Abstract
Background
Both protracted irinotecan and antiangiogenesis therapy have shown promising efficacy against Ewing sarcoma (EWS).
Methods
Patients diagnosed with recurrent or refractory EWS were enrolled and further categorized into cohort A (≥16 years) or cohort B (<16 years). In the dose‐defining phase Ib portion, anlotinib was given daily at a fixed dose, while a 3+3 design with dose de‐escalation was used to determine the dose of irinotecan. The next dose‐expanding phase II portion employed a conventional two‐stage study design model. The primary endpoint was objective response rate at 12 weeks (ORR12w).
Results
A total of 41 patients finally received the treatment regimen, including 29 in cohort A and 12 in cohort B. For cohort A, the first five patients were treated at the initial level of 20 mg/m2/d d × 5 × 2, and two of them subsequently a dose‐limiting toxicity (DLT). An additional six patients were then treated at 15 mg/m2 without any DLT, and the RP2D was determined. Notably, 23 out of 24 patients in cohort A were available for response evaluation at 12 weeks. ORR12w was determined to be 62.5%. For cohort B, no DLT was observed in the first six patients at the initial dose level. At last, 12 patients were included in cohort B. The ORR12w was 83.3%. The most frequently observed grade 3/4 adverse events were leukopenia (28.5%), neutropenia (24.4%), anemia (8.7%), and diarrhea (3.7%).
Conclusion
The combination of vincristine, irinotecan, and anlotinib demonstrated an acceptable toxicity profile and promising clinical efficacy in patients with advanced EWS.
Implications for Practice
This is the first trial to evaluate an irinotecan‐based regimen in combination with antiangiogenesis tyrosine kinase inhibitors in Ewing sarcoma (EWS). A 3+3 design with dose de‐escalation was used to determine the most appropriate dose of irinotecan in each cohort. The next dose‐expanding phase II portion employed a conventional two‐stage study design model. The objective response rate was 62.5% for adults and 83.3% for children. Median overall survival was not matured. This study shows that the combination of vincristine, irinotecan, and anlotinib demonstrates an acceptable toxicity profile and promising clinical efficacy in patients with advanced EWS.
Keywords: Anlotinib, Irinotecan, Ewing sarcoma
Short abstract
This article reports the results of a phase Ib/II trial to evaluate the safety and efficacy of anlotinib, vincristine, and irinotecan in patients with advanced Ewing sarcoma.
Background
Ewing sarcoma (EWS) is a small round blue cell tumor derived from primordial mesenchymal stem cells, which often originates in the bone marrow. The prognosis of patients with EWS refractory to first‐line treatment or recurring after radical therapy is relatively poor [1]. Although numerous potential drugs, including targeted therapy and immunotherapy, have been tested over the past 15 years [2, 3], the best therapeutic response was achieved by traditional irinotecan‐based regimens [4]. Compared to single‐bolus dose, a protracted administration of irinotecan for more than 5 days could lead to better outcomes in pediatric patients with sarcoma [4, 5].
Small molecular tyrosine kinase inhibitors (TKIs) targeting a broad spectrum of vascular endothelial growth factor receptor (VEGFR) have been introduced in patients with EWS, and promising results have been reported [6, 7, 8]. Anlotinib is a multikinase angiogenesis inhibitor that suppresses the activation of VEGFR2, platelet‐derived growth factor receptor beta (PDGFRβ), and fibroblast growth factor receptor 1 [9] and exhibits antitumor activity in several soft‐tissue sarcoma entities. According to previous reports in soft‐tissue sarcomas, we noticed that anlotinib may have better tolerance [10, 11]. It has been demonstrated that irinotecan can synergistically enhance the antiproliferative and proapoptotic effects of multikinase angiogenesis inhibitors in preclinical models [12]. The combination of multikinase angiogenesis inhibitors and irinotecan has been applied to colorectal cancer [13] and pancreatic cancer [12], which shows good therapeutic efficacy and low toxicity. Based on the evidence of this synergistic effect, we designed a multicenter, two‐cohort, phase Ib/II trial to evaluate the safety and efficacy of anlotinib, vincristine, and irinotecan (AVI) in patients with advanced EWS.
Subjects, Materials, and Methods
Study Design
This is a multicenter, two‐cohort, phase Ib/II clinical trial (NCT03416517). Patients were enrolled into different cohorts according to age at enrollment: cohort A (≥16 years) and cohort B (<16 years). Dose‐limiting toxicity (DLT) is defined by any of the following adverse events: (a) grade 4 neutropenia lasting >7 days or febrile neutropenia; (b) grade 4 thrombocytopenia (platelet <25,000/mm3); (c) grade 3 or 4 nonhematological adverse effects with the specific exclusion of grade 3 nausea or vomiting, grade 3 diarrhea or abdominal pain, and grade 3 hypertension; (d) delay in starting the next treatment cycle within 7 days or treatment discontinuation due to adverse events; and/or (e) treatment‐related death. Prophylactic use of granulocyte colony‐stimulating factor was permitted.
In the dose‐defining phase Ib portion, a 3+3 design with dose de‐escalation was used to evaluate the recommended level of irinotecan in each cohort. Specifically, a fixed anlotinib level of 12 mg was given to cohort A and 8 or 12 mg to cohort B (12 mg if body surface area [BSA] is ≥1.0 m2 or 8 mg if BSA is <1.0 m2). Different dose levels of irinotecan were predesigned according to previous literature and experience [4]. Recommended phase II dose (RP2D) was defined as the highest dose at which no more than 30% patients experienced a DLT during the first two courses. In each cohort, the first three patients were treated at a standard dose level 0, and three additional patients were treated at this level if one or no DLT was noticed. If no more than one DLT was observed in all the six patients, this level was then adopted as RP2D. If two or more of these patients experienced a DLT, the subsequent three patients were enrolled at a lower level.
In the next dose‐expanding phase II portion, RP2D defined in the previous phase Ib was adopted. A conventional two‐stage study design model was used to distinguish a favorable true response rate of 24% from a null rate of 5%. In stage 1, 12 patients were recruited. If one or more patients experienced objective responses, an additional 10 patients were enrolled into stage 2 to achieve a total population of 22 patients. Objective response rate (ORR) was defined as the proportion of patients with partial response (PR) and complete response (CR), ORR = CR + PR. If 3 or more of the 22 patients experienced objective responses, the regimen was considered effective. This statistical model assumed a null versus alternative response rate as 5% versus 24%, with a 9% type I error and 91% power. This design could reach a 54% probability of stopping early if the drug was ineffective. Patients who were initially treated at the RP2D level in phase Ib, with the presence of target lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, were directly transferred to the phase II portion.
This clinical trial was conducted after approval by the local institutional review board and independent ethics committee and done in accordance with the ethical principles derived from the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice Guidelines and locally applicable laws and regulations on January 22, 2018. Informed consent was obtained from each patient in Chinese.
Data available on request from the authors.
Inclusion and Exclusion Criteria
The inclusion criteria for phase Ib were as follows: (a) age ≥3 years; (b) histologically confirmed recurrent or refractory unresectable EWS, but EWS‐FLI 1 translocation by fluorescence in situ hybridization was not required in this study; (c) prior treatment consisted of standard EWS chemotherapy agents such as doxorubicin, vincristine, cyclophosphamide, ifosfamide, and etoposide; (d) adequate organ function; (e) an Eastern Cooperative Oncology Group performance status of 0 or 1; (f) an estimated life expectancy of ≥3 months; and (g) time elapsed from previous therapy must be ≥3 weeks for systemic therapy and ≥2 weeks for radiation therapy or major surgery. For the phase II portion, the existence of an evaluable lesion according to the RECIST version 1.1 was required in addition to the general criteria of phase Ib.
Patients were excluded if they had (a) poorly controlled hypertension; (b) metastasis to the central nervous system; (c) persistent clinically significant toxicities caused by previous cancer therapy; and/or (d) active hepatitis B, hepatitis C, or HIV.
Treatment Regimen
Protocol treatment was defined as a regimen of AVI. Specifically, the treatment consisted of a 90‐minute intravenous infusion of irinotecan at a dose of 20 mg/m2/d for 5 days at weeks 1 and 2 every 3 weeks (d × 5 × 2), vincristine given at a dose of 1.4 mg/m2 (maximum 2 mg) on days 1 and 8, and an oral administration of anlotinib once daily on days 1–14 within a 21‐day cycle. The treatment was continued until disease progression, unacceptable toxicity, withdrawal of consent, or discretion of the investigators. The whole regimen was delayed if the laboratory results on the first planned day of each cycle were absolute neutrophil count <1,000/mm3, platelets <75,000/mm3, hemoglobin <8 g/dL, serum transaminase >3 upper limit of normal (ULN), total bilirubin >1.5 ULN, common terminology criteria for adverse events (CTCAE) grade >2, as well as the occurrence of proteinuria or symptomatic toxicity. Irinotecan was obtained from Jiangsu Hengrui Medicine, Lianyungang, China, whereas anlotinib was supplied by Nanjing Zhengda Tianqing Pharmaceutical Co., Ltd.
Assessment and Follow‐Up
Pretreatment evaluation included a medical history; physical examination; complete blood cell count; serum biochemical tests; and chest, abdominal, and pelvic computed tomography (CT) scans or positron emission tomography CT (PET/CT). Clinical examination and laboratory tests were conducted before and during every treatment cycle. Tumor responses were evaluated every two courses according to the RECIST version 1.1. If PR was first recorded, it should be re‐evaluated and confirmed after two more courses. All adverse events during chemotherapy were evaluated every course using the National Cancer Institute CTCAE version 4.0. The minimum follow‐up period was 6 months for each patient.
Treatment Outcomes
For phase Ib, the aim was to determine the optimal RP2D of AVI in each cohort. For the phase II portion, the primary endpoint was ORR at week 12 in each cohort, and the secondary endpoints included OS, PFS, and FFS. PFS was defined as the time from study entry until documented progression, protocol violation determined at investigator's discretion, serious adverse effect, or death from any cause. FFS was defined as the time from study entry to first documented progression or death from any cause.
Results
From March 5, 2018 to October 5, 2018, 41 patients received the AVI treatment regimen, including 29 patients in cohort A and 12 in cohort B. The median follow‐up times were 18 months (interquartile range [IQR] 16–19) in cohort A and 20 months (IQR 18–22) in cohort B, respectively. A total of 36 patients were finally enrolled in the phase II portion (Table 1).
Table 1.
Demographics and baseline characteristics
| Patients | Cohort A (n = 24) | Cohort B (n = 12) |
|---|---|---|
| Age (years; mean ± SD) | 28 ± 9 | 11 ± 3 |
| Sex, n (%) | ||
| Male | 19 (79.2) | 5 (41.7) |
| Female | 5 (20.8) | 7 (58.3) |
| ECOG performance status at enrollment, n (%) | ||
| 0 | 19 (79.2) | 10 (100.0) |
| 1 | 5 (20.8) | 2 (16.7) |
| Presence of metastasis, n (%) | ||
| No (local advanced disease) | 1 (4.2) | 2 (16.7) |
| Yes | 23 (95.8) | 10 (83.3) |
| Primary tumor location, n (%) | ||
| Extremities | 9 (37.5) | 5 (41.7) |
| Axial skeleton | 6 (25.0) | 3 (25.0) |
| Other | 9 (37.5) | 4 (33.3) |
| Site of target and non‐target lesions, n (%) | ||
| Lung only | 11 (45.8) | 4 (33.3) |
| Other organs involved | 13 (54.2) | 8 (66.7) |
| Time from first diagnosis to enrollment, n (%) | ||
| ≤24 months | 22 (91.7) | 12 (100.0) |
| >24 months | 2 (8.3) | 0 (0.0) |
| Lines of previous chemotherapy, n (%) | ||
| 1 | 15 (62.5) | 8 (66.7) |
| ≥2 | 9 (37.5) | 4 (33.3) |
| EWS‐FLI1 translocation, n (%) | ||
| No | 1 (4.2) | 1 (8.3) |
| Yes | 11 (33.3) | 9 (75.0) |
| Not available | 12 (50.0) | 2 (16.7) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EWS, Ewing sarcoma.
Phase Ib Trial
For adults in cohort A, the first five patients were initially treated with 20 mg/m2 of irinotecan in phase Ib portion, and two of them subsequently experienced a DLT. Both of them had a delay in starting the next treatment cycle for more than 7 days or treatment discontinuation due to diarrhea. An additional six patients were then treated with a lower dose (15 mg/m2) of irinotecan, and no DLT was observed. These six patients were directly transferred to the phase II portion.
For children in cohort B, no DLT was found in the first six patients treated with a standard dose (20 mg/m2) of irinotecan. All these patients were directly transferred to the phase II portion.
Phase II Trial
Patient Enrollment
A total of 27 patients and 12 patients were assigned to cohorts A and B, respectively. The median number of cycles received per patient was 8 (range, 1–16). For the 27 patients in cohort A, two withdrew informed consent before treatment, one was diagnosed with hepatitis B virus infection and thus excluded, and one withdrew informed consent after the first assessment following two treatment courses, which recorded as unconfirmed PR. In total, 23 patients were available for response evaluation at week 12. For cohort B, subject recruitment was halted prematurely by the investigators because of slow enrollment. Six patients in phase Ib were transferred to phase II directly, and an additional six patients were enrolled. Table 1 summarizes the baseline demographics and treatment characteristics of the patients.
Objective Response
Of the 23 evaluable patients in cohort A, 1 CR, 14 PR, 2 stable disease (SD), and 6 progressive disease (PD) were observed (supplemental online Fig. 1). Although two patients with bone lesions showed complete resolution of 18F‐fludeoxyglucose uptake within tumor volume, abnormal lesions were still noticed on MR scan. Both of them were recorded as complete metabolic response (CMR) and classified into PR. The value of ORR was calculated to be 65%.
Figure 1.
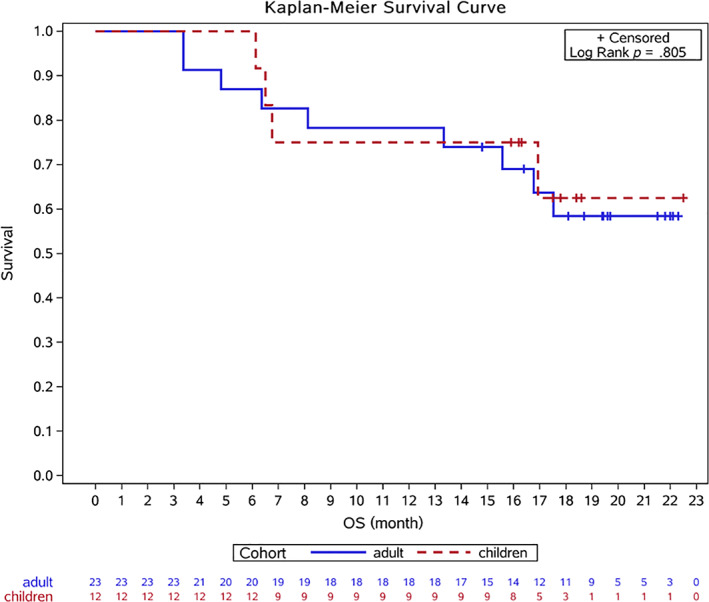
Overall survival. Abbreviation: OS, overall survival.
For cohort B, evaluation was available in all 12 patients. As shown in Table 2, there were four CR, six PR (including one CMR), and two PD. The ORR was 82%, and no SD was found in this cohort (supplemental online Fig. 2).
Table 2.
Efficacy of AVI in adult (n = 24) and child (n = 12) patients with advanced Ewing sarcoma
| Efficacy measurements | Adult (n =24) | Children (n = 12) |
|---|---|---|
| Response at 12 weeks | ||
| Complete response | 1 (4.2%) | 4 (33.3%) |
| Confirmed partial response a | 14 (58.3%) | 6 (50.0%) |
| Stable disease | 2 (8.3%) | 0 (0.0%) |
| Progressive disease | 6 (25.0%) | 2 (16.7%) |
| ORR at 12 weeks | 62.5% | 83.3% |
| ITT failure‐free survival | ||
| KM median, months (95% CI) | 10.2 (6.0, 14.2) | 8.7 (5.1, 14.4) |
| ITT overall survival | ||
| KM median | NR | NR |
| Patients' status at last follow‐up | ||
| NED | 3 (12.5%) | 3 (25.0%) |
| AWD | 12 (50.0%) | 5 (41.7%) |
| DOD | 9 (37.5%) | 4 (33.3%) |
One patient received only two cycles of AVI and was evaluated as unconfirmed PR.
Abbreviations: AVI, anlotinib, vincristine, and irinotecan; AWD, alive with disease; CI, confidence interval; DOD, died of disease; ITT, intention‐to‐treat population; KM, Kaplan‐Meier; NED, no evidence of disease; NR, not reached; ORR, overall response rate.
Figure 2.
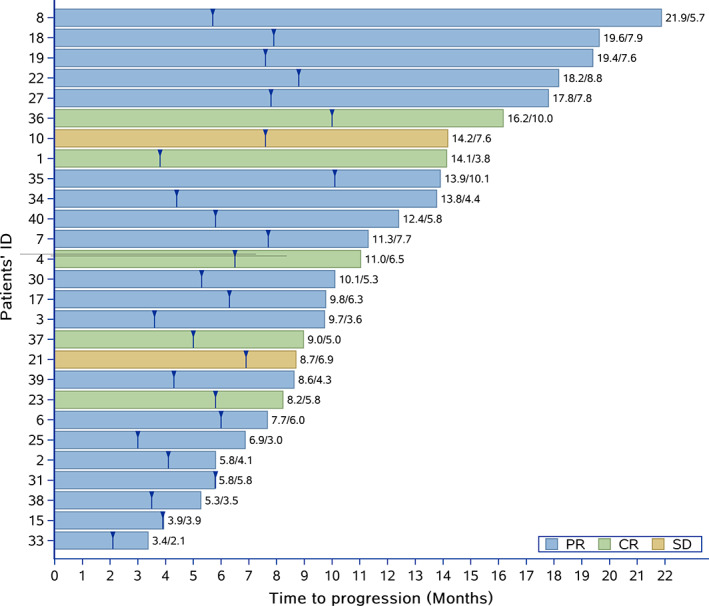
Duration of response for all patients (cohorts A and B). The numbers at the end of each line represent the duration of response. The number before the slant line means the whole period of time before progression, whereas the number after the slant line meansthe period of time from first evaluation of PR or CR to progression on treatment per protocol or the beginning of concurrent local treatment. Abbreviations: CR, complete response; PR, partial response; SD, stable disease.
Survival and Long‐Term Treatment Compliance
Considering the unexpected high efficacy, patients appealed for further local treatment to achieve better oncological outcome and better quality of life after obtaining CR or PR. For the benefit of patients, they were encouraged to discontinue standard AVI in the following situations: (a) whole lung radiation (WLI) if all pulmonary lesions were less than 3 mm in two consecutive CT scans; (b) surgical resection or radical radiation when a primary unresectable lesion became resectable and slow or no reduction in size was observed during the last assessment; (c) CR or nearly CR for longer than 6 months, and patients appealed for better quality of life, increased family involvement, and social work. Although the standard stick‐to‐protocol AVI was discontinued, a less dose‐intense regimen was adopted in the following treatment. All these situations of protocol violation were recorded as censored in the calculation of PFS. The times of events were recorded accordingly.
The median OS and PFS were not reached in both cohort A and cohort B (Fig. 1; supplemental online Fig. 3). The median FFS was 10 months (95% confidence interval [CI] 6–14) in cohort A and 8 months (95% CI 5–14) in cohort B (supplemental online Fig. 4). All 36 patients discontinued stick‐to‐ protocol AVI at last follow‐up: (a) Nine patients were recorded as PD at week 12. (b) Two patients were determined as PR at week 12 and then got PD subsequently. One of them developed a new bone lesion after two more cycles, and another patient showed progression of the primary lung lesion after four more cycles and eventually discontinued AVI. (c) Two patients discontinued AVI because of severe adverse effects (diarrhea at cycle 7 and fatigue at cycle 5). (d) One patient discontinued AVI at cycle 8 when one lesion showed progression, while other lesions displayed significant shrink. (e) All the other 22 (61.1%) patients discontinued AVI as decided by the investigators when they were still classified as CR or PR. Ten patients received WLI when their pulmonary lesions became smaller than 3 mm. Two patients received surgical resection and five patients underwent radical radiation. The remaining five patients received a less dose‐intense AVI after a median standard therapy of 8.8 months (Fig. 2).
Toxicity in Phase II Portion
Table 3 summarizes the toxicities (>1.0%) attributed to AVI in all assessable patients. The top three most common toxicities were leukopenia (70.1%), anemia (53.7%), and neutropenia (51.0%), and the most commonly reported grade 3/4 adverse effects were leukopenia (28.5%), neutropenia (24.5%), and anemia (8.7%).
Table 3.
Toxicities (>1.0%) attributed to therapy in assessable patients (n = 298 courses)
| Adverse event | All, n (%) | Grade 3/4, n (%) |
|---|---|---|
| Leukopenia | 209 (70.1) | 85 (28.5) |
| Anemia | 160 (53.7) | 26 (8.7) |
| Neutropenia | 152 (51.0) | 73 (24.5) |
| Hypokalemia | 79 (26.5) | 1 (0.3) |
| Hyponatremia | 79 (26.5) | 0 (0.0) |
| ALT increased | 75 (25.2) | 0 (0.0) |
| Diarrhea | 62 (20.8) | 11 (3.7) |
| Hypoalbuminemia | 61 (20.5) | 0 (0.0) |
| Hypertriglyceridemia | 51 (17.1) | 0 (0.0) |
| Hyperbilirubinemia | 40 (13.4) | 0 (0.0) |
| AST increased | 38 (12.8) | 0 (0.0) |
| Proteinuria | 32 (10.7) | 0 (0.0) |
| Hypothyroidism | 25 (8.4) | 0 (0.0) |
| Thrombocytopenia | 15 (5.0) | 2 (0.7) |
| Fatigue | 14 (4.7) | 4 (1.3) |
| Dyspepsia | 13 (4.4) | 0 (0.0) |
| Myalgia | 11 (3.7) | 0 (0.0) |
| Anorexia | 10 (3.4) | 2 (0.7) |
| Weight loss | 10 (3.4) | 2 (0.7) |
| Abdominal pain | 9 (3.0) | 3 (1.0) |
| Hypertension | 8 (2.7) | 2 (0.7) |
| DIC | 6 (2.0) | 0 (0.0) |
| Fever | 6 (2.0) | 1 (0.3) |
| Skin ulceration | 6 (2.0) | 3 (1.0) |
| Bone pain | 6 (2.0) | 0 (0.0) |
| Epistaxis | 5 (1.7) | 0 (0.0) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; DIC, disseminated intravascular coagulation.
Discussion
The results of this phase Ib/II study indicated the feasibility and efficacy of the multi‐targeted TKI anlotinib in combination with classical cytotoxic drugs irinotecan and vincristine in patients with recurrent or refractory EWS. In the phase Ib portion, dose de‐escalation of irinotecan was achieved without any DLT in pediatric patients with EWS. A lower irinotecan dose was adopted in adult patients due to diarrhea, the most common adverse effect caused by irinotecan. As for the dose of anlotinib in both cohorts and irinotecan in the children cohort, the classical dose of each drug was still adopted [10, 14, 15].
The irinotecan‐based regimen has been tested in patients with EWS in several prospective and retrospective trials. Several schedules of irinotecan administration have been studied. Compared with a high‐dose compressed regimen [16, 17], a protracted infusion of low‐dose irinotecan for more than 5 consecutive days showed better efficacy [18, 19, 20] in preclinical models [21] and clinical trials, especially in pediatric sarcomas. Frequent administration of low‐dose irinotecan should be considered to increase response rates in the clinic. Based on this point of view, we adopted the original schedule of d × 5 × 2 q3w. The combination of irinotecan and temozolomide with or without vincristine has been used to treat patients with EWS, with an ORR of 29%–63% [15, 22, 23, 24, 25]. In the recent rEECur trial, which is an international randomized controlled trial of chemotherapy for the treatment of recurrent and primary refractory Ewing sarcoma, the combination of irinotecan and temozolomide (d × 5 × 1, q3w) has shown an ORR of 20% versus 23% in topotecan/cyclophosphamide or ifosfamide group. However, 38% of patients discontinued before cycle 4 and were recorded as unevaluable, which may partly explain the relatively lower response rate [26]. In the phase II portion, compared with previously published data in the same group of heavily pretreated patients with EWS, we observed relatively high values of ORR in both cohorts. Although diarrhea was the main side effect of irinotecan, myelosuppression became a much more severe adverse effect of AVI regimen. The higher ORR and different spectrum of adverse events may be partly explained by the addition of anlotinib.
Antiangiogenic TKIs, such as cabozantinib [7], pazopanib [27], and apatinib [8], have shown meaningful activity in patients with EWS. As a multi‐targeted TKI, the target molecules (e.g., VEGFR 1‐3, EGFR, PDGFR‐α and ‐β, FGFR 1–3, and stem cell factor receptor) of anlotinib can contribute to the inhibition of tumor angiogenesis and consequently affect tumor cell growth function [9, 28]. The antitumor activity of anlotinib has been reported in many solid tumors, including non‐small cell lung cancer [29], thyroid cancer [30], and soft‐tissue sarcomas [10]. Previous studies showed that anlotinib was well tolerated in heavily treated patients [9, 10]. Synergistic effect of antiangiogenic TKI and irinotecan has been demonstrated in preclinical models [31], as well as in colorectal cancer, using sorafenib [13] or regorafenib [32]. However, an increased risk of diarrhea and neutropenia was observed in these trials. In this trial, we observed a higher ORR in the combination of AVI (65% in adults and 82% in children) compared with traditional irinotecan and vincristine (29%–63%) [15, 22, 23, 24].
In most trials, treatment will be continued unless there is disease progression or untolerated side effects. The eventual response rates in this trial were high, and the investigators attempted to cure more patients. To provide clinical benefits for patients, they were encouraged to receive WLI when all pulmonary lesions became too small to be evaluated, or surgical resection for primary unresectable lesions if surgeons feel confident regarding the adequacy of surgical margins. After local treatment (e.g., WLI), a less dose‐intense schedule was adopted in the subsequent treatment to improve their quality of life. All these data were recorded as endpoints in the calculation of PFS when they received local treatment or WLI. In our study, PFS was defined as the time from study entry until documented progression, protocol violation determined at investigator's discretion, serious adverse effect, or death from any cause. Based on such a high rate of protocol violation determined at investigator's discretion, the PFS in our study may be largely dependent on investigators (22/36, 66.1%), instead of disease progression (11/36, 30.6%) or severe adverse effects (2/36, 5.6%). FFS, where local treatment and WLI were allowed in the calculation, was believed to be the result of a comprehensive sequential treatment. Unfortunately, as a result of different sequential therapy, neither FFS nor PFS could reflect the process of tumor evaluation or biological resistance to AVI. The impacts of sequential therapy on final oncological outcome and long‐term prognosis will be discussed in our future article.
Given the high efficacy and good tolerance of AVI in patients with recurrent or refractory Ewing sarcoma, we believe it will become a promising choice in the future. The question is how to get long‐term disease‐free survival with good quality of life. In this study, we used WLI when all pulmonary lesions became too small to be evaluated and then stopped frequent chemotherapy or anlotinib. However, most of the patients experienced pulmonary recurrence later. We plan to design several maintenance tools, including anlotinib for at least half a year and stereotactic radiotherapy at an early stage rather than WLI at the end of chemotherapy, aiming at a longer recurrent‐free survival.
Conclusion
The combination of vincristine, irinotecan, and anlotinib is well tolerated and provides meaningful clinical efficacy in patients with relapsed or refractory EWS after first‐line chemotherapy. Future research is needed to establish the sequential treatment strategy for patients with advanced/recurrent EWS.
Author Contributions
Conception/design: Lu Xie, Jie Xu, Wei Guo
Provision of study material or patients: Wei Guo, Xiaodong Tang, Taiqiang Yan, Rongli Yang, Xin Sun
Collection and/or assembly of data: Lu Xie, Jie Xu, Xin Sun, Kuisheng Liu
Data analysis and interpretation: Kuisheng Liu, Lu Xie, Jie Xu, Wei Guo
Manuscript writing: Jie Xu
Final approval of manuscript: Jie Xu, Lu Xie, Wei Guo, Xin Sun, Kuisheng Liu, Xiaodong Tang, Taiqiang Yan, Rongli Yang
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information
Acknowledgments
This trial was funded by Chia‐tai Tianqing Pharmaceutical Co., Ltd. and Beijing Municipal Science and Technology Project (Z161100000116100). This trial was carried out in accordance with the Helsinki Declaration and was approved by the ethics committee of Peking University People's Hospital and Peking University Shougang Hospital. All patients gave written informed consent before study enrollment.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Alcindor T. Response of refractory Ewing sarcoma to pazopanib. Acta Oncol 2015;54:1063–1064. [DOI] [PubMed] [Google Scholar]
- 2. Leavey PJ, Mascarenhas L, Marina N et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi‐modality therapy: A report from the Children's Oncology Group. Pediatr Blood Cancer 2008;51:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stahl M, Ranft A, Paulussen M et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 2011;57:549–553. [DOI] [PubMed] [Google Scholar]
- 4. Xu J, Xie L, Sun X et al. Management of recurrent or refractory Ewing sarcoma: A systematic review of phase II clinical trials in the last 15 years. Oncol Lett 2019;18:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagner LM. Fifteen years of irinotecan therapy for pediatric sarcoma: Where to next? Clin Sarcoma Res 2015;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chuk MK, Widemann BC, Minard CG et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children's Oncology Group. Pediatr Blood Cancer 2018;65:e27077.29693796 [Google Scholar]
- 7. Italiano A, Mir O, Mathoulin‐Pelissier S et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2020;21:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie L, Guo W, Wang Y et al. Apatinib for advanced sarcoma: Results from multiple institutions' off‐label use in China. BMC Cancer 2018;18:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin B, Song X, Yang D et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018;654:77–86. [DOI] [PubMed] [Google Scholar]
- 10. Chi Y, Fang Z, Hong X et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft‐tissue sarcoma. Clin Cancer Res 2018;24:5233–5238. [DOI] [PubMed] [Google Scholar]
- 11. Tian Z, Liu H, Zhang F et al. Retrospective review of the activity and safety of apatinib and anlotinib in patients with advanced osteosarcoma and soft tissue sarcoma. Invest New Drugs 2020;38:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canu B, Fioravanti A, Orlandi P et al. Irinotecan synergistically enhances the antiproliferative and proapoptotic effects of axitinib in vitro and improves its anticancer activity in vivo. Neoplasia 2011;13:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samalin E, Bouché O, Thézenas S et al. Sorafenib and irinotecan (NEXIRI) as second‐ or later‐line treatment for patients with metastatic colorectal cancer and KRAS‐mutated tumours: A multicentre phase I/II trial. Br J Cancer 2014;110:1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dumont SN, Trent JC, Patel S et al. A phase II study of low‐dose protracted irinotecan in patients with advanced sarcomas. J Clin Oncol 2011;29(suppl 15):10064. [Google Scholar]
- 15. Raciborska A, Bilska K, Drabko K et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer 2013;60:1621–1625. [DOI] [PubMed] [Google Scholar]
- 16. Morland B, Platt K, Whelan JS. A phase II window study of irinotecan (CPT‐11) in high risk Ewing sarcoma: A Euro‐E.W.I.N.G. study. Pediatr Blood Cancer 2014;61:442–445. [DOI] [PubMed] [Google Scholar]
- 17. Bomgaars L, Kerr J, Berg S et al. A phase I study of irinotecan administered on a weekly schedule in pediatric patients. Pediatr Blood Cancer 2006;46:50–55. [DOI] [PubMed] [Google Scholar]
- 18. Bomgaars LR, Bernstein M, Krailo M et al. Phase II trial of irinotecan in children with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol 2007;25:4622–4627. [DOI] [PubMed] [Google Scholar]
- 19. Cosetti M, Wexler LH, Calleja E et al. Irinotecan for pediatric solid tumors: The Memorial Sloan‐Kettering experience. J Pediatr Hematol Oncol 2002;24:101–105. [DOI] [PubMed] [Google Scholar]
- 20. Mascarenhas L, Lyden ER, Breitfeld PP et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: A report from the Children's Oncology Group. J Clin Oncol 2010;28:4658–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vassal G, Boland I, Santos A et al. Potent therapeutic activity of irinotecan (CPT‐11) and its schedule dependency in medulloblastoma xenografts in nude mice. Int J Cancer 1997;73:156–163. [DOI] [PubMed] [Google Scholar]
- 22. Kurucu N, Sari N, Ilhan IE. Irinotecan and temozolamide treatment for relapsed Ewing sarcoma: A single‐center experience and review of the literature. Pediatr Hematol Oncol 2015;32:50–59. [DOI] [PubMed] [Google Scholar]
- 23. Wagner LM, McAllister N, Goldsby RE et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer 2007;48:132–139. [DOI] [PubMed] [Google Scholar]
- 24. Casey DA, Wexler LH, Merchant MS et al. Irinotecan and temozolomide for Ewing sarcoma: The Memorial Sloan‐Kettering experience. Pediatr Blood Cancer 2009;53:1029–1034. [DOI] [PubMed] [Google Scholar]
- 25. Palmerini E, Jones RL, Setola E et al. Irinotecan and temozolomide in recurrent Ewing sarcoma: An analysis in 51 adult and pediatric patients. Acta Oncol 2018;57:958–964. [DOI] [PubMed] [Google Scholar]
- 26. McCabe MG, Kirton L, Khan M et al. Results of the second interim assessment of rEECur, an international randomized controlled trial of chemotherapy for the treatment of recurrent and primary refractory Ewing sarcoma (RR‐ES). J Clin Oncol 2020;38(suppl 15):11502. [Google Scholar]
- 27. Mori Y, Kinoshita S, Kanamori T et al. The successful treatment of metastatic extraosseous Ewing sarcoma with pazopanib. Intern Med 2018;57:2753–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taurin S, Yang CH, Reyes M et al. Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer 2018;28:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han B, Li K, Wang Q et al. Effect of anlotinib as a third‐line or further treatment on overall survival of patients with advanced non‐small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol 2018;4:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dobbins DE, Swindall BT, Haddy FJ et al. Response of the forelimb vasculature to vasoactive agents: Effects of ouabain. Microcirc Endothelium Lymphatics 1985;2:517–549. [PubMed] [Google Scholar]
- 31. Mazard T, Causse A, Simony J et al. Sorafenib overcomes irinotecan resistance in colorectal cancer by inhibiting the ABCG2 drug‐efflux pump. Mol Cancer Ther 2013;12:2121–2134. [DOI] [PubMed] [Google Scholar]
- 32. Sanoff HK, Goldberg RM, Ivanova A et al. Multicenter, randomized, double‐blind phase 2 trial of FOLFIRI with regorafenib or placebo as second‐line therapy for metastatic colorectal cancer. Cancer 2018;124:3118–3126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information


