| Gapdh | AACTTTGGCATTGTGGAAGG | CACATTGGGGGTAGGACAC |
| Sox2 ChIP 1 | GAAAGAGCACAGAAGGACA | AGTACAGAACTGGCCCATCC |
| Sox2 ChIP 2 | GGCAGGCGAATTTCTGAGT | AGACCACAGTCGGATCGAGTT |
| Sox2 ChIP 3 | CTCCTCTACCAACAGGAACA | AAGGACCACCGATGGAGGGAGA |
| Sox2 ChIP 4 | GCTTTGCCTGCCATTTCTGT | GTCCATCCTGTTAGAGGTTCTG |
| Sox2 ChIP 5 | CCTGCCTACCGTACACAGCAGT | CCCATGAACTGCCTGTCAACAG |
Immunoprecipitation and Western Blot
Five hundred micrograms of protein extracts from Kit D814Y colony 1 or Kit wt ESCs were immuno-precipitated with anti Kit antibodies (custom made by ProteoGenix, Schiltigheim, France, based on the Kit sequence published in [46], 1:100); or with matched isotype immunoglobulins (IgGs, Sigma–Aldrich, Milan, Italy; I5006) overnight at 4°. Immunocomplexes were pulled down with ProteinA-Sepharose beads (Sigma–Aldrich, P3391), recovered by boiling beads in sample buffer and analyzed by Western blot. For Western blot analysis, ESCs, PGCs, and spermatogonia were harvested and washed 2 times with ice-cold PBS. Cell lysis was performed with Lysis solution (150 mM NaCl, 50 mM Tris HCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 0.5 M DTT, 0.5 M NaF, 100 mM PMSF, 1 M β-glycerophosphate, 0.5 M sodium orthovanadate and protease inhibitors). Proteins or immunocomplexes were separated by SDS-PAGE in 4%–20% gradient gels (Bio-Rad, Milan, Italy) or in uniform gels of 10% polyacrylamide and transferred to PVDF membrane (Amersham GE Healthcare Europe GmbH, Milan, Italy). The membrane was blocked in TBS-5% BSA for 1 hour at room temperature. Incubation with primary antibodies was carried out at 4°C o/n in TBST-5% BSA and then with the appropriate horseradish peroxidase-conjugated secondary antibody (SantaCruz, Heidelberg, Germany). Rabbit anti-Sox2 (Millipore, Milan, Italy; 1:1,000), rabbit anti-Actin (A2066, Sigma–Aldrich, 1:2,000), mouse anti-Oct4 (Santacruz sc-8,628; 1:100), rabbit anti-Nanog (Santacruz sc-376,915; 1:500), rabbit anti-Prdm14 (Abcam, ab139725, Cambridge, U.K.; 1:500), rabbit anti-GFP rabbit (Abcam ab6556; 1:1,000), mouse anti-tubulin (Sigma–Aldrich, T9026; 1:2,000), rabbit anti-Stra8 (Abcam ab49405; 1:1,000), rabbit anti-Kit (1:500), anti p-Tyr (Santacruz, PY20, SC508) anti-pSer9-GSK3β (Cell Signaling #9336, Milan, Italy) The horseradish peroxidase conjugate was detected by chemiluminescence with an ECL Kit (Clarity western ECL substrate Bio-Rad) and auto-fluorography.
Teratoma Assay
One million of D814Y Kit, wt (parental line 129iTL1) or p18-ESCs were resuspended in 50 μl of matrigel matrix (Corning, Turin, Italy) and injected into the flanks of CD1 nude immune-compromised mice (2 for D814Y Kit ESCs, 1 for p18/Oct4-DE). Teratomas were monitored and removed once the tumor size reached 1 cm. Tumors were then measured and processed for paraffin sectioning and hemaytoxylin and eosin staining analysis. Serial sections 5 μm thick were obtained at the microtome.
Immunofluorescence
For ICM, blastocysts from Kit-EGFP transgenic mice were adhered onto gelatin coated glass slides and allowed to outgrow for 24 hours in 2i condition. For whole mounts, blastocysts were cultured 24 hours in LIF or 2i-LIF conditions. Cells were then fixed for 15 minutes at room temperature in 4% PFA, washed twice in PBS, permeabilized for 10 minutes with PBS containing 0.1% Triton X-100 and incubated for 1 hour with PBS containing 0.5% BSA. Cells were then incubated o/n at 4°C with anti-Kit, anti-Sox2, anti-Nanog, or anti-Oct4 antibodies (all antibodies were used at 1:200 dilution) followed by 1 hour incubation in secondary antibody (Cy3-conjugated donkey anti-rabbit IgGs, from Millipore,) was added for 1 hour at room temperature. Slides were washed and mounted in 50% glycerol in PBS. Hoechst 33342 (Sigma–Aldrich) was used to counterstain nuclei. Deconvolution and microscopy inspections were performed on a Leica CTR6000 microscope.
Embryoid Bodies, Cardiac, and Neuronal Differentiation
Kit wt and Kit D814Y ESCs were detached and dissociated into single cells with 0.25% trypsin–EDTA and grown in hanging drops (200 cells per 25 μl KO-DMEM, 15% FBS) to obtain embryoid bodies. Day 3 embryoid bodies were plated onto gelatinized plates and cultured for 7 days in DMEM 15% FCS for cardiac differentiation and observed for the presence of beating foci. For neural differentiation day3 embryoid bodies were exposed to 10 μM all trans retinoic acid (ATRA) for further 5 days. Immunofluorescence was performed on cultures following 4% PFA fixation, using rabbit anti β3-Tubulin (T2200, Sigma–Aldrich; 1:200).
Chromatin Immunoprecipitation Assay
Pools of ESCs (1.2 × 107 total cells) were used for chromatin preparation as previously reported for PGCs [11]. Chromatin from approximately 6 × 106 cells was used in each immunoprecipitation experiment with mouse α-Sox2 (2 μg, SantaCruz sc-365,823) or with control IgG (2 μg SantaCruz sc-2025) antibodies in a total volume of 500 μl at 4°C. Semiquantitative PCR was performed on extracted DNA using the corresponding primers (Table 1); 35 cycles (95° 30 seconds, 58° 30 seconds, 72° 30 seconds) were performed for each DNA segment amplification.
Statistical Analysis
The Student’s t test has been used to assess the significance. All experiments were performed at least 3 times. Values are reported as mean and SD. Asterisks indicate the level of statistical significance (*, p < .05; **, p < .001).
Results
Kit Promoter Is Active in Preimplantation Embryos and in ESCs
To study the mechanisms that regulate Kit expression in early embryogenesis and during the process of ESC derivation, we took advantage of 3 transgenic mouse lines expressing EGFP under the control of different Kit-regulatory regions (Kit-EGFP p13, p18, and p70, Fig. 1A) that faithfully report Kit expression in the correct cell lineages ([8] and Supporting Information Fig. S1A). Two-cell stage embryos were obtained from wt females mated to transgenic males, to follow EGFP embryonic transcription, and cultured up to the blastocyst stage to generate ESCs. By fluorescence microscopy analysis we found that 2-cell embryos obtained from all the 3 transgenic lines were EGFP negative (not shown); however, starting from the morula stage they expressed EGFP at variable levels within the blastomeres (Supporting Information Fig. S1B). At the blastocyst stage EGFP was mainly expressed within the ICM in all the 3 lines, although at different levels (Fig. 1A). We found that the p18 line showed the brightest EGFP positivity among the 3 transgenic lines, thus we concentrated our studies on this line. In parallel, we obtained embryos from Oct4 distal enhancer-EGFP transgenic mice (Oct4-DE, a transgene which is known to be a pluripotency reporter [12]), that showed EGFP expression starting from 2/3 cell stage and variable EGFP distribution within the blastomeres at morula stage (Supporting Information Fig. S1B), similarly to p18 embryos.
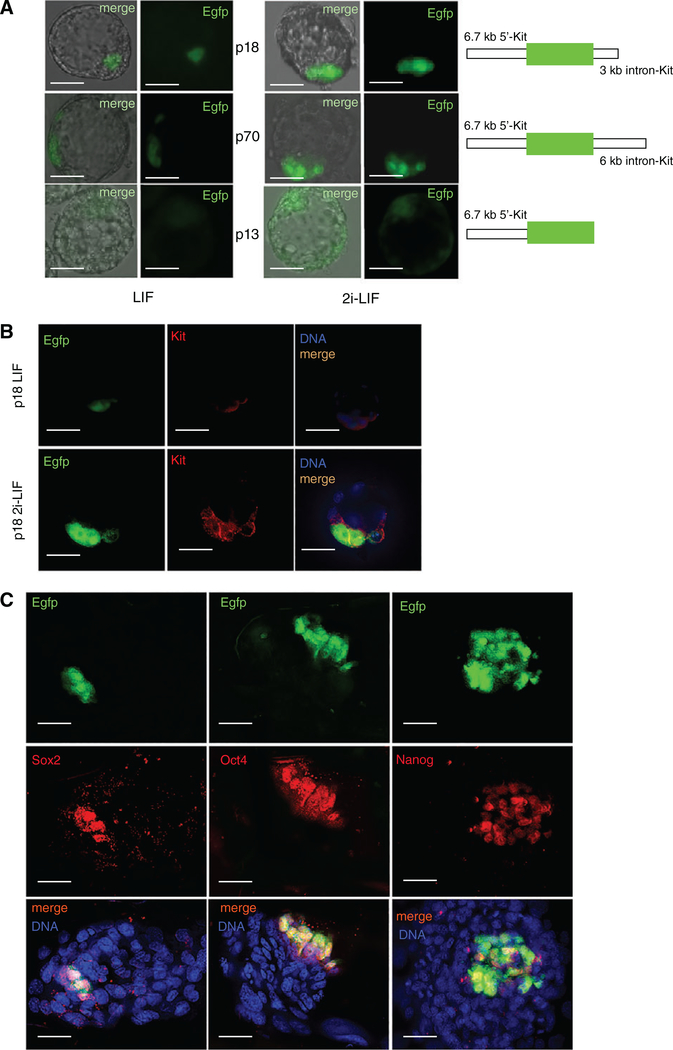
Figure 1.
Kit regulatory regions drive EGFP expression during early embryonic development. (A): Merged images of blastocysts in bright-field (left) and epifluorescence (right) microscopy obtained from p18 (superior panel), p70 (middle panel), and p13 (inferior panel) transgenic mice, following 24 hours culture in LIF or 2i-LIF condition. A scheme of the constructs used to generate transgenic animals is represented on the right. (B): Kit immuno-localization (red) in p18 (green) blastocysts following 24 hours culture in LIF or 2i-LIF condition. Note that EGFP signal is nuclear, due to the presence of a nuclear localization signal, whereas Kit has a membrane and cytoplasmic localization. (C): Oct4, Sox2, or Nanog immuno-localization (red) in p18 ICM (green) outgrowths.
We observed that not all the cells within the ICM of the blastocysts were EGFP positive when cultured in 15% fetal calf serum (FCS) and LIF (LIF condition, see “Materials and Methods” section); however, fluorescence always colocalized with Kit expression (Fig. 1B, top panels). When blastocysts were cultured in the presence of 2i and LIF to derive ESCs (2i-LIF condition, modified from [13], see “Materials and Methods” section), we found a homogeneous increase of Kit and EGFP fluorescence within the ICM compared with LIF condition (Fig. 1A, right panels; Fig. 1B, bottom panels). Co-localization experiments with the core pluripotency genes showed that Oct4, Sox2, and Nanog were all expressed in EGFP positive cells of the blastocyst outgrowths in 2i-LIF condition (Fig. 1C), indicating that Kit regulatory regions are active in the pluripotent cells of the ICM.
2i Condition Upregulates Kit and Pluripotency Gene Expression in ESCs
EGFP expression was enhanced by the 2i-LIF condition also in ESCs from all the 3 Kit-EGFP lines as observed by epifluorescence and cytofluorimetric analyses (Fig. 2A, 2B), similarly to what has been reported in Oct4-DE ESCs (Supporting Information Fig. S1C and [14]). By immunofluorescence we found that Kit and EGFP expression matched within the ESCs and p18 bright cells expressed higher levels of Kit and EGFP in the 2i-LIF condition (Fig. 2C). We confirmed that p18 and Oct4-DE ESCs were bona fide pluripotent stem cells, since they were able to form teratomas that contained all the 3 germ layer derivatives in the teratoma formation assay (Supporting Information Fig. S2). Since 2i-LIF condition increased EGFP intensity and its homogeneous distribution in p18 ESCs, we evaluated the mRNA and protein levels for Kit and EGFP and for the pluripotency factors Oct4, Sox2, Nanog, and Prdm14 in p18 and Oct4-DE ESC cultured in 2i-LIF or in LIF by qPCR and Western blot analysis. We found that mRNA and protein levels for all these genes but Oct4 were increased in 2i-LIF compared with LIF condition in p18 as well as in Oct4-DE ESCs (Fig. 2D, 2E). We found that Kit levels were upregulated in 2i-only condition with respect to LIF-only; however, LIF addition to 2i further increased Kit levels, indicating that both 2i and LIF increase Kit expression in ESCs (Fig. 2F).
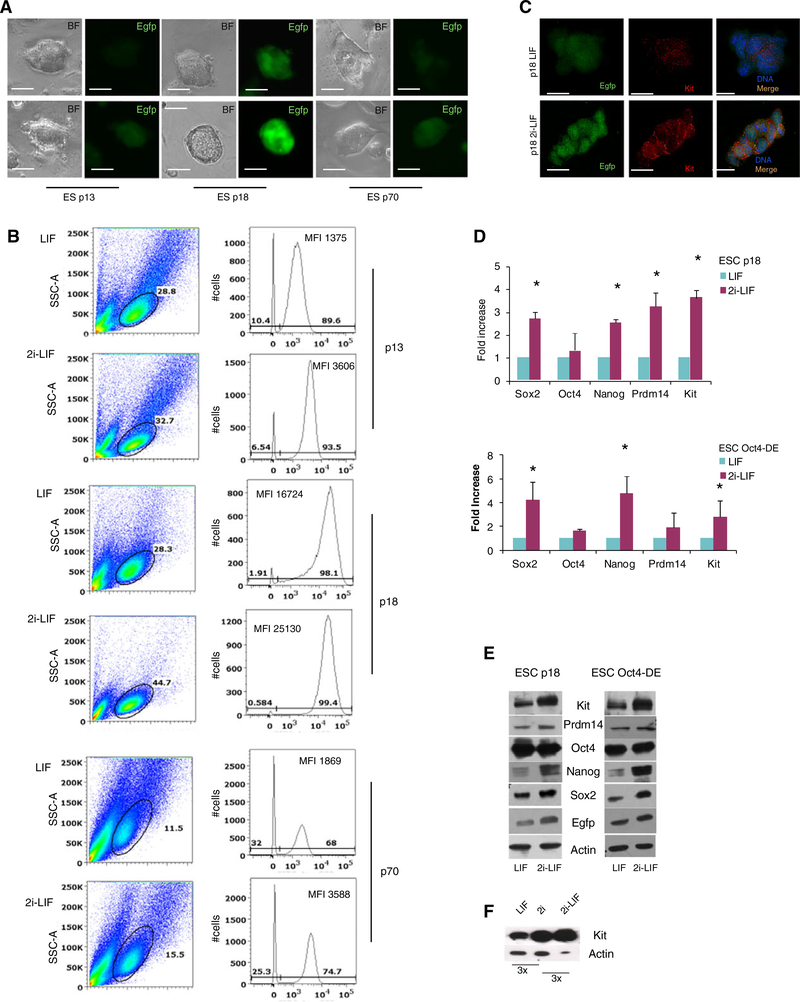
Figure 2.
2i-LIF culture upregulate EGFP, Kit, and the pluripotency genes Sox2, Prdm14, Nanog but not Oct4 expression in ESCs. (A): Brightfield and fluorescence images for EGFP on ESCs from different transgenic lines cultured in LIF or in 2i-LIF conditions. (B): Flow cytometry analysis of p13-EGFP, p18-EGFP, and p70-EGFP ESCs cultured in LIF compared with 2i-LIF. (C): Colocalization of Kit (red) and EGFP (green) in p18 ESCs cultured in LIF or in 2i-LIF conditions. (D): qRT-PCR and (E) Western blot analysis on extracts from p18 or OCT4-DE ESCs cultured in LIF or in 2i-LIF conditions. (F): Western blot analysis of Kit expression in p18 ESCs cultured in LIF, 2i-only, and 2i-LIF condition, and their relative increase measured by image quant analysis is indicated below. Bars represent ±SD; *, p < .05.
Kit Upregulation Occurs in the Process of PGC/EGC Transition
To assess if the culture conditions that upregulate Kit expression in ESCs might influence Kit levels also in fetal germ cells, we cultured PGCs isolated at 9.5 dpc, a developmental stage in which they are prone to derive EGCs, for 24 hours in LIF or 2i-LIF. We also tested the 2i-only, CHIR-LIF or PD-LIF conditions in a culture medium that could support PGC proliferation through the addition of forskolin, an agent that increases cAMP levels [15–17] see “Materials and Methods” section). By qPCR we did not find any increase of Kit nor of Oct4, Sox2, Nanog, and Prdm14 in any of the tested conditions (Fig. 3A). Also 13.5 dpc Kit expressing gonocytes, arrested in G0 of the cell cycle, which do not proliferate, did not upregulate neither Kit nor pluripotency gene expression in 2i-LIF condition (Fig. 3B, 3D). Similarly, 13.5 dpc oocytes, that downregulate pluripotency gene expression upon meiotic entry [18], showed very low levels of Nanog expression both in LIF or 2i-LIF conditions, whereas expressing the promeiotic gene Stra8 (Fig. 3C). Kit deregulated expression has been involved in the occurrence of teratocarcinoma [19], a tumor type originating from PGCs [20] that shares pluripotency gene expression with ESCs and EGCs [21]. To understand if 2i-LIF condition could upregulate Kit/EGFP expression during transition from PGC to EGC state, we cultured 9.5 dpc p18 PGCs in PGC medium on feeder layers for 2 days and then they were shifted to LIF or to 2i-LIF condition to derive EGCs. As shown in Figure 3E, we found that after 1 day from shifting to 2i-LIF condition, EGFP fluorescence intensity was increased in germ cell colonies that were in the process of transition to EGCs, compared with LIF condition. Moreover, the frequency of EGC colony formation was increased more than 3 times in 2i-LIF compared with LIF condition (not shown) in agreement to what has been previously reported [14, 15] and EGFP intensity was increased and more homogeneously distributed within cells (Fig. 3E). These results show that Kit transcriptional activity is upregulated in 2i-LIF condition when PGC become susceptible to be converted to EGCs.
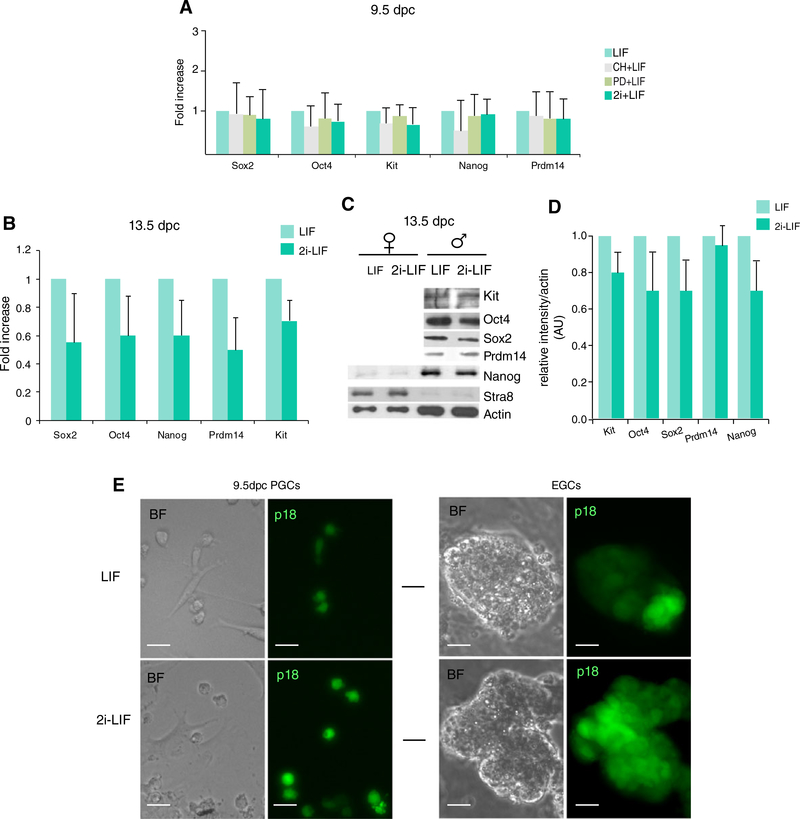
Figure 3.
PGCs and prospermatogonia are not sensitive to 2i-LIF culture conditions. (A): qRT-PCR analysis of Sox2, Oct4, Nanog, Prdm14, and Kit mRNA levels in 9.5 dpc p18 primordial germ cells cultured in LIF, CHIR (CH)+LIF, PD+LIF, or 2i+LIF. (B): qRT-PCR analysis and (C) Western blot analysis for Kit, pluripotency, or differentiation gene mRNA levels in 13.5 dpc p18 male and female germ cells cultured 24 hours in LIF or in 2i-LIF condition. (D): Densitometric analysis in arbitrary units of the intensity of the pluripotency gene bands reported in (C), normalized for the intensity of actin bands. (E): Brightfield and fluorescence images of p18 9.5 dpc germ cells that were previously cultured for 48 hours in PGC medium and then shifted to LIF (upper row) or 2i-LIF condition (lower row; left panels) and of EGCs obtained from the corresponding cultures (arrows pointing to right panels). Bars represent ±SD; **, p < .001 on at least 3 experiments.
Sox-2 Regulates Kit Expression in ESCs
We previously demonstrated that Sox2 regulates Kit expression in PGCs [11]. To understand if Sox2 might directly control Kit expression also in ESCs, we treated Sox2loxP/loxP/p18/Cre-ERT ESCs with 4-hydroxytamoxifen (4-HT) to induce Sox2 deletion. By qPCR we found that Sox2 mRNA levels strongly decreased after 48 hours following 4-HT treatment compared with untreated cells (Fig. 4A). Kit and EGFP expression as well as that of Oct4 were all decreased upon Sox2 deletion (Fig. 4B, 4C). To test if Sox2 was directly acting on Kit regulatory regions, we transfected a plasmid encoding for Sox2 in p18 or p70 ESCs. As shown in Figure 4D, 4E, Sox2 overexpression was able to induce Kit and, to a lesser extent, EGFP protein upregulation in ESCs from both genotypes. Within Kit promoter and the first intron, 4 Sox2 consensus sequences can be identified (ACAAAG; numbered 1–4, Fig. 4F [11]). To find out if Sox2 was able to bind these sequences, ChIP assays were carried out on chromatin isolated from pools of p18 ESC cultures. As shown in Figure 4G, we found at least 3 specific binding sites (bs), 2 within the promoter sequences (bs-2 and bs-3) and 1 in the first Kit intron (bs-4), that were specifically pulled down by Sox2 antibodies. The region containing the first Sox2 binding site (bs-1) gave ambiguous results and was not considered specific, whereas an unrelated sequence present within the 14th Kit exon (sequence 5) gave negative results. Altogether these results indicate that Kit is a transcriptional target of Sox2 in ESCs, as we previously demonstrated in PGCs [11].
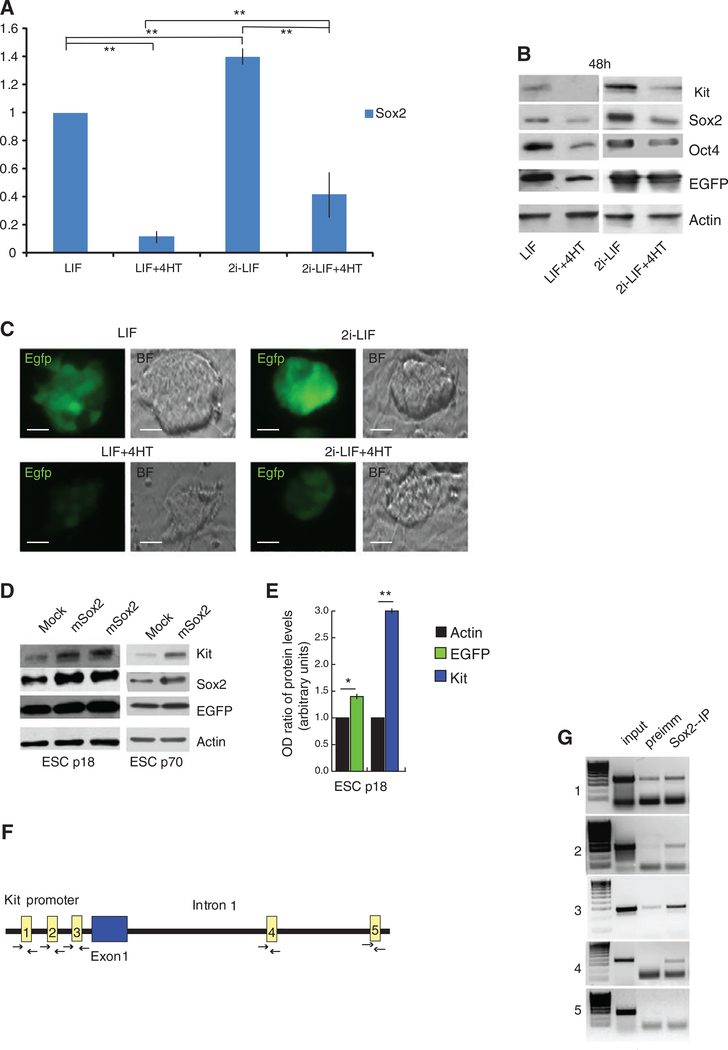
Figure 4.
Sox2 regulates Kit expression in ESCs. (A): qRT-PCR analysis for Sox2 expression in p18/Sox2loxP/loxP CreErt ESCs cultured in LIF or in 2i LIF culture condition, treated or untreated with 4-HT. (B): Western blot analysis for Sox2, Oct4, Kit, and EGFP in cell extracts obtained as in (A). (C): Brightfield and fluorescence images on p18/Sox2loxP/loxP CreErt ESCs treated as in (A). (D): Western blot analysis for Kit, Sox2, EGFP, and actin in p18 (2 representative transfections) or p70 ESCs extracts mock treated or transfected with a plasmid encoding for Sox2. (E): Densitometric analysis of the intensity of Kit and EGFP bands reported in (D; for p18 ESCs), normalized for the intensity of actin bands (arbitrary units). (F): Schematic representation of Sox2 binding sites (rectangles) in the Kit promoter and first intron. Arrows represent forward and reverse primer positions for each Sox2 potential binding site (numbered 1–4) or for the unrelated sequence 5. (G): Representative semiquantitative PCR analysis after chromatin immunoprecipitation using primers shown in (F). Bars represent ±SD; **, p < .001 on 3 experiments.
Kit Constitutive Activation Induces ESC Proliferation
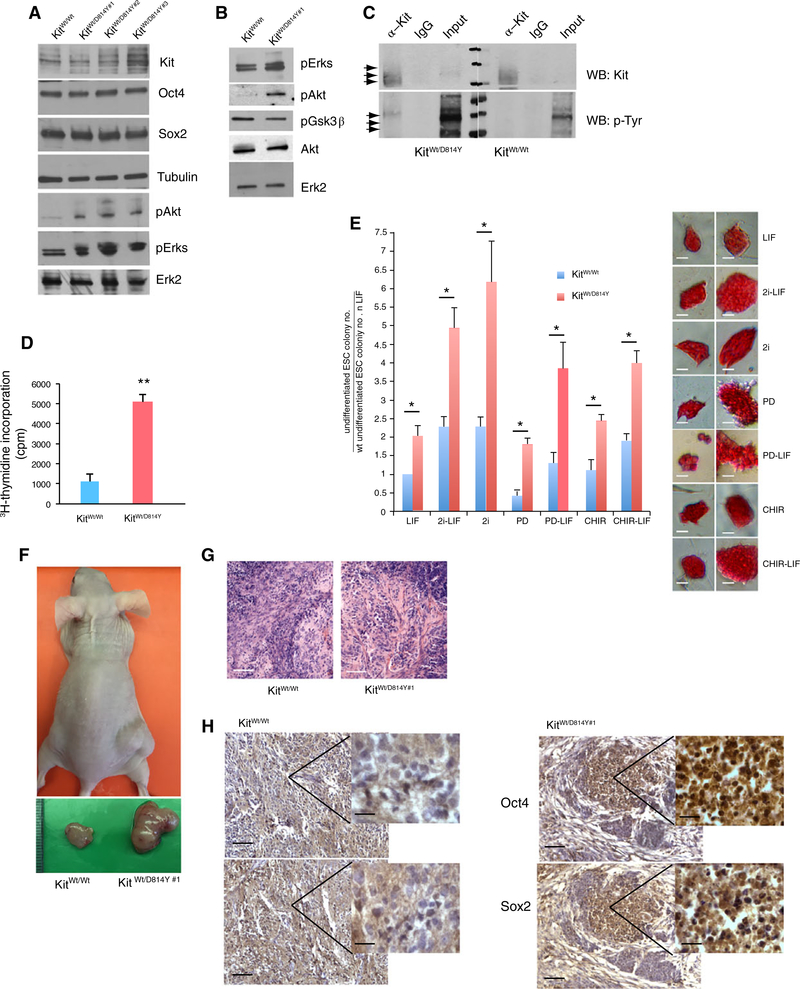
Kit controls cell proliferation and activating mutations in its kinase domain have been shown to induce cell transformation in vitro and in vivo [22, 23]. The evidence that Kit is coexpressed with pluripotency genes and it is upregulated by ground state culture conditions suggested that it might be involved in the control of ESC proliferation. To test this hypothesis and to overcome the influence of variably produced SCF by ESC feeder layers, we produced a dominant active mutation of Kit in ESCs. We generated 3 knockin ESC lines carrying the missense mutation D → Y at residue 814 (homologous to the human residue D816) of the Kit gene (Supporting Information Fig. S3A–S3C) that affects the tyrosine kinase domain of the receptor inducing its constitutive activation. We found that Kit was expressed in all the 3 Kit D814Y-ESC lines, although at different levels (Fig. 5A), and its constitutive activation was confirmed by the higher phosphorylation levels of Kit, MAPKs, and AKT compared with wt controls (Fig. 5A, 5C). All the 3 cell lines had normal karyotype (not shown), however, when injected into host blastocysts none of them yielded live chimeras (clone 6115: 55 blastocysts implanted/0 live pups; clone 6181: 65 blastocysts implanted/0 live pups; clone 6208: 35 blastocysts implanted/0 live pups). The levels of the pluripotency factors Sox2 and Oct4 in the 3 D814Y lines were similar to those in the parental wt-ESCs (Fig. 5A). We focused on one of the mutant ESC lines, line no. 6115 (colony #1, Fig. 5B), to further study Kit D814Y ESCs. By 3H-thymidine incorporation we found that mutant ESCs showed a higher proliferative activity compared with wt controls, when grown in 2i-LIF condition (Fig. 5D). In order to understand which pathway impacted on the proliferative advantage of Kit mutant cells, we assayed their ability to form colonies in the presence of 1, 2, or 3 components of the 2i-LIF condition with respect to wt cells. As shown in Figure 5E, we found that in all the culture conditions mutant ESCs were able to form more colonies and of larger size compared with wt ESCs after 4 days in culture. Moreover, while wt ESCs grew mostly as differentiated colonies in PD alone with respect to LIF condition, mutant ESCs gave more undifferentiated and larger colonies in the same conditions. When injected in immunodeficient CD-1 mice Kit mutant ESCs formed larger teratomas compared with parental wt ESCs; however, the histology of the 2 groups was similar and showed the presence of all the derivatives from the 3 embryonic germ layers (Fig. 5F, 5G). By immunohistochemistry, however, we found that mutant teratomas contained areas of Oct4/Sox2 positive cells, whereas control tumors contained only few scattered double positive cells (Fig. 5H), indicating that Kit mutant teratomas harbor undifferentiated teratocarcinoma cells. To further test their differentiation potential, we tested the ability of wt and mutant ESCs to differentiate along myocardial or neural lineages in vitro. Although we did not observe any difference in the ability to give rise to beating cells (not shown), when induced to in vitro differentiate into the neural lineage, Kit D814Y ESCs formed neuron-like TubJ positive cells much less efficiently and their axonal processes were shorter compared with wt ESCs (Supporting Information Fig. S3D).
Figure 5.
Constitutive Kit activation regulates ESC proliferation. (A, B): Western blot analysis for Kit, Sox2, Oct4, pERKs, and pAkt in targeted Kit D814Y ESC colonies. (C): Western blot analysis for p-Tyr in protein extracts from D814Y or wt ESCs immunoprecipitated for Kit that shows an increased level of tyrosine phosphorylation in mutant with respect to wt Kit. Arrows point to Kit bands that show different electrophoretic mobility depending on their glycosylation and phosphorylation levels. (D):3H-thimidine incorporation in Kit wt or in kit D814Y ESCs cultured in 2i-LIF condition. Values represent mean ± SD from triplicate experiments. (E): Left panel: colony assay on Kit wt and mutant ESCs cultured in 1, 2, or 3 components of 2i-LIF condition. Values represent the ratio between the colony numbers obtained in the different culture conditions with respect to the colony numbers of wt ESCs in LIF condition ± SD, from duplicate experiments. Right panel shows representative ESC morphologies and sizes under the corresponding culture conditions. (F): CD-1 nude mouse (upper panel) injected with wt (left flank) and Kit D814Y ESCs (right flank) under the skin (1 × 106 cells each). Isolated teratomas after 4 weeks from ESC injections (lower panel). (G): Histological analysis by hematoxylin–eosin staining of the teratomas showing tubular/gland differentiation. (H): Immunohistochemical analysis for Oct4 and Sox2 expression in sections of teratoma as in (F). Bars represent ±SD; *, p < .05 or **, p < .001 on 3 experiments.
Discussion
By exploiting transgenic mice expressing EGFP under the control of different regulatory regions of the Kit gene, we traced Kit expression during early embryogenesis and during the process of ESC derivation in vitro. We found that the 6.9 kb of Kit promoter, that is active in early PGCs [8], was sufficient to drive EGFP expression in the early mouse embryo starting from the morula stage up to blastocyst, within the ICM. EGFP expression colocalized with Kit within the ICM of the blastocyst, indicating that these regulatory regions correctly drive the expression of the reporter. We found that EGFP as well as Kit were continuously expressed in the ICM during the ESC derivation process and they were strongly upregulated in culture by the presence of MAPK and GSK3β inhibitors, suggesting that Kit regulatory regions are sensitive to the ground state conditions. By targeting EGFP into loci encoding for transcription factors such as Nanog [24], Esrrb (estrogen-related receptor β) [25], Rex1 [26], Tbx3 [27], and Prdm14 [28] or for maternal factors such as Stella [29], it has been shown that expression of the reporter and the corresponding proteins fluctuate both heterogeneously and dynamically and this is associated to pluripotency of the expressing cells. We found that EGFP and Kit were not uniformly expressed in the blastocysts at isolation, but they became homogeneously expressed and upregulated by 2i-LIF in all the blastomeres of the ICM, similarly to Sox2, Oct4, and Nanog. In the presence of 2i-LIF, ESCs maintain ground state pluripotency [30] and exhibit greater pluripotent gene expression than ESCs cultured in serum with LIF. Furthermore, 2i-LIF ESCs homogeneously express Nanog, that potentiates pluripotent gene transcription by creating a permissive chromatin structure [31]. Accordingly, we found that parallel to EGFP and Kit upregulation, also Nanog, Sox2, and PRDM14 expression were upregulated both at the mRNA and protein levels in p18 or Oct4-DE ESCs cultured in 2i-LIF. We found that Oct4 mRNA and protein levels were not modified in 2i-LIF condition; however, we detected an increase of EGFP brightness in Oct4-DE ESCs following 2i-LIF stimulation, in agreement with previous findings [14], indicating that the activity of the endogenous Oct4 locus was sensitive to naïve conditions. We found that 2i-only condition was able to increase Kit levels compared with LIF condition, but LIF addition to 2i further increased Kit protein levels, confirming that Kit expression is positively regulated by LIF in ESCs, as also reported previously [32]. From these evidences we hypothesized that Kit might share features of a pluripotency gene. Many of the pluripotency genes, as well as Kit, are also markers of PGCs and their expression correlates with their capacity to give rise to EGCs [15]. Although PGC conversion to EGCs can be obtained by direct exposure of PGCs to 2i-LIF [14], prior PGC culture for at least 2 days in proliferation medium leads to higher efficiency of EGC formation after shifting to naïve conditions [15]. We found that freshly isolated 9.5 dpc PGCs did not upregulate Kit, EGFP nor pluripotency genes in none of the culture conditions containing LIF, 2i or each the 2 inhibitor plus LIF. However, if we cultured PGCs under optimal culture conditions, such as in proliferative medium and in the presence of feeder layers, we found that Kit/EGFP intensity was significantly increased upon shifting the PGC cultures to 2i-LIF condition. These results indicate that PGC conversion to EGCs is accompanied by Kit up regulation, as we found also in ICM cells. Since we were not able to find any stimulation of freshly isolated PGCs, it is possible that the majority of them are not able to immediately undergo EGC conversion upon isolation, but they need to recover from a restrictive embryonic environment that prevents their uncontrolled growth. Indeed, PGCs undergo in vivo transformation, through a process similar to EGC conversion in vitro, that leads to the genesis of teratoma, if they do not complete correctly their migratory pathway toward the gonads and proliferate in ectopic sites [33]. What regulates Kit expression in ESCs? The evidences that: (a) Sox2 overexpression strongly upregulates Kit mRNA levels in ESCs ([34] and our results) and in PGCs [11]; (b) Sox2 deletion decrease both Kit and EGFP levels; and (c) Sox2 itself is upregulated by 2i-LIF, suggest that Sox2 directly regulates Kit in ESCs. Indeed, our results show that Sox2 specifically bound consensus sequences present within the promoter and first Kit intron and are in line with the ChIP profiling experiments that reported Sox2 binding within 8 kb upstream and downstream from Kit transcriptional start site [35]. The differences of induction levels between Kit and EGFP in the overexpression experiments might reflect either posttranscriptional regulation mechanisms or the involvement of additional DNA-binding sites present in the Kit locus not included in the p18 and p70 constructs. The latter hypothesis is supported by the evidence that regulatory regions that promote Kit expression in PGCs and in other stem cell types are spread over 200 kb upstream to Kit ORF [36, 37], indicating a complex level of regulation of Kit transcription. In agreement with our results, ChIP seq data showing Sox2 occupancy has been reported in close association to Oct4, Nanog, and Tcf3 within ±8 kb from the TSS of the Kit locus [35], suggesting a possible involvement of a pluripotency circuitry in the regulation of this gene. Since we previously found that only the first Kit intron region was occupied by Sox2 in PGC chromatin [11], our present results suggest that ESCs and PGCs share common transcription factors for the regulation of Kit transcription but that other partners might be involved to confer cell-specificity of Kit expression in the 2 cell types.
The potential involvement of Kit in pluripotency circuitries was assessed by generating a Kit gain of function mutation in ESCs that maintains the receptor in an active state and is able to transform hemopoietic cells [38]. We found that ESCs carrying a dominant active Kit allele (D814Y) show increased proliferation in vitro and that, when injected into carrier blastocysts, they never give rise to live births. These results suggest that their overgrowth might affect the normal development of chimaeric embryos. Both MAPK and PI3K pathways were constitutively activated, but not the Wnt pathway as indicated by unchanged levels of p-Ser9-GSK3β, that negatively regulates GSK3β enzymatic activity. Over-proliferation of mutant cells was observed either in LIF or 2i-LIF, although in this latter condition the proliferative effect was amplified. We observed that each component of the 2i-LIF condition (isolated inhibition of MAPK or GSK3β, or simple addition of LIF) significantly promoted the formation of undifferentiated colonies of mutant ESCs. Notably, while in PD-only condition most wt cells underwent differentiation, as previously reported [39] Kit mutant cells were able to form higher numbers of undifferentiated colonies. When injected into nude mice for teratoma formation assay, mutant Kit ESCs formed larger tumor masses compared with Kit wt ESCs and they showed similar differentiation pattern, although a large increase of undifferentiated teratocarcinoma foci was found in mutant teratomas. These results suggest that concomitant MAPK and PI3K pathway activation by Kit D814Y does not influence tumor lineage commitment, as it has been shown in ESCs harboring the HRas1Q61L mutation [40], but it supports the growth of undifferentiated tumor cells. However, Kit mutant cells showed an impaired ability to properly differentiate into neurons in vitro, which might explain, at least in part, their inability to give rise to live births when injected into carrier blastocysts.
Altogether our results show that, similarly to pluripotency genes, Kit transcription fluctuates in early embryos and in ESCs. Its expression is enhanced by 2i-LIF culture, by Sox2 overexpression and during the acquisition of the pluripotent state either from ICM to ESC or from PGC to EGC transition. Kit constitutive activation enhances ESC growth in vivo and in vitro, suggesting its involvement in promoting their tumorigenic potential. In conclusion, the role of Kit in ESCs and PGCs might be similar in supporting both a proliferative and a pluripotent state.
Supplementary Material
Significance Statement.
By exploiting transgenic mice carrying Kit different promoter regions fused to EGFP (Kit-EGFP) it was found that embryos initiate EGFP expression at morula stage. EGFP expression is then maintained in the blastocyst, within the ICM, and more importantly, its levels along with those of Kit increase in ground state culture conditions (MAPK and GSK3β inhibitors [2i] and LIF) compared with standard conditions (LIF only). Indeed, it was found that ESCs upregulated EGFP expression, as well as that of Sox2, Nanog, Prdm14 but not Oct4 in 2i-LIF, whereas they showed a nonhomogeneous EGFP expression pattern when cultured in LIF only condition. It was found that primordial germ cells, that share a similar transcriptional profile with ESCs, are sensitive to ground state culture conditions during the transition to EGC state. It was also found that Sox2 controls Kit and EGFP expression in ESCs. Finally, by producing a constitutive active Kit allele, it was found that mutant ESCs impaired early embryo development following their injection in host blastocyst and showed increased proliferation rate in vitro and in teratoma assays in vivo. The results show that Kit regulatory regions respond to ground state culture conditions and suggest a role of Kit in the regulation of ESC proliferation.
Acknowledgments
We thank G. Rossi for histology assistance, F. Lancia and S. Pucci for mouse husbandry. We also thank S. Ottolenghi and S. Nicolis (University of Milano, Bicocca) for supplying Kit-EGFP and Sox2-floxed/Cre-Ert2 transgenic lines. We also thank B.G.R. Jannini (University of Rome Torvergata, for animal genotyping). This work was supported by MIUR grants 2015XSNA83_006 to S.D., PRIN 2015XCR88M_001 and FIRB no. RBAP109BLT_004 to E.A.J.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Dolci S, Williams DE, Ernst MK et al. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 1991;352:809–811. [DOI] [PubMed] [Google Scholar]
- 2.Godin I, Deed R, Cooke J et al. Effects of the steel gene product on mouse primordial germ cells in culture. Nature 1991;352: 807–809. [DOI] [PubMed] [Google Scholar]
- 3.Omori I, Yamaguchi H, Miyake K et al. D816V mutation in the KIT gene activation loop has greater cell-proliferative and antiapoptotic ability than N822K mutation in core-binding factor acute myeloid leukemia. Exp Hematol 2017;52:56–64 e54. [DOI] [PubMed] [Google Scholar]
- 4.Manova K, Nocka K, Besmer P et al. Gonadal expression of c-kit encoded at the W locus of the mouse. Development 1990;110: 1057–1069. [DOI] [PubMed] [Google Scholar]
- 5.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154–156. [DOI] [PubMed] [Google Scholar]
- 6.Bashamboo A, Taylor AH, Samuel K et al. The survival of differentiating embryonic stem cells is dependent on the SCF-KIT pathway. J Cell Sci 2006;119:3039–3046. [DOI] [PubMed] [Google Scholar]
- 7.Manova K, Bachvarova RF. Expression of c-kit encoded at the W locus of mice in developing embryonic germ cells and presumptive melanoblasts. Dev Biol 1991;146:312–324. [DOI] [PubMed] [Google Scholar]
- 8.Cairns LA, Moroni E, Levantini E et al. Kit regulatory elements required for expression in developing hematopoietic and germ cell lineages. Blood 2003;102:3954–3962. [DOI] [PubMed] [Google Scholar]
- 9.Barrios F, Filipponi D, Campolo F et al. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci 2012;125:1455–1464. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini M, Filipponi D, Gori M et al. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle 2008;7:3878–3888. [DOI] [PubMed] [Google Scholar]
- 11.Campolo F, Gori M, Favaro R et al. Essential role of Sox2 for the establishment and maintenance of the germ cell line. Stem Cells 2013;31:1408–1421. [DOI] [PubMed] [Google Scholar]
- 12.Yeom YI, Fuhrmann G, Ovitt CE et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 1996;122:881–894. [DOI] [PubMed] [Google Scholar]
- 13.Ying QL, Wray J, Nichols J et al. The ground state of embryonic stem cell self-renewal. Nature 2008;453:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitch HG, Blair K, Mansfield W et al. Embryonic germ cells from mice and rats exhibit properties consistent with a generic pluripotent ground state. Development 2010; 137:2279–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leitch HG, Nichols J, Humphreys P et al. Rebuilding pluripotency from primordial germ cells. Stem Cell Rep 2013;1:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Felici M, Dolci S, Pesce M. Proliferation of mouse primordial germ cells in vitro: A key role for cAMP. Dev Biol 1993;157: 277–280. [DOI] [PubMed] [Google Scholar]
- 17.Farini D, Scaldaferri ML, Iona S et al. Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Dev Biol 2005;285:49–56. [DOI] [PubMed] [Google Scholar]
- 18.Western P, Maldonado-Saldivia J, van den Bergen J et al. Analysis of Esg1 expression in pluripotent cells and the germline reveals similarities with Oct4 and Sox2 and differences between human pluripotent cell lines. STEM CELLS 2005;23:1436–1442. [DOI] [PubMed] [Google Scholar]
- 19.Sihto H, Sarlomo-Rikala M, Tynninen O et al. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol 2005;23:49–57. [DOI] [PubMed] [Google Scholar]
- 20.Dolci S, Campolo F, De Felici M. Gonadal development and germ cell tumors in mouse and humans. Semin Cell Dev Biol 2015;45: 114–123. [DOI] [PubMed] [Google Scholar]
- 21.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 2005;5:210–222. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Melenhorst JJ, Alemu L et al. KIT with D816 mutations cooperates with CBFB-MYH11 for leukemogenesis in mice. Blood 2012;119:1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnittger S, Kohl TM, Haferlach T et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood 2006;107: 1791–1799. [DOI] [PubMed] [Google Scholar]
- 24.Chambers I, Silva J, Colby et al. Nanog safeguards pluripotency and mediates germline development. Nature 2007;450: 1230–1234. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg DL, Zhang W, Yates A et al. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol 2008;28:5986–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyooka Y, Shimosato D, Murakami K et al. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 2008;135:909–918. [DOI] [PubMed] [Google Scholar]
- 27.Niwa H, Ogawa K, Shimosato D et al. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 2009;460:118–122. [DOI] [PubMed] [Google Scholar]
- 28.Yamaji M, Ueda J, Hayashi K et al. PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell 2013;12:368–382. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi K, de Sousa Lopes SMC, Tang F et al. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 2008;3:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva J, Barrandon O, Nichols J et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol 2008;6:e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva J, Nichols J, Theunissen TW et al. Nanog is the gateway to the pluripotent ground state. Cell 2009;138:722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martello G, Bertone P, Smith A. Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J 2013;32:2561–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Felici M, Dolci S. From testis to teratomas: A brief history of male germ cells in mammals. Int J Dev Biol 2013;57:115–121. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama A, Xin L, Sharov AA et al. Uncovering early response of gene regulatory networks in ESCs by systematic induction of transcription factors. Cell Stem Cell 2009;5: 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marson A, Levine SS, Cole MF et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 2008;134:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berrozpe G, Agosti V, Tucker C et al. A distant upstream locus control region is critical for expression of the Kit receptor gene in mast cells. Mol Cell Biol 2006;26:5850–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Siena S, Gimmelli R, Nori SL et al. Activated c-Kit receptor in the heart promotes cardiac repair and regeneration after injury. Cell Death Dis 2016;7:e2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, Zeng S, Metcalfe DD et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood 2002;99: 1741–1744. [DOI] [PubMed] [Google Scholar]
- 39.Wray J, Kalkan T, Smith AG. The ground state of pluripotency. Biochem Soc Trans 2010;38:1027–1032. [DOI] [PubMed] [Google Scholar]
- 40.Lu CW, Yabuuchi A, Chen L et al. Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet 2008;40:921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Favaro R, Valotta M, Ferri AL et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci 2009;12: 1248–1256. [DOI] [PubMed] [Google Scholar]
- 42.Court DL, Swaminathan S, Yu D et al. Mini-lambda: A tractable system for chromosome and BAC engineering. Gene 2003;315:63–69. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Sharan SK. A simple two-step, ’hit and fix’ method to generate subtle mutations in BACs using short denatured PCR fragments. Nucl Acids Res 2003;31: e80–e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majumdar MK, Feng L, Medlock E et al. Identification and mutation of primary and secondary proteolytic cleavage sites in murine stem cell factor cDNA yields biologically active, cell-associated protein. J Biol Chem 1994;269:1237–1242. [PubMed] [Google Scholar]
- 45.Viglietto G, Dolci S, Bruni P et al. Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int J Oncol 2000;16: 689–694. [DOI] [PubMed] [Google Scholar]
- 46.Albanesi C, Geremia R, Giorgio M et al. A cell- and developmental stage-specific promoter drives the expression of a truncated c-kit protein during mouse spermatid elongation. Development 1996;122: 1291–1302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.