PURPOSE
In men with localized prostate cancer, the addition of androgen-deprivation therapy (ADT) or a brachytherapy boost (BT) to external beam radiotherapy (EBRT) have been shown to improve various oncologic end points. Practice patterns indicate that those who receive BT are significantly less likely to receive ADT, and thus we sought to perform a network meta-analysis to compare the predicted outcomes of a randomized trial of EBRT plus ADT versus EBRT plus BT.
MATERIALS AND METHODS
A systematic review identified published randomized trials comparing EBRT with or without ADT, or EBRT (with or without ADT) with or without BT, that reported on overall survival (OS). Standard fixed-effects meta-analyses were performed for each comparison, and a meta-regression was conducted to adjust for use and duration of ADT. Network meta-analyses were performed to compare EBRT plus ADT versus EBRT plus BT. Bayesian analyses were also performed, and a rank was assigned to each treatment after Markov Chain Monte Carlo analyses to create a surface under the cumulative ranking curve.
RESULTS
Six trials compared EBRT with or without ADT (n = 4,663), and 3 compared EBRT with or without BT (n = 718). The addition of ADT to EBRT improved OS (hazard ratio [HR], 0.71 [95% CI, 0.62 to 0.81]), whereas the addition of BT did not significantly improve OS (HR, 1.03 [95% CI, 0.78 to 1.36]). In a network meta-analysis, EBRT plus ADT had improved OS compared with EBRT plus BT (HR, 0.68 [95% CI, 0.52 to 0.89]). Bayesian modeling demonstrated an 88% probability that EBRT plus ADT resulted in superior OS compared with EBRT plus BT.
CONCLUSION
Our findings suggest that current practice patterns of omitting ADT with EBRT plus BT may result in inferior OS compared with EBRT plus ADT in men with intermediate- and high-risk prostate cancer. ADT for these men should remain a critical component of treatment regardless of radiotherapy delivery method until randomized evidence demonstrates otherwise.
INTRODUCTION
For men with unfavorable intermediate- and high-risk prostate cancer, external beam radiotherapy (EBRT) alone has been shown to be inferior to combination treatment approaches for various end points. Two methods of treatment intensification for men treated with EBRT have been assessed in multiple independent randomized clinical trials: the addition of androgen-deprivation therapy (ADT) or the addition of a brachytherapy boost.1-11 The addition of ADT to EBRT has been shown in multiple randomized trials to reduce local treatment failure, improve biochemical control, reduce the incidence of metastases, and improve overall survival (OS) in men with intermediate- and high-risk prostate cancer.1-3,5,7,9 The addition of a brachytherapy boost to EBRT serves as a form of local treatment intensification and has been shown to reduce local recurrence and improve biochemical control in multiple randomized trials, but not to significantly reduce the development of metastatic disease or improve survival.4,6,8,10
CONTEXT
Key Objectives
To compare the predicted outcomes of external beam radiotherapy plus androgen deprivation therapy to external beam radiotherapy plus a brachytherapy boost in men with intermediate and high-risk prostate cancer.
Knowledge Generated
We demonstrate with high probability that external beam radiation therapy plus androgen deprivation therapy results in improved survival compared to external beam radiation therapy plus a brachytherapy boost in men with intermediate and high-risk prostate cancer.
Relevance
Androgen deprivation therapy should not be omitted on the basis of available evidence in men with intermediate- or high-risk prostate cancer receiving external beam radiation therapy plus a brachytherapy boost.
It has been hypothesized that with radiotherapeutic dose escalation, the benefit of ADT would be abrogated. However, in EORTC 22991, ADT showed a similar benefit among all patients, independent of the radiation dose delivered (interaction P value = .82).1 Whether ADT provides the same benefits in the setting of extreme dose escalation with the addition of a brachytherapy boost has never been tested.12 Despite the lack of evidence supporting the omission of ADT in men with intermediate- and high-risk prostate cancer receiving EBRT plus a brachytherapy boost, practice patterns demonstrate that receipt of brachytherapy is independently associated with omission of ADT. In both intermediate- and high-risk prostate cancer, there is a 30% to 40% decreased rate of ADT use in these men.13,14
The decreased use of ADT in men treated with EBRT plus a brachytherapy boost suggests that physicians may feel that only one form of treatment intensification is needed in addition to EBRT in men with intermediate- and high-risk prostate cancer. The addition of ADT or a brachytherapy boost to EBRT has never been directly compared in a clinical trial, and which form of treatment intensification is superior remains unclear. We therefore sought to perform a network meta-analysis to compare the predicted outcome of EBRT plus ADT versus EBRT plus a brachytherapy boost to help guide treatment decision making in men with intermediate- and high-risk prostate cancer.
MATERIALS AND METHODS
Search Strategy and Study Selection
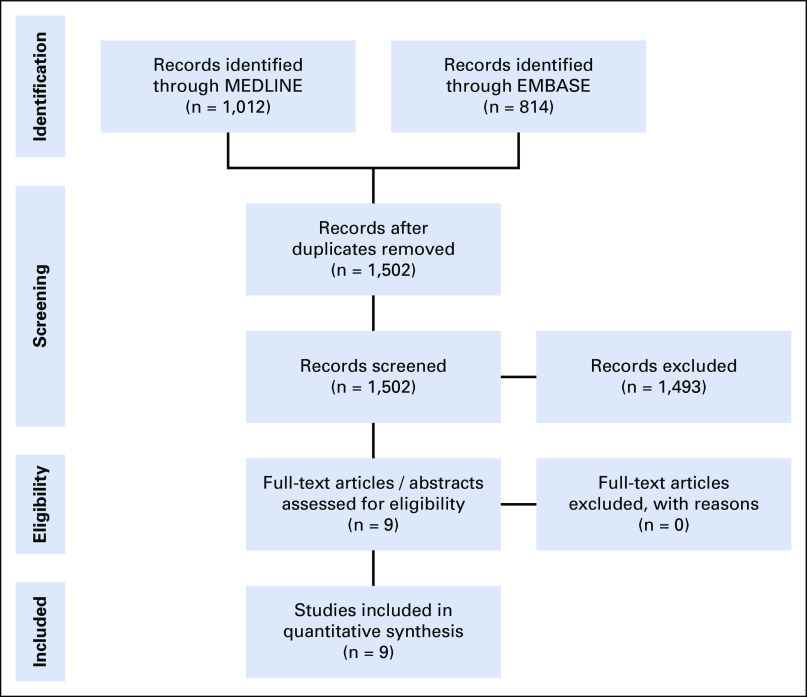
A systematic review was performed of published randomized trials comparing EBRT with or without ADT, as well as trials assessing EBRT (with or without ADT) with or without a brachytherapy boost. This was in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.15 To be included, trials were required to have reported on biochemical recurrence-free survival (bRFS) and OS and to have been published in English between January 1980 and June 2018. Metastasis-free survival data were not collected because they were not reported consistently among the brachytherapy boost trials. The search was performed using the EMBASE and MEDLINE electronic databases. A PRISMA flow diagram can be found in Figure 1, and a basic diagram of the network meta-analysis performed can be found in the Data Supplement (online only).
FIG 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Data Extraction and End Points
Data were extracted from the 9 identified randomized trials by W.C.J and D.E.S. Trial level data extracted included the years the trial accrued, median follow-up, patient age, National Comprehensive Cancer Network risk group, clinical T stage, pretreatment prostate-specific antigen, Gleason score, duration of ADT if administered, and hazard ratios (HRs) with corresponding 95% CIs for bRFS and OS.
Statistical Methods
Weighted descriptive statistics were calculated using study sample size as the weight. HRs for the randomized brachytherapy boost trial reported by Hoskin et al6 were estimated from Kaplan-Meier curves using methods from Tierney et al.16 The standard error of the log HRs was estimated from the log-rank P value provided and the estimated HRs. Data were extracted from the Kaplan Meier curve using WebPlotDigitizer17 using the “X Step w/ Interpolation” setting, and points were extracted along regular intervals for each curve. With the extracted data, median follow-up, recruitment time, and number of patients in each arm, the HR was estimated.16 Spearman correlation was calculated for ADT duration and log(HRs), thus examining the correlation between the value ranks so that trends could be nonlinear. HRs for the meta-analysis where only indirect comparisons were available were calculated using the consistency equation (where θ is a log HR estimate).18 To perform a network meta-analysis, the consistency equation is assumed to be true. Frequentist analysis using the meta package in R version 3.4.2 was performed. A generic inverse variance meta-analysis with fixed effects was used to estimate the HR for EBRT plus ADT versus EBRT alone and the HR for EBRT plus a brachytherapy boost versus EBRT alone. We also conducted a meta-regression using an exponential model of ADT effect to adjust for ADT use and duration. We used the consistency equation to compare EBRT plus ADT with EBRT plus a brachytherapy boost. Cochran’s Q test was used to examine heterogeneity.
Bayesian analyses were performed using rjags for network meta-analysis with fixed effects and a continuous outcome (log[HR] as the outcome).18,19 Regression using an exponential model of ADT effect to adjust for ADT duration was performed. We ran a Markov Chain Monte Carlo with 20,000 burn-in iterations and 20,000 iterations for each analysis. Autocorrelation was checked graphically. The consistency equation was used to estimate the HR for EBRT plus ADT versus EBRT plus a brachytherapy boost. The median was used as the sample estimate, and the 2.5th percentile and 97.5th percentile were used for the credible interval. The probability of the estimated HR being less than 1 is the percentage of the 20,000 iterations that were below 1.
For each iteration, the ranking of EBRT alone, EBRT plus ADT, and EBRT plus a brachytherapy boost was determined using the HRs from that iteration. Surface under the cumulative ranking curve (SUCRA) was calculated from these rankings20 by summing the cumulative probabilities of all the ranks divided by the number of ranks minus 1. This statistic has no known distribution and is a means of summarizing treatment rankings. If a treatment always ranks 1, with a value of 1 denoting the most preferred treatment, then the SUCRA = 1. If a treatment always ranks last, then the SUCRA = 0, with a value of 0 denoting the least preferred treatment.
We estimated the sample sizes necessary to demonstrate statistical differences in OS between treatment modalities using a sample size equation for one arm:
where are the standard normal cumulative functions for or the type I error and power specified, is the event rate, and is the log HR.
Cumulative incidence curves were estimated by extracting the percentage of survival values for each year from the EBRT plus ADT Kaplan-Meier curve from EORTC 229911 using WebPlotDigitizer. Cumulative incidence was calculated as 1% survival. A locally weighted scatter-plot smoother curve was fit over the cumulative incidence points using the stat package included in the base packages in R version 3.4.2.
RESULTS
Trial and Patient Characteristics
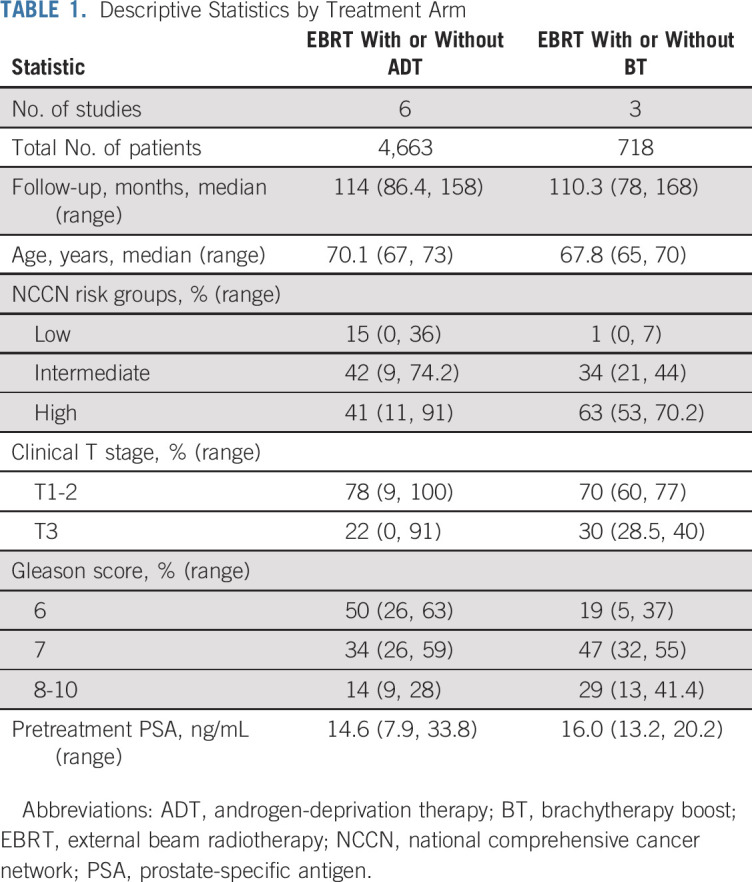
Six trials were identified that compared EBRT with or without ADT in 4,663 men, and three trials were identified that compared EBRT with or without a brachytherapy boost in 718 men. The Data Supplement lists the included trials, with summary statistics for the included trials and descriptive statistics by treatment in Table 1. The majority of all men included had intermediate- or high-risk prostate cancer (84%). In two of the trials assessing the addition of a brachytherapy boost to EBRT, patients in both arms received ADT, either systematically in ASCENDE-RT or nonsystematically in the trial reported by Hoskin et al.6,8
TABLE 1.
Descriptive Statistics by Treatment Arm
Network Meta-Analysis
In the meta-analysis of EBRT plus ADT versus EBRT alone, the Cochran’s Q test for heterogeneity was significant without adjustment for ADT duration. After adjusting for ADT duration, this was no longer significant. In an analysis of trials comparing EBRT plus a brachytherapy boost versus EBRT alone, the test for heterogeneity was not significant, and as such, no adjustment for ADT use or duration was made (Cochran’s Q = 2.61; P = .3). Compared with EBRT alone, the addition of ADT to EBRT improved OS (HR, 0.70; 95% CI, 0.62 to 0.81). Although this HR estimate is independent of baseline risk group, as a sensitivity analysis we repeated the analysis in the subset of randomized trials treating mostly men with high-risk prostate cancer (RTOG 8610, EORTC 22863, and TROG 9601) as well as in the remaining trials that were mostly men with intermediate-risk prostate cancer. This resulted in similar estimates of the HR for EBRT alone versus EBRT plus ADT (high-risk: HR, 0.66; 95% CI, 0.38 to 1.15; intermediate-risk: HR, 0.73; 95% CI, 0.51 to 1.04, with a P value of .74 for comparison of HRs). Conversely, compared with EBRT (with or without ADT), the addition of a brachytherapy boost to EBRT did not significantly improve OS (HR, 0.94; 95% CI, 0.70 to 1.27).
In a network meta-analysis using fixed-effects modeling, EBRT plus ADT was associated with improved OS compared with EBRT plus a brachytherapy boost (HR, 0.68; 95% CI, 0.52 to 0.89; Fig 2). Network meta-analysis findings were similar when only the brachytherapy boost trial reported by Sathya et al,10 the only brachytherapy boost trial to exclude the use of ADT, was included. A cumulative incidence curve of overall mortality using our calculated HR (0.68) comparing EBRT plus ADT to EBRT plus a brachytherapy boost assuming proportional hazards can be found in the Data Supplement, with the EBRT plus ADT curve estimated from EORTC 22991.1 The estimated 10-year cumulative incidence of overall mortality was 28% for EBRT plus ADT versus 41% for EBRT plus a brachytherapy boost.
FIG 2.
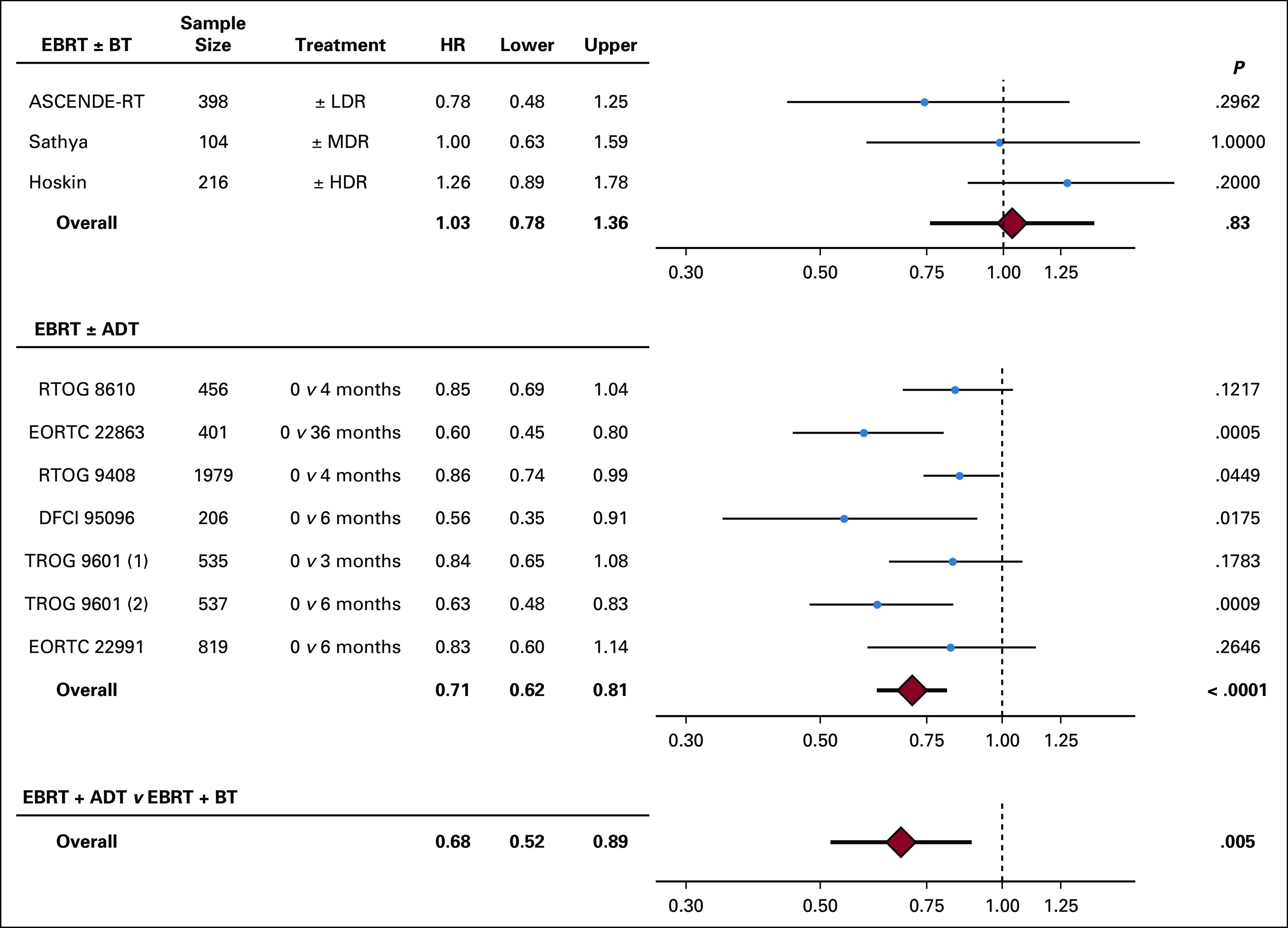
Network meta-analysis of overall survival.6,10 Meta-analysis from individual trials for overall survival for external beam radiotherapy (EBRT) with or without brachytherapy boost (BT) and EBRT with or without androgen-deprivation therapy (ADT), as well as results from fixed-effects network meta-analysis comparing EBRT plus ADT with EBRT plus BT. HDR, high-dose rate; HR, hazard ratio; LDR, low-dose rate; MDR, medium-dose rate.
Given the estimated magnitude of the OS benefit of EBRT plus ADT compared with EBRT plus a brachytherapy boost, we estimated the number of men that would be required to be randomly assigned between these two treatments to have 80% power to detect an OS benefit of this magnitude in an attempt to inform future clinical trial design. Assuming an α of 0.05, an estimated 832 men would need to be randomly assigned to have 80% power to demonstrate a statistically significant difference in OS between these two treatment modalities, assuming a 20% event rate.
Bayesian Modeling
We next sought to compare EBRT plus ADT with EBRT plus a brachytherapy boost using Bayesian modeling. With this approach, there was an 88% probability that EBRT plus ADT would result in superior OS compared with EBRT plus a brachytherapy boost. Plots of the distributions of HRs from the Bayesian analysis can be found in the Data Supplement.
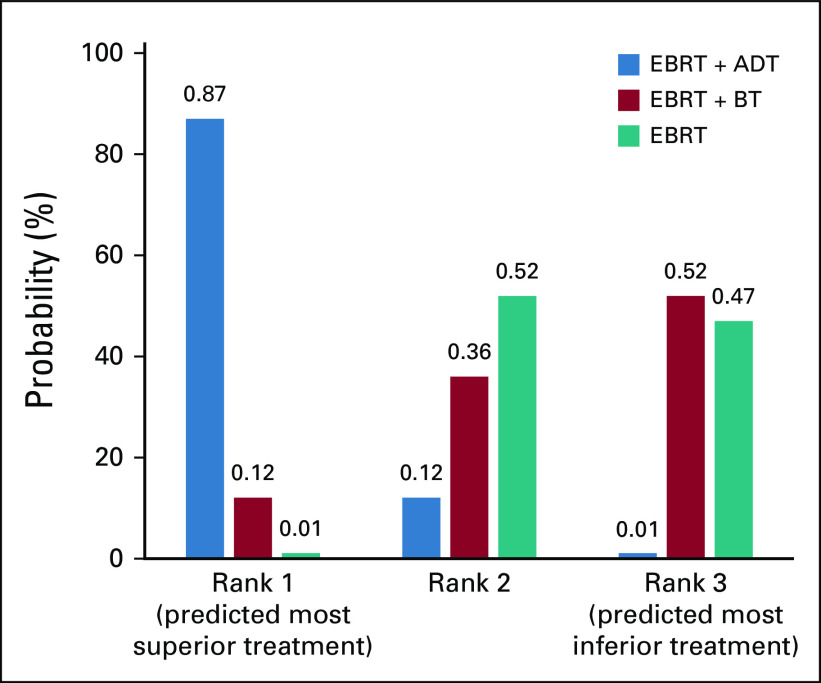
Last, we ranked the probability that each treatment option (EBRT alone, EBRT plus brachytherapy boost, and EBRT plus ADT) resulted in superior OS using the SUCRA. The SUCRA for EBRT alone, EBRT plus a brachytherapy boost, and EBRT plus ADT were 27%, 30%, and 90%, respectively, for OS. Overall, with this approach, there was an 87% probability that EBRT plus ADT ranked first in terms of superior OS outcomes among the three treatment methods (Fig 3). All bRFS analyses can be found in the Data Supplement.
FIG 3.
Predicted treatment rankings for overall survival. ADT, androgen deprivation therapy; BT, brachytherapy boost; EBRT, external beam radiotherapy.
DISCUSSION
Herein, we demonstrate a high probability that in men with mostly intermediate- and high-risk prostate cancer treated with EBRT the addition of ADT results in superior OS compared with the addition of a brachytherapy boost. As such, if only these two options are considered, our findings suggest that the addition of ADT provides a greater oncologic benefit than a brachytherapy boost. This said, recent evidence from ASCENDE-RT8 suggests that, compared with EBRT with ADT, the addition of a brachytherapy boost further improves biochemical and local control. Thus, if a brachytherapy boost is to be used for intermediate- or high-risk disease, our data suggest that the addition of ADT is required to prevent inferior survival compared with EBRT with ADT alone. On the basis of our findings, there is currently insufficient evidence to support the omission of ADT in men with intermediate- or high-risk prostate cancer treated with any form of definitive radiation therapy, including brachytherapy.
A recent review from the American Brachytherapy Society outlined the lack of clinical trials and the paucity of data assessing the benefit of ADT with brachytherapy.12 Retrospective studies do not demonstrate a benefit from the addition of ADT to brachytherapy.12 However, many retrospective or observational studies also show no benefit of adding ADT to EBRT, in stark contrast to multiple randomized controlled trials. Despite the lack of quality data to omit ADT with brachytherapy, one of the strongest factors that predicts the omission of ADT in intermediate- and high-risk prostate cancer is the concurrent use of brachytherapy.14 Many have advocated that the use of brachytherapy can spare patients from ADT or shorten ADT duration, despite the lack of evidence for this approach. Our data question the validity of this practice and suggest that the use of EBRT plus ADT provides a high probability of superior OS in comparison with EBRT plus a brachytherapy boost without ADT.
To the best of our knowledge, only two small randomized trials have assessed the role of ADT in men treated with brachytherapy, one assessing 3 months of neoadjuvant ADT before low-dose rate monotherapy, and the second assessing combination EBRT plus a high-dose rate brachytherapy boost with or without ADT, with the duration of ADT not reported.21,22 Both trials have only been published in abstract form, and neither was powered to assess the impact of ADT on metastasis-free survival or OS.21,22 As such, these trials will not elucidate the role of ADT for men receiving either brachytherapy as monotherapy or as a boost. In addition, although the yet to be reported RTOG 0815, a randomized trial of dose-escalated radiation therapy with or without short-term ADT, allowed for men to receive a brachytherapy boost as a component of their treatment, this trial was not powered to assess the effect of ADT solely in men receiving a brachytherapy boost. Thus, there is a great need for trials to be performed to understand the role of brachytherapy with the use of ADT in contemporary practice and with the potential integration of newer systemic treatment options.
Distant failure from pre-existing micrometastatic disease, which will not be eradicated through local treatment intensification, remains an important mode of failure for high-risk men. Thus, one plausible hypothesis for the etiology of our findings is that although the addition of a brachytherapy boost enhances local eradication of disease, ADT has a greater survival impact by sterilizing both local and distant sites. Multimodality approaches are likely necessary for optimal outcomes. This can be appreciated in ASCENDE-RT. Despite improved bRFS with the addition of a brachytherapy boost to EBRT plus ADT, rates of distant metastases were nearly identical, driven by similar rates in both arms of early metastases.8 Importantly, this trial did not use long-term adjuvant ADT, which may have prevented or delayed the time to develop these early metastatic events. Conversely, nearly all trials included in this analysis assessing the addition of ADT to EBRT demonstrated a reduction in the development of distant metastases. On the basis of the available evidence, there is clearly a need for ADT or other forms of systemic treatment intensification, even in the context of effective local therapies.
Our analyses have limitations. First, we are limited by the number and sample size of randomized trials assessing the addition of a brachytherapy boost to EBRT. Furthermore, we were unable to control for total dose delivered in our analysis. Two of the three brachytherapy trials used radiation treatment doses that are lower than those currently used in clinical practice. Nonetheless, these are the only randomized data that currently exist to support this treatment method, and as such, they form the basis for the role of a brachytherapy boost in the treatment of men with intermediate- and high-risk prostate cancer. In addition, we were unable to meaningfully compare treatment-related toxicity between EBRT plus ADT and EBRT plus a brachytherapy boost, given the nonoverlapping toxicity profiles of these two forms of treatment intensification. Future trials comparing ADT and brachytherapy boost should include patient-reported quality of life as a secondary outcome. Another limitation is that we were unable to assess the impact of ADT or a brachytherapy boost on distant metastases, because the brachytherapy boost trial reported by Hoskin et al6 reported only bRFS and OS. Last, two of the three randomized trials of EBRT with or without a brachytherapy boost either selectively or uniformly included ADT. In the randomized trial from the United Kingdom reported by Hoskin et al,6 patients nonsystematically received neoadjuvant ADT followed by an additional 6 to 36 months of ADT, with 76% of men receiving ADT. ADT was administered to men with more aggressive disease, likely accounting for the finding in the multivariable analysis that ADT use predicted for worse bRFS. Conversely, all men in the ASCENDE-RT trial were to receive ADT; however, the timing and duration of ADT were nonstandard. ADT was given neoadjuvantly for 8 months, and then all men received an additional 4 months of ADT with and after their radiation therapy. As such, we are unable to provide insight on the potential benefits or appropriate duration of ADT in men treated with EBRT plus a brachytherapy boost. It is unlikely that the use of ADT in two of the three trials assessing the use of a brachytherapy boost confounded our findings because there was no heterogeneity among the 3 trials, although the power to detect heterogeneity is likely low. Furthermore, the results from the trial reported by Sathya et al,10 the only brachytherapy boost trial to exclude ADT, were similar to the meta-analyzed results of the three brachytherapy boost trials, and the network-meta analysis findings were similar when excluding the two brachytherapy boost trials in which patients received ADT.
In our analysis of men with intermediate- or high-risk prostate cancer, the addition of ADT to EBRT seems to improve survival, whereas the addition of a brachytherapy boost did not, and this remained true on meta-analysis. Our findings demonstrate that there is a high probability that EBRT plus ADT provides superior OS compared with EBRT plus a brachytherapy boost in men with intermediate- and high-risk prostate cancer. Our study suggests that ADT should not be omitted on the basis of available evidence in men with intermediate- or high-risk prostate cancer receiving EBRT plus a brachytherapy boost. Randomized trials are needed if the omission of ADT is to be recommended with EBRT and a brachytherapy boost.
SUPPORT
Supported in part by National Institutes of Health Grant T32 CA-083654, the Prostate Cancer Foundation (D.E.S.), P50 CA186786 (D.E.S.), and generous philanthropic gifts from patients.
W.C.J. and H.E.H. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: William C. Jackson, Robert T. Dess, Payal D. Soni, Amar U. Kishan, Rohit Mehra, Peter J. Hoskin, Daniel E. Spratt
Financial support: Daniel E. Spratt
Administrative support: Daniel E. Spratt
Provision of study material or patients: Mack Roach III
Collection and assembly of data: William C. Jackson, Holly E. Hartman, Sam R. Birer, Payal D. Soni, Daniel A. Krauss, Howard M. Sandler, Peter J. Hoskin, Daniel E. Spratt
Data analysis and interpretation: William C. Jackson, Holly E. Hartman, Robert T. Dess, Payal D. Soni, Jason W. D. Hearn, Zachary R. Reichert, Amar U. Kishan, Brandon A. Mahal, Zachary S. Zumsteg, Jason A. Efstathiou, Samuel Kaffenberger, Todd M. Morgan, Timothy N. Showalter, Paul L Nguyen, Matthew J. Schipper, Felix Y. Feng, Peter J. Hoskin, Mack Roach III, Daniel E. Spratt
Manuscript writing All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Addition of Androgen-Deprivation Therapy or Brachytherapy Boost to External Beam Radiotherapy for Localized Prostate Cancer: A Network Meta-Analysis of Randomized Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Zachery R. Reichert
Consulting or Advisory Role: Dendreon
Research Funding: AstraZeneca (Inst)
Amar U. Kishan
Honoraria: Varian Medical Systems, ViewRay
Consulting or Advisory Role: Janssen
Zachary S. Zumsteg
Consulting or Advisory Role: EMD Serono, Scripps Proton Therapy Center
Other Relationship: King and Spalding LLP (I)
Jason A. Efstathiou
Consulting or Advisory Role: Blue Earth Diagnostics, Taris BioMedical, Janssen, AstraZeneca, Boston Scientific
Samuel Kaffenberger
Consulting or Advisory Role: MDxHealth, Clovis Oncology
Travel, Accommodations, Expenses: BMS
Todd M. Morgan
Consulting or Advisory Role: Myriad Genetics, TerumoBCT
Research Funding: Myriad Genetics (Inst), MDxHealth (Inst), GenomeDx (Inst)
Timothy N. Showalter
Research Funding: Varian Medical Systems
Paul L. Nguyen
Stock and Other Ownership Interests: Volatilyx (I)
Stock and Other Ownership Interests: Augmenix
Consulting or Advisory Role: Medivation, GenomeDx, Ferring, Nanobiotix, Dendreon, Augmenix, GigaGen (I), Biogen (I), Bayer, Astellas Pharma, Blue Earth Diagnostics, Coda, Boston Scientific, Janssen Oncology
Research Funding: Astellas Pharma, Nanobiotix, Janssen, Wako Diagnostics (I), Bayer
Patents, Royalties, Other Intellectual Property: Wife has a patent on volatile diagnostics of infections (I)
Travel, Accommodations, Expenses: Ferring
Matthew J. Schipper
Consulting or Advisory Role: Innovative Analytics
Felix Y. Feng
Leadership: PFS Genomics
Stock and Other Ownership Interests: PFS Genomics, Nutcracker Therapeutics, SerImmune
Honoraria: Genentech
Consulting or Advisory Role: Bayer, Blue Earth Diagnostics, Celgene, Medivation/Astellas, Sanofi Genzyme, EMD Serono, Janssen Biotech
Research Funding: Zenith Epigenetics
Patents, Royalties, Other Intellectual Property: I helped develop a molecular signature to predict radiation resistance in breast cancer, and this signature was patented by the University of Michigan, my employer; it is in the process of being licensed to PFS Genomics, a company that I helped found (Inst)
Howard M. Sandler
Stock and Other Ownership Interests: Radiogel
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
Peter J. Hoskin
Research Funding: Varian Medical Systems (Inst), Astellas Pharma (Inst), Bayer (Inst)
Travel, Accommodations, Expenses: Eleka
Mack Roach III
Honoraria: Bayer, Blue Earth Diagnostics, Ferring, Myriad Genetics, Tolmar
Consulting or Advisory Role: Ferring, Myriad Genetics, Bayer, Blue Earth Diagnostics, Accuray, Tolmar, Noxopharm, Genomic Health, Janssen
Expert Testimony: Ferring
Travel, Accommodations, Expenses: Bayer, Ferring, Myriad Genetics, Blue Earth Diagnostics, Tolmar
Other Relationship: TYME
Daniel F. Spratt
Consulting or Advisory Role: Blue Earth Diagnostics, Janssen Oncology, AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bolla M, Maingon P, Carrie C, et al. : Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC Trial 22991. J Clin Oncol 34:1748-1756, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Van Tienhoven G, Warde P, et al. : External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11:1066-1073, 2010 [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Chen MH, Renshaw A, et al. : Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA 314:1291-1293, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Dayes IS, Parpia S, Gilbert J, et al. : Long-term results of a randomized trial comparing iridium implant plus external beam radiation therapy with external beam radiation therapy alone in node-negative locally advanced cancer of the prostate. Int J Radiat Oncol Biol Phys 99:90-93, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Denham JW, Steigler A, Lamb DS, et al. : Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12:451-459, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Hoskin PJ, Rojas AM, Bownes PJ, et al. : Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 103:217-222, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Jones CU, Hunt D, McGowan DG, et al. : Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365:107-118, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Morris WJ, Tyldesley S, Rodda S, et al. : Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 98:275-285, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Roach M, III, Bae K, Speight J, et al. : Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol 26:585-591, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Sathya JR, Davis IR, Julian JA, et al. : Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 23:1192-1199, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Dess RT, Soni PD, Jackson WC, et al. : The current state of randomized clinical trial evidence for prostate brachytherapy. Urol Oncol 37:599-610, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Keyes M, Merrick G, Frank SJ, et al. : American Brachytherapy Society Task Group Report: Use of androgen deprivation therapy with prostate brachytherapy-A systematic literature review. Brachytherapy 16:245-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YW, Muralidhar V, Mahal BA, et al. : Factors associated with the omission of androgen deprivation therapy in radiation-managed high-risk prostate cancer. Brachytherapy 15:695-700, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Mohiuddin JJ, Narayan V, Venigalla S, et al. : Variations in patterns of concurrent androgen deprivation therapy use based on dose escalation with external beam radiotherapy vs. brachytherapy boost for prostate cancer. Brachytherapy 18:322-331, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339:b2700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, et al. : Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WebPlotDigitizer. https://automeris.io/WebPlotDigitizer.
- 18.Greco T, Landoni G, Biondi-Zoccai G, et al. : A Bayesian network meta-analysis for binary outcome: How to do it. Stat Methods Med Res 25:1757-1773, 2016 [DOI] [PubMed] [Google Scholar]
- 19. Jonas DE, Wilkins TM, Bangdiwala S, et al (eds): AHRQ Methods for Effective Health Care, in Findings of Bayesian Mixed Treatment Comparison Meta-Analyses: Comparison and Exploration Using Real-World Trial Data and Simulation. Rockville, MD, Agency for Healthcare Research and Quality, 2013, Appendix A-1 to A-2. [PubMed] [Google Scholar]
- 20.Salanti G, Ades AE, Ioannidis JP: Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol 64:163-171, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Cui X, Li Q, Xu JJ, et al. : Application of neoadjuvant hormonal therapy in (125)I permanent seed implantation for prostate cancer [in Chinese]. Zhonghua Yi Xue Za Zhi 92:2710-2712, 2012 [PubMed] [Google Scholar]
- 22.García Blanco A, Anchuelo Latorre J, Payá Barceló G, et al. : Brachytherapy in localized prostate cancer with or without androgen deprivation. Rep Pract Oncol Radiother 18:S142, 2013 [Google Scholar]