Abstract
Cell volume is one of the most aggressively defended physiological set points in biology. Changes in intracellular ion and water concentrations, which are induced by changes in metabolism or environmental exposures, disrupt protein folding, enzymatic activity, and macromolecular assemblies. To counter these challenges, cells and organisms have evolved multifaceted, evolutionarily conserved molecular mechanisms to restore cell volume and repair stress induced damage. However, many unanswered questions remain regarding the nature of cell volume ‘sensing’ as well as the molecular signaling pathways involved in activating physiological response mechanisms. Unbiased genetic screening in the model organism C. elegans is providing new and unexpected insights into these questions, particularly questions relating to the hypertonic stress response (HTSR) pathway. One surprising characteristic of the HTSR pathway in C. elegans is that it is under strong negative regulation by proteins involved in protein homeostasis and the extracellular matrix (ECM). The role of the ECM in particular highlights the importance of studying the HTSR in the context of a live organism where native ECM-tissue associations are preserved. A second novel and recently discovered characteristic is that the HTSR is regulated at the post-transcriptional level. The goal of this review is to describe these discoveries, to provide context for their implications, and to raise outstanding questions to guide future research.
Keywords: hypertonic stress, osmoregulation, stress response, compatible solute
Introduction
Intracellular water and solute content, and therefore cell volume, are directly influenced by extracellular osmolarity. Fluctuations in extracellular osmolarity is a common stress faced by all cells [1]. For example, cells are exposed to changes in extracellular osmolarity during the switch from diuresis to anti-diuresis in the case of the mammalian kidney [2]. Organisms moving through their natural environment, such as the nematode C. elegans, also encounter variable levels of salinity. Virtually all cells have evolved mechanisms to acclimate to and survive changes in extracellular osmolarity [1]. In particular, the hypertonic stress response (HTSR) is a conserved cellular stress response that protects cells from cell volume loss due to increased extracellular osmolarity, or hypertonic stress (HTS). While the response to hypotonic stress and increases in cell volume also have physiological significance, this review will only focus on the hypertonic stress response.
A critical feature of the HTSR is the intracellular accumulation of compatible osmolytes. Compatible osmolytes are small organic molecules [3]. They include carbohydrates (i.e. glycerol, sorbitol), amino acids (i.e. betaine, taurine), and methylamines (i.e. glycine-betaine, TMAO). Osmolyte intracellular concentrations track with extracellular solute concentration to maintain appropriate intracellular water content and therefore cell volume. There is significant chemical diversity in compatible osmolytes and the particular compatible osmolyte a cell uses depends on the organism and cell type [4, 5]. Most compatible osmolytes have a net neutral charge and therefore exhibit mostly neutral interactions with charged cell metabolites or other cellular components [5]. Their lack of reactivity allows compatible osmolytes to accumulate to molar concentrations [3, 6] within the cell without negatively interfering with cellular function [3, 6, 7]. In fact, many compatible osmolytes actually function as chemical chaperones to promote proper protein folding by interacting unfavorably with the peptide backbone and therefore increasing the Gibbs free energy required to shift a protein from the folded to unfolded state [8–11].
In virtually all organisms and cells types, the concentration of intracellular compatible osmolytes is increased via osmolyte biosynthetic enzymes or osmolyte transporter proteins whose expression is upregulated by HTS [12, 13]. These compatible osmolyte accumulation genes are part of a larger group of osmotically regulated genes that are transcriptionally upregulated during the HTSR to protect cells from cell shrinkage and facilitate acclimation. Mammals have more than one hundred osmotically regulated genes. In addition to osmolyte biosynthetic enzymes and transporter proteins, these osmotically regulated genes include heat shock proteins, paracrine and autocrine factors, and extracellular matrix (ECM) associated proteins [14].
Prior cellular studies provided important first insights into mechanisms of osmotic gene regulation. Tonicity element binding protein (TonEBP) / nuclear factor of activated T cells 5 (NFAT5) is a transcription factor that is activated by HTS and induces the transcriptional upregulation of many mammalian osmotically regulated genes, including the compatible osmolyte accumulation genes aldose reductase and sodium / myo-inositol cotransporter SMIT [15–17]. Some studies suggest that p38 mitogen activated protein kinases (MAPKs) phosphorylate and activate TonEBP/NFAT5, while other studies suggest that p38 MAPK signaling is dispensable for activation [18–22]. Together, these cellular studies show that the regulation of TonEBP/NFAT5 is complex. Although TonEBP/NFAT5 has been the major focus of studies investigating the mammalian HTSR, it is certainly not the only signaling mechanism and pathway involved in the HTSR. Genetic studies in particular offer an opportunity to identify new signaling mechanisms associated with cell physiology in an unbiased manner. While the HTSR has been studied for decades, it is only in the last ~10 years that unbiased genetic screening approaches have been deployed to identify HTSR genes and pathways in multicellular organisms.
The small nematode C. elegans has emerged as a relatively new platform for investigating how osmotically regulated genes, in particular osmolyte accumulation genes, are upregulated during the HTSR. Several experimental attributes make C. elegans an ideal system in which to investigate HTSR mechanisms using unbiased genetic approaches. First, the entire 100 Mb C. elegans genome of roughly 20,000 protein coding and 1,300 non-protein coding genes has been sequenced and thoroughly annotated [23]. ~ 80% of C. elegans genes have human homologs, suggesting that many aspects of this organism’s physiology are conserved across evolution [24]. The compact and stable nature of the genome, along with inexpensive whole-genome resequencing, allows investigators to rapidly identify phenotype causing mutations isolated in unbiased genetic screens. A publically available repository maintains and distributes thousands of mutant strains, which greatly facilitates genetic studies [25]. Additionally, C. elegans is compatible with the full range of CRISPR/Cas9 approaches, which makes generation of custom alleles and/or tagged proteins at the endogenous locus rapid and inexpensive [26, 27].
Second, the reproductive biology of C. elegans facilitates large-scale genetic approaches to address questions in physiology. C. elegans exists as two sexes - hermaphrodites and males. A single hermaphrodite self-fertilizes to produce 200 – 300 offspring that are genetically representative of the maternal genotype. These characteristics make it simple to identify and propagate single animals exhibiting rare mutant phenotypes from genetic screens through hermaphrodite ‘selfing’. At the same time, crossing hermaphrodites to males allows mutated genes to be introduced into a population. While most mutations studied in C. elegans are recessive homozygous loss/reduction of function (LOF) mutations (e.g. there is very little haploinsufficiency), rare gain-of-function (GOF) mutants can also be identified and maintained.
A third benefit of C. elegans for unbiased genetic methods is that genes can be knocked down via feeding-based RNAi. Genome-wide RNAi screens are enabled through the commercial availability of bacterial RNAi feeding libraries targeting ~90% of C. elegans protein coding genes [28]. One important advantage of this approach is that RNAi screening can be initiated post-developmentally, which allows these screens to test the function of genes that are otherwise essential for development. Such genes are not isolated from typical forward genetic screens, which usually demand that mutants exhibit viability and fertility. Another advantage of RNAi is the ability to functionally silence highly homologous gene duplicates, which can reveal phenotypes for recently duplicated genes. Such phenotypes would be missed in mutagen-based genetic screens which are unlikely to induce SNPs in both genes simultaneously. On the other hand, RNAi screens only reveal loss/reduction of function mutant phenotypes. Unusual alleles affecting specific gene functions (i.e. missense LOF or GOF alleles) are not revealed through RNAi screening. Therefore, RNAi screening and forward genetic mutagenesis screening play complementary roles in defining genetic pathways.
The final and perhaps greatest of advantage of C. elegans is its optical transparency throughout its entire lifecycle. This makes it possible to use fluorescent reporters to view gene and protein expression in all tissues of live animals without disrupting native tissue or ECM integrity. As a result, genetic screens based on the isolation of single, live mutant hermaphrodites with altered GFP expression is a commonly utilized screening approach in C. elegans [29–34]. Such screens are virtually impossible in other systems due to their lack of optical transparency. Despite its advantages, there are still considerable limitations with C. elegans, such as its small size (which complicates biochemical approaches), lack of immortal cell lines, and differences in functional anatomy. Nevertheless, this system provides a wealth of new opportunities to investigate molecular mechanisms of the HTSR in the context of a live multicellular animal.
The C. elegans HTSR
HTS is induced in C. elegans by increasing the osmolarity of its environment. True isotonicity for C. elegans is not known. However, most studies of the C. elegans HTSR consider standard Nematode Growth Media (NGM), which contains 51 mM NaCl and has an osmolarity of ~170 mOsm, to be isotonic. Raising NaCl concentrations is typically how HTS is elicited. HTS causes at least three distinct organismal phenotypes in C. elegans. First, acute HTS (>500 mM NaCl) can lead to death of the organism within 24 hours. Second, acute exposure to non-lethal HTS (i.e. acclimation, 200 mM NaCl) allows animals to subsequently survive a normally lethal HTS for naïve animals [35]. Third, chronic exposure to non-lethal HTS (250 mM NaCl) elicits acclimation mechanisms that enable growth and development over multiple generations [34]. Specific and sometimes overlapping molecular mechanisms mediate each of these organismal responses.
Immediately upon exposure to HTS, the body cavity of C. elegans shrinks as water leaves [35]. This HTS - induced cell shrinkage activates the conserved ‘With No Lysine’ protein kinase 1 (WNK-1) pathway [36]. The WNK-1 cell volume recovery pathway is the initial cellular response to HTS that mediates the influx of ions (and osmotically obliged water) into the cell to quickly restore cell volume. In mammals, cell shrinkage activates auto-phosphorylation of WNK-1. Phosphorylated WNK-1 subsequently phosphorylates and activates the Ste20/SPS1-related proline/alanine-rich kinase (SPAK) and oxidative stress responsive kinase 1 (OSR1). Finally, SPAK and OSR1 phosphorylate and activate the Na – K – Cl cotransporter NKCC1, which mediates the influx of sodium and water to correct cell shrinkage [37, 38]. The WNK-1 pathway is also activated in C. elegans following HTS [36]. Although the WNK-1 pathway acutely restores cell volume, the activation of WNK-1 targets increases intracellular ionic strength due to the influx of sodium ions. Increased intracellular ionic strength interferes with many cellular processes such as protein folding and enzyme activity [6, 39]. While the WNK1 pathway is primarily important for the acute phase of the HTSR, other pathways mediate long-term aspects of the HTSR that replace inorganic ions with compatible solutes.
Glycerol is the primary compatible osmolyte used by laboratory-reared C. elegans to acclimate to HTS [35]. This is similar to yeast, which also utilize glycerol [40]. Although mammals do not utilize glycerol as an osmolyte, they do take a similar metabolic approach to osmolyte production by breaking down glucose to produce sorbitol [41]. In this respect, C. elegans may represent an evolutionarily intermediate between yeast and mammals in terms of mechanisms of HTS acclimation. Intracellular glycerol concentration tracts extracellular osmolarity and accumulates on the order of hours in C. elegans [35, 42]. When C. elegans acclimated to a hypertonic environment is transferred back to normal growth conditions, glycerol levels drop due to glycerol efflux [35]. Therefore, glycerol accumulation in C. elegans is dynamic.
C. elegans biosynthesizes glycerol via transcriptional upregulation of a glycerol-3-phosphate dehydrogenase homolog (gpdh) [35]. GPDH catalyzes the rate-limiting step in glycerol biosynthesis. Therefore, upregulation of gpdh enhances the rate of glycerol production [43]. There are two classes of eukaryotic GPDH enzymes, a cytosolic NADH-dependent form and a mitochondrial FAD-dependent form. NADH – and FAD – dependent GPDHs together make up the ‘glycerol – phosphate shuttle’ that is critical for cellular glucose metabolism [44, 45]. NADH – dependent GPDH reduces dihydroxyacetone (DAP) to glycerol-3-phosphate (G3P) while oxidizing NADH to NAD. FAD – dependent GPDH oxidizes G3P to DAP while reducing FAD to FADH2 [45]. Only NADH – dependent GPDH is involved in the HTSR [35].
There are two NADH-dependent gpdh genes in the C. elegans genome encoded by gpdh-1 and gpdh-2 [35, 42]. During the HTSR, gpdh-2 exhibits little to no upregulation [42]. This contrasts with gpdh-1, which is upregulated > 20 – 50 – fold within the first three hours of HTS exposure [34, 46]. Notably, loss of gpdh-1 does not significantly reduce steady state whole animal glycerol levels in response to HTS, although the rate of glycerol accumulation is slowed [42]. gpdh-1 mutants do exhibit a mild defect in their ability to acutely acclimate to HTS, showing that gpdh-1 does play a functionally significant role the HTSR [34]. However, animals lacking both gpdh-1 and gpdh-2 exhibit a ~50% reduction in steady-state glycerol levels [42]. This suggests that gpdh-2 can function redundantly with gpdh-1 in HTS-induced glycerol production. It also suggests the existence of gpdh-independent mechanisms for glycerol production.
A transcriptional reporter for gpdh-2 is constitutively expressed in the intestine, hypodermis, and excretory cell and is not substantially upregulated by HTS [42]. On the other hand, a gpdh-1 transcriptional reporter is virtually undetectable under control conditions but is strongly induced in the hypodermis and intestine during HTS. This reporter is specifically induced by HTS since it is not induced by other cellular stressors, such as heat shock, ER stress, or oxidative stress. Interestingly, the gpdh-1 reporter is not activated in other tissues, such as the muscle, neurons, or germline [42]. This suggests a model in which the hypodermis and intestine, which are environmentally exposed epithelial tissues that are the first to encounter HTS, are the primary sites of glycerol production. Glycerol is then shunted out of the basolateral membranes into the pseudocoelomic space, where it can be accumulated by non-glycerol producing cells, such as muscle and neurons, via passive uptake mechanisms. In support of such a model, glycerol permeant aquaglyceroporins are localized to the basolateral epithelial membrane, while water permeant aquaporins are present on the apical membrane [47]. This could provide a permeability pathway for the efflux of glycerol out of the intestine as well as an influx pathway for osmotically driven water movement.
gpdh-1 is one of 324 osmotically regulated genes in C. elegans. Several of these upregulated osmotic genes suggest there may be other interesting physiological mechanisms involved in the C. elegans HTSR. For example, the H+-coupled myo-inositol transporter hmit-1.1 is upregulated >100-fold by HTS. The kinetics of hmit-1.1 upregulation differ from those of gpdh-1 in that hmit-1.1 upregulation peaks at later timepoints than gpdh-1 [46]. While myo-inositol is a major osmolyte in mammalian cells and is accumulated via transcriptional upregulation of the sodium-coupled myo-inositol transporter SMIT, there is no evidence that C. elegans accumulate myo-inositol under laboratory conditions [48, 49]. However, myo-inositol is a major breakdown product of plant organic material, which is present in many of the sites C. elegans are known to inhabit [50]. Therefore, the upregulation of hmit-1.1 may indicate that C. elegans in the wild can utilize myo-inositol as an osmolyte in addition to glycerol, but that it is unable to use myo-inositol in the laboratory since it is absent from the cultivation conditions.
Many genes upregulated by HTS are also upregulated during bacterial and fungal infection in C. elegans. This does not include gpdh-1 or hmit-1.1 which appear to be exclusively upregulated by HTS. Many of these co-regulated genes are components of the C. elegans innate immune response, such as members of the neuropeptide-like protein (nlp) and caenicin (cnc) gene families [46]. Some pathogens, such as the fungal pathogen Drechmeria coniospora, physically penetrate the cuticle as part of the infection process [31, 51]. Since both pathogens and HTS impinge on the specialized C. elegans ECM that forms the cuticle, this supports the hypothesis that upregulation of some osmotically regulated genes may be triggered via hypertonicity- or pathogen-induced disruptions in the cuticle. Consistent with this hypothesis, several mutants affecting structural components of the cuticle, including many collagen-encoding dpy genes (i.e. dpy-7, -8, -9, and -10), constitutively activate the C. elegans HTSR [42, 46, 52]. One possible interpretation of these genetic findings is that the cuticle is a water impermeant cell wall-like structure that slows the movement of water out of the underlying tissues during the HTSR. However, the cuticle is permeable to many small molecule dyes [53], suggesting that smaller molecules, such as water, can move through this structure with relative ease, although such permeability could be dynamically regulated by HTS. Moreover, enhancing cuticle permeability does not alter the osmotic stress phenotype of C. elegans mutants [53]. Another possible hypothesis that is consistent with such observations is that the cuticle functions as a mechanical osmosensor whose shape, tension, and/or connections to the underlying hypodermis are influenced by changes in the hypodermal tissue volume. Whether or not HTS itself leads to specific disruption of the cuticle and how such disruptions might couple to signaling pathways that activate osmotically regulated genes is not yet known.
Although the HTSR was described in C. elegans over a decade ago, many interesting questions can now be addressed due to the recent availability of whole-genome resequencing for mutant identification and CRISPR/Cas9 genome engineering. For example, what are the signaling mechanisms that allow HTS to upregulate osmolyte biosynthesis genes like gpdh-1 in vivo? Where and when do these pathways function? Are these mechanisms / pathways conserved in other animals, including mammals? Forward genetic screens are providing unanticipated answers to these questions. Below, we highlight data describing mechanisms of transcriptional regulation, as well as newly discovered and unanticipated mechanisms involving post-transcriptional regulation.
Transcriptional regulation of the C. elegans HTSR
The initial genetic screen for regulators of the HTSR utilized genome-wide RNAi screening to identify genes that when inhibited caused upregulation of gpdh-1 under isotonic conditions [42]. This screen identified over one hundred genes negatively regulating gpdh-1 and other osmotically regulated gene expression at the transcriptional level. Surprisingly, most of the genes identified in this screen fell into two transcriptional pathways (Figure 1).
Figure 1. Hypertonic stress response pathways in C. elegans.
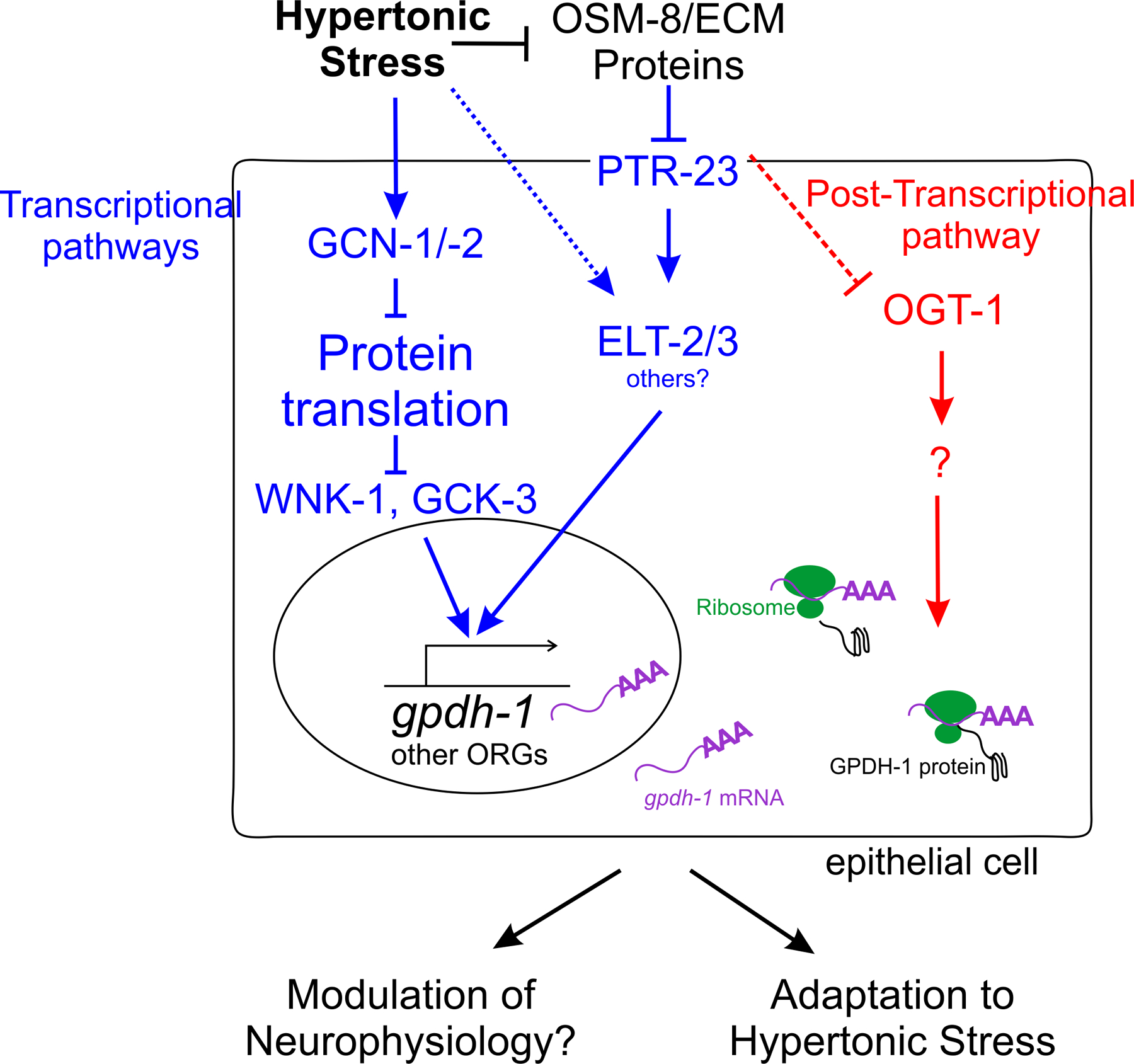
The induction of osmotically regulated genes, such as gpdh-1, during hypertonic stress (HTS) is regulated through both transcriptional (blue) and post-transcriptional (red) mechanisms. At least three pathways regulate the transcriptional induction of gpdh-1. First, inhibition of proteins that maintain protein folding and new protein synthesis activate gpdh-1 expression. HTS-induced decreases in protein translation lead to increased gpdh-1 transcription through a gcn-1/2 and wnk-1/gck-3 dependent pathway. Additionally, while significant work shows that HTS causes unique types of protein damage and inhibition of protein homeostasis genes activates the HTSR, the specific signaling mechanisms linking HTS-induced protein damage to gpdh-1 mRNA upregulation are not known. Second, the HTSR transcriptional response is negatively regulated by extracellular proteins that function upstream of the transmembrane protein PTR-23/patched-related protein 23, and the GATA erythroid-like transcription factors ELT-2 and ELT-3. However, ptr-23 is not required for all osmotically regulated gene expression and as such at least one ptr-23 independent pathway must exist. It is unknown if this ptr-23 independent pathway functions through the GATA transcription factors. It also remains unknown if HTS itself can activate ELT-2/-3 through an extracellular protein and PTR-23 independent pathway. Finally, the O-GlcNAc transferase OGT-1 regulates GPDH-1 protein translation through a post-transcriptional pathway. ogt-1 is required for osm-8 and osm-11 phenotypes, suggesting there is some crosstalk between the extracellular protein transcriptional pathway and the ogt-1 post-transcriptional pathway. The precise mechanism by which ogt-1 induces GPDH-1 protein expression is unknown, but it could include regulation of mRNA cleavage, 3’ UTR usage, mRNA export, initiation factor interactions or ribosomal elongation. The output of both the transcriptional and post-transcriptional pathways are two-fold. First, they mediate acclimation that enable animals to survive and reproduce in hypertonic environments. Second, these pathways may also modulate the function of osmosensory neurons such that once acclimation has occurred, behavioral avoidance to hypertonic stimuli is no longer necessary.
The first class of osmotic regulators included protein homeostasis genes involved in RNA processing, protein synthesis, protein folding, and protein degradation [42]. One hypothesis to explain this discovery is that cells sense HTS and upregulate gpdh-1 expression through detection of stress-induced protein damage. While protein damage can be replicated by inhibition of protein homeostasis genes, other stressors, such as heat shock, also cause protein damage but nevertheless fail to upregulate gpdh-1 expression [42]. This suggests the possibility that HTS causes a type of protein damage that differs from other types of protein damage and specifically activates the HTSR without activating other stress response pathways. In support of this hypothesis is the observation that HTS causes a model protein (polyQ) to form aggregates. These polyQ aggregates differ from aggregates caused by other stressors in both C. elegans and mammalian cells in their morphology, solubility, and ubiquitination characteristics, suggesting they are a unique aggregate species [54, 55]. Additional studies show that endosomal sorting pathways and lysosomes clear existing protein damage and new protein synthesis is decreased to facilitate survival and acclimation during HTS [56, 57]. However, when genes involved in degradation or translation regulation are inhibited, protein damage accumulates and C. elegans can no longer survive or acclimate to HTS [55, 56]. It remains unknown if specific degradative pathways, such as the ERAD (endoplasmic-reticulum-associated protein degradation) pathway are activated during HTS. Nevertheless, these data clearly reveal an important role for maintenance of the proteome in the regulation of the C. elegans HTSR.
In a mechanism likely related to HTSR-induced accumulation of damaged proteins, regulation of protein translation also has a critical role in the regulation of the HTSR. Global protein translation is inhibited ~50% by mild HTS (200 mM NaCl) [58]. Moreover, pharmacological inhibitors of translation led to mild upregulation of gpdh-1 mRNA even in the absence of HTS, suggesting a direct signaling link between HTS-induced translational inhibition and regulation of the osmotically regulated gene gpdh-1 [58]. HTS-induced translational inhibition requires phosphorylation of eIF2α by the kinase GCN-2 and its accessory protein GCN-1 and loss of gcn-1 or gcn-2 reduced HTS-induced gpdh-1 expression by ~50% [58]. GCN-1/-2 appear to act in the same pathway as the WNK-1 and GCK-3 kinases since gcn-1 mutants are non-additive with wnk-1/gck-3 knockdown [58] (Figure 1). How this pathway for HTS-induced translational inhibition is linked to transcriptional regulation of gpdh-1 is currently unknown.
The second large class of genes that negatively regulate gpdh-1 gene expression and the HTSR are genes encoding extracellular proteins (Figure 1). While all of these proteins are predicted to be secreted, many are synthesized by the hypodermis and secreted from the apical membrane to generate the specialized C. elegans ECM called the cuticle. This ECM is shed and resynthesized during each of the four larval molts that occur in C. elegans development. The precise organization and makeup of the cuticle differs between these developmental stages. The adult cuticle contains about 500 ring-like annular furrows that form circumferential-oriented ingressions along the length of the animals [59]. One of the most abundant structural components of the cuticle are collagen proteins, which are encoded by many genes in C. elegans [60]. Mutations in several of these collagen genes lead to alterations in body shape. Interestingly, mutations in the collagen genes dpy-7, -8, -9, and -10 that disrupt the furrow, but not other collagens, activate gpdh-1 expression under isotonic conditions and cause the accumulation of glycerol to levels that are similar to levels seen in acclimation. As a result, these mutants retain motility in extremely hypertonic environments (500 mM NaCl), whereas wild type animals rapidly paralyze [42, 52]. Many other detoxification genes which are regulated by HTS and other stressors are also responsive to these extracellular protein mutations, although the effect size is significantly more modest than that observed for osmotically regulated genes [52, 61]. Nevertheless, the osmotic stress resistance of these extracellular protein mutants suggests that the furrow plays an important role is the C. elegans HTSR. However, other secreted proteins, such as the mucin-like protein OSM-8, the notch ligands OSM-7 and OSM-11, and the novel secreted protein OSR-1 exhibit qualitatively normal furrows but also activate gpdh-1 expression, accumulate glycerol, and are osmotic stress resistant [42, 46, 53, 62–64]. One possibility is that these non-furrow disrupting extracellular proteins act to couple furrow-based osmosensing to the underlying hypodermis through detection of HTS-induced structural changes. Direct tests of this mechanically- based osmosensing model are still needed.
The genetic origins of several HTSR-regulating genes encoding extracellular proteins reveal connections between whole-animal HTS acclimation and behavioral responses that allow C. elegans to avoid hypertonic environments. osm-7, -8, and -11 were isolated in a forward genetic screen for osmotic avoidance abnormal (osm) mutants [65]. While wild type animals crawl away from hypertonic stimuli, osm mutants fail to avoid these stimuli. Hypertonic avoidance behavior depends on the ciliated ASH sensory neurons [66]. There are three classes of osm genes that differ based on their effects on the ASH neuron. The first and second class of osm genes function cell autonomously in the ASH neurons to regulate cilia formation and cell signaling respectively [67, 68]. In contrast, the third class of osm genes, which includes osm-7, -8, and -11, is not expressed in the ASH neurons. Instead, these three osm genes are expressed in the hypodermis, where they also function to inhibit the expression of gpdh-1 and glycerol accumulation [53, 62]. The hypodermal expression of these class three osm genes suggests that unidentified paracrine factors signal from the hypodermis to the ASH neurons during HTS to modulate behavior. One possibility is that this paracrine factor is glycerol itself because glycerol could blunt amphid neuron volume changes upon hypertonic exposure when present at high levels. In addition, there is some evidence that the ASH neurons signal to osmosensitive tissues to modify the HTSR. The class two osm gene, osm-9, encodes a transient receptor potential channel, TRPV, that is expressed in ASH neurons to facilitate ASH-dependent signaling [67]. Surprisingly, osm-9 mutants are not only behaviorally osm, but they also constitutively upregulate the osmotically regulated gene aqp-8 in osmosensitive tissues and have improved acute survival during HTS due to decreased protein damage [69, 70]. Therefore, unidentified paracrine factors signal from the ASH neurons to the osmosensitive tissues of osm-9 mutants to modulate cellular physiology. The osm paradigm provides a unique opportunity to investigate how hypodermis – based physiological information is integrated to modulate neuronal – based behavior and vice versa. It also highlights the importance of investigating the HTSR at the organismal level, where behavior and physiology can be studied together.
The class three gene osm-8 has been studied in significant molecular detail to understand its role in the HTSR. osm-8 encodes a small hypodermally – secreted mucin protein. Like the other class three osm mutants, osm-8 mutants constitutively induce osmotically regulated genes, including gpdh-1, accumulate large amounts of glycerol during control conditions, and are resistant to normally lethal levels of HTS [53]. To identify genes that may function downstream of the secreted osm-8 gene to transduce signals from outside the cell to the nucleus, we performed an unbiased RNAi screen for suppressors of osm-8 mutants. Inhibition of the multi-pass transmembrane patched – related protein 23 (ptr-23) completely suppressed many osm-8 phenotypes, including constitutive gpdh-1 induction, glycerol accumulation, and the osr phenotype. However, ptr-23 did not suppress the induction of all osmotically regulated genes, since many innate immunity genes upregulated in osm-8 mutants were similarly upregulated in osm-8; ptr-23 double mutants. Therefore, a subset of osmotically regulated genes must be regulated in a ptr-23 – independent manner. Furthermore, ptr-23;osm-8 double mutants still induce the gpdh-1 transcriptional reporter during HTS, suggesting that the HTSR does not exclusively require ptr-23 [53]. These two observations indicate that there are multiple redundant pathways regulating the HTSR and at least one of them is independent of ptr-23 (Figure 1).
While unbiased genetic screens have revealed valuable information about the HTSR, the specific signaling pathways that control upregulation of gpdh-1 and/or other osmotically regulated genes have not yet been identified. In yeast and mammals, MAPK – dependent signaling pathways are known to regulate HTS – dependent transcription (Table 1). The p38 MAPK activates hypertonic induction of the mammalian transcription factor TonEBP/NFAT5 and the p38 MAPK homolog, HOG1, regulates the transcription of osmotically regulated genes during HTS in yeast [18, 19, 71]. Despite the roles of MAPK signaling in the yeast and mammalian HTSR, there is little evidence that MAPK signaling is involved in the C. elegans HTSR. MAPK signaling components have thus far not been isolated in genetic screens for regulators of the HTSR, although we note that such screens have not yet reached saturation. Moreover, knockdown of p38 signaling pathway components in osm-7 and osm-11 mutants has no effect on acute or chronic OSR [62]. Similarly, p38 MAPK signaling is not involved in induction of the osmotically regulated gene, nlp-29, by HTS despite being required for induction of nlp-29 during fungal infection [72]. However, MAPK signaling is involved in the chronic survival of osr-1 mutants during HTS and the survival of wild type animals during desiccation [64, 73]. Therefore, MAPKs may be involved in the C. elegans HTSR, but they are unlikely to be a major contributor to the transcriptional upregulation of osmotically regulated genes during HTS.
Table 1. Comparison of the hypertonic stress response in eukaryotic organisms.
The compatible osmolytes and mechanisms of transcriptional induction, post-transcriptional induction, and the transcriptional repression of the hypertonic stress response (HSTR) are compared among S. cerevisiae, C. elegans, and mammals.
| Characteristic of hypertonic stress response | S. cerevisiae | C. elegans | Mammals |
|---|---|---|---|
| Compatible osmolytes | Glycerol, trehalose | Glycerol | Sorbitol, myo-inositol, betaine, glycerophosphocholine (GPC), taurine |
| Osmolyte accumulation proteins | GPD1, GPD2, TPS1, TPS2 | GPDH-1,-2 | Aldose reductase, SMIT, BGS1, GPC1, TAUT |
| Transcriptional upregulation? | Yes (MAP kinase dependent) | Yes (MAP kinase independent) | Yes (partially MAP kinase dependent) |
| Transcription factors | Hog1, Sko1, Hot1, others | GATA factors • elt-2,-3 |
Rel-type factors
|
| Post-transcriptional upregulation? | Unknown | Yes (OGT-1 dependent) | Unknown |
| Extracellular proteins required for transcriptional inhibition | Mucins
|
Mucin-like
|
Unknown |
| Dependence of transcriptional inhibition on canonical induction pathways? | Yes
|
Yes
|
Unknown |
| Activation of osmolyte accumulation by inhibition of protein translation? | Unknown | Yes
|
Unknown |
| Induction of protein misfolding by HTS? | Unknown | Yes | Yes |
In mammals, the rel family transcription factor TonEBP / NFAT5 is directly responsible for the transcriptional upregulation of many genes by HTS [14]. However, the C. elegans genome does not contain rel family transcription factors. While the tonicity-responsive transcription factor in C. elegans is currently unknown, promoter analysis of C. elegans osmotically regulated genes has provided some candidates. The promoters of C. elegans osmotically regulated genes are highly enriched for GATA-type transcription factors [46]. RNAi screening identified the GATA erythroid-like factors elt-2 and elt-3 as being required for HTS-induced upregulation of a gpdh-1 transcriptional reporter [46]. elt-2 is expressed in the intestine and mediates intestinal upregulation of gpdh-1, while elt-3 is expressed in the hypodermis and is required for hypodermal gpdh-1 upregulation. Both of these transcription factors function downstream of the extracellular proteins that negatively regulate the HTSR, but it is unknown if they are required for all HTSR pathways [46]. An alternative hypothesis is that elt-2 and elt-3 are required for intestinal and hypodermal differentiation and development but are not the physiological targets of the HTSR [74, 75]. Future studies using CRISPR/Cas9 to tag native alleles of elt-2 and elt-3 followed by cell biological and biochemical analysis in the presence and absence of HTS are needed to determine if HTS leads to changes in the localization and/or activity of elt-2/-3, as has been shown for other stress-responsive transcription factors [76, 77].
In conclusion, the maintenance of protein homeostasis and extracellular proteins inhibit HTSR-induced transcriptional responses. There is conflicting evidence as to whether these HTSR regulators function in distinct tissues or pathways. osm-7 and osm-8 mutants suppress HTS and age induced protein aggregation in the intestine, whereas osm-11 mutants do not suppress either age or HTS induced protein aggregation in muscle cells [54, 56]. OSM-8 functions through PTR-23 and the GATA transcription factors ELT-2 and ELT-3. While it is tempting to speculate that increased protein damage functions as the illusive “osmosensor” during HTS, it is unlikely to be the primary way cells sense HTS because it is prominent only at extremely high levels of HTS [54, 55]. Another hypothesis is that cells sense HTS through changes in ECM structure. The annular furrow in the ECM has been linked to the HTSR, but it remains unknown if changes in its structure during HTS trigger the HTSR [52]. Finally, in addition to understanding how cells sense HTS, a transcription factor(s) specific to osmotically regulated gene induction remains to be identified. ELT-2 and ELT-3 are required for intestinal and hypodermal osmotically regulated gene induction respectively. However, since they are also generally required for transcription in these tissues, it is unknown whether they themselves are activated in a specific way by HTS or function with other HTS – specific factors [74, 75]. Continued unbiased genetic screening efforts, which is the greatest strength of the C. elegans model system, should help to identify HTSR relevant transcription factors, signaling pathways, and other proteins involved in signaling the transcriptional response to HTS.
Post-transcriptional regulation of the C. elegans HTSR
Another surprising discovery that has emerged from unbiased forward genetic screening for regulators of the C. elegans HTSR is that this pathway is also under post-transcriptional regulation (Figure 1). In an unbiased forward genetic screen for mutants with no induction of osmolyte biosynthesis gene expression (nio mutants), we discovered two alleles of the gene nio-2 that block hypertonic GPDH-1 protein induction and acclimation to HTS [34]. Both nio-2 alleles encode nonsense mutations in the highly conserved O-GlcNAc transferase ogt-1 (nio-2 is subsequently referred to as ogt-1). ogt-1 null mutants are defective in their ability to upregulate GPDH-1 protein. However, gpdh-1 mRNA (as well as several other osmotically regulated mRNAs) continue to be upregulated to wild type levels by HTS. In addition to their post-transcriptional effects on osmotically regulated gene expression, ogt-1 mutants also exhibit defects in acute and chronic HTS acclimation and are thus unable to propagate in even mildly hypertonic environments (250 mM NaCl). ogt-1 is required in the hypodermis to mediate these phenotypes and functions downstream of extracellular proteins such as osm-8 and osm-11 [34]. Therefore, ogt-1 acts in the same tissue as extracellular proteins, the transmembrane protein PTR-23, and the GATA transcription factor ELT-3 to regulate the HTSR. Together, these data suggest that the hypodermis is where HTS is sensed and signaled in C. elegans and that ogt-1 plays a critical role in this pathway.
OGT is a highly conserved enzyme found exclusively in metazoans and expressed ubiquitously in somatic tissue and localized primarily to the nucleus [34, 78]. Structurally, OGT-1 is made up of three primary domains; 1) an N-terminal tetratricopeptide repeat (TPR) domain that is involved in substrate recognition and binding, 2) a nuclear localization signal, and 3) a C-terminal catalytic domain [79–81]. OGT is known to have at least three distinct functions. The major known biochemical function of OGT is to catalyze post-translational O-GlcNAcylation of serine and threonine residues of nucleocytoplasmic proteins [82–84]. OGT can also proteolytically cleave and activate host cell factor 1 (HCF-1) in mammals [85, 86]. Finally, OGT can function through a non-catalytic mechanism to assemble multiprotein complexes in in both nuclear and non-nuclear locations [87–89]. Through its three known functions, OGT is involved in diverse aspects of cell physiology, including stress response pathways.
Surprisingly, the most well studied function of OGT, post-translational O-GlcNAcylation, is not required for the C. elegans HTSR [34]. In contrast, C. elegans expressing OGT-1 without a TPR domain have defective GPDH-1 induction and acclimation to HTS. This non-catalytic function of OGT-1 is clearly important since the HTSR phenotype of C. elegans ogt-1 mutants can be partially rescued by expression of either wild type or catalytically inhibited human OGT. The TPR domain is known to be involved in other O-GlcNAcylation-independent functions of OGT, although its precise role in this respect is unknown [89]. One possibility is that the OGT-1 TPR domain acts as a scaffolding protein to assemble signaling complexes that respond to HTS. Consistent with an acute role in signaling, inhibition of OGT-1 after the completion of development, but just prior to exposure to HTS, is sufficient to block gpdh-1 reporter induction [34]. In other paradigms, mammalian OGT is known to regulate the activity of the transcriptional regulator mSin3A through a non-catalytic mechanism requiring the TPR domain of OGT [89]. Whether or not C. elegans OGT-1 functions via regulation of a mSin3A-like mechanism or another pathway is a critical hypothesis to address in order to gain a better understanding of the non-catalytic OGT-1 function(s), which are very poorly understood.
OGT is an essential gene in all animals except for C. elegans [90, 91]. In mammalian systems, OGT knockout cells are not viable because they are unable to divide [92]. Therefore, it is not possible to identify genes like OGT in screens for regulators of the mammalian HTSR, even using the most sophisticated and state-of-the-art CRISPR/Cas9 based screening methods. Identification of ogt-1 as a regulator of the HTSR was only possible in C. elegans due to its unique biology. Furthermore, there are likely many other Nio genes involved in the HTSR that can only be identified through forward genetic screens in C. elegans. For example, the null phenotype for thousands of genes is lethal or sterile, which would prevent the recovery of such alleles in most screens. However, mutagenesis screens, such as the Nio screen we performed to identify ogt-1, also generate unusual non-null missense alleles that allow the essential requirements of the gene to be fulfilled but disrupt other more specific physiological roles. Such examples demonstrate the power of using genetic approaches in C. elegans to dissect apart cellular pathways in ways that can significantly complement and extend our understanding of conserved cell physiological pathways such as the HTSR.
Conclusion
Intracellular compatible osmolyte accumulation is the primary mechanism by which all cells maintain appropriate cell volume during HTS. Compatible osmolytes are accumulated via the transcriptional upregulation of biosynthetic enzymes or transporter proteins. These compatible osmolyte accumulation genes are part of a larger group of osmotically regulated genes that are transcriptionally upregulated during the HTSR. C. elegans synthesizes the compatible osmolyte glycerol in hypodermal and intestinal cells during HTS by upregulating the biosynthetic enzyme GPDH-1. Glycerol accumulation facilitates the survival and acclimation of C. elegans in hypertonic environments.
Study of the HTSR in C. elegans, primarily through unbiased genetic screens, has revealed both transcriptional and post-transcriptional regulation of osmosensitive gene expression (Figure 1). Transcriptionally, the HTSR is under strong negative regulation. Regulation of protein homeostasis genes, which oppose protein damage and synthesis, negatively regulate the HTSR transcriptional response through gcn-1/-2 and gck-3/wnk-1 signaling pathways. Extracellular proteins inhibit gpdh-1 transcriptional induction under isotonic conditions through a pathway involving a transmembrane protein and GATA-type transcription factors. Additional independent pathways also regulate the transcription of other osmotically regulated genes. The HTSR is also regulated post-transcriptionally through the enzyme OGT. Therefore, genetic screens performed to date have revealed the existence of at least three basic HTSR pathways in C. elegans (Figure 1).
One notable finding from these studies of the C. elegans HTSR is that the genes and mechanisms identified in this relatively simple organism are not ‘worm-specific’. Rather, they are ancient and highly conserved cellular pathways that impinge on critical aspects of cell physiology such as protein synthesis and folding, extracellular matrices, and novel functions of highly conserved proteins, such as OGT-1. However, much remains unknown about these HTSR regulatory pathways. The mechanisms by which cells sense HTS have yet to be described. Furthermore, in the transcriptional pathways, a transcription factor specific to the HTSR has not been identified and the signaling mechanism(s) by which this transcription factor becomes activated is unknown. One possibility is that HTS is sensed by the cell through the ECM. This paradigm occurs in the yeast Sho1 branch of the high osmolarity glycerol (HOG) pathway, where the OSM-8 – like, extracellular mucin domain – containing proteins, Msb2 and Hkr1 sense HTS to ultimately activate the master regulator of the yeast HTSR, Hog1 (Table 1) [93].
The post-transcriptional pathway through which OGT-1 functions to regulate the HTSR is also uncharacterized. OGT-1 could regulate GPDH-1 protein expression through mRNA cleavage, 3’ UTR usage, mRNA export, initiation factor interactions or ribosomal elongation. While the TPR domain, and not catalytic activity, of OGT-1 is required to regulate GPDH-1 protein expression, the mechanism(s) by which this domain regulates the HTSR is currently unknown. Continued unbiased genetic screens, complemented by targeted biochemical and cell biological studies, will be instrumental towards further defining the cellular pathways regulating the C. elegans HTSR. The success of the genetic screening approaches used to characterize the role of extracellular proteins, PTR-23, protein homeostasis, and OGT-1 in the HTSR indicate that such approaches are well suited to study this cellular stress response. Capturing unique missense alleles, identifying new genes, and describing new loss of function phenotypes through forward genetic screens in C. elegans will not only provide insight into the protein functions and cellular pathways regulating the HTSR, but will also reveal novel cell signaling paradigms that can be applied to other aspects of cellular physiology.
Main Insights.
C. elegans accumulate the compatible osmolyte glycerol during hypertonic stress by upregulating glycerol-3-phosphate dehydrogenase (gpdh-1) in hypodermal and intestinal epithelial cells.
In addition to gpdh-1, hypertonic stress differentially regulates 324 genes, including many antimicrobial peptides normally induced by pathogenic infection.
- At least three separate pathways regulate osmosensitive gene expression during hypertonic stress in C. elegans.
- Extracellular proteins, including several structural collagens and osm genes, inhibit the transcriptional induction of a subset of osmosensitive genes, including gpdh-1, in isotonic conditions.
- Protein damage triggered by inhibition of protein translation and/or protein folding genes activates the transcription of gpdh-1.
- The O-GlcNAc transferase OGT-1 acutely activates the hypertonic induction of GPDH-1 protein through a O-GlcNAc-independent, post-transcriptional mechanism.
Acknowledgements
Funding sources: S.U. - Research Advisory Council grant from the Children’s Hospital of Pittsburgh, T.L. - NIH R01GM135577
Footnotes
Disclosure statement
The authors have no conflicts of interest to declare.
Statement of ethics: The authors have no ethical conflicts to disclose.
References
- 1.Burg MB: Molecular basis of osmotic regulation. Am J Physiol 1995;268:F983–996. [DOI] [PubMed] [Google Scholar]
- 2.Burg MB, Ferraris JD, Dmitrieva NI: Cellular Response to Hyperosmotic Stresses. Physiological Reviews 2007;87:1441–1474. [DOI] [PubMed] [Google Scholar]
- 3.Yancey PH: Compatible and counteracting solutes: protecting cells from the Dead Sea to the deep sea. Sci Prog 2004;87:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancey PH: Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 2005;208:2819–2830. [DOI] [PubMed] [Google Scholar]
- 5.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN: Living with water stress: evolution of osmolyte systems. Science 1982;217:1214–1222. [DOI] [PubMed] [Google Scholar]
- 6.Borowitzka LJ, Brown AD: The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch Mikrobiol 1974;96:37–52. [DOI] [PubMed] [Google Scholar]
- 7.Brown AD, Simpson JR: Water relations of sugar-tolerant yeasts: the role of intracellular polyols. J Gen Microbiol 1972;72:589–591. [DOI] [PubMed] [Google Scholar]
- 8.Gekko K, Timasheff SN: Thermodynamic and kinetic examination of protein stabilization by glycerol. Biochemistry 1981;20:4677–4686. [DOI] [PubMed] [Google Scholar]
- 9.Back JF, Oakenfull D, Smith MB: Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry 1979;18:5191–5196. [DOI] [PubMed] [Google Scholar]
- 10.Tatzelt J, Prusiner SB, Welch WJ: Chemical chaperones interfere with the formation of scrapie prion protein. Embo j 1996;15:6363–6373. [PMC free article] [PubMed] [Google Scholar]
- 11.Arakawa T, Timasheff SN: The stabilization of proteins by osmolytes. Biophys J 1985;47:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burg MB, Ferraris JD: Intracellular organic osmolytes: function and regulation. J Biol Chem 2008;283:7309–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon HM, Handler JS: Cell volume regulated transporters of compatible osmolytes. Curr Opin Cell Biol 1995;7:465–471. [DOI] [PubMed] [Google Scholar]
- 14.Lee SD, Choi SY, Lim SW, Lamitina ST, Ho SN, Go WY, Kwon HM: TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: organic osmolyte-dependent and -independent pathways. Am J Physiol Renal Physiol 2011;300:F707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Rodríguez C, Aramburu J, Rakeman AS, Rao A: NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci U S A 1999;96:7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyakawa H, Woo SK, Chen CP, Dahl SC, Handler JS, Kwon HM: Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am J Physiol 1998;274:F753–761. [DOI] [PubMed] [Google Scholar]
- 17.Rim JS, Atta MG, Dahl SC, Berry GT, Handler JS, Kwon HM: Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 5’-flanking region. J Biol Chem 1998;273:20615–20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadkarni V, Gabbay KH, Bohren KM, Sheikh-Hamad D: Osmotic response element enhancer activity. Regulation through p38 kinase and mitogen-activated extracellular signal-regulated kinase kinase. J Biol Chem 1999;274:20185–20190. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh-Hamad D, Di Mari J, Suki WN, Safirstein R, Watts BA, 3rd, Rouse D: p38 kinase activity is essential for osmotic induction of mRNAs for HSP70 and transporter for organic solute betaine in Madin-Darby canine kidney cells. J Biol Chem 1998;273:1832–1837. [DOI] [PubMed] [Google Scholar]
- 20.Ko BC, Lam AK, Kapus A, Fan L, Chung SK, Chung SS: Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP). J Biol Chem 2002;277:46085–46092. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Naguro I, Ichijo H, Watanabe K: Mitogen-activated protein kinases as key players in osmotic stress signaling. Biochim Biophys Acta 2016;1860:2037–2052. [DOI] [PubMed] [Google Scholar]
- 22.Dahl SC, Handler JS, Kwon HM: Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol Cell Physiol 2001;280:C248–253. [DOI] [PubMed] [Google Scholar]
- 23.Hillier LW, Coulson A, Murray JI, Bao Z, Sulston JE, Waterston RH: Genomics in C. elegans: so many genes, such a little worm. Genome Res 2005;15:1651–1660. [DOI] [PubMed] [Google Scholar]
- 24.Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W: Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res 2000;10:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stiernagle T: Maintenance of C. elegans. WormBook 2006:1–11. [DOI] [PMC free article] [PubMed]
- 26.Dokshin GA, Ghanta KS, Piscopo KM, Mello CC: Robust Genome Editing with Short Single-Stranded and Long, Partially Single-Stranded DNA Donors in Caenorhabditis elegans. Genetics 2018;210:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghanta KS, Mello CC: Melting dsDNA Donor Molecules Greatly Improves Precision Genome Editing in Caenorhabditis elegans. Genetics 2020;216:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamath RS, Ahringer J: Genome-wide RNAi screening in Caenorhabditis elegans. Methods 2003;30:313–321. [DOI] [PubMed] [Google Scholar]
- 29.Zugasti O, Thakur N, Belougne J, Squiban B, Kurz CL, Soule J, Omi S, Tichit L, Pujol N, Ewbank JJ: A quantitative genome-wide RNAi screen in C. elegans for antifungal innate immunity genes. BMC Biol 2016;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labed SA, Omi S, Gut M, Ewbank JJ, Pujol N: The pseudokinase NIPI-4 is a novel regulator of antimicrobial peptide gene expression. PloS one 2012;7:e33887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pujol N, Cypowyj S, Ziegler K, Millet A, Astrain A, Goncharov A, Jin Y, Chisholm AD, Ewbank JJ: Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr Biol 2008;18:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D: IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002;415:92–96. [DOI] [PubMed] [Google Scholar]
- 33.Choe KP, Przybysz AJ, Strange K: The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol 2009;29:2704–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urso SJ, Comly M, Hanover JA, Lamitina T: The O-GlcNAc transferase OGT is a conserved and essential regulator of the cellular and organismal response to hypertonic stress. PLoS Genet 2020;16:e1008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamitina ST, Morrison R, Moeckel GW, Strange K: Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol Cell Physiol 2004;286:C785–791. [DOI] [PubMed] [Google Scholar]
- 36.Choe KP, Strange K: Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol 2007;293:C915–927. [DOI] [PubMed] [Google Scholar]
- 37.Zagórska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Thastrup J, Deak M, Campbell DG, Morrice NA, Prescott AR, Alessi DR: Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol 2007;176:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH: Properties of WNK1 and implications for other family members. J Biol Chem 2005;280:26653–26658. [DOI] [PubMed] [Google Scholar]
- 39.Bowlus RD, Somero GN: Solute compatibility with enzyme function and structure: rationales for the selection of osmotic agents and end-products of anaerobic metabolism in marine invertebrates. J Exp Zool 1979;208:137–151. [DOI] [PubMed] [Google Scholar]
- 40.Albertyn J, Hohmann S, Prior BA: Characterization of the osmotic-stress response in Saccharomyces cerevisiae: osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr Genet 1994;25:12–18. [DOI] [PubMed] [Google Scholar]
- 41.Grunewald RW, Wagner M, Schubert I, Franz HE, Müller GA, Steffgen J: Rat renal expression of mRNA coding for aldose reductase and sorbitol dehydrogenase and its osmotic regulation in inner medullary collecting duct cells. Cell Physiol Biochem 1998;8:293–303. [DOI] [PubMed] [Google Scholar]
- 42.Lamitina T, Huang CG, Strange K: Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci U S A 2006;103:12173–12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remize F, Barnavon L, Dequin S: Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab Eng 2001;3:301–312. [DOI] [PubMed] [Google Scholar]
- 44.Albertyn J, van Tonder A, Prior BA: Purification and characterization of glycerol-3-phosphate dehydrogenase of Saccharomyces cerevisiae. FEBS Lett 1992;308:130–132. [DOI] [PubMed] [Google Scholar]
- 45.Mráček T, Drahota Z, Houštěk J: The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta 2013;1827:401–410. [DOI] [PubMed] [Google Scholar]
- 46.Rohlfing AK, Miteva Y, Hannenhalli S, Lamitina T: Genetic and physiological activation of osmosensitive gene expression mimics transcriptional signatures of pathogen infection in C. elegans. PloS one 2010;5:e9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang CG, Lamitina T, Agre P, Strange K: Functional analysis of the aquaporin gene family in Caenorhabditis elegans. Am J Physiol Cell Physiol 2007;292:C1867–1873. [DOI] [PubMed] [Google Scholar]
- 48.Kwon HM, Yamauchi A, Uchida S, Preston AS, Garcia-Perez A, Burg MB, Handler JS: Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem 1992;267:6297–6301. [PubMed] [Google Scholar]
- 49.Yamauchi A, Uchida S, Preston AS, Kwon HM, Handler JS: Hypertonicity stimulates transcription of gene for Na(+)-myo-inositol cotransporter in MDCK cells. Am J Physiol 1993;264:F20–23. [DOI] [PubMed] [Google Scholar]
- 50.Turner BL, Papházy MJ, Haygarth PM, McKelvie ID: Inositol phosphates in the environment. Philos Trans R Soc Lond B Biol Sci 2002;357:449–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dijksterhuis J, Veenhuis M, Harder W: Ultrastructural study of adhesion and initial stages of infection of nematodes by conidia of Drechmeria coniospora. Mycological Research 1990;94:1–8. [Google Scholar]
- 52.Dodd W, Tang L, Lone JC, Wimberly K, Wu CW, Consalvo C, Wright JE, Pujol N, Choe KP: A Damage Sensor Associated with the Cuticle Coordinates Three Core Environmental Stress Responses in Caenorhabditis elegans. Genetics 2018;208:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohlfing AK, Miteva Y, Moronetti L, He L, Lamitina T: The Caenorhabditis elegans mucin-like protein OSM-8 negatively regulates osmosensitive physiology via the transmembrane protein PTR-23. PLoS Genet 2011;7:e1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moronetti Mazzeo LE, Dersh D, Boccitto M, Kalb RG, Lamitina T: Stress and aging induce distinct polyQ protein aggregation states. Proc Natl Acad Sci U S A 2012;109:10587–10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burkewitz K, Choe K, Strange K: Hypertonic stress induces rapid and widespread protein damage in C. elegans. Am J Physiol Cell Physiol 2011;301:C566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burkewitz K, Choe KP, Lee EC, Deonarine A, Strange K: Characterization of the proteostasis roles of glycerol accumulation, protein degradation and protein synthesis during osmotic stress in C. elegans. PloS one 2012;7:e34153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choe KP, Strange K: Genome-wide RNAi screen and in vivo protein aggregation reporters identify degradation of damaged proteins as an essential hypertonic stress response. Am J Physiol Cell Physiol 2008;295:C1488–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee EC, Strange K: GCN-2 dependent inhibition of protein synthesis activates osmosensitive gene transcription via WNK and Ste20 kinase signaling. Am J Physiol Cell Physiol 2012;303:C1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnstone IL: The cuticle of the nematode Caenorhabditis elegans: a complex collagen structure. Bioessays 1994;16:171–178. [DOI] [PubMed] [Google Scholar]
- 60.Cox GN, Kramer JM, Hirsh D: Number and organization of collagen genes in Caenorhabditis elegans. Mol Cell Biol 1984;4:2389–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dresen A, Finkbeiner S, Dottermusch M, Beume JS, Li Y, Walz G, Neumann-Haefelin E: Caenorhabditis elegans OSM-11 signaling regulates SKN-1/Nrf during embryonic development and adult longevity and stress response. Dev Biol 2015;400:118–131. [DOI] [PubMed] [Google Scholar]
- 62.Wheeler JM, Thomas JH: Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans. Genetics 2006;174:1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komatsu H, Chao MY, Larkins-Ford J, Corkins ME, Somers GA, Tucey T, Dionne HM, White JQ, Wani K, Boxem M, Hart AC: OSM-11 facilitates LIN-12 Notch signaling during Caenorhabditis elegans vulval development. PLoS Biol 2008;6:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solomon A, Bandhakavi S, Jabbar S, Shah R, Beitel GJ, Morimoto RI: Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 2004;167:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Culotti JG, Russell RL: Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics 1978;90:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bargmann CI, Thomas JH, Horvitz HR: Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol 1990;55:529–538. [DOI] [PubMed] [Google Scholar]
- 67.Colbert HA, Smith TL, Bargmann CI: OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 1997;17:8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RH, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR: Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev 2004;18:1630–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Igual Gil C, Jarius M, von Kries JP, Rohlfing AK: Neuronal Chemosensation and Osmotic Stress Response Converge in the Regulation of aqp-8 in C. elegans. Front Physiol 2017;8:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee EC, Kim H, Ditano J, Manion D, King BL, Strange K: Abnormal Osmotic Avoidance Behavior in C. elegans Is Associated with Increased Hypertonic Stress Resistance and Improved Proteostasis. PloS one 2016;11:e0154156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC: An osmosensing signal transduction pathway in yeast. Science 1993;259:1760–1763. [DOI] [PubMed] [Google Scholar]
- 72.Pujol N, Zugasti O, Wong D, Couillault C, Kurz CL, Schulenburg H, Ewbank JJ: Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog 2008;4:e1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banton MC, Tunnacliffe A: MAPK phosphorylation is implicated in the adaptation to desiccation stress in nematodes. J Exp Biol 2012;215:4288–4298. [DOI] [PubMed] [Google Scholar]
- 74.McGhee JD, Fukushige T, Krause MW, Minnema SE, Goszczynski B, Gaudet J, Kohara Y, Bossinger O, Zhao Y, Khattra J, Hirst M, Jones SJ, Marra MA, Ruzanov P, Warner A, Zapf R, Moerman DG, Kalb JM: ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult. Dev Biol 2009;327:551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilleard JS, McGhee JD: Activation of hypodermal differentiation in the Caenorhabditis elegans embryo by GATA transcription factors ELT-1 and ELT-3. Mol Cell Biol 2001;21:2533–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morton EA, Lamitina T: Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 2013;12:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.An JH, Blackwell TK: SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev 2003;17:1882–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rahe DP, Hobert O: Restriction of Cellular Plasticity of Differentiated Cells Mediated by Chromatin Modifiers, Transcription Factors and Protein Kinases. G3 (Bethesda) 2019;9:2287–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lubas WA, Hanover JA: Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem 2000;275:10983–10988. [DOI] [PubMed] [Google Scholar]
- 80.Seo HG, Kim HB, Kang MJ, Ryum JH, Yi EC, Cho JW: Identification of the nuclear localisation signal of O-GlcNAc transferase and its nuclear import regulation. Sci Rep 2016;6:34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S: Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 2011;469:564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torres CR, Hart GW: Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 1984;259:3308–3317. [PubMed] [Google Scholar]
- 83.Kreppel LK, Blomberg MA, Hart GW: Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 1997;272:9308–9315. [DOI] [PubMed] [Google Scholar]
- 84.Lubas WA, Frank DW, Krause M, Hanover JA: O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem 1997;272:9316–9324. [DOI] [PubMed] [Google Scholar]
- 85.Daou S, Mashtalir N, Hammond-Martel I, Pak H, Yu H, Sui G, Vogel JL, Kristie TM, Affar el B: Crosstalk between O-GlcNAcylation and proteolytic cleavage regulates the host cell factor-1 maturation pathway. Proc Natl Acad Sci U S A 2011;108:2747–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Capotosti F, Guernier S, Lammers F, Waridel P, Cai Y, Jin J, Conaway JW, Conaway RC, Herr W: O-GlcNAc transferase catalyzes site-specific proteolysis of HCF-1. Cell 2011;144:376–388. [DOI] [PubMed] [Google Scholar]
- 87.Giles AC, Desbois M, Opperman KJ, Tavora R, Maroni MJ, Grill B: A complex containing the O-GlcNAc transferase OGT-1 and the ubiquitin ligase EEL-1 regulates GABA neuron function. J Biol Chem 2019;294:6843–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu H, Gu Y, Qi J, Han C, Zhang X, Bi C, Yu W: Inhibition of E-cadherin/catenin complex formation by O-linked N-acetylglucosamine transferase is partially independent of its catalytic activity. Molecular medicine reports 2016;13:1851–1860. [DOI] [PubMed] [Google Scholar]
- 89.Yang X, Zhang F, Kudlow JE: Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 2002;110:69–80. [DOI] [PubMed] [Google Scholar]
- 90.Ingham PW: A gene that regulates the bithorax complex differentially in larval and adult cells of Drosophila. Cell 1984;37:815–823. [DOI] [PubMed] [Google Scholar]
- 91.Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, Hart GW, Marth JD: The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A 2000;97:5735–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Donnell N, Zachara NE, Hart GW, Marth JD: Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 2004;24:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saito H, Posas F: Response to hyperosmotic stress. Genetics 2012;192:289–318. [DOI] [PMC free article] [PubMed] [Google Scholar]


