Supplemental Digital Content is available in the text
Keywords: acute respiratory distress syndrome, aerosol generating procedure, airway, anesthesia, ARDS, Coronavirus, COVID-19, critical care, ICU, mortality, multicenter, multidisciplinary teams, multi-institutional, pandemic, patient safety, personal protective equipment, PPE, resource allocation, respiratory failure, SARS-CoV-2, surgical technique, timing, tracheostomy, tracheotomy, ventilation
Abstract
Objective:
The aim of this study was to assess the outcomes of tracheostomy in patients with COVID-19 respiratory failure.
Summary Background Data:
Tracheostomy has an essential role in managing COVID-19 patients with respiratory failure who require prolonged mechanical ventilation. However, limited data are available on how tracheostomy affects COVID-19 outcomes, and uncertainty surrounding risk of infectious transmission has led to divergent recommendations and practices.
Methods:
It is a multicenter, retrospective study; data were collected on all tracheostomies performed in COVID-19 patients at 7 hospitals in 5 tertiary academic medical systems from February 1, 2020 to September 4, 2020.
Result:
Tracheotomy was performed in 118 patients with median time from intubation to tracheostomy of 22 days (Q1–Q3: 18–25). All tracheostomies were performed employing measures to minimize aerosol generation, 78.0% by percutaneous technique, and 95.8% at bedside in negative pressure rooms. Seventy-eight (66.1%) patients were weaned from the ventilator and 18 (15.3%) patients died from causes unrelated to tracheostomy. No major procedural complications occurred. Early tracheostomy (≤14 days) was associated with decreased ventilator days; median ventilator days (Q1–Q3) among patients weaned from the ventilator in the early, middle and late groups were 21 (21–31), 34 (26.5–42), and 37 (32–41) days, respectively with P = 0.030. Compared to surgical tracheostomy, percutaneous technique was associated with faster weaning for patients weaned off the ventilator [median (Q1–Q3): 34 (29–39) vs 39 (34–51) days, P = 0.038]; decreased ventilator-associated pneumonia (58.7% vs 80.8%, P = 0.039); and among patients who were discharged, shorter intensive care unit duration [median (Q1–Q3): 33 (27–42) vs 47 (33–64) days, P = 0.009]; and shorter hospital length of stay [median (Q1–Q3): 46 (33–59) vs 59.5 (48–80) days, P = 0.001].
Conclusion:
Early, percutaneous tracheostomy was associated with improved outcomes compared to surgical tracheostomy in a multi-institutional series of ventilated patients with COVID-19.
Coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has taken a staggering toll around the world, with most of the mortality attributable to respiratory failure and acute respiratory distress syndrome (ARDS).1,2 Approximately 10% to 15% of COVID-19 patients develop respiratory failure and require prolonged invasive mechanical ventilation.1,3 Previous studies have shown that tracheostomy leads to decreased need for sedation, earlier weaning from mechanical ventilation, and decreased ventilator-associated pneumonia in patients with prolonged respiratory failure.4,5 Tracheostomy also improves pulmonary hygiene, reduces intensive care unit (ICU) capacity strain, decreases risk of chronic laryngeal injury, and expedites rehabilitation.6 However, either performing tracheotomy or providing post-procedure patient care may lead to aerosolization of respiratory secretions that contain SARS-CoV-2, thereby posing a risk of infection to medical staff.7
During the pandemic, multiple professional organizations and groups issued guidelines about the timing and performance of tracheostomy.8–11 Many of the early guidelines recommended performing tracheostomy after 21 days of mechanical ventilation, based on the assumption that the delay would allow for lower viral load.8,11 However, others supported the procedure after 10 days to leverage the anticipated advantages of early tracheostomy.9 The lack of evidence around the timing of tracheostomy is widely acknowledged, and some expert panel consensus statements have refrained from providing any specific recommendations.10 Controversy also exists about the preferred technique, with advocates divided between surgical and percutaneous methods.12–14 Thus, institutions are following different guidelines, and the resulting variations in practice likely translate into overall lower quality care.15,16 As the subsequent waves of the pandemic unfold, data remain limited about the best approach to manage prolonged COVID-19 respiratory failure. The aim of the present study was to assess tracheostomy-related practices in a multicenter cohort of COVID-19 patients in the United States (US). We also investigated whether different timing and techniques of tracheostomy were associated with better patient outcomes.
METHODS
All adult patients with COVID-19 respiratory failure who required tracheostomy between February 1, 2020 and September 4, 2020 across 5 systems in United States were included. Data on outcomes were collected and reviewed last on January 15, 2021. Tracheostomies were performed by several specialties: interventional pulmonology, general surgery, thoracic surgery, otorhinolaryngology, and neurocritical care intensivists. Participating institutions included Duke University (Durham, NC), University of Michigan (Ann Arbor, MI), University of California at San Diego (San Diego, CA), Emory University (Atlanta, GA), and Virginia Commonwealth University (VCU; Richmond, VA). Institutional review board (IRB) approval was obtained from every institution (Duke IRB protocol # 00106067). Data were extracted from review of electronic medical records and included patient and disease characteristics; intra and post-procedural data; hospital course including ventilator weaning and length of stay; and outcomes including adverse events and survival. All data were entered into a REDCap database maintained by Duke University for secure web-based data capture from different institutions.
Statistical Analysis
Continuous data are presented as the median with 25th and 75th percentiles (Q1-Q3), whereas categorical data are displayed as counts with percentages. Comparisons between continuous variables were made using the nonparametric Wilcoxon rank sum test, and the Kruskal-Wallis test was used if >2 groups were compared, as none of the variables met normality assumptions for parametric tests. The Wilcoxon signed rank test was used to compare matched pairs. Categorical variables were assessed using the χ2 test. SAS version 9.4 (SAS Institute, Inc., Cary, NC) was used for all analyses, and a two-sided P value <0.05 was considered statistically significant.
RESULTS
During the study time frame, 2793 adult patients were admitted with COVID-19 to the seven hospitals in 5 academic medical systems, 966 (34.5%) were admitted to the intensive care units, and 668 (23.9%) required invasive mechanical ventilation. Tracheostomy was performed in 118 (4.2% of total admitted) mechanically ventilated patients. Patients on invasive mechanical ventilation who did not undergo tracheostomy either stayed on the ventilator for <2 weeks or died. Patient characteristics are shown in Table 1. Most patients were obese, with a median body mass index (BMI) of 32.6 (Q1–Q3: 27.9–37.4). Acute respiratory distress syndrome (ARDS) was present in all patients during the ICU admission, and based on Berlin definition,17 63.6% of patients met criteria for severe ARDS. Thirty patients (25%) were treated with extracorporeal membrane oxygenation (ECMO). Median time from intubation to tracheostomy was 22 days (Q1–Q3: 18–25), and only 9 (7.6%) patients had tracheostomy within 14 days (Table 2). Percutaneous technique was used for 78.0% of procedures, whereas the remainder were performed with open surgical technique—primarily because of anatomical concerns that precluded percutaneous approach. Tracheostomy was performed with standard percutaneous and surgical techniques, with addition of personal protective equipment and measures to decrease aerosolization.4,9 Most of the tracheostomies (61.0%) were performed by interventional pulmonologists. Five (4.2%) surgical tracheostomies were done in the operating room, mainly due to complexity of the patient airway and surgeon preference.
TABLE 1.
Patient Characteristics (N = 118)
| Demographics | |
| Age, y, median (Q1–Q3) | 54 (42.5–65.0) |
| Sex, N (%) | |
| Male | 75 (63.6%) |
| Female | 43 (36.4%) |
| Race, N (%) | |
| African American | 54 (45.7%) |
| White | 26 (22%) |
| Hispanic | 27 (22.9%) |
| Others | 11 (9.3%) |
| BMI, median (Q1–Q3) | 32.6 (27.9–37.4) |
| Comorbidities, N (%) | |
| Diabetes | 49 (41.5%) |
| Hypertension | 53 (44.9%) |
| Cardiovascular disease | 13 (11%) |
| Cerebrovascular disease | 5 (4.2%) |
| Chronic obstructive pulmonary disease | 2 (1.7%) |
| Asthma | 16 (13.6%) |
| Liver disease | 3 (2.5%) |
| Renal disorder | 26 (22%) |
| Malignancy | 2 (1.7%) |
| Hospitalization data | |
| Mode of COVID-19 diagnosis, N (%) | |
| Nasopharyngeal swab | 118 (100%) |
| Time in days from COVID-19 diagnosis to intubation, median (Q1–Q3) | 2 (0–5) |
| ARDS∗, N (%) | |
| Any | 118 (100%) |
| Mild | 10 (8.5%) |
| Moderate | 33 (28.0%) |
| Severe | 75 (63.6%) |
| SAPS II Score on admission to ICU, median (Q1–Q3) | 35 (29–45) |
| ECMO support, N (%) | 30 (25.4%) |
| Respiratory data on day of tracheostomy | |
| PEEP on tracheostomy day, median (Q1–Q3), cm H2O | 10 (6–12) |
| FiO2 on tracheostomy day, median (Q1-Q3) | 0.4 (0.4–0.5) |
| PaO2 on tracheostomy day, median (Q1–Q3), mm Hg | 81 (72–107) |
| PaO2/FiO2 ratio on tracheostomy day, median (Q1–Q3) | 195.8 (144.3–273.3) |
ARDS defined according to Berlin definition where PaO2/FiO2 ratio of 201 to 300 is mild, 101–200 is moderate, and ≤100 is severe ARDS.
ARDS indicates acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; PEEP, positive end-expiratory pressure.
TABLE 2.
Tracheostomy Procedural Data
| Time from intubation to tracheostomy, median (Q1–Q3), days | 22 (18–25) |
| Tracheostomy timing, N (%) | |
| Early (0–14 days) | 9 (7.6%) |
| Middle (15–21 days) | 48 (40.7%) |
| Late (>21 days) | 61 (51.7%) |
| Type of tracheostomy procedure, N (%) | |
| Percutaneous | 92 (78.0%) |
| Surgical or open | 26 (22.0%) |
| Procedural team, N (%) | |
| Interventional pulmonary | 72 (61.0%) |
| General surgery | 23 (19.5%) |
| Thoracic surgery | 8 (6.8%) |
| Otorhinolaryngology | 10 (8.5%) |
| Neurocritical care | 5 (4.2%) |
| Tracheostomy tube size, N (%) | |
| 6 | 35 (29.7%) |
| 7 | 4 (3.4%) |
| 7.5 | 1 (0.8%) |
| 8 | 77 (65.3%) |
| 8.5 | 1 (0.8%) |
| Paralytic used, N (%) | 118 (100%) |
| Ventilator pause, N (%) | 118 (100%) |
| Ultrasound use during tracheostomy, N (%) | |
| Yes | 56 (47.5%) |
| No | 62 (52.5%) |
| Bronchoscope, N (%) | |
| Disposable | 105 (89.0%) |
| Nondisposable | 13 (11.0%) |
| Personal protective equipment, N (%) | |
| PAPR | 70 (59.3%) |
| CAPR | 14 (11.8%) |
| N95 with face-shield | 54 (45.8%) |
| Caps | 118 (100%) |
| Gowns | 118 (100%) |
| Gloves | 118 (100%) |
| Location of procedure, N (%) | |
| Bedside | 113 (95.8%) |
| Operating room | 5 (4.2%) |
| Negative pressure room, N (%) | 117 (99.2%) |
| Medications when performing tracheostomy, N (%) | |
| Aspirin | 15 (12.7%) |
| Clopidogrel | 0 (0%) |
| Heparin | |
| Subcutaneous | 8 (6.8%) |
| Intravenous-held | 77 (90.6%) |
| Enoxaparin- held | 21 (18.4%) |
| Direct thrombin inhibitors-held | 12 (10.3%) |
| Vasopressors | 43 (36.4%) |
CPAR indicates controlled air-purifying respirator; PAPR, powered air-purifying respirator.
Direct thrombin inhibitors included bivalirudin and argatroban.
Seventy-eight (66.1%) patients were weaned from the ventilator by the time of last data review, with a median of 36 days (Q1–Q3: 30–41) on ventilator (Table 3). The median sedation days, defined as days with Richmond Agitation-Sedation Scale (RASS) <0, were 18 days (Q1–Q3: 14–22) pre-tracheostomy versus 7 days (Q1–Q3: 3–13) post-tracheostomy (P < 0.001). Tracheostomy tubes were decannulated in 40 (33.9%) patients. Ninety-eight patients (83.8%) were discharged from ICU, with median ICU length of stay of 35.5 days (Q1–Q3: 28–45.5). Ultimately, 100 patients (84.7%) were discharged from the hospital, with median length of stay of 49 days (Q1–Q3: 37–61). Eighteen (15.3%) patients died in the hospital, and no deaths were attributable to tracheostomy. Non–life-threatening complications seen with tracheostomy are listed in Table 3. There were no significant differences in complications when stratified by timing, technique of tracheostomy, or patient BMI. Seventy-five (63.6%) patients had ventilator-associated pneumonia (VAP) defined as clinical suspicion of ventilator associated pneumonia with positive respiratory cultures that necessitated antibiotic administration. Most VAPs were caused by gram-negative bacilli (39.8%) followed by Staphylococcus aureus (22.9%).
TABLE 3.
Patient Outcomes, N = 118
| Sedation days, median (Q1–Q3) | |
| Total | 26 (21–36) |
| Pre-tracheostomy | 18 (14–22) |
| Post-tracheostomy | 7 (3–13) |
| Ventilator weaned, N (%) | 78 (66.1%) |
| Total ventilator days for patients weaned, median (Q1–Q3) (n = 78) | 36 (30–41) |
| Days from tracheostomy to ventilator weaning, median (Q1–Q3) (n = 78) | 12 (6–20) |
| ECMO cohort, N = 30 | |
| ECMO weaned, N (% total on ECMO) | 25 (83.3%) |
| Days from intubation to ECMO weaning, median (Q1–Q3), days (n = 25) | 26 (23–31) |
| Days from tracheostomy to ECMO weaning, median (Q1–Q3), days (n = 25) | 5 (3–8) |
| Post tracheostomy care | |
| Tracheostomy decannulated, N (%) | 40 (33.9%) |
| Days from tracheostomy to decannulation, median (Q1–Q3) (n = 40) | 23.5 (19.5–46.5) |
| Tracheostomy tube changed, N (%) | 65 (55.1%) |
| Days from tracheostomy to tube change, median (Q1–Q3) (n = 65) | 20 (14–27.5) |
| Ventilator-associated pneumonia, N (%) | 75 (63.6%) |
| Ventilator associated pneumonia organisms, N (%) (n = 118) | |
| Methicillin-sensitive Staphylococcus aureus | 17 (14.4%) |
| Methicillin-resistant Staphylococcus aureus | 10 (8.5%) |
| Stenotrophomonas | 3 (2.5%) |
| Gram-negative bacilli | 47 (39.8%) |
| Pseudomonas | 15 (12.7%) |
| Klebsiella | 11 (9.3%) |
| Escherichia coli | 10 (8.5%) |
| Enterobacter | 10 (8.5%) |
| Serratia | 6 (5.1%) |
| Proteus | 3 (2.5%) |
| Citrobacter | 3 (2.5%) |
| Acinetobacter | 1 (0.8%) |
| Burkholderia | 1 (0.8%) |
| Length of stay and survival outcome metrics | |
| Patients discharged from ICU, N (%) | 98 (83.8%) |
| ICU LOS, median (Q1–Q3), days (n = 98) | 35.5 (28–46) |
| Patients discharged from hospital, N (%) | 100 (84.7%) |
| Hospital LOS, median (Q1–Q3), days (n = 100) | 49 (37–61) |
| In-hospital mortality, N (%) | 18 (15.3%) |
| Complications of tracheostomy, N (%) | |
| Total patients with complications | 18 (15.3%) |
| Bleeding∗ | 10 (8.5%) |
| Pneumothorax | 2 (1.7%) |
| Dislodgement | 3 (2.5%) |
| Cellulitis | 2 (1.7%) |
| Air-leak requiring tube change | 3 (2.5%) |
| Tube breakage | 1 (0.8%) |
| Site Ulcer | 1 (0.8%) |
Bleeding defined as bleeding at the tracheostomy site requiring local hemostatic measures and cessation of anticoagulation.
ECMO indicates extracorporeal membrane oxygenation; ICU, intensive care unit; LOS, length of stay.
For comparing the effect of timing of tracheostomy to outcomes, we classified the timing of tracheostomy into early (≤14 days), middle (15–21 days), and late (>21 days) groups, as shown in Figure 1 and Table 4. This classification was based on the range of timing for performing tracheostomy recommended in protocols at the participating institutions as well as guidelines and publications.8–10,18–21 Among the patients who were weaned from the ventilator, the early tracheostomy group had fewer days on ventilator; median ventilator days (Q1–Q3) among patients weaned from the ventilator in the early, middle, and late groups were 21 (21–31), 34 (26.5–42), and 37 (32–41) days, respectively with P = 0.030. Different patient factors like age, BMI, diabetes, and ARDS were not associated with ventilator duration, as shown in Supplemental Table 1.
FIGURE 1.
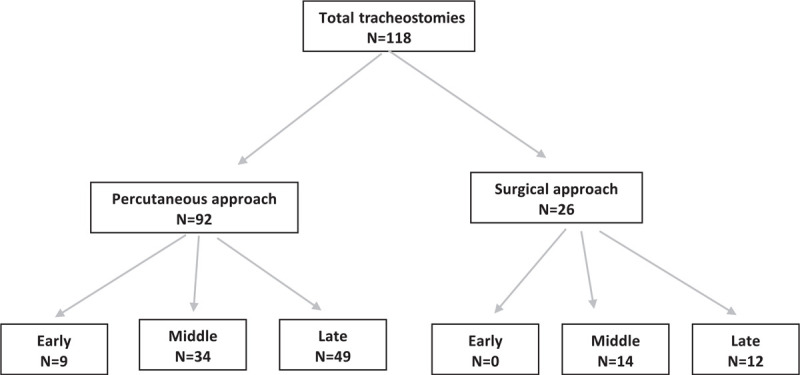
Flow diagram of tracheostomy technique and timing.
TABLE 4.
Patient Outcomes Based on Timing of Tracheostomy
| Early tracheostomy,† N = 9 (7.6%) | Middle tracheostomy,† N = 48 (40.7%) | Late tracheostomy,† N = 61 (51.7%) | P∗ | |
| Sedation days, median (Q1–Q3) | ||||
| Total | 20 (18–22) | 25.5 (20–32) | 29 (22–36) | .091 |
| Pre-tracheostomy | 10 (9–14) | 17 (14–19.5) | 21 (17–25) | <.001 |
| Post-tracheostomy | 11 (7–13) | 8.5 (3–14.5) | 6 (3–11) | .145 |
| Ventilator-related outcomes | ||||
| Weaned from ventilator, N (%) | 5 (55.6%) | 33 (68.8%) | 40 (65.6%) | .739 |
| Total ventilator days for patients weaned, median (Q1–Q3), n = 78 | 21 (21–31) | 34 (26.5–42) | 37 (32–41) | .030 |
| Days from tracheostomy to ventilator weaning (if weaned), median (Q1–Q3), n = 78 | 8 (7–18) | 15.5 (7.5–23.5) | 10.5 (5–16) | .188 |
| Complications, N (%) | ||||
| Ventilator associated pneumonia | 4 (44.4%) | 28 (58.3%) | 43 (70.5%) | .197 |
| Patients with complications of tracheostomy | 2 (22.2%) | 9 (18.8%) | 7 (11.5%) | .481 |
| Overall outcomes | ||||
| Discharged from ICU, N (%) | 6 (75.0%) | 40 (83.3%) | 52 (85.2%) | .757 |
| ICU days for patients discharged, median (Q1–Q3), n = 98 | 31 (22–41) | 33.5 (27–45.5) | 37.5 (30–47.5) | .357 |
| Discharged from hospital, N (%) | 7 (77.8%) | 42 (87.5%) | 51 (83.6%) | .711 |
| Hospital days for patients discharged, median (Q1–Q3), n = 100 | 49.5 (33–57) | 47.5 (35–60) | 51 (39–64) | .792 |
| Death, N (%) | 2 (22.2%) | 6 (12.5%) | 10 (16.4%) | .711 |
ICU indicates intensive care unit.
P value calculated using χ2 test or Kruskal–Wallis test, as appropriate.
Early defined as tracheostomy up to 14 days following intubation; Middle defined as tracheostomy between days 15 and 21 following intubation; Late defined as tracheostomy 21 days following intubation.
Comparing percutaneous vs. surgical technique, 92 patients (78.0%) had percutaneous tracheostomy, and 26 patients (22.0%) had surgical tracheostomy (Fig. 1 and Table 5). Among the patients weaned, compared to those who underwent surgical tracheostomies, patients with percutaneous tracheostomies had decreased ventilator days [median (Q1–Q3): 34 (29–39) vs 39 (34–51) days; P = 0.038] and fewer ventilator-associated pneumonias (58.7% vs 80.8%; P = 0.039). Among patients who were discharged, compared to surgical patients, percutaneous tracheostomy patients had shorter ICU length of stay [median (Q1–Q3): 33 (27–42) vs 47 (33–64) days; P = 0.009] and shorter hospital length of stay [median (Q1–Q3): 46 (33–59) vs 59.5 (48–80) days; P = 0.001]. There was no significant difference in the BMI between percutaneous and surgical groups (P = 0.827).
TABLE 5.
Patient Outcomes Based on Tracheostomy Technique
| Percutaneous, N = 92 (78.0%) | Surgical, N = 26 (22.0%) | P∗ | |
| Sedation in days, median (Q1–Q3) | |||
| Total | 27 (21–35) | 25.5 (21–37) | .972 |
| Pre-tracheostomy | 19 (14–22.5) | 17 (14–20) | .126 |
| Post-tracheostomy | 7 (3–12.5) | 8.5 (4–14) | .332 |
| Ventilator-related outcomes | |||
| Weaned from the ventilator, N (%) | 57 (62.0%) | 21 (80.8%) | .074 |
| Total ventilator days for patients weaned, median (Q1–Q3), n = 78 | 34 (29–39) | 39 (34–51) | .038 |
| Days from intubation to tracheostomy, median (Q1–Q3), n = 118 | 22 (18–25.5) | 21 (18–24) | .480 |
| Days from tracheostomy to ventilator weaning, median (Q1–Q3), n = 78 | 11 (5–18) | 16 (10–23) | .024 |
| Complications | |||
| Ventilator-associated pneumonia, N (%) | 54 (58.7%) | 21 (80.8%) | .039 |
| Patients with complications of tracheostomy, N (%) | 11 (12.0%) | 7 (26.9%) | .168 |
| Bleeding from tracheostomy | 6 (6.5%) | 4 (15.4%) | .152 |
| Dislodgement of tracheostomy | 3 (3.3%) | 0 (0.0%) | .351 |
| Cellulitis of tracheostomy site | 1 (1.1%) | 1 (3.8%) | .341 |
| Pneumothorax | 2 (2.2%) | 0 (0.0%) | .448 |
| Air-leak | 1 (1.1%) | 2 (7.7%) | .059 |
| Other | 1 (1.1%) | 1 (3.8%) | .910 |
| Overall outcomes | |||
| Patients discharged from ICU, N (%) | 77 (84.6%) | 21 (80.8%) | .639 |
| ICU days for patients discharged, median (Q1–Q3), n = 98 | 33 (27–42) | 47 (33–64) | .009 |
| Discharged from hospital, N (%) | 78 (84.8%) | 22 (84.6%) | .983 |
| Hospital days for patients discharged, median (Q1–Q3), n = 100 | 46 (33–59) | 59.5 (48–80) | .001 |
| Death, N (%) | 14 (15.2%) | 4 (15.4%) | .983 |
P value calculated using χ2 test or Wilcoxon Rank sum test, as appropriate.
ICU indicates intensive care unit.
DISCUSSION
This study is the first to report on multicenter and multidisciplinary experience of tracheostomy in COVID-19 patients with acute respiratory failure in the United States. Differences in protocols at institutions allowed us to identify sources of variation and to investigate outcomes associated with different approaches. Almost all the tracheostomies were performed at bedside in negative pressure rooms in the intensive care units, using enhanced personal protective equipment. Percutaneous tracheostomy was the first-line technique, with surgical tracheostomy reserved for patients with difficult anatomy. Most tracheostomies were performed after >2 weeks of mechanical ventilation, and limited bleeding was the most common complication.
Percutaneous tracheostomy, as compared to surgical tracheostomy, was associated with decreased total ventilator days, earlier weaning from the ventilator post-tracheostomy, and shorter ICU and hospital length of stay. This outcome contrasts with a randomized controlled trial by two surgeons at a single institution that compared percutaneous versus surgical tracheostomy in 67 patients with COVID-19 and found no difference between the 2 techniques.21 The decreased total ventilator days may have led to lower incidence of VAP in the percutaneous group when compared to the surgical cohort (59% vs 81%, P = 0.039), as other patient characteristics like BMI were similar in both groups. Lower VAP rate can also explain other improved outcomes seen with percutaneous tracheostomy.
There was no significant difference in complications between percutaneous and surgical techniques. The most common complication associated with tracheostomy was non–life-threatening bleeding (n = 10, 8.5%). This incidence of bleeding falls within the range reported in previous COVID-19 cohorts of 2% to 30%.18,19,21,22 Tracheostomy-associated bleeding in patients with COVID-19 could be explained by the high use of anticoagulants in COVID-19 patients due to their prothrombotic state23 and ECMO cannula maintenance. Additional factors that might have predisposed to bleeding risk include the lack of standardized use of ultrasonography between centers to avoid vascular structures and the avoidance of electrocautery use.
There is a significant controversy as to which tracheostomy technique should be first-line in COVID-19 patients.12,13 Proponents of percutaneous tracheostomy cite lower incidence of infection and bleeding,24 whereas advocates of surgical tracheostomy point to previous experience with severe acute respiratory syndrome (SARS) outbreak and possibly decreased aerosolization.13,14 Such recommendations are likely influenced by not only the available evidence in the literature but also the experience and expertise of the operators.22,25 In our study, we observed that percutaneous technique was the routine first-line procedure, with surgical tracheostomy reserved for patients whose anatomy was not conducive to percutaneous method. This approach was also the standard practice at all institutions before the COVID-19 pandemic, indicating that institutions may maintain their practice with additional safety measures to minimize aerosolization.
No previous studies have compared different timings of tracheostomy in COVID-19 patients. An important caveat in this study was our use of a definition for what constituted early (≤14 days), middle (15–21 days), and late (>21 days) tracheostomy groups based on time from intubation. Although several publications during the pandemic have alluded to timing of tracheostomy within 14 days,18–20 or 21 days,8,10,11,21,26 the guideline with broadest stakeholder engagement suggests that tracheostomy need only be delayed until day 10 of invasive mechanical ventilation.9 In our cohort, relatively few patients underwent tracheostomy before 14 days. We observed that the early tracheostomy group was associated with decreased ventilator days compared to late groups in patients who were weaned, but there was no significant association between tracheostomy timing and the rate of VAP. Nonetheless, a significant body of literature attests to the salutary effect of early tracheostomy on reducing days on ventilator and ventilator associated pneumonia.6,27,28 In addition, we observed that early tracheostomy was associated with non-significant increase in complications. A larger cohort will be needed to delineate possible benefits from earlier tracheostomy in COVID-19 respiratory failure.
Outcomes of COVID-19 patients who underwent tracheostomy have been reported with varying levels of detail.18–22,25,26,29,30 Detailed outcomes, such as comparison of different techniques, sedation duration, and mechanical ventilator parameters, were not reported. In our study, the ventilator weaning rate of 66.1% was similar to other studies, and ECMO weaning rate was 83.3%. About 25% of our patients were on ECMO, after failing the routine ventilator support and proning, as our institutions were tertiary care centers specializing in ECMO care. Tracheostomy was associated with accelerated weaning from sedation, consistent with studies predating the pandemic.5 In our cohort, 15.3% of patients died, corresponding to a mortality rate similar to the other large reported studies.19,26,30 We observed a 63.6% incidence of ventilator-associated pneumonia (VAP), which is notable since few data are available on VAP in COVID-19 patients on mechanical ventilation.
The strengths of our study include its multicenter design; performance of the tracheostomy by a variety of specialists with both percutaneous and surgical techniques; and identification of significant associations with patient outcomes, which are missing in some larger registries.19,30 Our study is limited by its observational design and small sample size, precluding causal inferences regarding tracheostomy technique and outcomes. We observed that the early tracheostomy group was associated with decreased ventilator days compared to late groups in patients who were weaned, but there was no significant association between tracheostomy timing and the rate of VAP. We also hypothesize that systematic differences in patient selection, rather than effects of procedural technique, may account for the association of percutaneous tracheostomy with reduced time on ventilator, VAP, and length of stay. Although performance of tracheostomy by different specialties increased the heterogeneity of the data, it reflected the real world, multidisciplinary practice at the institutions and increased the generalizability of the findings.
Percutaneous tracheostomy is a safe and effective procedure for patients with COVID-19 respiratory failure and has become a first line approach at several institutions, with surgical tracheostomy often reserved for patients with challenging anatomy. The outcomes of early percutaneous tracheostomy versus surgical tracheostomy should be evaluated in prospective, randomized trials assessing tracheostomy timing and technique in COVID-19 respiratory failure.
Supplementary Material
Acknowledgments
The authors recognize their interprofessional frontline health care workers, patients, and their families for steadfast courage and dedication during the pandemic.
Footnotes
The authors report no conflicts of interest.
Author contribution: K.M. and M.J.B. wrote the manuscript which was reviewed, edited and approved by all authors; K.M., G.Z.C., K.V.N., S.S., M.J.B., and J.D.C. designed the study; K.M., K.V.N., M.A., M.T.W., J.S. and D.N. collected the data; A.P. and C.L.G. analyzed the data; K.M. and M.J.B. take full responsibility for the integrity of the manuscript.
REFERENCES
- 1.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med 2020; 201:1560–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics OF AND IMPORTANT LESSONS FROm the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmood K, Wahidi MM. The changing role for tracheostomy in patients requiring mechanical ventilation. Clin Chest Med 2016; 37:741–751. [DOI] [PubMed] [Google Scholar]
- 5.Young D, Harrison DA, Cuthbertson BH, et al. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA 2013; 309:2121–2129. [DOI] [PubMed] [Google Scholar]
- 6.Rumbak MJ, Newton M, Truncale T, et al. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med 2004; 32:1689–1694. [DOI] [PubMed] [Google Scholar]
- 7.Jackson T, Deibert D, Wyatt G, et al. Classification of aerosol-generating procedures: a rapid systematic review. BMJ Open Respir Res 2020; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith D, Montagne J, Raices M, et al. Tracheostomy in the intensive care unit: Guidelines during COVID-19 worldwide pandemic. Am J Otolaryngol 2020; 41:102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med 2020; 8:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb CR, Desai NR, Angel L, et al. Use of tracheostomy during the COVID-19 pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology Program Directors Expert Panel Report. Chest 2020; 158:1499–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao TN, Braslow BM, Martin ND, et al. Tracheotomy in ventilated patients with COVID-19. Ann Surg 2020; 272:e30–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz P, Morvan JB, Fakhry N, et al. French consensus regarding precautions during tracheostomy and post-tracheostomy care in the context of COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis 2020; 137:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer DD, Engels PT, Weitzel EK, et al. Recommendations from the CSO-HNS taskforce on performance of tracheotomy during the COVID-19 pandemic. J Otolaryngol Head Neck Surg 2020; 49:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay JK, Khoo ML, Loh WS. Surgical considerations for tracheostomy during the COVID-19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg 2020; 146:517–518. [DOI] [PubMed] [Google Scholar]
- 15.Schultz MJ, Teng MS, Brenner MJ. Timing of tracheostomy for patients with COVID-19 in the ICU-setting precedent in unprecedented times. JAMA Otolaryngol Head Neck Surg 2020; 146:887–888. [DOI] [PubMed] [Google Scholar]
- 16.Bier-Laning C, Cramer JD, Roy S, et al. Tracheostomy during the COVID-19 pandemic: comparison of international perioperative care protocols and practices in 26 countries. Otolaryngol Head Neck Surg 2020; 194599820961985. [DOI] [PubMed] [Google Scholar]
- 17.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 18.Angel L, Kon ZN, Chang SH, et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg 2020; 110:1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Villares C, Perez Molina-Ramirez C, Bartolome-Benito M, et al. Outcome of 1890 tracheostomies for critical COVID-19 patients: a national cohort study in Spain. Eur Arch Otorhinolaryngol 2021; 278:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riestra-Ayora J, Yanes-Diaz J, Penuelas O, et al. Safety and prognosis in percutaneous vs surgical tracheostomy in 27 patients with COVID-19. Otolaryngol Head Neck Surg 2020; 163:462–464. [DOI] [PubMed] [Google Scholar]
- 21.Long SM, Chern A, Feit NZ, et al. Percutaneous and open tracheostomy in patients with COVID-19: comparison and outcomes of an institutional series in New York City. Ann Surg 2021; 273:403–409. [DOI] [PubMed] [Google Scholar]
- 22.Zuazua-Gonzalez A, Collazo-Lorduy T, Coello-Casariego G, et al. Surgical tracheostomies in COVID-19 patients: indications, technique, and results in a second-level Spanish hospital. OTO Open 2020; 4:2473974X20957636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol 2020; 13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putensen C, Theuerkauf N, Guenther U, et al. Percutaneous and surgical tracheostomy in critically ill adult patients: a meta-analysis. Crit Care 2014; 18:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picetti E, Fornaciari A, Taccone FS, et al. Safety of bedside surgical tracheostomy during COVID-19 pandemic: a retrospective observational study. PLoS One 2020; 15:e0240014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao TN, Harbison SP, Braslow BM, et al. Outcomes after Tracheostomy in COVID-19 Patients. Ann Surg 2010; 272:1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA 2010; 303:1483–1489. [DOI] [PubMed] [Google Scholar]
- 28.Siempos II, Ntaidou TK, Filippidis FT, et al. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med 2015; 3:150–158. [DOI] [PubMed] [Google Scholar]
- 29.Queen Elizabeth Hospital Birmingham COVID-19 airway team. Safety and 30-day outcomes of tracheostomy for COVID-19: a prospective observational cohort study. Br J Anaesth 2020; 125:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.COVIDTrach collaborative. COVIDTrach; the outcomes of mechanically ventilated COVID-19 patients undergoing tracheostomy in the UK: Interim Report. Br J Surg 2020; 17:e583–e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


