Abstract
Background
Menarche is an important milestone in a feminine reproductive life, and regular menstrual cycles reflect normal functioning of the hypothalamic-pituitary-ovarian axis, a vital sign of women’s general health.
Aim of the study
We explored the age at menarche and the following menstrual cycles characteristics among 85 unmarried Transfusion-Dependent β-Thalassemia (TDT) women, born between 1965 and 1995, concerning iron chelation therapy (ICT) with desferrioxamine (DFO) and nutritional status, assessed by body mass index (BMI).
Results
53 adolescents who had begun ICT before the age of 10 years experienced menarche at 13,7 ± 1,6 years (mean ± DS), whereas 32 who began treatment after ten years experienced menarche significantly later (15.5 ± 1.9 yrs; p: 0.001). At the age of menarche: BMI-Z score (n= 67, −0,09 ±1) was inversely correlated with both age at starting ICT (r = −0,39; p = 0001) and age at menarche (−0,45, p = 0,0001). Serum ferritin levels (SF) were significantly correlated with the age at starting chelation therapy (n = 79; r = 0,34; p = 0,022). In 56 TDT adolescents who developed secondary amenorrhea (SA), the SF levels were significantly higher (4,098 ± 1,907 ng/mL) compared to 23 TDT adolescents with regular menstrual cycles (2,913±782 ng/mL; p = 0,005). Nutritional status of “thinness” at menarche was associated with a lower prevalence of subsequent regular menstrual cycles and a higher prevalence of early SA.
Conclusion
An early ICT in TDT patients was associated with a normal “tempo” of pubertal onset and a higher frequency of subsequent regular menstrual cycles. In TDT patients, who developed SA, a diagnosis of acquired central hypogonadism was made, mainly due to the chronic exposure to iron overload, however other potential causes linked to nutritional status, deficient levels of circulating nutrients, and the chronic disease itself cannot be fully excluded.
Keywords: Transfusion-dependent β-thalassemia, Menarche, Menstrual cycles, Secondary amenorrhea, Iron overload, Nutritional status
Introduction
The hemoglobinopathies (mainly thalassemias and sickle-cell disease) are the most frequent inherited genetic disorders worldwide, with some 300,000 infants born annually with major hemoglobinopathies. Approximately 80% of these births occur in low- and middle-income countries where the control and management of hemoglobinopathies are meager.1 At present, thalassemia diseases are classified into transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia (NTDT).2 Current treatment of TDT consists of regular transfusions that lead to iron overload, requiring iron chelation to prevent iron-related organ toxicity. Iron overload-related complications include involvement of the heart, liver, and endocrine glands.3
Iron accumulates at different rates in various organs, and these organs show different susceptibility to the damage induced by reactive iron species such as non-transferrin bound iron (NTBI) and the intracellular labile iron pool (LIP).4–6
In clinical practice, serum ferritin (SF) measurement and non-invasive imaging techniques are available to diagnose iron overload, quantify its extent in different organs, and monitor clinical response to therapy.
Three iron chelators (ICT) are currently available to treat iron overload: desferrioxamine (DFO), deferiprone (DFP) and deferasirox (DFX). DFO is administered subcutaneously (s.c.) over 8–24 hours, 6–7 nights per week, DFP and DFX are given orally as a monotherapy or in combination with DFO.7,8
Overall, ICT results in better overall survival, primarily if it is instituted early and the compliance is maintained.
Menarche is one of the markers of puberty and is considered an essential milestone in a woman’s reproductive life. Irregular and prolonged menstrual cycles, often attributed to the functional disruption of the hypothalamic-pituitary-ovarian axis, are, however, common among women of reproductive age. They have been associated with a greater risk of non-communicable diseases, including ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems.9
Few reports on the relationship between the age at the beginning of ICT and age at menarche in patients with TDT are reported in the literature,10 and practically nothing, to the best of our knowledge, of their menstrual history.
Our retrospective study aimed to collect data on age at menarche, body mass index (BMI), and the menstrual characteristics in a group of unmarried Italian and Cypriot TDT women treated with s.c. DFO, given before and after 10 years (yrs), from the 70s to 2000s.
Patients and Study Design
Clinical data were obtained from medical records of 85 TDT adolescent girls, followed from 1978 to 2018 at the Thalassemia Centers of Ferrara (Italy) (72 patients) and Nicosia (Cyprus) (13 patients). In Ferrara, the study was started by VDS in January 1977 and was completed in 2018, by the same physician, at the Pediatric and Adolescent Outpatient Quisisana Clinic of Ferrara (Italy).
Exclusion criteria were: 1) NTDT; 2) mental illness (depression, anxiety disorders, eating disorders, and addictive behaviors); 3) chronic kidney diseases; 4) history of severe head injury; 5) bone marrow transplanted patients; 7) HIV positivity; 8) TDT patients with poor or inconsistent control of associated endocrine complications; 9) autoimmune diseases, chromosomal abnormality, gonadotoxic treatment by systemic chemo-or radiation therapy; 10) smoking of more than 15 cigarettes/day or alcohol abuse, and 11) patients with a clinical menstrual history of isolated menarche. The latter condition was reported in 10 TDT patients.
The study was restricted to the analysis of the following available data: patients’ age at menarche (n = 85), menstrual characteristics (n = 79) and persistence of menstrual cycles at the last clinical examination (n = 23), age at starting ICT and duration of treatment (n = 85). In addition, nutritional status, assessed by BMI, was available at menarche in 20 patients with subsequent preserved menstrual cycles and in 47 patients who developed secondary amenorrhea (SA), at the age of SA in 61 patients and at last examination in 14 patients with preserved menses.
Serum ferritin levels (SF) were available at menarche in 56 patients who developed SA and in 23 with preserved menstrual cycles, at the age of SA in 56 patients, and at last examination in 14 with preserved menstrual cycles. Therefore, the data of 55 patients with SA were coupled with those registered at menarche.
The regular tempo of menarche was defined arbitrarily as the time interval from 11 to 14 yrs of age, derived from the ± 1,25 standard deviations (SD) of the average age of 12,4 ± 1,3 yrs of Italian and Greek population.11,12 Menses were defined as regular13 if menstrual cycle length was between 21 and 35 days (45 days during the first post-menarcheal year); irregular (oligomenorrhea) if the menstrual interval was > 35 days and < 6 months. SA was defined as the absence of menstruation for 6 months and over at any time after menarche.14 The number of “gynecologic years” was considered the interval time between age at menarche and the duration of menstrual history. It was determined in 23 patients with preserved menstrual cycles up to the last examination and in 62 patients from menarche to the age of SA.
BMI and BMI Z-Score were calculated with the formula weight in kg/ height in m2 (Kg/Ht m2) based on the Center for Disease Control (CDC) growth charts [http://zscore.research.chop.edu]. The BMI cut-off, by age and sex, was defined according to Cole et al.15 Thus, BMI values between 18.5 and 25 (Kg/Ht m2) were classified as normal, between 17 – 18.5 as mild thinness, between 16 – 17 as moderate thinness, < 16 as severe thinness and overweight/obesity if > 25–30, and over (Kg/Ht m2).
Patients with SA had been evaluated for pituitary–ovarian axis integrity (luteinizing hormone–LH and follicle-stimulating hormone–FSH before and after stimulation with gonadotropin-releasing hormone (Gn-RH stimulation test), prolactin, and 17 β-estradiol. In addition, blood samples were assayed for FSH and LH before and 20, 40, 60, and 120 minutes after injection.
A lack of gonadotropin release to the Gn-RH test (1-fold to 1.5-fold increase from the baseline level) associated with a negative medroxyprogesterone acetate test (MAP test: 10 mg given orally for 7 days), in the presence of a normal PRL and TSH, was considered as an index of hypothalamic and/or pituitary SA. In addition, hyperprolactinemia was defined by a high level of serum prolactin (PRL) above the standard upper limit of the normal range.
Pelvic ultrasonography was performed to determine the pelvic organs’ morphology and maturity and exclude pathologies.
The patients’ iron overload was assessed by SF. The manufacturer’s normal reference range values in females were 15–150 ng/mL. Iron overload was arbitrarily classified as mild (SF < 1,000 ng/mL), moderate (SF: >1,000 ng/mL and < 2,000 ng/mL) or severe (SF: >2,000 ng/mL).16
Ethical approval for the study was obtained in accordance with local institutional requirements according to the Declaration of Helsinki [http://www.wma.net].
Statistical analysis
Differences for continuous variables were analyzed with the independent-sample t-test for normally distributed variables and with the Mann-Whitney test in case of non-normality. Qualitative variables were compared using the chi-squared test. Finally, a comparison of regression lines by ANOVA was performed to examine the relationship between different parameters. The Stat-Graph XVII software for Windows was used for all statistical analyses. A p-value < 0.05 was considered statistically significant.
Results
Clinical characteristics and menstrual history of TDT patients
Before 1972, blood transfusions were given when anemia was severe enough to cause symptoms. After that, patients were regularly transfused every 2–3 weeks to maintain the mean pre-transfusional hemoglobin (Hb) level around 9.5 g/dL. Treatment with intramuscular DFO, at a dose of 20 mg/kg body weight (b.w.), was available for most patients since 1969. Regular subcutaneous (s.c.) DFO infusion was started in 1978 in patients older than 2 years. Initially, the recommended DFO dose was 20 mg/kg body weight administered daily at night by infusion pump over 10 hours. Based on transfusional iron input the dose increased to 40 mg/kg body weight (b.w.) in 1982 and up to 60 mg/ kg b.w. in 1984. Ascorbic acid was added orally at a dose of 2–5 mg/kg (maximum dose 200 mg) in a selected group of patients.
Since 1995, the oral chelator DFP has been available; it was given at a dose of 75 mg/kg/b.w. to some patients over 11 years, as monotherapy or combined with DFO. In 2007, DFX was introduced at a dose of 25–30 mg/kg b.w. for patients whose treatment with DFO was contraindicated or inadequate.
Our patients’ mean age at the start of chelation was 7,6 ±3,8 yrs. Twenty-four of them started therapy before the age of 5 (2,8 ± 1,2 yrs).
At the last clinical examination in 2018, the mean age of 85 TDT patients was 36,5 ± 5,7 years. Over the years, the age at menarche ranged from 11 to 19,5 years (mean age of 14,3 ± 1,8 yrs). At last observation, 23 patients were still menstruating, whereas 62 (72,9 %) had lost the menstrual periods (SA), at a mean age of 18,0 ± 3,5 yrs. In patients with preserved menstruations, the age at menarche was lower (13,4 ± 0,8 yrs) than in patients who developed SA (14,6 ± 2,0 yrs; p = 0,007). No statistical significant difference was present between their mean chronological age: 33,8 ± 7,2 yrs versus 37,0 ± 5,3 yrs, respectively (p = 0,15).
The duration of “gynecological years” of the whole group of subjects correlated significantly with the years at birth (n = 85, r = 0,25; p = 0,02). At the last clinical examination, the duration of “gynecological years” in the 23 women with preserved menses was 21,2 ± 5,9 yrs, whereas in the 62 women who experienced SA was 3,5 ± 3,0 yrs ( p = 0,0001).
BMI-Z score at menarche was 0,5 ± 0,9 in the 23 patients with preserved menstruations and – 0,32 ± 1,0 in 50 patients who developed SA (p = 0,002).
Iron chelation therapy (ICT) in relation to menstrual history
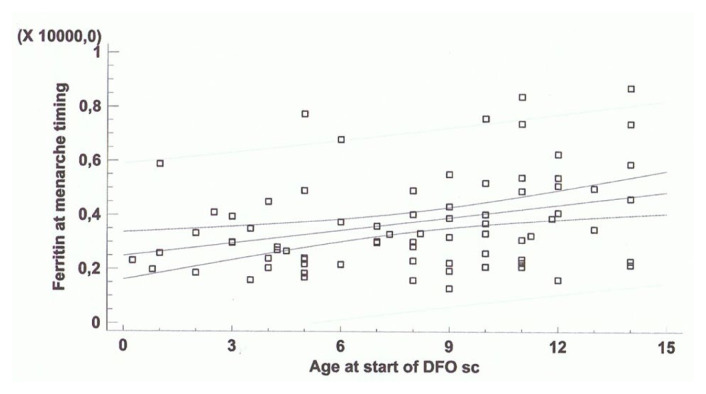
At menarche, the mean SF level in 79 TDT patients was 3,753 ± 1,741 ng/mL. A significant correlation was present between the starting ICT age and SF at menarche (Figure 1). The subgroup of the 23 TDT patients with preserved menstrual cycles had a mean SF level at menarche significantly lower compared to 55 patients who developed SA (2,913 ± 782 ng/mL versus 4,098 ± 1,907 ng/mL; p = 0,005), although in the latter group of patients, the mean SF level at SA was lower (3,163 ± 2,125 ng/mL) compared to the values registered at menarche (4,098 ± 1,907 ng/mL; p = 0,01).
Figure 1.
Correlation between age at start of chelation therapy with DFO (yrs) and serum ferritin levels at menarche (ng/ml) (n = 79, r = 0,34; p = 0, 002).
Despite an early iron chelation therapy (mean age: 2,8 ± 1,2 yrs), 11 out of 24 of our TDT patients developed a precocious SA, at a mean age of 19,5 ± 5,9 years with a mean SF level of 1,758 ± 846 ng/mL.
Finally, the mean SF at last examination in 14 women with preserved menses, was significantly lower (1,938 ± 837 ng/mL) compared to 55 TDT patients with SA (3,163 ± 2,125 ng/mL; p = 0,03).
Menarcheal age in relation to ICT and BMI
The patients’ year of birth was inversely correlated with the age at menarche (n = 85, r = . 0,27; p: 0,01) and the age at start of ICT (n = 85, r = . 0,86; p: 0,00001).
The TDT patients who had begun ICT before the age of 10 years (n = 53) experienced menarche at 13,7 ± 1,6 yrs, whereas those who begun treatment after ( n = 32), at a significantly later age (15,5 ± 1,9 yrs; p: 0,001). Table 1 summarizes the distribution of patients’ mean age at menarche (≤ or > 14 years) in relation to the age at ICT start with DFO; the frequencies were significantly different (χ2 = 0,016).
Table 1.
Ages at menarche (≤ or > 14 yrs) distributed in relation to the age at start of ICT with DFO.
| Age at menarche (yrs) | ≤ 14 | > 14 | Row total |
|---|---|---|---|
|
| |||
| Age at start of s.c. ICT with DFO: < 5 yrs | 17 | 7 | 24 |
| (%) | 71% | 29% | |
|
| |||
| Age at start of s.c. ICT with DFO: 5 – 12.5 yrs | 29 | 24 | 53 |
| (%) | 54,7% | 45,3% | |
|
| |||
| Age at start of s.c. ICT with DFO: > 12.5 years | 1 | 7 | 8 |
| (%) | 12,5% | 87,5% | |
|
| |||
| Column total | 47 | 38 | 85 |
| (%) | 55,3% | 44,7% | 100% |
|
| |||
| χ2 test, p: 0,016 | |||
At menarche, the average BMI-Z score of the whole group of patients was within the normal range (−0,09 ± 1), whereas, in 47 TDT adolescents with age at menarche ≤ 14 yrs, it was 0.3 ± 0.85. The difference with patients who had menarche at age > 14 yrs was statistically significant (n = 38, −0,53 ± 1,1; p = 0,001).
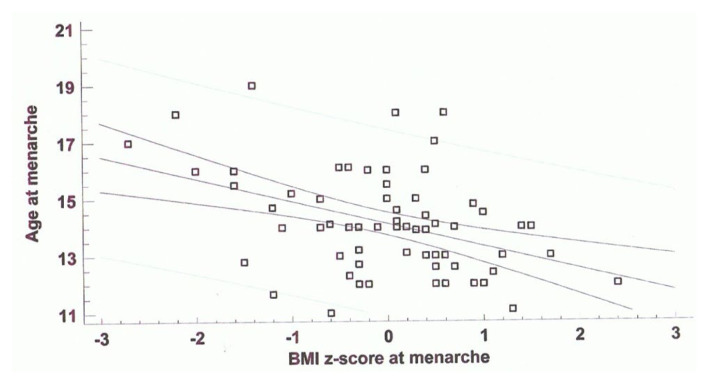
Finally, BMI-Z score was inversely correlated with age at start of ICT (n = 67, r = −039; p = 0,0001) and age at menarche (Figure 2).
Figure 2.
Correlation between age at menarche (yrs) and BMI-Z score at menarche timing (n = 67, r = −0,45 ; p = 0,00001).
Menstrual characteristics and duration of menstrual history in relation to BMI and ICT
The 50 TDT patients with subsequent regular menstrual cycles had a menarche age lower than 29 patients with irregular menstrual cycles (13,9 ± 1,8 vs. 14,7 ± 1,9 yrs; p: 0.028). The prevalence of regular and irregular menstrual cycles, in relation to the age at menarche ≤ or > 14 years, is reported in table 2. Age at menarche at or below 14 yrs was associated with a lower risk of irregular menstrual cycles (38% vs. 62% at age > 14 years, χ2 = 0,005).
Table 2.
Prevalence of regular and irregular menstrual cycles in relation to the age at menarche (≤ or > 14 yrs).
| Regular | Irregular | Rowtotal | |
|---|---|---|---|
| Menarche ≤ 14 yrs (%) | 35 (70) | 11 (38) | 46 (58.2) |
| Menarche > 14 yrs (%) | 15 (30) | 18 (62) | 33 (41.8) |
| Column total (%) | 50 (63,3) | 29 (36,7) | 79 (100) |
| χ2 test, p: 0,005 |
Nutritional status of “thinness” at menarche was associated with a lower prevalence of subsequent regular menstrual cycles (χ2 test; p: 0.02). Moreover, the BMI-Z score at menarche was directly correlated to the number of years of menstruations (n = 67, r = 0,36, p = 0,003), and the prevalence of regular menstrual cycles in patients with normal BMI was higher (68,7%) compared to those with thinness or overweight (38,4%, χ2 test; p: 0,02).
The condition of thinness in 13 TDT patients (2 mild, 7 moderate, and 4 severe) was associated with a higher prevalence of SA than in the patients with normal BMI (92%, χ2 test, p: 0,02).
The duration of menstrual history in the whole group of patients was strictly correlated to the age at the start of s.c. ICT with DFO. In particular it was 12,5 ± 8,9 yrs in 24 patients who started the chelation therapy < 5 years (13 with preserved menses and 11 with SA), 7,2 ± 8,8 yrs in 54 patients who started treatment from 5 to 12,5 years, and 3,1 ± 2,3 yrs in 8 patients who started treatment > 12,5 yrs (p= < 0,01).
However, it should be noted that precocious SA is still present in 11 out of 24 TDT patients who started ICT with DFO at the early age of 2,8 ± 1,2 yrs.
SA developed at a mean age of 19,5 ± 5,9 years, when SF was 1,676 ± 996 ng/mL. These values were not significantly lower compared to patients with preserved menses at last examination (1,938 ± 837 ng/mL, p = 0,08), but significantly lower compared to 51 TDT patients with SA who started ICT at later age (3,235 ± 2,154 ng/mL; p = 0,03). Finally, their BMI-Z score (0,84 ± 1,27) was not statistically different compared to BMIZ score of 8 patients with preserved menses who started ICT before 5 yrs (0,5 ± 0,8, p = 0,52).
Discussion
Timing of puberty, menarche, and menstrual patterns are considered indicators of overall health and self-perception of well-being. They are influenced by multiple variables such as: genetics, geographic location, climate, psychological factors, socioeconomic status, body weight and height, nutrition, body fat, and exercise, as well as the presence of chronic diseases.17,18
In our patients, the mean age at menarche was associated with an earlier age at the start of ICT with DFO, lower SF levels, and an average BMI-Z score, and a higher prevalence of regular subsequent menstrual cycles and duration of menstrual history.
The significant correlation, observed in our patients, between age at starting of s.c. ICT and SF at menarche confirm the importance of early onset of chelation therapy.19 Our patients started the iron chelation therapy with DFO at a mean age of 7,6 ±3,8 yrs and twenty-four of them before the age of 5 (2,8 ± 1,2 yrs). Nevertheless, approximately one-third of patients suffered from some forms of menstrual irregularities (light or infrequent menstruation) before developing SA. An absent correlation was observed between age at SA (mean age of 18,1 ± 3,5 yrs ; range: 12 to 30 yrs) and severity of overload (SF: 3,163 ± 2,125 ng/mL) (p = 0,51).
Patients with regular menstrual cycles had a lower mean age at menarche. Interestingly, age at menarche (≤ 14 yrs) was associated with a lower risk of irregular menstrual cycles (38% vs. 62% at age > 14 years, χ2 = 0,005). Moreover, lower age at menarche was associated with a better BMI-Z score, whereas the nutritional status of thinness was associated with a lower frequency of regular menstrual cycles history and a higher frequency of SA. However, the SF and BMI-Z score in 11 out of 62 TDT patients with SA, who started ICT before 5 years of age, resulted not different compared to patients with preserved menses. The small number of patients may have influenced the significance between the two groups, but other factors cannot be excluded.
In all TDT patients who developed SA, acquired central hypogonadism was diagnosed, mainly linked to chronic exposure to iron overload. At the SA time, their mean SF level was 3,163 ± 2,125 ng/mL, whereas, at the last examination, the SF in patients with preserved menses was 1,938 ± 837 ng/mL (p = 0,03). Hypogonadotropic hypogonadism (HH), also called central hypogonadism or secondary hypogonadism, is characterized by deficient Gn-RH secretion from the hypothalamus leading to decreased LH and FSH secretion from the pituitary gland and inadequate testosterone (in males) or estradiol (in females) production.
It has been reported that iron deposition in the pituitary gland starts in the first decade of life and has a cytotoxic effect, leading to hypogonadotropic hypogonadism.20,21
After approximately one year of transfusions, iron is deposited in parenchymal tissues, where it may cause significant toxicity compared to that within reticuloendothelial cells. Furthermore, as iron loading progresses, the capacity of serum transferrin, the main transport protein of iron, to bind and detoxify iron may be exceeded. Therefore, non–transferrin-bound iron is readily transported through calcium channels into the liver (hepatocytes), heart (cardiac myocytes), and endocrine glands, leading to the different clinical complications of iron overload. In addition, reactive oxygen species produced by the metabolism of non–transferrin-bound iron contribute to cellular dysfunction, apoptosis, and necrosis in target organs.22,23
Susceptibility to HH development seems to be associated with genotype, as patients with severe underlying molecular defects have a greater rate of iron loading and probably a different vulnerability to free radical damage.24
Very little is known on the effects of oral ICT on age at menarche,25 and nothing is available in the recent literature on the menstrual history of TDT patients during the treatment with s.c. DFO. Moreover, the relationship of menstrual history with nutritional status has not been investigated before.
Despite an early iron chelation therapy (mean age: 2,8 ± 1,2 yrs), 11 out of 24 of our TDT patients developed a precocious SA, at a mean age of 19,5 ± 5,9 years with a mean SF level of 1,758 ± 846 ng/mL. Intensification of treatment or improvement of patients’ compliance to iron chelation therapy did not induce a reversal of H-P-G dysfunction. In contrast to our observations, Farmaki et al.26 reported in four out of 10 TDT patients with SA an improvement or reversal of pituitary-gonadal dysfunction after a very intensive combined chelation with DFO and DFP (the SF in the entire group of patients was 99 ± 37 ng/mL). Two of them became pregnant spontaneously and two after in vitro fertilization (IVF). However, we do not know how long we can maintain children and adolescents at normal levels of iron stores without side effects from the over-chelation of iron.
Interestingly, we found an inverse correlation between the BMI-Z score and age at menarche and a direct relationship between the BMI-Z score and years of menstrual cycles. It is well known that an optimal nutritional status is important for normal growth and pubertal development.27
A decreased body fat results in a leptin concentration reduction and gonadotropin deficiency. Leptin is a polypeptide hormone produced in fat cells due to the expression of the ob gene. In girls, leptin levels dramatically increase, as puberty develops, and stimulate the H-P-G axis, and which is a permissive factor in puberty onset.28
The nutritional history of our patients did not document relevant deficiencies; however, we cannot fully exclude a reduced intake of macro and micronutrients. Goldberg et al.29 have reported that many TDT patients who consumed an adequate dietary intake had deficient levels of circulating nutrients. Liver iron concentration in TDT patients displayed a significant negative relationship with vitamins C, E, and zinc, indicating that in iron-overloaded patients, these nutrients are either endogenously consumed at higher rates or sequestered within the liver resulting in a functional nutrient deficiency. Therefore, even a marginal zinc deficiency could affect leptin secretion and serum leptin concentrations, as documented in zinc-deficient rats.30 A potential additional factor leading to impaired leptin secretion may be the iron toxicity in adipose tissue, as reported by Perrone et al..31
Although our paper has some limitations, due to the retrospective nature of the study, the limited number of patients included in the study, the use of surrogate indices for assessing iron overload, and the lack of comparison with other iron chelators, it has the strength of standardized documentation of clinical data, the rigorous selection of patients and the description, for the first time, of a long-term menstrual history in two groups of TDT patients who started early and late ICT with DFO.
In conclusion, early ICT in TDT patients was associated with a regular “tempo” of pubertal onset and a higher frequency of subsequent regular menstrual cycles. However, SA remains a frequent morbidity and obstacle for having children. Patients with transfusional iron overload begin to develop pituitary iron overload in the first decade of life; however, clinically significant volume loss was not observed until the second decade of life.20,21
The serum ferritin levels in our patients with SA were consistent with a non-adequate ICT.
Therefore optimization of iron burden is mandatory, particularly in subjects uncompliant to therapy or no access to optimal medical care.
The present data could represent a reference for future studies in order to compare also the efficacy of different iron chelation regimes on the long-term maintenance of a normal function of an H-P-G axis and may generate future studies for investigating whether body mass percentile and trace elements could be potential additional factors responsible for menstrual cycle irregularities in TDT patients.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Weatherall DJ. Thalassemia as a global health problem: recent progress toward its control in the developing countries. Ann N Y Acad Sci. 2010;1202:17–23. doi: 10.1111/j.1749-6632.2010.05546.x. [DOI] [PubMed] [Google Scholar]
- 2.Viprakasit V, Ekwattanakit S. Clinical Classification, Screening and Diagnosis for Thalassemia. Hematol Oncol Clin North Am. 2018;32:193–211. doi: 10.1016/j.hoc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Thalassaemia International Federation. Guidelines for the management of transfusion dependent thalassaemia (TDT) 3rd ed. 2014. [Accessed 15 June 2018]. Available from www.thalassaemia.org.cy. [PubMed]
- 4.Kanbour I, Chandra P, Soliman A, De Sanctis V, Nashwan A, Abusamaan S, Moustafa A, Yassin MA. Severe Liver Iron Concentrations (LIC) in 24 Patients with β-Thalassemia Major: Correlations with Serum Ferritin, Liver Enzymes and Endocrine Complications. Mediterr J Hematol Infect Dis. 2018;10(1):e2018062. doi: 10.4084/MJHID.2018.062. doi: 10.4084/MJHID.2018.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St Pierre TG, El-Beshlawy A, Elalfy M, Al Jefri A, Al Zir K, Daar S, Habr D, Kriemler-Krahn U, Taher A. Multicenter validation of spin-density projection-assisted R2-MRI for the non invasive measurement of liver iron concentration. Magn Reson Med. 2014;71:2215–2223. doi: 10.1002/mrm.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassaemia. Blood. 1997;89:739–761. doi: 10.1182/blood.V89.3.739. [DOI] [PubMed] [Google Scholar]
- 8.Berdoukas V, Farmaki K, Carson S, Wood J, Coates T. Treating thalassemia major-related iron overload: the role of deferiprone. J Blood Med. 2012;3:119–129. doi: 10.2147/JBM.S27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YX, Arvizu M, Rich-Edwards JW, Stuart JJ, Manson JE, Missmer SA, Pan A, Chavarro JE. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ. 2020;371:m3464. doi: 10.1136/bmj.m3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronspiegel-Weintrob N, Olivieri NF, Tyler B, Andrews DF, Freedman MH, Holland FJ. Effect of age at the start of iron chelation therapy on gonadal function in β-thalassemia major. N Engl J Med. 1990;323:713–719. doi: 10.1056/NEJM199009133231104. [DOI] [PubMed] [Google Scholar]
- 11.De Sanctis V, Bernasconi S, Bianchin L, Bona G, Bozzola M, Buzi F, De Sanctis C, Rigon F, Tatò L, Tonini G, Perissinotto E. Onset of menstrual cycle and menses features among secondary school girls in Italy: a questionnaire study on 3783 students. Indian J Endocrinol Metab. 2014;18(suppl 1):S84–S92. doi: 10.4103/2230-8210.140251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadimitriou A, Fytanidis G, Douros K, Bakoula C, Nicolaidou P, Fretzayas A. Age at menarche in contemporary Greek girls: evidence for levelling-off of the secular trend. Acta Paediatr. 2008;97:812–815. doi: 10.1111/j.1651-2227.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfield RL, Cooke DW, Radovick S. Sperling MA Pediatric Endocrinology. fourth edition. ELSEVIER Saunders; Philadelphia: 2014. Puberty and its disorders in the female; pp. 694–696. [DOI] [Google Scholar]
- 14.De Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniatis M, Karimi M, Yassin MA, El Awwa A, Stoeva I, Raiola G, Galati MC, Bedair EM, Fiscina B, El Kholy M. Growth and endocrine disorders in thalassemia: the International network on endocrine complications in thalassemia (I-CET) position statement and guidelines. Indian J Endocrinol Metab. 2013;17:8–18. doi: 10.4103/2230-8210.107808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body Mass Index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335(7612):194. doi: 10.1136/bmj.39238.399444.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casale M, Meloni A, Filosa A, Cuccia L, Caruso V, Palazzi G, Gamberini MR, Pitrolo L, Putti MC, D’Ascola DG, Casini T, Quarta A, Maggio A, Neri MG, Positano V, Salvatori C, Toia P, Valeri G, Midiri M, Pepe A. Multiparametric Cardiac Magnetic Resonance Survey in Children With Thalassemia Major: A Multicenter Study. Circ Cardiovasc Imaging. 2015;8(8):e003230. doi: 10.1161/CIRCIMAGING.115.003230. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Dang S, Xing Y, Li Q, Yan H. Correlation of body mass index levels with menarche in adolescent girls in Shaanxi, China: a cross sectional study. BMC Womens Health. 2016;16:61. doi: 10.1186/s12905-016-0340-4. doi: 10.1186/s12905-016-0340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palel L. Growth and Chronic Disease. Ann Nestlé [Engl] 2007;65:129–136. doi: 10.1159/000112235. [DOI] [Google Scholar]
- 19.Bronspiegel-Weintrob N, Olivieri NF, Tyler B, David F, Andrews DF, Melvin H, Freedman MH, Holland FJ. Effect of age at the start of iron chelation therapy on gonadal function in β-thalassemia major. N Engl J Med. 1990;323:713–719. doi: 10.1056/NEJM199009133231104. [DOI] [PubMed] [Google Scholar]
- 20.Wood JC. Guidelines for quantifying iron overload. Hematology (Am Soc Hematol Educ Program) 2014;2014:210–215. doi: 10.1182/asheducation-2014.1.210. [DOI] [PubMed] [Google Scholar]
- 21.Hekmatnia A, Radmard AR, Rahmani AA, Adibi A, Khademi H. Magnetic resonance imaging signal reduction may precede volume loss in the pituitary gland of transfusion-dependent beta-thalassemic patients. Acta Radiol. 2010;51:71–77. doi: 10.3109/02841850903292743. [DOI] [PubMed] [Google Scholar]
- 22.Pippard M, Johnson D, Callender S, Finch C. Ferrioxamine excretion in iron loaded man. Blood. 1982;60:288–294. doi: 10.1182/blood.V60.2.288.bloodjournal602288. [DOI] [PubMed] [Google Scholar]
- 23.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21:219–222. doi: 10.1097/GCO.0b013e32832924ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skordis N, Gourni M, Kanaris C, Toumba M, Kleanthous M, Karatzia N, Pavlides N, Angastiniotis M. The impact of iron overload and genotype on gonadal function in women with thalassaemia major. Pediatr Endocrinol Rev. 2004;2:292–295. [PubMed] [Google Scholar]
- 25.Origa R, Danjou F, Orecchia V, Zappu A, Dessì C, Foschini ML, Leoni GB, Moi P, Morittu M, Demurtas A, Loche S. Current growth patterns in children and adolescents with thalassemia major. Blood. 2016;128:2580–2582. doi: 10.1182/blood-2016-05-712695. [DOI] [PubMed] [Google Scholar]
- 26.Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol. 2010;148:466–475. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- 27.P, Sanguansermsri T, Fuchs GJ. Malnutrition and growth abnormalities in children with beta thalassemia major. Southeast Asian J Trop Med Public Health. 1996;27:356–361. [PubMed] [Google Scholar]
- 28.Kiess W, Reich A, Meyer K, Glasgow A, Deutscher J, Klammt J, Yang Y, Müller G, Kratzsch J. A role for leptin in sexual maturation and puberty? Horm Res. 1999;51(S3):55–63. doi: 10.1159/000053163. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg EK, Neogi S, Lal A, Higa A, Fung E. Nutritional deficiencies are common in patients with Transfusion-Dependent Thalassemia and associated with iron overload. J Food Nutr Res (Newark) 2018;6:674–681. doi: 10.12691/jfnr-6-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ott ES, Shay NF. Zinc deficiency reduces leptin gene expression and leptin secretion in rat adipocytes. ExpBiol Med (Maywood) 2001;226:841–846. doi: 10.1177/153537020122600906. [DOI] [PubMed] [Google Scholar]
- 31.Perrone L, Perrotta S, Raimondo P, Mucerino J, De Rosa C, Siciliani MC, Santoro N, Miraglia del Giudice E. Inappropriate leptin secretion in thalassemia: a potential cofactor of pubertal timing derangement. J Pediatr Endocrinol Metab. 2002;16:877–881. doi: 10.1515/JPEM.2003.16.6.877. [DOI] [PubMed] [Google Scholar]