Abstract
COVID‐19 is highly transmissible; however, its severity varies from one individual to another. Variability among different isolates of the virus and among its receptor (ACE2) may contribute to this severity, but comorbidity plays a major role on disease prognosis. Many comorbidities have been reported to be associated with severe COVID‐19 patients. We have collected data from retrospective studies which include clinical and epidemiological features of patients and categorize them into severe/mild, ICU/non‐ICU and survivors/dead patients. In this review, we give an update about SARS‐CoV‐2 structure with emphasis on the possible reasons for the severity of the virus in patients. We also collected information and patients’ data to highlight the relation between COVID‐19 patients and comorbidities.
Keywords: comorbidities, COVID‐19, risk factors, SARS‐CoV‐2, Severity
Abbreviations
- 3CLpro
3‐Chymotrypsin‐Like Protease
- (SR)‐rich
Serine‐Arginine rich
- ACE2
Angiotensin‐Converting Enzyme 2
- ARDS
Acute Respiratory Distress Syndrome
- COVID 19
Coronavirus Disease 2019
- CVDs
Cardiovascular Diseases
- DM
Diabetes Mellitus
- DPP4
Dipeptidyl Peptidase 4
- ER
Endoplasmic Reticulum
- GA
Golgi Apparatus
- IBD
Inflammatory Bowel Disease
- ICU
Intensive Care Unit
- Ifβ
Interferon β
- LKR
Linker Region
- MERS‐CoV
Middle East Respiratory Syndrome coronavirus
- MODS
Multiple Organ Dysfunction Syndrome
- MS
Multiple Sclerosis
- NsP1
Non‐structural Polyprotein 1
- PAMP
Pathogen‐Associated Molecular Patterns
- PRRs
Pattern Recognition Receptors
- RdRP
RNA‐dependent RNA Polymerase
- SARS‐CoV
Severe Acute Respiratory Syndrome Coronavirus
- SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus 2
- TB
Total Bilirubin
- TLR2/4
Toll‐Like Receptor 2 and 4
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The outbreak of the pandemic COVID‐19 originated form Wuhan city, China, in December 19. 1 Until 9th of October 2020, a total number of 36,754,395 confirmed cases and over a million deaths were reported worldwide. 2 Patients with COVID‐19 exhibit common symptoms that can be divided into two groups: systematic disorders and respiratory disorders. Systematic disorders include fever, headache and gastrointestinal distress, while respiratory disorders include cough, dyspnoea, bilateral ground‐glass opacities and pneumonia. 3 Distinguishably, COVID‐19 showed some unshared symptoms with beta‐coronavirus diseases such as sneezing and rhinorrhoea. 4 , 5 The rapid spread and expansion of SARS‐CoV‐2 lies on the asymptomatic carriers because they unknowingly transmit the virus to others. 6 SARS‐CoV‐2 is an RNA virus with a genomic size of around 30 kb and categorized under the beta‐coronavirus genera under the coronaviridae family. 7 The natural host reservoir for SARS‐CoV‐2 is believed to be bats as the novel virus was found to have 96% sequence similarity with bat's coronavirus. 8 Nevertheless, human infection is unlikely to be directly caused from bats; instead, an intermediate host is essential for crossing the species barrier. The exact intermediate reservoir for SARS‐CoV‐2 is yet to be discovered.
The whole genome is composed of 14 open reading frames encode for 27 proteins 9 (Figure 1). It includes four structural proteins: the spike surface glycoprotein (S), the envelope protein (E), the nucleocapsid protein (N) and the membrane protein (M). Structural proteins are essential for virus attachment, entry, assembly and pathogenesis. The rest of the encoded proteins are both non‐structural and accessory proteins. 7 The non‐structural proteins are essential in virus transcription and replication whereas accessory proteins play a role in virus pathogenesis as they interfere with the host immune response, however, many of them their functions are still under investigations. 10 , 11
FIGURE 1.
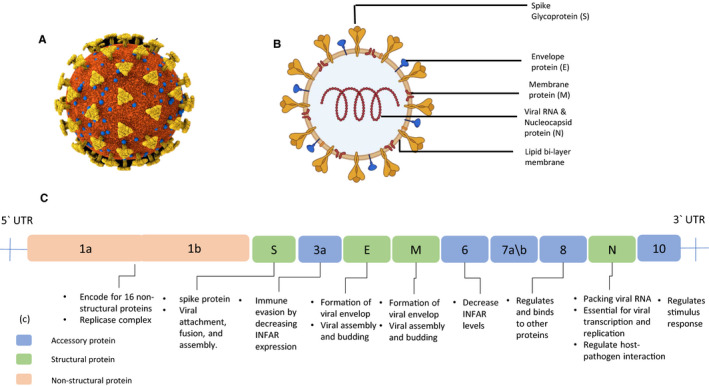
Schematic diagram for the SARS‐CoV‐2 structure and genome. (a) SARS‐CoV‐2 structure with viral components created with BioRender.com (b) 3D structure for SARS‐CoV‐2 created with Cinema 4D (c) SARS‐CoV‐2 genome organization: the viral genome yields structural, non‐structural and accessory proteins as shown in the ligand. The proteins functions are illustrated downstream their locations
N protein has essential roles in viral replication and assembly. It binds to the viral RNA producing helical ribonucleoprotein core and co‐localizes with the replicase complex; therefore, it is needed in viral replication and RNA synthesis. In addition, N protein helps in virus assembly by interacting with other structure proteins and aids virion formation and release. One of the factors that increase SARS‐CoV‐2 pathogenesis is that it uses the N protein as antagonist that inhibits the production of Interferon β (IFβ) as well as serving as an antigen for SARS‐CoV‐2 to be detected by IgM, IgA and IgG. 12 , 13 The spike protein shared by coronaviruses is a trimeric transmembrane protein composed of a large ectodomain, a single‐pass transmembrane anchor, and a short intracellular tail. The ectodomain is divided into main subunits, S1 and S2, in which S1 domain is responsible for virus‐receptor attachment while S2 is responsible for virus‐membrane fusion to the host cells. 14 , 15
SARS‐CoV‐2 uses angiotensin‐converting enzyme 2 (ACE2) receptor to enter their hosts. ACE2 receptors are found not only in lung tissue but also in the renal tubular epithelium, the Leydig cells in the testes, smooth muscle cells and the vascular smooth muscle cells of the blood vessels, in addition to expression in the kidney, liver and gastrointestinal tract cells. 16 , 17 Consequently, the virus has potential to infect multiple organs, mainly the respiratory epithelia and lung tissue. Once the virus attaches and enters the host cell, the process of uncoating begins, releasing the viral genome into cell cytoplasm. The translation begins with the expression of replicase polyproteins pp1a and 1ab, which undergo proteolytic cleavage by 3‐chymotrypsin‐like protease (3CLpro) and papain‐like proteases (PLpro) yielding sixteen non‐structural proteins to form the replication‐translation complex and RNA‐dependent RNA Polymerase (RdRP). 18 , 19 The synthesized polymerase produces sub‐genomic mRNAs by using the transcription regulating sequence that lie on the 5` end of each gene as SARS‐CoV. 20 , 21 Concomitantly, structural proteins S, N, E and M as well as other viral proteins begin to translate. 20 , 22 Finally, the proteins and the viral genome are assembled into virions in the endoplasmic reticulum (ER) along with the aid of Golgi apparatus (GA) that helps the complete virus exit the cell through vesicles. 23 Focussing on any of the previous events can be an effective approach for constraining the viral spread.
So far most of the studies done on SARS‐Cov‐2 patients are retrospective and meta‐analysis studies aiming to understand the clinical features of the patients and assess different factors of poor prognosis. The available prospective studies are testing previously made drugs non‐specific antivirals and repurposing them to control the symptoms. There has been several treatment regimens used with hospitalized and ICU patients to alleviate critical complications like acute respiratory dysfunction (ARDS) and multiple organ dysfunction (MODS) among others caused by several factors of disease severity such as viral load, cytokine storm and underlying comorbidities. 24 These were restricted to oxygen therapy, corticosteroids and broad‐spectrum antibiotics for possible secondary bacterial infection. 25 In other cases, direct acting antivirals were used such as Remdesivir which is an inhibitor to viral RNA‐dependent RNA polymerase that proved efficient against SARS‐CoV‐1 and MERS virus in‐vitro. 26 Chloroquine and hydroxychloroquine used for malaria treatment as well as an immunosuppressor for rheumatoid arthritis was also included in certain guidelines. 27 All these regimens, however, vary by case due to the diverse array of symptoms caused by the virus. 25 Two companies Pfizer‐BioNTech and Moderna have launched mRNA vaccines against SARS‐CoV‐2. 28 They utilize a modified mRNA encoding the spike gene for SARS‐CoV‐2 locked in perfusion conformation to elicit specific neutralizing antibodies and T cell immune responses. 29 On December 3, the CDC recommended 3 phases for vaccine administration; phase 1a for healthcare personnel, phase 1b was recommended on December 22 to include frontline essential workers and people aged 75 and older. Finally phase 1c to be administered for people aged 65‐75 and 16 and younger with underlying comorbidities. 30
Age has been a contributing factor of disease severity where consistently the older proportion of patients (commonly > 60 years of age) constituted the higher percentage of patients with poor prognosis and ICU admission. Old age is also associated with comorbidities such as hypertension, cardiovascular diseases and diabetes. Even in the younger proportion of patients associated with these comorbidities were also suffering from severe symptoms. 31 , 32 , 33 In this review, we aim to tackle the most important information of SARS‐Cov‐2 in terms of genetics, mode of infection and lifecycle with a focus on the host immune response and viral immune evasion. Finally, we address the different risk factors in addition to the common and uncommon comorbidities factors such as pregnancy, smoking and nutrient/vitamin deficiency associated with disease severity.
2. IMMUNE RESPONSE TO SARS‐COV‐2 INFECTION
The innate immune response is the first line of defence against pathogens including viral infections. For COVID‐19 patients, the body becomes sensitized upon attachment of the S1 subunit of the spike protein to ACE2 receptor of the alveolar epithelial cells, which is the same receptor for SARS‐CoV. 34 ACE‐2 is an enzyme that helps maintaining the balance between two forms of angiotensin namely angiotensin II, which causes inflammation and proliferation of cells, and angiotensin‐(1‐7) that promotes apoptosis and limit inflammation. ACE2 as a part of an axis called the ACE2/Ang‐(1‐7) axis is balanced by the ACE1/ angiotensin II axis. 35 ACE2 converts angiotensin II to Ang‐(1‐7) to maintain balance between angiotensin II mediated vasoconstriction, inflammation, and proliferation versus the vasodilation, anti‐inflammatory and apoptotic effects caused by small peptides of Ang‐(1‐7). 36
The entry of SARS‐CoV‐2 reportedly reduces ACE‐2 receptors as they appear to enter the cell with the membrane receptor causing it to lose its function and result in an elevated angiotensin II level, leading to impaired lung function. 36 , 37 The elevation of angiotensin II not only leads to lung dysfunction but has also been associated with old age, obesity, diabetes, CVD and hypertension and heart failure. 38
Following attachment, the viral RNA which is considered a pathogen‐associated molecular patterns (PAMP), produced during the replication process, is recognized by the innate immune system through pattern recognition receptors (PRRs). 39 PRRs are divided into three types according to their localization in the host cell: membrane and cytoplasmic types. The membrane type includes toll‐like receptor 2 and 4 (TLR2/4), whereas the cytoplasmic type includes RIG‐I, MDA5, TLR3, 7 and 8. 40 RIG‐I and MDA5 identify the viral genome in the endosomal compartment; in contrast, the TLR3 and TLR7 identify the RNA when exposed to the cytoplasm. 41 Recognition by any of these receptors triggers a cascade of events involving the MyD88 pathway and cGAS /STING pathway activating the NF‐KB transcription factors, which in turn induces the expression of type I interferons (IFN‐α /β). 42 Interferons I and III inhibit viral replication and induce the release of non‐specific pro‐inflammatory cytokines such as IL 1, IL 2, IL 4, IL 6, IL 7, IL 10 and MCP‐1, MIP1a, IFN‐γ, and TNF‐alpha and chemokines (CCL2, CXCL10, CXCL9, IL‐8 and IP‐10, through neutrophils, macrophages. 34
The presence of macrophage‐induced cytokines such as IL‐1 and IL‐6 induce the recruitment of cytotoxic T cells and neutrophils. 43 In mild cases of COVID‐19, cytotoxic CD8 T cells, which recognize the viral peptide presented on by MHC class I molecules, kill the cells infected with the virus via perforin and granzymes and provide immunity against secondary infections. As the disease progresses, the number of exhausted CD8 + T cells is higher than the non‐exhausted. Humoral immune response by B cells is activated by helper T cells and macrophage‐derived IL‐6. 43 When the virus or viral particles are presented by MHC class II molecules for a short period on antigen presenting cells such as dendritic cells and macrophage releasing first IgM before neutralizing IgG causing prolonged protection from the virus. In severe cases of COVID‐19, autoantigen presentation occurs and CD4 T cells become activated by CD154 and pro‐inflammatory CCR6 + Th17 in CD4 T cells, which leads to hyperactivation and release of pro‐inflammatory cytokines. 44 Also, the cytotoxicity of CD8 T cells is higher in severe cases. Another important observation is that the number of naïve CD4 T cells increases in comparison with a decrease in memory CD4 T cells in the peripheral blood of severe cases of COVID‐19 as well as a decrease in T regulatory cells which all collectively contribute to immune dysregulation. 42
A cytokine storm also known as hypercytokinaemia leads to dysregulation of the immune response. In mild cases, a balance exists between the pro‐ and anti‐inflammatory immune responses. The release of pro‐inflammatory cytokines is high at the start of infection and is then lowered during recovery as interferon I inhibits viral replication and specific adaptive immunity eliminates the infected cells. 45 A delay in the specific acquired immune response, which could be caused by interference with IFN‐α /β signals and lymphopenia in severe COVID‐19, can cause rapid viral replication that can lead to hyperactivation of macrophages and neutrophils in the lung parenchyma leading to over‐production of pro‐inflammatory cytokines, chemokines and leukotrienes. This leads to release of free radicals causing tissue damage in lung epithelial and endothelial cells followed by symptoms of pneumonia, acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS), which leads to increased mortality. 43 , 45
Elevation in IL‐6 has been reported to be associated with cases of ARDS, owed to its recruitment of other immune modulators such as neutrophils and cytotoxic T cells as well as other cytokines. 46 Elevation of IL‐6 could be a hallmark for severe COVID‐19 prognosis and treatment; however, it is not the sole agent causing complications and mortality. 43 This was evidenced by cases that when administered IL‐6 blocking drugs such as tocilizumab or siltuximab, more than half the patients did not recover from hyperinflammation. 47 One hypothesis suggests that this is due to constant release of IL‐6 and other cytokines through macrophages as the primary response of infection, which likely causes evasion from blockade. 48
The release of IL‐1b and IL‐18 is mainly lead by inflammasome activation. 49 In addition, IL1b also causes local neutrophil cytotoxicity and pyroptosis, which is a form of cell death by causing an increase in circulating lactate dehydrogenase. The continued release of interleukin 1 from macrophage and especially IL‐1b causes lung inflammation and fibrosis and eventually severe respiratory problems. 50
It was also reported that severe cases exhibit a lowered count of regulatory T cells, which functions to balance the activation of pro‐inflammatory function of CD4 T cells, CD8 T cells, B cells and natural killer cells. While the count of T and B lymphocytes is low due to activation of apoptosis and P53 signalling pathway in lymphocytes, their hyperactivation causes the release of multiple inflammatory cytokines such as CCR6 + th17 in CD4 T cells, IL‐2R, IL‐6, IL‐7, IL‐8, IL‐17, IL‐10, IFN‐ɣ, MCP‐1, MIP‐1A, TNF‐α, chemokines IP10 and CD8 T cells’ cytotoxins, which cause damage to lungs and eventually other organs. 51 In addition, amplification of naïve T helper cells is observed with a decrease in memory B and T cells. In conclusion, severe cases of SARS‐CoV 2 report higher counts of neutrophils, c‐reactive proteins and pro‐inflammatory cytokines and lower count of hyperactivated lymphocytes (CD4 and CD8 T cells). In addition, signs of hyperinflammation include an increasing level of ferritin and erythrocyte sedimentation rate and C‐reactive protein. 52
Coronaviruses use different methods to evade those PRRs hence, guarantee successful replication. In order for the virus to protect its RNA from being recognized by the immune sensors, it either forms a double‐membrane vesicle called replication organelle (RO) or it mimics the 5`cap structure of the host mRNA. 10 , 39 Sometimes, viruses intentionally destroy their own genome to evade the cytoplasmic sensors. This can be achieved by two methods. First, the virus genome encodes for non‐structural protein has an endonuclease activity. Second, the virus can undergo the non‐sense mediated decay, used continuously by the host cell, by shifting its reading frame concomitantly producing a premature stop codon. 39 Moreover, SARS‐CoV and potentially SARS‐CoV‐2 can halt the cellular translation machinery process using its 5`terminal subunit of the non‐structural polyprotein 1(NsP1). NsP1 binds to the 40S subunit of the ribosome subunit hindering it from translocation hence protein synthesis. 53 , 54
In addition, the genome of SARS‐CoV encodes for eight antagonists that regulate the production of type I interferon (INF), which is used by the host cells to inhibit the virus replication. 55 This can be achieved by downregulating the phosphorylation of STAT1 that will reduce the induction of INF. Moreover, it interferes with the downstream cascade of interferon induction by ubiquitinating hence degradation of RNA sensor molecules. 56 MERS‐CoV was found to downregulate the expression of viral antigen presentation‐related genes, as a way of adaptive immune system evasion, hence, decrease the detection by the adaptive immune system. 57 Unlike SARS‐CoV and MERS‐CoV, the O‐linked glycan that was predicted in SARS‐CoV‐2 may serve as a mucin‐like domain used by the virus to hide its recognition sites, that is epitopes from the immune system. 58 Such phenomenon was found to be used by Ebola and hepatitis C viruses for the same immunity‐response impairment purposes. 59 , 60
In the next section, different risk factors and comorbidities that could affect the severity of COVID‐19 will be covered.
3. RISK FACTORS ASSOCIATED WITH COVID‐19 SEVERITY
Studies on COVID‐19 continuously report the higher risk of diseases severity and poor prognosis for elderly patients with underlying diseases or comorbidities. A meta‐analysis study reported that the most prevalent comorbidities in COVID‐19 patients are hypertension (18.6%), cardiovascular diseases (14.4%) and diabetes (11.9%). 32 Other factors such as liver diseases, smoking, pregnancy, autoimmune disease and nutrition are not as common nor as impactful on poor prognosis. In this section, we will cover the relation between each factor and SARS‐CoV‐2 whether a direct relationship to the virus or an indirect one. Also, we collected data from clinical studies from several scientific databases and sources on each factor and their prevalence in severe/ICU admitted/Death patients compared to Mild/non‐ICU/Survivor patients (Supplementary Table 1).
We used scientific databases (Pubmed and lit. Covid) and searched with terms ‘nCov, Covid‐19, SARS‐Cov‐2, clinical features, clinical risk factors, comorbidities’, and then, we adjusted filters to include clinical trials, observational studies, multi‐center, randomized trials and excluded meta‐analysis, systematic reviews and reviews. We further went through the publication to include the ones that had required data such as comorbidities, severe, non‐severe groups, ICU, non‐ICU groups, survivors and death groups. We also included any additional studies found in references. We then searched for specific meta‐analysis publications that included COVID‐19 with risk factors that were minimally or not found in the common comorbidities or patient criteria such as pregnancy, nutrient, vitamin deficiency and autoimmune diseases. The outcome results are presented in Supplementary Table 1.
3.1. Age‐associated risk
There has been compiling evidence of the higher risk imposed on elders compared to children in terms of contracting the virus and the fitness of the immune response. The relation between ACE2 expression level and age remains controversial. 61 It was reported that children have a lower baseline expression level of ACE2 in nasal epithelium when compared to older people, used a cohort of 305 patients aged 4 to 60 but did not compare with older patients. 62 While other studies proposed the opposite with a significant decline of ACE2 expression with age and associating the decrease with the age‐associated androgen and oestrogen downregulation when analysing the ACE2 expression level in mice models. 63 , 64 We conclude from these confounding results that perhaps the protective effect of ACE2 decreases with age and its function in angiotensin II conversion diminishes with age while its presence allows for easy entry of SARS‐CoV2. Another indisputable fact is the age‐associated comorbidities, which are normally associated with an elevated level of angiotensin II leading to an increased risk of severity when compounded with SARS‐CoV‐2 infection due to the impact of angiotensin II on pro‐inflammatory cytokines, vasoconstriction and lung impairment. 38 These results were also further validated by several epidemiological studies where the percentage of SARS‐Cov‐2 infections were higher in people with older age. 65 , 66 In terms of immunity, children have a stronger innate immune response with a higher level of lymphocytes, naïve T cells, B cells and natural killer cells, which will strengthen the response to COVID‐19. Additionally, they have a lower expression of pro‐inflammatory cytokines lowering the risk of developing a cytokine storm. 67 Moreover, due to multiple exposure of children to respiratory diseases and an intense vaccination regime, their bodies develop a trained immunity that may help them combat the disease better than elders. 68 The higher probability of children attracting the virus from 2nd and 3rd generation was also suggested as a contributing factor to milder symptoms. 69 It is important to note that the earlier hypothesis about children not being possible targets of the virus was refuted. The previously mentioned reports show that children are equally to contract the virus but likely show mild symptoms and have shown a better immune response to the virus, while in one case study children from an infected family repeatedly shown negative PCR but cellular immunity demonstrated presence of SARS‐Cov‐2 antibodies with very mild symptoms. 70 , 71 As shown in Supplementary Table 1, the difference between the average of age medians is 10 years between severe and in mild. In the next sections, we will cover different chronic diseases that could pose a risk on patients infected with SARS‐CoV‐2. Although hypertension and diabetes are also age‐related diseases, we will tackle studies and theories on how the effect of each disease on the body could affect SARS‐Cov‐2 prognosis.
3.2. Hypertension, cardiovascular disease and coagulopathy
There is a correlation between the COVID‐19 and myocardial and vascular dysfunction. One arm of this correlation could be the multifunctional ACE2. The renin‐angiotensin system (RAS) is a system that maintains vascular homeostasis and controls blood flow and among other functions. ACE and ACE2 are two essential and sometimes opposing components of this system. Although they share structure similarity, they trigger different events. ACE converts angiotensin I into angiotensin II causing vasoconstriction, while ACE2 which shares structural homology with ACE converts angiotensin II into ang (1‐7) promoting vasodilation. 72 After infection with SARS‐Cov2, the transmembrane enzyme binds to the spike protein causing an increase in the level of angiotensin II in circulation due to dysfunction of ACE2. 38 In addition to the effect SARS‐Cov‐2 has on lung injury and inflammation, it also contributes to myocardial and heart damage. Patients with chronic hypertension, cardiovascular diseases (CVD) and cardiac hypertrophy seem to have a reduced ACE2 and Ang 1‐7 leading to an increased level of circulating angiotensin II which speeds up the progression to heart failure. 35 , 36 There is also an increase in Troponin I /T and creatine kinase in hospitalized patients which are markers for cardiac‐related diseases. 73 In two meta‐analysis study tracking the elevation of troponin I/T or creatine kinase in hospitalized patients as a marker for underlying CVD from clinical trials and case reports, the proportion of patients with underlying hypertension and cardia‐cerebrovascular diseases was higher in ICU versus non‐ICU admitted patients. 74 , 75
The reported over‐production of angiotensin II and IL‐6 caused by SARS‐CoV‐2 lead to endothelial activation and hyperinflammation which are contributing factors to coagulation activation and coagulopathy. 76 It is important to mention that about 70% of hospitalized patients have reported coagulation abnormalities like hypercoagulation, venous thrombosis (VT) and disseminated intravascular coagulation (DIC) with VT and pulmonary embolism being the causative factors behind almost 58% deaths. 77 This has also been validated by the elevated D‐dimer reported in patients who develop severe symptoms. As a result, the steep and sudden decrease in fibrinogen levels causes an increase in consumptive coagulopathy in deceased patients. 78 The molecular mechanism that leads to SARS‐CoV‐2 mediated coagulopathy is not yet know. The increased risk of severity has been reported in both animal models and humans and serves as an important prognosis and a recommendation to use anticoagulants to prevent adverse outcomes. 79
Hypertension and CVD are the most commonly found comorbidities or underlying diseases in retrospective studies when analysing clinical features of COVID‐19 patients as is apparent in Supplementary Table I. A large Chinese meta‐analysis study with 44,672 cases reported 10.5% increased fatality rate for patients with CVD and 6% for patients with hypertension. 80 Additionally, it was found that COVID‐19 patients with no previous chronic diseases could develop cardiac injury. It is important to note that ACE inhibitors and angiotensin receptor blockers are recommended as a first line of treatment for patients with hypertension. There were recommendations for discontinuation of the ACE inhibitors for risk of altering ACE2 expression and affecting SARS‐CoV‐2 virulence. 81 Although animal studies showed confounding effects of ACE inhibitors and ARBs on ACE2 expression, human studies have shown intravenous administration of these drugs for patients with hypertension or heart failure had no direct effect of on ACE2 expression or production of angiotensin 1‐7 or angiotensin 1‐9. 82 One Japanese study observed urinary increase in ACE2 after long‐term treatment with ARB Olmesartan but not with ACE inhibitors. 83 A very large population study aimed to test the effect of ACE inhibitors and ARBs on increased risk of infection and/or admission in ICU. The study showed an overall reduced risk of SARS‐CoV2 positive PCR with adjusted administration of the drugs, while it proved no significant correlation with the risk of ICU admission. 84 However, a direct correlation between heart dysfunction and COVID‐19 induced death is not yet established. Moreover, there is no proof that patients with hypertension or any cardiac‐related comorbidities were more susceptible to infection. 75 , 85 In Supplementary Table I out of 5138 patients, the average of severe case patients with hypertension is about double the average in mild cases. In patients with CVD, the average is approx. 20% in severe and 10% in mild.
3.3. Diabetes
Both type I and type II diabetes mellitus (DM) are chronic diseases that put the body under immune stress. 86 Diabetes can be considered as a hyperinflammation state, which could lead to rapid worsening of patients with COVID‐19 who already have an elevated level of cytokines like IL‐6 and IL‐1b leading to ARDS and mortality. 87 It is important to note that diabetes is as with hypertension an age‐associated disease and in most retrospective studies is present in the older age groups. Patients with DM also exhibit lowered expression of ACE2 and high angiotensin II in circulation, reportedly due to glycosylation of the ACE2. 88 Nonetheless to reverse the effect of increased angiotensin II, patients with DM are prescribed with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers ACEI/ARB as part of their treatment as an anti‐hypertensive drug. These drugs increase the level of ACE2 that is used by SARS‐Cov‐2 as a receptor for entry, leading to a vicious cycle. 89 Diabetes mellitus is also associated with increased levels of serum ferritin and D‐dimer, which are two indicators of inflammation. In addition, an increase in blood clot formation or hypercoagulation is elevated in COVID‐19 patients. 90 , 91 Due to the abundance of ACE2 in the pancreatic cells, SARS causes the reduction or dysfunction of the pancreatic islet cells, leading to reduced glycemic control. 92 In addition, the presence of a pro‐inflammatory environment or a cytokine storm further reduces the insulin sensitivity in type I and type II DM. 87 Several trials recorded the percentage of COVID‐19 patients with DM in severe and non‐severe cases. In several studies in China, diabetes patients represented up to 20% of the COVID‐19 patients. 90 , 93 , 94 Even higher percentage of COVID‐19 induced death and ICU cases suffered from DM was reported, in some cases up to 35%. 95 The data collected from studies in Supplementary Table I also conclude that the percentage of patients with DM is higher (almost double) in severe cases than in mild cases.
3.4. Liver dysfunction
SARS‐Cov‐2 has been reported to affect liver functions such as the AST and ALT levels as well as a slight elevation in total bilirubin (TB). 96 The effect of COVID‐19 on liver has been controversial, with some studies correlating abnormal AST and ALT levels with severity of infection, while others report no difference between mild and severe cases. A large meta‐analysis study with 3428 patient records of liver function analysis pooled from 20 prospective studies reported liver injury incidence marked by AST, ALT and bilirubin elevation at 58% to 78%. 97 Another study specified elevated ALT or AST levels in severe and mild cases, respectively. 98 It is undecided whether the cause of liver abnormalities is due to the viral infection or the inflammation caused by cytokine storm. Although it is comparably low, ACE2 is found in liver and bile duct cells as reported in a recent ACE2 expression profiling study comparing the expression across different cell types. 99 Therefore, the damage observed in liver functions could be owed to SARS‐CoV‐2 effect on ACE2 expression. However, the expression is about 20 times higher in bile duct cells than in hepatocytes and histopathological analysis of hepatocytes show minimal damage, which leads us back to acute inflammatory reaction and multiple organ dysfunction. 44 , 100 The cytokine storm caused by the virus in severe cases leads to severe inflammation, apoptosis and necrosis of cells and eventually leads to multiple organ dysfunction syndrome (MODS). Another important issue is the side effects of the drugs used for treatment. Most of the drugs used to alleviate the symptoms contains acetaminophen, which has proven to cause liver damage. 101 The use of different non‐specific antivirals such as oseltamivir or lopinavir also affect the liver. 100
Chronic liver diseases are widespread worldwide and are especially more common in the elderly. People with underlying liver disease and cirrhosis are more vulnerable to severe COVID‐19 manifestation due to systemic immunodeficiency. There are currently no reports of increased risk of infection due to pre‐existing liver diseases. 102 , 103 According to data collected in Supplementary Table I, over 30% of the studies did not contain data on liver disease and the average between severe and mild cases show a slight increase with no confirmed causality between severity and liver dysfunction. Until now, there have been no reports of patients undergoing treatment for liver hepatitis B or C having an increased or decreased risk of COVID‐19.
3.5. Autoimmune disease and organ transplantation
The increased level of pro‐inflammatory cytokines such as IL‐1 and IL‐6 and c‐reactive protein accompanied by lymphopenia in severe cases of COVID‐19 can be found closely related to symptoms in other diseases such as rheumatoid arthritis, systemic Lupus, multiple sclerosis (MS) and inflammatory bowel disease (IBD), which could lead to confusion in diagnosis. 104 There are two major prespectives when looking at the relation between SARS‐CoV‐2 and autoimmune disease; one is that people with autoimmune diseases are greatly susceptible to viral infection due to decreased adaptive immunity and because of increased intake of immunosuppressants such as corticosteroids as part of their treatment regimen. 105 The other prospective is that these immunosuppressants may help contain the cytokine storm cause by COVID‐19, which eventually leads to ARDS and death. The latter has been used to test drugs with immunosuppressive nature normally consumed by rheumatic patients such as chloroquine, hydroxychloroquine and IL‐6 blockers in treatment regimens of mild and severe COVID‐19 cases with variable results. 106 , 107 , 108 We found that most of the randomized cohort studies assessing clinical features of COVID‐19 cases did not include autoimmune disease as a comorbidity, but rather were presented in case‐controlled studies and case reports as the main inclusion criteria. One trial following up with 111 patients of previous organ transplantation and undergoing long‐term immunosuppressive treatment did not show correlation between immunosuppression and increased risk of infection. 109 A follow‐up study with 20 kidney transplants patients who had developed SARS‐CoV‐2 induced pneumonia. All patients were taken off their immunosuppressive drug tocilizumab (IL‐6 blocker) and started antiviral treatment. Out of 20 admitted patients, 14 were severe cases, including five deaths, and six patients were moderate cases. 110
At the beginning of the outbreak in China and Italy, preliminary data reported lower risk of infection in patients with inflammatory bowel disease (IBD). 111 , 112 With the increase in COVID‐19 spread, severe and fatal cases of IBD (ulcerative colitis or Crohn's disease) infected patients started to appear. 113 A retrospective observational study included 79 IBD patients with COVID‐19 to study their clinical features and outcome were reported. This study found that patients with active IBD and treated for flare‐ups developed COVID‐19 related pneumonia and showed increased ICU admission as well as fatality. 114 In the data gathered in Supplementary Table I, only six studies contained data on patients with autoimmune diseases, but even then, the number of patients was very small.
3.6. Pregnancy‐associated risk
Monitoring pregnant women with SARS‐Cov2 report relatively similar symptoms of fever, coughing and diarrhoea across the sample size but with higher severity compared to non‐pregnant women. 115 More importantly, several cases of premature birth were reported experiencing other complications such as premature membrane rupture, irregular contractions, growth restriction and some stillbirths. 116 The newborns manifested shortness of breath, vomiting and rashes, and some had an elevated level of IgM specific to SARS‐CoV‐2. 66 However, tests for viral RNA from amniotic fluid, serum and breast milk came out negative for SARS‐CoV‐2. 117 Therefore, there is no proof of vertical transmission from the mother, but there is a suggestion that the viral infection affects the pregnancy and the general health of the newborn. Pregnancies go through different phases in immune response between pro‐inflammatory and anti‐inflammatory with a strong innate immune response and a decrease in the number of T and B cells. The high level of oestrogen and progesterone tends to cause swollen upper respiratory tract. 117 Another systematic review of pregnant women infected with CoVs (MERS‐CoV, SARS‐CoV, and SARS‐CoV‐2) found the increased risk of severe symptoms in pregnant women to be higher in SARS‐CoV and MERS‐CoV than in SARS‐CoV‐2. Of the COVID‐19 infected women, pregnant women showed a higher rate of premature birth and neonatal ICU admission, with 2.4% rate of stillbirths and 2.4% of neonatal death. 118 However, other studies showed that pregnant women with COVID‐19 who are treated had the same pattern of clinical features as non‐pregnant women with no reported deaths. 119 , 120 Overall, the number of studies monitoring infection in pregnant women is limited without a significant pattern for severe prognosis between pregnant and non‐pregnant women. 121 , 122 In a US study, they reported a total of 91,412 women infected with SARS‐CoV‐2 at the reproductive age. In this study, among the 8,207 pregnant women about 31% were hospitalized and 1.5% admitted to ICU while in non‐pregnant women only 5.8% were hospitalized and 0.9% admitted to ICU. 123 Notably, the percentage of pregnant women admitted to ICU was higher in the older age range (34%) compared to younger women (29.3%), and compared to non‐pregnant women they were more likely to have comorbidities such as DM (15.3% versus 6.4%), CVD (14% versus 7%) and immunocompromised condition (3.5 versus 2.8). 123 It is important to note that pregnancies are often associated with hypertension, respiratory problems, gestational diabetes and an immunosuppressive state that may complicate their situation when they get infected. 120 , 124
3.7. Health and nutrition
Comorbidities resulting from smoking, obesity and nutrient deficiencies affect the severity level, treatment regimen and complications of the viral infection. Smoking and obesity lead to diseases such as hypertension, cardiovascular and respiratory diseases. Studies comparing intensity of COVID‐19 symptoms between patients with different comorbidities and healthier patients. People with comorbidities were more vulnerable and developed more critical symptoms and even a higher mortality rate. Bad habits such as smoking and accumulating saturated fatty acids in morbid obesity lead to a lowered adaptive immunity and an increased innate immunity. 125 , 126 The WHO released a review including results from 26 observational studies and eight meta‐analyses that reported a correlation between smoking and hospitalized COVID‐19 patients. 127 The review reports no evidence of increased risk of infection but a prevalence of 1.4%‐18.5% of smokers among hospitalized patients. 128 Vitamin D has also been included in the treatment protocol of COVID‐19 patients with suggested higher risk in patients with vitamin D deficiency. Vitamin D has been known to enhance adaptive immune response, which would hasten specific immunity against COVID‐19 and decrease cytokine storm. 129 With the current restriction of outdoor activity, this could affect the vitamin D intake caused by lack of exposure to the sun. It is worth noting that the number of studies monitoring health habits such as smoking, obesity or nutrient/ vitamin deficiency is not common.
3.8. Viral load
Although not a host related factor, it is imperative to mention one of the first and most effective factors affecting the prognosis of the disease is the initial viral load. Primarily this was observed with SARS where it was reported that higher expression of ACE2 mRNA was concurrent with higher levels of SARS‐CoV RNA in patient plasma and serum. 130 , 131 Several retrospective studies have recorded high viral load just before or at the time of symptoms onset. These studies monitor the quantitative viral load in serum and plasma and viral shedding in stool. Higher viral load leads to erratic shedding in stool with symptoms appearing on day 3 to 5 followed by a decrease in the viral load. 132 One study reported detectable levels of SARS‐CoV‐2 in the serum of critically ill patients as opposed to mild and severe patients with positive nasal swabs. This study proposed a correlation between higher load of SARS‐CoV2 in serum and a sharp elevation in IL‐6 in critically ill patients (almost 5 times the level in severe patients). 133 It was also reported that quantitative viral load measurements differentiates between patients who survived versus patients who died from SARS‐CoV‐2. 134
4. CONCLUSION
The severity of the SARS‐CoV‐2 infection is variable among patients. This can be on the virus level, the host genetics level or the host health condition level. Therefore, different isolates of the virus, different ACE2 variants and their abundance, or different comorbidities may participate in this variation in the COVID‐19 severity among patients. Once the virus enters the cell via spike protein (S), it induces the activation of the innate immune system and macrophage‐derived cytokines are released. The virus then through mechanisms not yet definite such as interference with interferon signalling causes a delay in specific adaptive response, lymphopenia and over‐expression of inflammatory cytokines namely, IL‐ 2R, IL‐6, IL‐7, IL‐8, IL‐17, IL‐10, IFN‐ɣ, MCP‐1, MIP‐1A and TNF‐α, chemokines IP10. This condition is known as a cytokine storm, and it causes severe outcomes in the patients ranging from ARDS, pneumonia to multiple organ dysfunction and mortality. The virus also causes a downregulation of ACE2 in the infected cells. ACE2 is the enzyme that converts angiotensin II to ang 1‐7 keeping the homeostasis of ACE2/Ang1‐7 axis, which is anti‐inflammatory and proliferative, and ACE1/angiotensin II, which is pro‐inflammatory and apoptotic that further pushing the body towards tissue necrosis and dysfunction. Until this date, the virus has been medically managed through drug repurposing and symptom alleviation with non‐specific antivirals, antibiotics, analgesics, immunosuppressive drugs and vitamins. The variability in patient symptoms called for classification of the disease into mild, moderate and severe depending on multiple aspects. Viral load being the predominant cause followed by factors in the patient history record. Age being the most predominant and prevalent factor, where the higher percentage of severe outcomes belongs to older patients who tend to have weakened immune system and underlying comorbidities. In this review, we collected data from studies with specific criteria as mentioned in the methods section, displaying a total of 5138 patient clinical features (Supplementary Table I). The review emphasizes the level of severity relevant to age, comorbidities, pregnancy and smoking. In agreement with previous meta‐analysis studies, the highest percentage in the severe group belonged to patients with hypertension, followed by diabetes, CVD and liver dysfunction. Data on autoimmune diseases, pregnancy and smoking factors were very limited. The cause of the high percentage of severity can be linked to exacerbation of symptoms of underlying chronic disease either due to ACE2 downregulation or the acute inflammatory response. Whether the infection was mild or severe, early diagnosis, prognosis and personalized treatment greatly affects the outcome for the patient. Also monitoring the side effects of the drugs given in terms of hepatotoxicity and inflammation is essential to avoid complications.
CONFLICT OF INTEREST
The authors declare that the article was prepared in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHORS’ CONTRIBUTIONS
FN performed literature review, wrote and edited the manuscript; AE performed literature review and co‐wrote the manuscript; STZ designed, edited and directed the study.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL
Not applicable.
Supporting information
Table S1
Fadl N, Ali E, Salem TZ. COVID‐19: Risk Factors Associated with Infectivity and Severity. Scand J Immunol. 2021;93:e13039. 10.1111/sji.13039
Funding information
This work is supported by the internal fund ZC 016‐2019 and in part with the Science and Technology Development Fund (STDF) ID 22976.
DATA AVAILABILITY STATEMENT
All data sets, on which the conclusions of the manuscript rely, are presented in the main paper and the supplementary Table.
REFERENCES
- 1. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020:497‐501. 10.1016/j.bja.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Coronavirus Disease (COVID‐19) Dashboard | WHO Coronavirus Disease (COVID‐19) Dashboard. Available from: https://covid19.who.int/?gclid=EAIaIQobChMIxom87sCY6wIVA7TVCh2EPQwyEAAYASAAEgJbrfD_BwE [accessed August 13, 2020]
- 3. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020. 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986‐1994. 10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- 5. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752‐761. 10.1016/S1473-3099(13)70204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang DE, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA ‐ J Am Med Assoc. 2020;1092‐1093. 10.1001/jama.2020.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID‐19): current status and future perspectives. Int J Antimicrob Agents. 2020;55(5): 10.1016/j.ijantimicag.2020.105951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dawood AA. Mutated COVID‐19 may foretell a great risk for mankind in the future. New Microbes New Infect. 2020:100673. 10.1016/j.nmni.2020.100673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saxena SK, Kumar S, Maurya VK, et al. Current insight into the novel coronavirus disease 2019 (COVID‐19). Singapore: Springer; 2020:1‐8. [Google Scholar]
- 11. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. (COVID‐19) outbreak‐ an update on the status. Mil Med Res. 2019;2020:1‐10. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McBride R, van Zyl M, Fielding B. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6(8):2991‐3018. 10.3390/v6082991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng W, Liu G, Ma H, et al. Biochemical characterization of SARS‐CoV‐2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527(3):618‐623. 10.1016/j.bbrc.2020.04.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3(1):237‐261. 10.1146/annurev-virology-110615-042301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Douglas GC, O’Bryan MK, Hedger MP, et al. The novel Angiotensin‐Converting Enzyme (ACE) homolog, ACE2, is selectively expressed by adult leydig cells of the testis. Endocrinology. 2004;145(10):4703‐4711. 10.1210/en.2004-0443 [DOI] [PubMed] [Google Scholar]
- 17. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tahir ul Qamar M, Alqahtani SM, Alamri MA, Chen L‐L. Structural basis of SARS‐CoV‐2 3CLpro and anti‐COVID‐19 drug discovery from medicinal plants. J Pharm Anal. 2020. 10.1016/j.jpha.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toraih EA, Elshazli RM, Hussein MH, et al. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID‐19 patients: a meta‐regression and Decision tree analysis. J Med Virol. 2020:jmv.26166. 10.1002/jmv.26166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim D, Lee J‐Y, Yang J‐S, Kim JW, Kim VN, Chang H. The Architecture of SARS‐CoV‐2 transcriptome. Cell. 2020;181(4):914‐921.e10. 10.1016/j.cell.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marra MA. The genome sequence of the SARS‐associated coronavirus. Science (80‐ ). 2003;300(5624):1399‐1404. 10.1126/science.1085953 [DOI] [PubMed] [Google Scholar]
- 22. Abd El‐Aziz TM, Stockand JD. Recent progress and challenges in drug development against COVID‐19 coronavirus (SARS‐CoV‐2) ‐ an update on the status. Infect Genet Evol. 2020;83:104327. 10.1016/j.meegid.2020.104327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID‐19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91‐98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nitulescu G, Paunescu H, Moschos S, et al. Comprehensive analysis of drugs to treat SARS‐CoV‐2 infection: mechanistic insights into current COVID‐19 therapies (Review). Int J Mol Med. 2020: 467‐488. 10.3892/ijmm.2020.4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iacob S, Iacob DG. SARS‐CoV‐2 treatment approaches: numerous options, no certainty for a versatile virus. Front Pharmacol. 2020:1224. 10.3389/fphar.2020.01224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 — final report. N Engl J Med. 2020;383(19):1813‐1826. 10.1056/nejmoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khuroo MS. Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID‐19). Facts, fiction and the hype: a critical appraisal. Int J Antimicrob Agents. 2020:106101. 10.1016/j.ijantimicag.2020.106101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Different COVID‐19 Vaccines | CDC. Available from: https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/different‐vaccines.html [accessed December 29, 2020]
- 29. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020:NEJMoa2034577. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. When Vaccine is Limited, Who Gets Vaccinated First? | CDC. Available from: https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/recommendations.html [accessed December 29, 2020]
- 31. Chen G, Wu DI, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020:101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol. 2020;92(6):568‐576. 10.1002/jmv.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tufan A, Avanoğlu GA, Matucci‐Cerinic M. Covid‐19, immune system response, hyperinflammation and repurposinantirheumatic drugs. Turkish J Med Sci. 2020;620‐632. 10.3906/sag-2004-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/Angiotensin‐(1–7)/Mas axis of the renin‐angiotensin system: focus on angiotensin‐(1–7). Physiol Rev. 2018: 505‐553. 10.1152/physrev.00023.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo J, Huang Z, Lin L, Lv J, Disease C. (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2019;2020:e016219. 10.1161/JAHA.120.016219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. 10.1016/j.ejim.2020.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kikkert M. Innate immune evasion by human respiratory RNA viruses. J Innate Immun. 2020;12(1):4‐20. 10.1159/000503030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li K, Hao Z, Zhao X, Du J, Zhou Y. SARS‐CoV‐2 infection‐induced immune responses: friends or foes? Scand J Immunol. 2020;92(2). 10.1111/sji.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133(1):101‐112. 10.1016/j.virusres.2007.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564‐1581. 10.1111/all.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vardhana SA, Wolchok JD. The many faces of the anti‐COVID immune response. J Exp Med. 2020;217(6). 10.1084/jem.20200678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID‐19 pneumonia. Cytokine Growth Factor Rev. 2020. 10.1016/j.cytogfr.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gritti G, Raimondi F, Ripamonti D, et al. Use of siltuximab in patients with COVID‐19 pneumonia requiring ventilatory support. MedRxiv. 2020:2020.04.01.20048561. 10.1101/2020.04.01.20048561 [DOI] [Google Scholar]
- 48. Tate MD, Ong JDH, Dowling JK, et al. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Rep. 2016;6. 10.1038/srep27912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2). 10.23812/CONTI-E [DOI] [PubMed] [Google Scholar]
- 50. Rayamajhi M, Zhang Y, Miao EA. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods in Molecular Biology (Clifton, N.J.). 2013;1040, NIH Public Access:85‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid‐19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1). 10.1136/bmjdrc-2020-001343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;1033‐1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kamitani W, Huang C, Narayanan K, Lokugamage KG, Makino S. A two‐pronged strategy to suppress host protein synthesis by SARS coronavirus Nsp1 protein. Nat Struct Mol Biol. 2009;16(11):1134‐1140. 10.1038/nsmb.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lokugamage KG, Narayanan K, Huang C, Makino S. Severe acute respiratory syndrome coronavirus protein nsp1 is a novel eukaryotic translation inhibitor that represses multiple steps of translation initiation. J Virol. 2012;86(24):13598‐13608. 10.1128/jvi.01958-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Totura AL, Baric RS. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol. 2012;2(3):264‐275. 10.1016/j.coviro.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol. 2020;1‐9. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 57. Menachery VD, Schäfer A, Burnum‐Johnson KE, et al. MERS‐CoV and H5N1 influenza virus antagonize antigen presentation by altering the epigenetic landscape. Proc Natl Acad Sci. 2018;115(5):E1012‐E1021. 10.1073/pnas.1706928115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS‐CoV‐2. Nat Med. 2020;26(4):450‐452. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Francica JR, Varela‐Rohena A, Medvec A, Plesa G, Riley JL, Bates P. Steric shielding of surface epitopes and impaired immune recognition induced by the ebola virus glycoprotein. PLoS Pathog. 2010;6(9):e1001098. 10.1371/journal.ppat.1001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Helle F, Duverlie G, Dubuisson J. The hepatitis C virus glycan shield and evasion of the humoral immune response. Viruses. 2011;1909‐1932. 10.3390/v3101909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schouten LR, van Kaam AH, Kohse F, et al. Age‐dependent differences in pulmonary host responses in ARDS: a prospective observational cohort study. Ann Intensive Care. 2019;9(1):55. 10.1186/s13613-019-0529-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin‐converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427. 10.1001/jama.2020.8707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xudong X, Junzhu C, Xingxiang W, Furong Z, Yanrong L. Age‐ and gender‐related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166‐2171. 10.1016/j.lfs.2005.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS‐CoV‐2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19(7). 10.1111/acel.13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sun D, Li H, Lu X‐X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020;16(3):251‐259. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zimmermann P, Curtis N. COVID‐19 in children, pregnancy and neonates. Pediatr Infect Dis J. 2020;39(6):469‐477. 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;74‐98. 10.1128/CMR.00032-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mantovani A, Netea MG. Trained innate immunity, epigenetics, and Covid‐19. N Engl J Med. 2020;383(11):1078‐1080. 10.1056/nejmcibr2011679 [DOI] [PubMed] [Google Scholar]
- 69. Dijkman R, Jebbink MF, El Idrissi NB, et al. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol. 2008;46(7):2368‐2373. 10.1128/JCM.00533-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tosif S, Neeland MR, Sutton P, et al. Immune responses to SARS‐CoV‐2 in three children of parents with symptomatic COVID‐19. Nat Commun. 2020;11(1):1‐8. 10.1038/s41467-020-19545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID‐19: why children fare better than adults? Indian J Pediatr. 2020;1‐10. 10.1007/s12098-020-03322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tikellis C, Thomas MC. Angiotensin‐converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012. 10.1155/2012/256294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang L, He WB, Yu XM, Liu HF, Zhou WJ, Jiang H. Prognostic value of myocardial injury in patients with COVID‐19. Zhonghua Yan Ke Za Zhi. 2020;56:E009. 10.3760/cma.j.cn112148-20200313-00202 [DOI] [PubMed] [Google Scholar]
- 74. Zhao B‐C, Liu W‐F, Lei S‐H, et al. Prevalence and prognostic value of elevated troponins in patients hospitalised for coronavirus disease 2019: a systematic review and meta‐analysis. J Intensive Care. 2020;8(1):88. 10.1186/s40560-020-00508-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li BO, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;531‐538. 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Biswas I, Khan GA. Coagulation disorders in COVID‐19: role of toll‐like receptors J Inflamm Res. 2020;13:823‐828. 10.2147/JIR.S271768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wichmann D, Sperhake J‐P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID‐19: addressing a pharmacological challenge by targeting pathways triggered by SARS‐CoV‐2. Signal Transduct Target Ther. 2020;1‐10. 10.1038/s41392-020-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Klok FA, Kruip MJH, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cheng JL, Huang C, Zhang GJ, et al. Epidemiological characteristics of novel coronavirus pneumonia in Henan. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E027. 10.3760/cma.j.cn112147-20200222-00148 [DOI] [PubMed] [Google Scholar]
- 81. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020:e21. 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382(17):1653‐1659. 10.1056/nejmsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin‐converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15‐21. 10.1093/ajh/hpu086 [DOI] [PubMed] [Google Scholar]
- 84. Hippisley‐Cox J, Young D, Coupland C, et al. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503‐1511. 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pal R, Bhadada SK. COVID‐19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr. 2020;14(4):513‐517. 10.1016/j.dsx.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kassir R. Risk of COVID‐19 for patients with obesity. Obes Rev. 2020. 10.1111/obr.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pal R, Bhansali A. COVID‐19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020:108132. 10.1016/j.diabres.2020.108132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cure E, Cumhur CM. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID‐19 pandemic. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):349‐350. 10.1016/j.dsx.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020. 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dunn E, Grant P. Type 2 diabetes: an atherothrombotic syndrome. Curr Mol Med. 2005;5(3):323‐332. 10.2174/1566524053766059 [DOI] [PubMed] [Google Scholar]
- 92. Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623‐628. 10.1111/j.1464-5491.2006.01861.x [DOI] [PubMed] [Google Scholar]
- 93. Singh AK, Gupta R, Misra A. Comorbidities in COVID‐19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):283‐287. 10.1016/j.dsx.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Guan W‐J, Liang W‐H, Zhao YI, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5). 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA ‐ J Am Med Assoc. 2020;1775‐1776. 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 96. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020. 10.1111/liv.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Youssef M, Hussein M, Attia AS, et al. COVID‐19 and liver dysfunction: a systematic review and meta‐analysis of retrospective studies. J Med Virol. 2020. 10.1002/jmv.26055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1). 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Feng G, Zheng KI, Yan Q‐Q, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8(1):1‐7. 10.14218/jcth.2020.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Krenzelok EP. The FDA acetaminophen advisory committee meeting what is the future of acetaminophen in the United States the perspective of a committee member future of APAP in the United States. Clin Toxicol. 2009;784‐789. 10.1080/15563650903232345 [DOI] [PubMed] [Google Scholar]
- 102. Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Reports. 2020;2(3):100113. 10.1016/j.jhepr.2020.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID‐19: A comprehensive review. World J Gastroenterol. 2020;26(19):2323‐2332. 10.3748/wjg.v26.i19.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID‐19) and potential therapeutic targets. Clin Rheumatol. 2020:1. 10.1007/s10067-020-05073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Figueroa‐Parra G, Aguirre‐Garcia GM, Gamboa‐Alonso CM, Camacho‐Ortiz A, Galarza‐Delgado DA. Are my patients with rheumatic diseases at higher risk of COVID‐19? Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-217322 [DOI] [PubMed] [Google Scholar]
- 106. Sinha N, Balayla G. Hydroxychloroquine and covid‐19. Postgrad Med J. 2020. 10.1136/postgradmedj-2020-137785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhou D, Dai S‐M, Tong Q. COVID‐19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75(7):1667‐1670. 10.1093/jac/dkaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Emmi G, Bettiol A, Mattioli I, et al. SARS‐CoV‐2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020:102575. 10.1016/j.autrev.2020.102575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;532‐533. 10.1016/S2468-1253(20)30116-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;1083‐1088. 10.1016/j.kint.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Norsa L, Indriolo A, Sansotta N, Cosimo P, Greco S, D’Antiga L. Uneventful course in patients with inflammatory bowel disease during the severe acute respiratory syndrome coronavirus 2 outbreak in Northern Italy. Gastroenterology. 2020. 10.1053/j.gastro.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. An P, Ji M, Ren H, et al. Prevention of COVID‐19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol. 2020;525‐527. 10.1016/S2468-1253(20)30121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mazza S, Sorce A, Peyvandi F, Vecchi M, Caprioli F. A fatal case of COVID‐19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut. 2020. 10.1136/gutjnl-2020-321183 [DOI] [PubMed] [Google Scholar]
- 114. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID‐19 in 79 patients with IBD in Italy: an IG‐IBD study. Gut. 2020. 10.1136/gutjnl-2020-321411 [DOI] [PubMed] [Google Scholar]
- 115. Lam CM, Wong SF, Leung TN, et al. A case‐controlled study comparing clinical course and outcomes of pregnant and non‐pregnant women with severe acute respiratory syndrome. BJOG An Int J Obstet Gynaecol. 2004;111(8):771‐774. 10.1111/j.1471-0528.2004.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Liu H, Wang LL, Zhao SJ, Kwak‐Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID‐19? an immunological viewpoint. J Reprod Immunol. 2020:103122. 10.1016/j.jri.2020.103122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Aghaeepour N, Ganio EA, Mcilwain D, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2(15). 10.1126/sciimmunol.aan2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID‐19) pneumonia: a preliminary analysis. Am J Roentgenol. 2020;215(1):127‐132. 10.2214/AJR.20.23072 [DOI] [PubMed] [Google Scholar]
- 120. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Muhidin S, Behboodi MZ, Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019‐nCoV; a systematic review. Arch Acad Emerg Med. 2020;8(1):e49. 10.22037/aaem.v8i1.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020. 10.1111/aogs.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status — United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769‐775. 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26(6):1335‐1336. 10.3201/eid2606.200287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Butler MJ, Barrientos RM. The impact of nutrition on COVID‐19 susceptibility and long‐term consequences. Brain Behav Immun. 2020. 10.1016/j.bbi.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Smoking and COVID‐19. Available from: https://www.who.int/news‐room/commentaries/detail/smoking‐and‐covid‐19 [accessed February 24, 2021]
- 128. Patanavanich R, Glantz SA. Smoking is associated with COVID‐19 progression: a meta‐analysis. Nicotine Tob Res. 2020;1653‐1656. 10.1093/ntr/ntaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12(1). 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chu CM, Poon LLM, Cheng VCC, et al. Initial viral load and the outcomes of SARS. CMAJ. 2004;171(11):1349‐1352. 10.1503/cmaj.1040398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ng EKO, Hui DS, Chan KCA, et al. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49(12):1976‐1980. 10.1373/clinchem.2003.024125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta‐analysis. The Lancet Microbe. 2020. 10.1016/s2666-5247(20)30172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically Ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(8):1937‐1942. 10.1093/cid/ciaa449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pujadas E, Chaudhry F, McBride R, Richter F, Zhao S, Wajnberg A, et al. SARS‐CoV‐2 viral load predicts COVID‐19 mortality. 2020. 10.1002/jmv.25988 [DOI] [PMC free article] [PubMed]
- 135. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA ‐ J Am Med Assoc. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742‐1752. 10.1111/all.14309 [DOI] [PubMed] [Google Scholar]
- 137. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Xu X‐W, Wu X‐X, Jiang X‐G, et al. novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2019;2020:368. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wan S, Xiang YI, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797‐806. 10.1002/jmv.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380‐1388. 10.1164/rccm.202002-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhou Y, He Y, Yang H, et al. Development and validation a nomogram for predicting the risk of severe COVID‐19: a multi‐center study in Sichuan, China. PLoS One. 2020;15(5):e0233328. 10.1371/journal.pone.0233328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Huang Q, Deng X, Li Y, et al. Clinical characteristics and drug therapies in patients with the common‐type coronavirus disease 2019 in Hunan, China. Int J Clin Pharm. 2020;42(3):837‐845. 10.1007/s11096-020-01031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Huang R, Zhuid L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu Province, China: a retrospective, multi‐center study. PLoS Negl Trop Dis. 2020;14(5):1‐14. 10.1371/journal.pntd.0008280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhang R, Ouyang H, Fu L, et al. CT features of SARS‐CoV‐2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol. 2020;30(8):4417‐4426. 10.1007/s00330-020-06854-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. 10.1016/j.jcv.2020.104364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen C‐P, Lin Y‐C, Chen T‐C, et al. A multicenter, randomized, open‐label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID‐19). MedRxiv. 2020:2020.07.08.20148841. 10.1101/2020.07.08.20148841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Du Rong‐Hui C, Liu L‐M, Yin W, Wang W, Guan L‐L, Yuan M‐L, et al. Hospitalization and critical care of 109 decedents with COVID‐19 pneumonia in Wuhan 2020. 10.1513/AnnalsATS.202003-225OC [DOI] [PMC free article] [PubMed]
- 149. Zhang J‐J, Dong X, Cao Y‐Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 150. Wei Y‐Y, Wang R‐R, Zhang D‐W, et al. Risk factors for severe COVID‐19: evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020. 10.1016/j.jinf.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zachariah P, Johnson CL, Halabi KC, et al. (COVID‐19) in a children’s hospital in New York city, New York. JAMA Pediatr. 2019;2020:E202430. 10.1001/jamapediatrics.2020.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol. 2020;92(10):2055‐2066. 10.1002/jmv.25966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Liu F, Li L, Xu MDA, et al. Prognostic value of interleukin‐6, C‐reactive protein, and procalcitonin in patients with COVID‐19. J Clin Virol. 2020;127:104370. 10.1016/j.jcv.2020.104370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Buckner FS, McCulloch DJ, Atluri V, et al. Clinical features and outcomes of 105 hospitalized patients with COVID‐19 in Seattle, Washington. Clin Infect Dis. 2020. 10.1093/cid/ciaa632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Pan L, Mu MI, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766‐773. 10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
All data sets, on which the conclusions of the manuscript rely, are presented in the main paper and the supplementary Table.


