Abstract
Background
Basal cell carcinoma (BCC) is a common skin cancer often curable by excision; however, for patients with BCC around the eye, excision places visual organs and function at risk. In this article, we test the hypothesis that use of the hedgehog inhibitor vismodegib will improve vision‐related outcomes in patients with orbital and extensive periocular BCC (opBCC).
Materials and Methods
In this open‐label, nonrandomized phase IV trial, we enrolled patients with globe‐ and lacrimal drainage system–threatening opBCC. To assess visual function in the context of invasive periorbital and lacrimal disease, we used a novel Visual Assessment Weighted Score (VAWS) in addition to standard ophthalmic exams. Primary endpoint was VAWS with a score of 21/50 (or greater) considered successful, signifying globe preservation. Tumor response was evaluated using RECIST v1.1. Surgical specimens were examined histologically by dermatopathologists.
Results
In 34 patients with opBCC, mean VAWS was 44/50 at baseline, 46/50 at 3 months, and 47/50 at 12 months or postsurgery. In total, 100% of patients maintained successful VAWS outcome at study endpoint. Compared with baseline, 3% (95% confidence interval [CI], 0.1–15.3) experienced major score decline (5+ points), 14.7% (95% CI, 5 to 31.1) experienced a minor decline (2–4 points), and 79.4% experienced a stable or improved score (95% CI, 62.1–91.3). A total of 56% (19) of patients demonstrated complete tumor regression by physical examination, and 47% (16) had complete regression by MRI/CT. A total of 79.4% (27) of patients underwent surgery, of which 67% (18) had no histologic evidence of disease, 22% (6) had residual disease with clear margins, and 11% (3) had residual disease extending to margins.
Conclusion
Vismodegib treatment, primary or neoadjuvant, preserves globe and visual function in patients with opBCC. Clinical trail identification number.NCT02436408.
Implications for Practice
Use of the antihedgehog inhibitor vismodegib resulted in preservation of end‐organ function, specifically with regard to preservation of the eye and lacrimal apparatus when treating extensive periocular basal cell carcinoma. Vismodegib as a neoadjuvant also maximized clinical benefit while minimizing toxic side effects. This is the first prospective clinical trial to demonstrate efficacy of neoadjuvant antihedgehog therapy for locally advanced periocular basal cell carcinoma, and the first such trial to demonstrate end‐organ preservation.
Keywords: Basal cell, Cancer, Patched, Smoothened, SMO, PTCH, Hedgehog, Orbit, Orbital, Tumor, Cancer, Lacrimal, Epiphora, Visual function
Short abstract
This article reports the results of a prospective clinical trial of vismodegib for patients with basal cell carcinoma occurring in the orbital and periocular regions to assess whether such treatment helps to preserve visual organs and function.
Introduction
Basal cell carcinoma (BCC) is the most common skin cancer, occurring more frequently than all other cancer types combined [1]. Up to 80% of BCCs occur in the head and neck region, with 20% of those occurring on the eyelid [2]. Although the majority of BCCs can be cured via excision [3], for locally advanced BCCs occurring in the orbital and periocular regions (opBCC), recurrence rates are high and excision may result in loss of visual organs/function [4, 5, 6, 7, 8, 9]. This presents a clinical conundrum: curative surgical treatment of the cancer may result in worse visual functional outcomes than noncurative treatments that retain significant tumor burden.
BCC is driven by mutations in the hedgehog signaling pathway. Up to 90% of BCCs are caused by mutations in the receptor Patched1 (PTCH1), with the remaining 10 + % caused by mutations in Smoothened (SMO) or other downstream factors [10, 11]. Vismodegib (GDC‐0449, Genentech Inc., South San Francisco, CA) is a molecular SMO‐inhibitor approved for the treatment of advanced and metastatic BCC [12]. Phase I and II trials reported a clinical response in 30%–58% of patients, with complete response in 0.6%–46% of patients [13, 14, 15, 16, 17]. The pivotal study, a prospective nonrandomized clinical trial, revealed a 43% response rate among patients with locally advanced BCC and 30% response rate among patients with metastatic BCC, with a median duration of response of 7.6 months [8]. Prospective trials have assessed vismodegib as a neoadjuvant in a variety of body locations [17, 18, 19], and several case studies have highlighted the efficacy of vismodegib for preserving vision in patients with orbital BCC [20, 21, 22, 23, 24, 25]. More recently, post hoc prospective analysis of the multisite multicountry Safety Events in Vismodegib study data showed either complete or partial response to vismodegib in 67% of patients [25]. However, to date, no prospective trials have specifically assessed whether vismodegib treatment preserves visual function. In 2015, we initiated a prospective clinical trial of vismodegib for patients with opBCC to assess whether vismodegib treatment helps to preserve visual organs and function.
Materials and Methods
Study Design
This was a prospective phase IV single‐center open‐label trial approved by the institutional review board of the University of Michigan Rogel Cancer Center (NCT02436408). All patients provided signed informed consent prior to enrollment. We followed the interventional standard of care use of vismodegib in patients with locally advanced globe‐threatening or lacrimal drainage system‐threatening BCC. An amount of 150 mg vismodegib was taken orally daily for up to 12 months or until disease progression or unacceptable toxicity, upon which surgical excision was recommended. An interim analysis for descriptive purpose was planned for midstudy.
Every patient underwent an ophthalmic exam, including lacrimal probing, and exam elements were recorded in a Velos database (Velos L.L.C., Freemont, CA). The primary endpoint was visual function, as measured by the VAWS, used as a compendium of standard ophthalmic exam elements. The VAWS consists of eight items related to preservation of visual organs, acuity, extraocular motility, and lacrimal drainage (Table 1). A total score of 21 was considered a positive outcome, because it suggests globe preservation (20 points) along with one additional aspect of visual function (1+ points). Tumor measurements and response were assessed using the RECIST guidelines v1.1 [26]. For patients who elected to undergo surgical excision, pathologic evaluation was performed by our study dermatopathologists. Patients were followed for 1 year after initiation of vismodegib. The final visit for patients who elected to undergo excision was 1 month (±1 month) following surgery. Importantly, this study was not designed or powered to assess for recurrence, which for BCC can take years for detection [5, 7]. Because the majority of patients underwent Mohs surgery, we anticipate that recurrence rates will be comparable to published rates for Mohs surgery for BCC.
Table 1.
Visual assessment weighted score
| Clinical Evaluation Item | Score (no = 0; yes = 1) | Weighted score |
|---|---|---|
| Intact globe (no enucleation or evisceration) | 0/1 | 0/20 |
| VA within 4 Snellen lines of baseline VA or better | 0/1 | 0/5 |
| VA at 20/200 or better | 0/1 | 0/5 |
| No binocular diplopia in primary gaze | 0/1 | 0/2 |
| Fusion (Fly) ± prism | 0/1 | 0/2 |
| No symptomatic tearing | 0/1 | 0/3 |
| Intact lacrimal system by probing/irrigation (either canaliculus to NLD) | 0/1 | 0/3 |
| Patient pleased with visual function (poor, fair, good) | 0/1/2 | 0/5/10 |
| Total possible | 9 | 50 |
Abbreviationss: NLD, nasolacrimal duct; VA, visual acuity.
Study Oversight
All data collection, management, and analyses were performed by investigators on site, and a Medical Monitor was assigned for regular reviews. The study was approved by the institutional review board at the University of Michigan (HUM00082579). Funding for the study was provided in part through an investigator‐initiated study grant from Genentech, Inc.; the granting agency received periodic reports from the principal investigator but had no control over the conduct of the study.
Eligibility
We recruited adult patients older than 18 years of age with locally advanced or recurrent opBCC with orbital invasion, or medial canthal BCC that threatens the lacrimal drainage system (within 7 mm of lacrimal apparatus). Clinical photography, magnetic resonance imaging (MRI)/computed tomography (CT) imaging, and positive biopsy were also required. Patients were excluded for the following: inability or willingness to swallow capsules; inability to comply with study protocol; pregnant, lactating, or breastfeeding women; women of childbearing potential; uncontrolled medical illnesses; and dementia or significantly altered mental status that would prohibit the understanding of the protocol.
Data Collection
At 3 months, and every 3 months thereafter, a standard oculoplastic eye examination was performed and VAWS was calculated. MRI or CT with contrast was obtained prior to onset of treatment and at 5 and 8 months (±1 month) after treatment initiation. Tumor cross sectional sizes were measured by physical examination every 3 months in the greatest coordinates using a metric ruler by the treating physician or trained designee. Adverse event data were collected through study completion.
Statistical Analysis
The study was powered based on the previously published treatment success rate of 43% and intolerable side effect rate of 50% [13]. With a type 1 error probability of 0.05, enrollment was aimed at 38 patients, of which 16 were expected to respond to vismodegib therapy. Of the 16 responders, 8–10 would be expected to develop intolerable side effects and be candidates for organ‐preserving surgery. An exact binomial one‐sided test was used to compare success rate of the primary outcome (VAWS) with a benchmark rate of 30% (a literature‐ and practice‐based estimate of the loss of visual and lacrimal function after treatment of locally advanced opBCC). Kendall's tau‐b was used to measure associations between VAWS components. After 23 patients, we performed an interim analysis for descriptive purposes. This revealed far greater rates of clinical response and intolerable side effects than originally anticipated. The study was terminated early as a consequence of the therapeutic and administrative challenges posed by the novel coronavirus pandemic and because the interim analysis revealed that the study at the point of termination was sufficient to achieve statistical significance.
Results
Patients
A total of 34 patients were enrolled between July 2015 and May 2019. Our initial study design aimed to enroll 50 patients; however, the study was halted early in part as a result of the novel coronavirus pandemic and in part for benefit (see Statistical Analysis section). All 34 patients had biopsy‐positive locally advanced opBCC. The median age of patients was 68.5 years at screening (Table 2). One patient had two distinct orbital lesions; 33 patients had one lesion. Of 35 tumors, 22 were in the medial canthal region, 3 were lateral canthal, 8 were lower lid, and 2 were brow/orbit junction (Table 2). Median tumor size (largest dimension) was 22 mm. In total, 19 patients presented with lesions whose complete excision with clear margins would have likely required exenteration. The remaining 15 patients’ lesions would have qualified for globe‐sparing surgery; however, to achieve clear margins, such surgery would have resulted in loss of lacrimal drainage apparatus function (4 patients), extraocular motility (1 patient), or both (10 patients) (Table 2). Median treatment duration was 261 days (Fig. 1B). In total, 27 of 34 patients (79.4%) elected to undergo excision prior to completion of the 1‐year course of treatment because of poor tolerance of vismodegib (Fig. 1B). One patient died prior to completing the study.
Table 2.
Baseline patient characteristics
| Characteristic | Locally advanced orbital/periocular basal cell carcinoma |
|---|---|
| Number of subjects | 34 |
| Age, years | 67.1 ± 12.2 |
| Median | 68.5 |
| Range | 48‐95 |
| Sex, n (%) | |
| Male | 19 (56) |
| Female | 15 (44) |
| Predicted surgical outcome at baseline, n (%) | |
| Exenteration | 19 (56) |
| Globe‐Sparing | 15 (44) |
| Lacrimal damage only | 4 (12) |
| EOM damage only | 1 (3) |
| Lacrimal and EOM damage | 10 (29) |
| Number of tumors | 35 |
| Tumor location, n (%) | |
| Medial canthus | 22 (63) |
| Lateral canthus | 3 (8.5) |
| Lower lid | 8 (23) |
| Brow/orbit | 2 (5.5) |
| Tumor size, mm | |
| Median | 21.5 |
| Range | 10‐60 |
Abbreviation: EOM, extraocular muscle.
Figure 1.
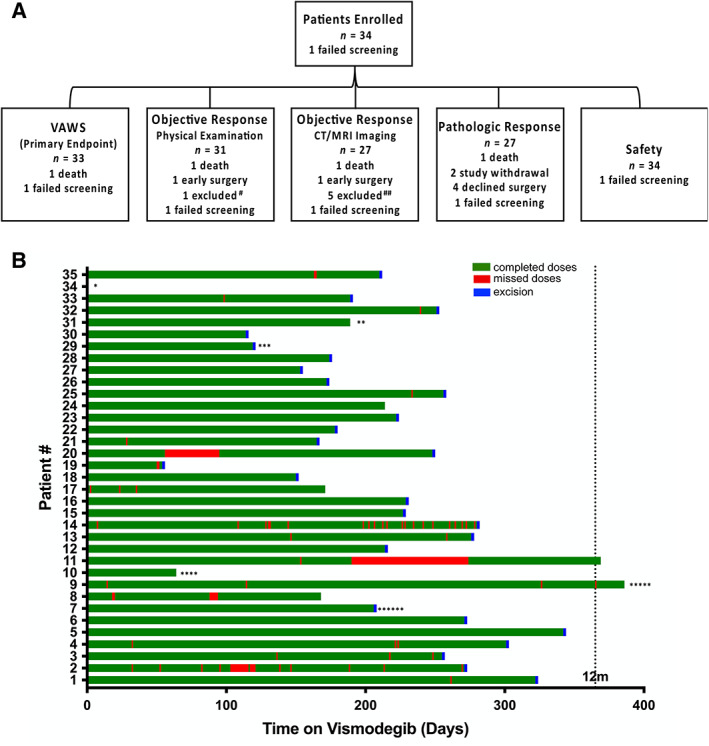
Patient disposition and treatment duration. (A): Schematic highlighting patient disposition. # Patient's tumor histological subtype (infiltrative) prevented physical exam (PE) and MRI measurements. ##No MRI/CT imaging available. (B): Treatment duration (green), missed doses (red), and excision (blue) for all patients. *Failed screening. **Lost to follow‐up. ***Missing drug diaries. ****Died prior to completing study. *****Patient's tumor histological subtype (infiltrative) prevented PE and MRI measurements. ******Left study prior to excision. Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; VAWS, Visual Assessment Weighted Score.
Visual Function
Upon screening, we evaluated patients’ visual function using both an ophthalmic exam and a newly developed tool: the VAWS, which assigns weights to key exam elements. We developed the VAWS specifically for this project, as existing tools focus on visual acuity and do not focus on orbital and lacrimal function (e.g., National Eye Institute Visual Function Index, i.e., NEI‐VFI). The VAWS contains elements present in a standard ophthalmic evaluation, and additional elements were also tracked, such as punctate epithelial keratopathy, eyelid margin‐to‐reflex measurements, and a slit‐lamp biomicroscopy exam. Thirty‐three (100%) patients maintained a VAWS score of >21 at 12 months or postoperatively (p < .0001). The mean VAWS scores were 43.9, 45.8, 45.8, 46, and 46.63 at screening, 3, 6, 9, and 12 months (or postoperative), respectively (Fig. 2B). Thirty‐three (100%) patients maintained an intact globe through study completion (Fig. 2A). Thirty‐two (97%) patients maintained visual acuity within four Snellen lines of screening at study completion (Fig. 2A). Thirty‐one (94%) patients maintained visual acuity of at least 20/200 (Fig. 2A). Thirty‐two (97%) patients were experiencing no diplopia and had good stereopsis at study completion (Fig. 2A). Seven (21%) patients were experiencing symptomatic tearing at study completion (Fig. 2A). Twenty‐one (64%) patients had an intact lacrimal system by probing or irrigation (Fig. 2A). Twenty‐six (79%) patients reported their vision as “good,” three (9%) reported “fair,” and one (3%) reported “poor” at study completion (Fig. 2A). Compared with screening scores, one (3%) patient had a major score decline (5+ points), five (15%) patients had a minor decline (2–4 points), 27 (82%) patients had a stable or improved score (one‐point decline, no change, or improvement), and one patient was not evaluable (unrelated mortality; Fig. 2C, D). An association analysis revealed overall correlation between individual elements of the VAWS and the total score (supplemental online Fig. 1; see Discussion).
Figure 2.

Visual function preservation during vismodegib treatment. (A): VAWS component status at screening, 3, 6, 9, and 12 months/postoperative (yes/good, green; fair, yellow; no/poor, red). (B): Average total VAWS score for all patients at screening, 3, 6, 9, 12 months/postoperative. (C): ∆VAWS 12 months or postoperative versus screening. (D): ∆VAWS 12 months/postoperative vs. screening by patient. (>5 point reduction, red; 2–4 point reduction, orange; <1 point reduction or improved score, blue; error bars indicate 95% confidence interval) Abbreviations: LAC, lacrimal; NE, not evaluable; VA, visual acuity; VAWS, Visual Assessment Weighted Score.
Tumor Response
Regardless of tumor location, many patients displayed either complete or near‐complete clinical response to vismodegib (Fig. 3A). Based on physical examination measurements, cross sectional tumor sizes were on average 44% of baseline size after 3 months of vismodegib and 22% of baseline after 6 months (Fig. 3B–D). For the 10 patients who had not undergone surgery by 9 months, tumors were on average 22% of baseline. For the three patients who remained on vismodegib through the end of study (12 months), mean cross‐sectional tumor size was 20% of baseline (Fig. 3B, C). A total of 19 (56%) patients achieved complete response (CR), 10 (29%) achieved partial response (PR), 2 (6%) achieved stable disease (SD), and none achieved progressive disease (PD) based on best physical exam (PE) measurement (Fig. 3D; Table 3). For MRI/CT measurements, tumors showed maximum response at 6 months (18.5% of baseline) (Fig. 3C). Sixteen (47%) patients showed CR, nine (26.5%) showed PR, two (6%) showed SD, and zero showed PD (Table 3).
Figure 3.
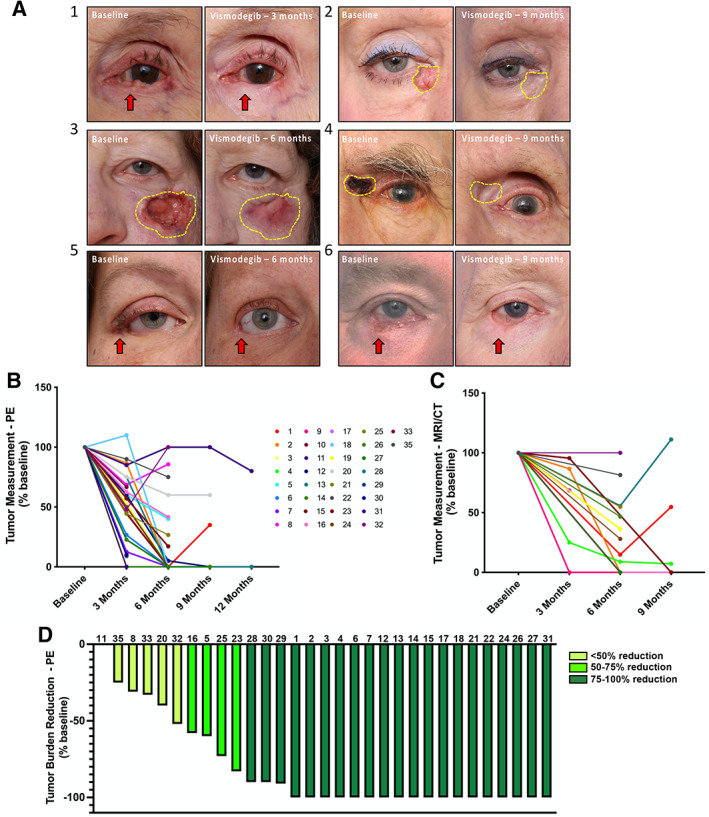
Tumor response to vismodegib treatment. (A‐1): Patient 30 (female, age 92) with left lower eyelid basal cell carcinoma (BCC) that obliterated her eyelid margin and canaliculus. (A‐2): Patient 25 (female, age 69) with recurrent left periocular BCC with perineural spread that invaded the orbit. (A‐3): Patient 18 (female, age 58) with long‐standing right lower eyelid BCC involving the lateral canthus, anchored to bone, with orbital extension, causing lower eyelid retraction and upper eyelid cicatricial ptosis. (A‐4): Patient 14 (female, age 65) with nodular BCC of her right medial canthus. They also had independent BCC tumors at the left medial canthus and central forehead. All three tumors responded to vismodegib therapy. (A‐5): Patient 1 (male, age 62) with BCC of the left medial canthus, with anchoring to bone. (A‐6): Patient 3 (male, age 69) with BCC of the right lower eyelid invading the anterior orbit. (B, C): PE (B) and MRI/CT (C) tumor measurement (percentage baseline) at 3, 6, 9, and 12 months after vismodegib treatment. (D): Tumor burden reduction (PE percentage baseline) at 12 months post treatment or presurgery. Abbreviations: MRI/CT, magnetic resonance imaging/computed tomography; PE, physical exam.
Table 3.
Treatment outcomes and adverse events summary
| Treatment Response | Number of patients (%) n = 34 (100) |
|---|---|
| Clinical response, PE / MRI | 34 (100) / 34 (100) |
| Complete response | 19 (56) / 16 (47) |
| Partial response | 10 (29) / 9 (26.5) |
| Stable disease | 2 (6) / 2 (6) |
| Progressive disease | 0 (0) / 0 (0) |
| Not evaluable | 3 (9) / 7 (20.5) |
| Histological response | 27 (100) |
| No sign of disease | 18 (67) |
| Disease present, clear margins | 6 (22) |
| Disease present, extending to margins | 3 (11) |
| Surgical response | 27 (100) |
| No evidence of disease | 24 (89) |
| Disease present | 3 (11) |
| Adverse events | 34 (100) |
| All AEs, any cause | 33 (97) |
| Treatment‐related AE | 33 (97) |
| Grade 3–4 AE | 7 (21.2) |
| Treatment‐related grade 3–4 AE | 3 (8.8) |
| Grade 5 AE | 1 (2.9) |
| Treatment‐related grade 5 AE | 0 (0) |
| Serious adverse event | 8 (23.5) |
| Treatment‐related SAE | 3 (8.8) |
| AS leading to treatment suspension | 2 (5.9) |
| Deaths | 1 (2.9) |
Abbreviations: AE, adverse event; AS, adverse symptoms; MRI, magnetic resonance imaging; PE, physical exam; SAE, serious adverse event.
Surgical specimens from the 27 patients who elected to undergo excision underwent routine (independent) histologic evaluation by two board‐certified dermatopathologists to assess for the presence of residual BCC. The specimens demonstrated substantial reductions in tumor load with residual foci of degenerating tumor cells, inflammation, and fibrous replacement as previously described [20]. In total, 67% (18) of patients who underwent excision showed complete histologic clearance, six (22%) had evidence of residual disease but with clear margins, and three (11%) patients had disease extending to the surgical margins (Table 3). The three patients with positive microscopic involvement of the margins are being followed clinically. Taken together, based on both clinical tumor measurements and pathology results, 25 patients were able to achieve complete response with either vismodegib alone (2 patients) or as a neoadjuvant to excision (24 patients). Following completion of the study, all patients have been followed routinely per standard of care. On such long‐term follow‐up (outside the study), two patients experienced local recurrence, prompting additional excision for both and consideration of adjuvant radiation therapy for one. These recurrences were detected 2 years following completion of the study. All patients have retained their affected globe. Importantly, this study was designed to assess preservation of visual and lacrimal functions and not rates of recurrence, which are expected to conform to rates of recurrence following Mohs surgery.
Adverse Events
Adverse events are summarized in Table 3. Thirty‐three (97%) patients experienced at least one treatment‐related adverse event (AE). Three (9%) patients experienced grade 3–4 treatment‐related AEs. Three (9%) patients experienced treatment‐related serious AEs. Two (6%) patients experienced AEs resulting in treatment suspension in consultation with the study team. The most common treatment‐related AEs were dysgeusia (25, 74%), myalgia (23, 67%), and alopecia (16, 47%; Fig. 4A). One patient experienced an unrelated serious adverse event, which led to death.
Figure 4.
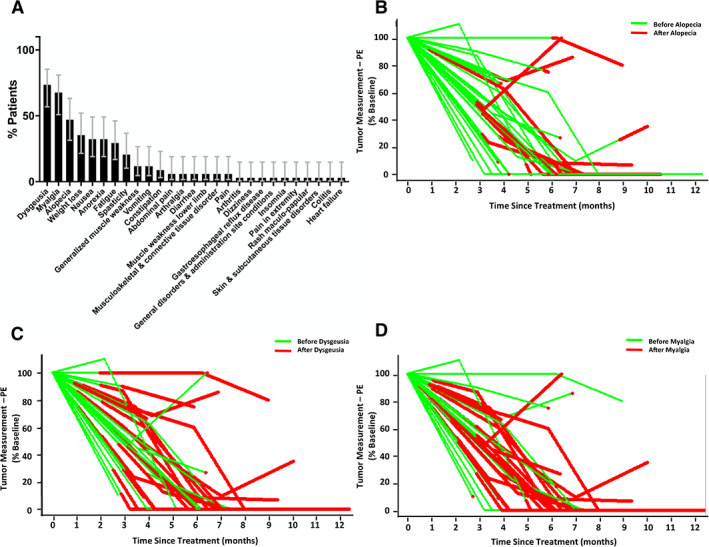
Adverse events related to vismodegib treatment. (A): Adverse events ranked by frequency of occurrence (error bars indicate 95% confidence intervals). (B): Tumor measurement versus alopecia onset. (C): Tumor measurement versus dysgeusia onset. (D): Tumor measurement versus myalgia onset. Abbreviation: PE, physical exam.
Discussion
BCC is a cutaneous malignancy associated with sun exposure. It is the most common malignancy in the U.S. [1] and among white individuals in general [27, 28], with a particular predilection for the eyelids [2]. For most patients with BCC, excision with clear margins is curative, but for patients with orbital or extensive periocular BCC (opBCC), excision can be disfiguring and may place visual organs and function at risk.
The VISORB study was designed to test whether vismodegib treatment, either as a monotherapy or as a neoadjuvant therapy to excision, contributes to preservation of visual organs and function in patients with advanced and/or recurrent opBCC. In our study, prior to treatment, 56% of patients had tumors with orbital involvement of extraocular muscle insertions and physical contact with sclera, for whom excision for a cure with clear margins would have required orbital exenteration. For the remaining 44% of patients, excision would have resulted in lacrimal drainage apparatus and/or extraocular muscle damage that would have limited visual function. Remarkably, 100% of patients who completed the study were spared exenteration of the affected globe. In addition, 100% of patients maintained a successful visual function outcome, as documented in serial ophthalmic exams and measured by the VAWS.
The tumor responses to vismodegib that we observed aligned with previous studies. In total, 56% of patients achieved complete responses based on clinical physical measurements. In addition, 67% of patients who underwent excision following vismodegib treatment showed no histopathological signs of disease in the surgical specimens. Nevertheless, it is important to note that we previously observed residual clusters of tumor cells of uncertain viability in tissue after vismodegib treatment [20]. Furthermore, the inflammation and fibrosis due to successful vismodegib treatment may mask residual small microtumors or single cells from histopathological assessment of surgical margins. It is also possible that portions of tumors exhibiting altered phenotype, such as squamous cell differentiation [20], may also be resistant to vismodegib and remain as a nidus of residual neoplasm causing later tumor regrowth. Molecular genetic studies are ongoing to address these questions and will be published at a later date. These questions do not negate the observed preservation of visual function achieved through use of vismodegib but underlie the clinical need for continued surveillance of patients treated using vismodegib with or without subsequent residual tumor excision.
The inability of vismodegib to achieve a complete histological cure is not a novel observation. Studies in animal models clearly highlight this phenomenon [29]. In addition, trials of vismodegib in patients with Gorlin Syndrome (with vismodegib‐sensitive PTCH1 mutations) showed that despite complete clinical response, tumors regrow rapidly following treatment withdrawal [15]. These studies, coupled with our own, provide compelling evidence that despite high clinical “cure” rates with vismodegib monotherapy, vismodegib as a neoadjuvant to surgery or in combination with other treatments is more likely to achieve a true cure.
The VAWS was created to address a void in research tools for studying diseases that affect orbital function. The bony orbit houses the ocular globe and associated extraocular muscles, nerves, blood vessels, conjunctival lining, lacrimal gland, and lacrimal drainage system. The tissues of the orbit are protected by mobile eyelids that cover the ocular surface, providing physical protection, oxygenation, and lubrication while enabling visual function through the palpebral fissure during waking hours. To assess the success or failure of treatment, all aspects of orbital structure function must be assessed. The VAWS elements are by design redundant, to provide internal validation of each measure. These include the presence of the globe, absolute and change in visual acuity, the absence of diplopia and presence of stereopsis (which require both good extraocular motility and fusion of images in the visual cortex), the presence or absence of epiphora along with objective assessment of the lacrimal drainage apparatus, and a patient‐reported assessment of overall visual function. Assessment of the redundant elements within the VAWS reveal that they track together, providing internal confirmation of validity. The strongest associations were between “VA versus 20/200,” “fusion,” and “satisfaction” and between “no tearing” and “lacrimal intact” (supplemental online Fig. 1). It is important to note that four components, “intact globe,” “VA versus baseline,” “VA vs. 20/200,” and “no diplopia,” displayed great imbalance in score distribution, which precludes confident assessment of association (supplemental online Table 1). For the purpose of this trial, preservation of the globe was given the highest weight. However, different studies may distribute the weights differently, depending on the goals of the trial. Additional studies are warranted to test and validate the VAWS in a variety of trials on orbital structure and function. However, this study also documented the individual elements of the ophthalmic exam, which were tracked throughout the study in a standard fashion.
Our trial patients experienced similar toxicity profiles as published in other reports. Nearly 80% of patients had to undergo surgery prior to completion of a 1‐year course of treatment with vismodegib. Importantly, by the time common adverse reactions were reported, most tumors had already responded (Fig. 4B–D), meaning that globe‐ and function‐sparing surgery was now possible, usually via Mohs micrographic surgery, which enabled careful assessment of both peripheral and deep margins. Thus, vismodegib may be most useful as a neoadjuvant therapy, with a 3–6‐month course of medical therapy preceding surgical excision for histologic clearance and potential cure. Such an approach would maximize utility of the drug for organ preservation while minimizing toxicity and adverse events.
Vismodegib is “indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation.” [12] Because patients with orbital BCC are technically candidates for surgery, even if that surgery requires exenteration and results in loss of visual function, vismodegib is often overlooked for these patients. Our data suggest that vismodegib is highly effective in preserving essential ocular structures and visual acuity in patients with advanced opBCC. Exenteration results in loss of the eye and causes major facial deformity. Multiple studies have shown that patients experience a significant reduction in quality of life following exenteration [30, 31]. Furthermore, for locally advanced tumors, even globe‐sparing surgery frequently results in irreversible loss of visual function because of ocular misalignment and surface disorders, which often require multiple reconstructive surgeries that are both costly and disfiguring [5, 32]. Hence, preservation of the globe and visual function should be the ultimate goal of treatment for patients with opBCC that threatens the globe and associated essential ocular structures such as extraocular muscles, full thickness eyelid tissue, and the lacrimal drainage system. Based on our results, we also would advocate for using vismodegib when surgery would result in total loss of an eyelid, particularly the upper eyelid, which would require extensive reconstructive surgeries following surgical excision and rarely provide fully satisfactory results. Instead, use of vismodegib as a neoadjuvant for 3–6 months provides tumor shrinkage, facilitating a more limited surgical removal of the residual tumor and potentially greater preservation of eyelid structures and functions. With careful clinical surveillance, these patients can maintain their eye and quality of life, while being monitored for the possibility of further therapy should tumor recur despite histologic clearance.
The tumor response rate in this study was quite high, with 25 of 34 patients (74%) achieving a complete clinical response on medical treatment alone (2) or in combination with surgery (23). This rate is higher than previously published for treatment of locally advanced BCC throughout the body. It is important to note that although locally advanced BCCs throughout the body may achieve a size measured in centimeters, most visually significant opBCCs are <2 cm in size and are considered locally advanced only because they occur in a particularly sensitive region of the face. It is quite conceivable that opBCCs are particularly sensitive to antihedgehog therapy because they are still relatively small on an absolute scale and have not had a chance to accumulate resistance mutations. Hence, although neoadjuvant therapy with a hedgehog inhibitor appears to be effective for opBCC, our results cannot be easily extrapolated to other areas of the body. This is also a single‐center study, which limits potential confounding variables inherent in previous multicentered studies.
Conclusion
This trial is the first prospective clinical trial to solely assess end‐organ functional preservation with vismodegib treatment for BCC. Our findings reveal that vismodegib is effective in protecting the eye and visual function, either alone or more commonly as a neoadjuvant therapy. Use of vismodegib as a neoadjuvant therapy was effective at reducing the tumor burden prior to surgery while reducing the toxicity. Such treatment should be considered for opBCC that might otherwise require orbital exenteration or surgeries that cause significant loss of function in addition to facial deformities.
Author Contributions
Conception/design: Alon Kahana, Shelby Unsworth, Francis Worden, Christopher Andrews
Provision of study material or patients: Alon Kahana, May P. Chan, Scott C. Bresler, Christopher K. Bichakjian, Alison B. Durham, Hakan Demirci, Victor Elner, Christine C. Nelson, Denise S. Kim, Shannon S. Joseph, Paul L. Swiecicki, Francis P. Worden
Collection and/or assembly of data: Alon Kahana, Shelby P. Unsworth, Christopher A. Andrews, May P. Chan, Scott C. Bresler
Data analysis and interpretation: Alon Kahana, Shelby P. Unsworth, Christopher A. Andrews
Manuscript writing: Alon Kahana, Shelby P. Unsworth, Christopher A. Andrews
Final approval of manuscript: Alon Kahana, Shelby P. Unsworth, Christopher A. Andrews, May P. Chan, Scott C. Bresler, Christopher K. Bichakjian, Alison B. Durham, Hakan Demirci, Victor Elner, Christine C. Nelson, Denise S. Kim, Shannon S. Joseph, Paul L. Swiecicki, Francis P. Worden
Disclosures
Alon Kahana: Genentech (RF, C/A); Shannon S. Joseph: Horizon Therapeutics (C/A); Francis P. Worden: Merck Sharp & Dohme, Eisai, Bristol‐Myers Squibb, LOXO, Bayer, CUE Biopharma (H), Merck, Loxo, Bristol‐Myers Squibb, Eisai, Bayer, CUE Biopharma, Rakuten, Exilexis (C/A), Bristol‐Myers Squibb, Loxo, Oragenics, Eli Lilly & Co., Pfizer, Merck, Eisai (RF), Merck Sharp & Dohme, Bayer (Other–Travel, Accommodations, Expenses). The other authors indicate no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.
Acknowledgments
The study was funded by Genentech National Cancer Institute: Head Neck SPORE, Research to Prevent Blindness, University of Michigan Rogel Cancer Center and University of Michigan Kellogg Eye Center.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Rogers HW, Weinstock MA, Feldman SR et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015;151:1081–1086. [DOI] [PubMed] [Google Scholar]
- 2. Saleh GM, Desai P, O Collin JR et al. Incidence of eyelid basal cell carcinoma in England: 2000‐2010. Br J Ophthalmol 2017;101:209–212. [DOI] [PubMed] [Google Scholar]
- 3. Smeets NW, Krekels GAM, Ostertag JU et al. Surgical excision vs Mohs’ micrographic surgery for basal‐cell carcinoma of the face: Randomised controlled trial. Lancet 2004;364:1766–1772. [DOI] [PubMed] [Google Scholar]
- 4. Weesie F, Naus NC, Vasilic D et al. Recurrence of periocular basal cell carcinoma and squamous cell carcinoma after Mohs micrographic surgery: A retrospective cohort study. Br J Dermatol 2019;180:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard GR, Nerad JA, Carter KD et al. Clinical characteristics associated with orbital invasion of cutaneous basal cell and squamous cell tumors of the eyelid. Am J Ophthalmol 1992;113:123–133. [DOI] [PubMed] [Google Scholar]
- 6. Leibovitch I, McNab A, Sullivan T et al. Orbital invasion by periocular basal cell carcinoma. Ophthalmology 2005;112:717–723. [DOI] [PubMed] [Google Scholar]
- 7. Furdova A, Lukacko P. Periocular basal cell carcinoma predictors for recurrence and infiltration of the orbit. J Craniofac Surg 2017;28:e84–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun MT, Wu A, Figuiera E et al. Management of periorbital basal cell carcinoma with orbital invasion. Future Oncol 2015;11:3003–3010. [DOI] [PubMed] [Google Scholar]
- 9. Shi Y, Jia R, Fan X. Ocular basal cell carcinoma: A brief literature review of clinical diagnosis and treatment. Onco Targets Ther 2017;10:2483–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson RL, Rothman AL, Xie J et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996. 272:1668–1671. [DOI] [PubMed] [Google Scholar]
- 11. Xie J, Murone M, Luoh SM et al. Activating Smoothened mutations in sporadic basal‐cell carcinoma. Nature 1998;391:90–92. [DOI] [PubMed] [Google Scholar]
- 12. Axelson M, Liu K, Jiang X et al. U.S. Food and Drug Administration approval: Vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res 2013;19:2289–2293. [DOI] [PubMed] [Google Scholar]
- 13. Sekulic A, Migden MR, Oro AE et al. Efficacy and safety of vismodegib in advanced basal‐cell carcinoma. N Engl J Med 2012;366:2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Von Hoff DD, LoRusso PM, Rudin CM et al. Inhibition of the hedgehog pathway in advanced basal‐cell carcinoma. N Engl J Med 2009;361:1164–1172. [DOI] [PubMed] [Google Scholar]
- 15. Tang JY, Mackay‐Wiggan JM, Aszterbaum M et al. Inhibiting the hedgehog pathway in patients with the basal‐cell nevus syndrome. N Engl J Med 2012;366:2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. LoRusso, PM , Rudin CM, Reddy JC et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC‐0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res 2011;17:2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ally MS, Aasi S, Wysong A et al. An investigator‐initiated open‐label clinical trial of vismodegib as a neoadjuvant to surgery for high‐risk basal cell carcinoma. J Am Acad Dermatol 2014;71:904–911.e1. [DOI] [PubMed] [Google Scholar]
- 18. Kwon GP, Ally MS, Bailey‐Healy I et al. Update to an open‐label clinical trial of vismodegib as neoadjuvant before surgery for high‐risk basal cell carcinoma (BCC). J Am Acad Dermatol 2016;75:213–215. [DOI] [PubMed] [Google Scholar]
- 19. Sofen H, Gross KG, Goldberg LH et al. A phase II, multicenter, open‐label, 3‐cohort trial evaluating the efficacy and safety of vismodegib in operable basal cell carcinoma. J Am Acad Dermatol 2015;73:99–105.e1. [DOI] [PubMed] [Google Scholar]
- 20. Kahana A, Worden FP, Elner VM. Vismodegib as eye‐sparing adjuvant treatment for orbital basal cell carcinoma. JAMA Ophthalmol 2013;131:1364–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. González AR, Etchichury D, Gill ME et al. Neoadjuvant Vismodegib and mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg 2019;35:56–61. [DOI] [PubMed] [Google Scholar]
- 22. Eiger‐Moscovich M, Reich E, Tauber G et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol 2019;207:62–70. [DOI] [PubMed] [Google Scholar]
- 23. Sagiv O, Nagarajan P, Ferrarotto R et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol 2019;103:775–780. [DOI] [PubMed] [Google Scholar]
- 24. Gill HS, Moscato EE, Chang ALC et al. Vismodegib for periocular and orbital basal cell carcinoma. JAMA Ophthalmol 2013;131:1591–1594. [DOI] [PubMed] [Google Scholar]
- 25. Ben Ishai M, Tiosano A, Fenig E et al. Outcomes of vismodegib for periocular locally advanced basal cell carcinoma from an open‐label trial. JAMA Ophthalmol 2020;138:749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 27. Hoban PR, Ramachandran S, Strange RC. Environment, phenotype and genetics: Risk factors associated with BCC of the skin. Expert Rev Anticancer Ther 2002;2:570–579. [DOI] [PubMed] [Google Scholar]
- 28. Nan H, Kraft P, Hunter DJ et al. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer 2009;125:909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eberl M, Mangelberger D, Swanson JB et al. Tumor architecture and Notch signaling modulate drug response in basal cell carcinoma. Cancer Cell 2018;33:229–243.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rasmussen ML, Ekholm O, Prause JU et al. Quality of life of eye amputated patients. Acta Ophthalmol 2012;90:435–440. [DOI] [PubMed] [Google Scholar]
- 31. Ye J, Lou L, Jin K et al. Vision‐related quality of life and appearance concerns are associated with anxiety and depression after eye enucleation: A cross‐sectional study. PLoS One 2015;10:e0136460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerring RC, Ott CT, Curry JM et al. Orbital exenteration for advanced periorbital non‐melanoma skin cancer: Prognostic factors and survival. Eye (Lond) 2017;31:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.


