Abstract
Lessons Learned
Radiotherapy plus anti–PD‐1 antibody as first‐line therapy is safe and feasible in locally advanced esophageal squamous cell carcinoma (ESCC).
Tumor‐infiltrating and peripheral lymphocytes were associated with patient survival.
Further studies combining chemoradiotherapy with immunotherapy in locally advanced ESCC and exploration of predictive biomarkers are warranted.
Background
We conducted a phase Ib study of radiotherapy plus programmed cell death protein 1 (PD‐1) monoclonal antibody camrelizumab as first‐line treatment for locally advanced esophageal squamous cell carcinoma (ESCC).
Methods
We planned to enroll 20 patients with newly diagnosed locally advanced ESCC. Patients received 60 Gy radiation (2.0 Gy/fraction, 5 fractions/week), with camrelizumab (200 mg every 2 weeks) starting with radiotherapy and continuing for 32 weeks (i.e., for 16 cycles). The primary endpoints were safety and feasibility. Secondary endpoints were rates of radiologic and pathologic response, overall survival (OS), and progression‐free survival (PFS). Study data were collected by the week during radiotherapy (RT), every month during the maintenance camrelizumab treatment, and every 3 months after treatment. Tumor microenvironment and peripheral blood were monitored at baseline and after 40 Gy radiation for association with efficacy.
Results
Twenty patients were enrolled and received treatment. One patient (patient 10) was excluded upon discovery of a second tumor in the bladder during treatment, leaving 19 patients for analysis. Toxicity was deemed tolerable. Fourteen (74%) patients had assessed objective response. At a median follow‐up time of 31.0 months (95% confidence interval [CI], 27.0–35.1), median OS and PFS times were 16.7 months (95% CI, 5.9–27.9) and 11.7 months (95% CI, 0–30.3), respectively. OS and PFS rates at 24 months were 31.6% and 35.5%, respectively. Kaplan‐Meier analysis revealed associations between the following factors and OS/PFS: tumor programmed cell death ligand 1 (PD‐L1) expression, PD‐1+CD8+, PD‐1+CD4+ T cells, and PD‐L1+CD4+ T cells; peripheral blood CD4+, CD8+, CD4+ regulatory T cells, and their subsets.
Conclusion
Radiotherapy plus camrelizumab had manageable toxicity and antitumor efficacy for locally advanced ESCC. Several biomarkers were associated with clinical benefit and deserve further study.
Keywords: Radiotherapy, Immunotherapy, PD‐1, Camrelizumab, Esophageal cancer
Discussion
Camrelizumab (SHR‐1210) is a selective, humanized, IgG4‐kappa PD‐1 monoclonal antibody. It was developed by Jiangsu Hengrui Medicine Co. Ltd. and received conditional approval in China for the treatment of relapsed or refractory classical Hodgkin lymphoma in 2019. In 2020, another three indications were added for camrelizumab in China: (a) patients with advanced hepatocellular carcinoma who have previously received sorafenib and/or oxaliplatin‐based systemic chemotherapy; (b) combined with pemetrexed and carboplatin as the first‐line treatment of locally advanced or metastatic nonsquamous non‐small cell lung cancer that has no EGFR or ALK mutation; and (c) patients with locally advanced or metastatic ESCC as second‐line treatment. In this phase Ib study, we investigated the safety and feasibility of definitive RT plus camrelizumab as first‐line therapy for patients with locally advanced ESCC who were either ineligible for or had refused concurrent chemoradiotherapy (CCRT).
Of 19 patients enrolled from July 24, 2017, through January 25, 2018, 18 completed RT; 13 (68%) completed full cycles of camrelizumab. Objective responses were observed in 14 (74%) patients (2 [11%] complete responses and 12 [63%] partial responses). Among the 10 patients with PD‐L1–positive tumors, 5 experienced a major pathologic response (indicated by ≤1% viable residual tumor cells in the tumor specimen) (Fig. 1A).
Figure 1.
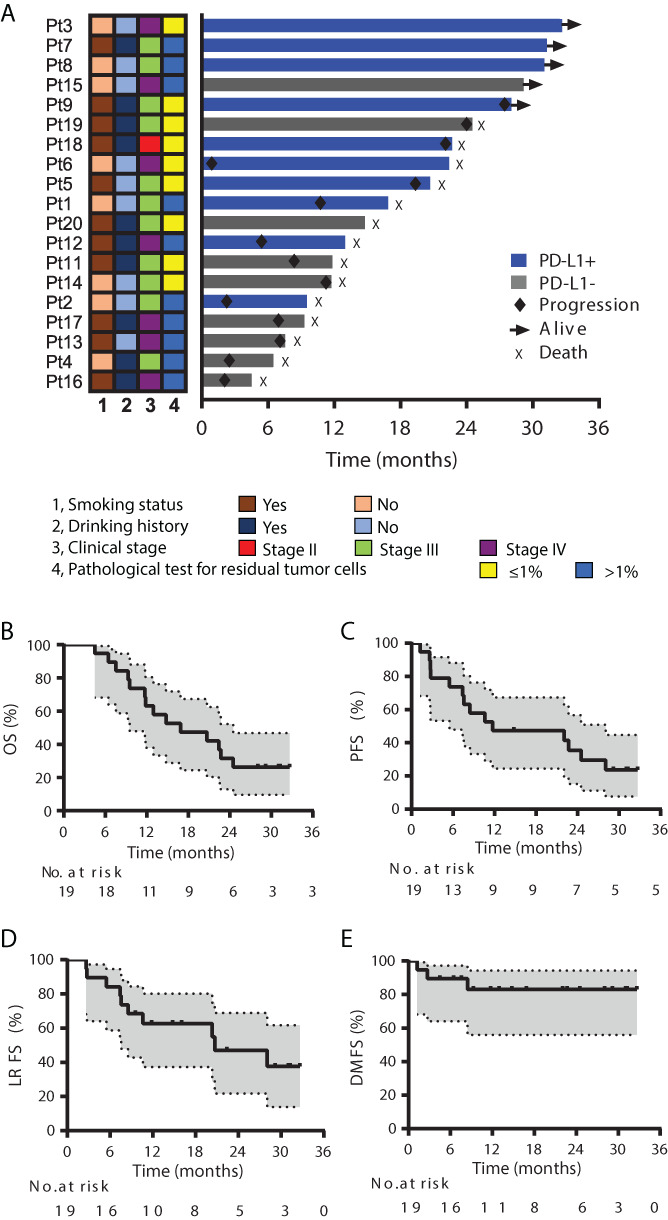
Antitumor efficacy of combining radiotherapy and SHR‐1210. (A): Treatment exposure and response duration. The length of each bar represents the time to the last radiographic assessment according to RECIST version 1.1. Clinical and pathological features (smoking status, drinking history, clinical disease stage, and pathological test for residual tumor cells after 40 Gy) are shown for each patient (per RECIST version 1.1 by investigator review). (B): Overall survival. (C): Progression‐free survival. (D): Local recurrence–free survival. (E): Distant metastasis–free survival. Abbreviations: DMFS, distant metastasis–free survival; LRFS, local recurrence–free survival; OS, overall survival; PD‐L1, programmed cell death ligand 1; PFS, progression‐free survival; Pt, patient.
The target result expected was a median OS of 14 months. Our results showed median OS time at 16.7 months (95% CI, 5.9–27.9); 12‐month and 24‐month OS rates were 63.2% and 31.6% (Fig. 1B). Median PFS time was 11.7 months (95% CI, 0–30.3); 12‐month and 24‐month PFS rates were 47.4% and 35.5% (Fig. 1C). The relative shorter 24‐month OS rate compared with 24‐month PFS rate was because one patient died from cerebral infarction after 14.8 months of enrollment. Rates of locoregional recurrence–free survival were 62.7% at 12 months and 48.8% at 24 months (Fig. 1D), and corresponding distant metastasis–free survival rates were both 83.1% (Fig. 1E). At 24 months, locoregional recurrence occurred in nine (47%) patients, and distant metastasis occurred in three (16%) patients (two in liver and one in bone). Although CCRT is considered standard therapy for locally advanced ESCC, median OS times are only about 18.1–19 months. For patients who are intolerant to or refuse chemotherapy, RT is the main treatment. However, the median OS was 12 months. The median OS in our study was comparable to that after CCRT and improved compared with RT alone [1, 2, 3].
All patients experienced some form of treatment‐related adverse events (AEs), but most were grade 1–2, and no grade 5 events were reported. The toxicity profile after RT plus camrelizumab was similar to previous reports of either modality given alone. Cutaneous capillary hemangioma was observed in 17 (89%) patients (15 [79%] with grade 1; 2 [10%] with grade 2), which was the immune‐related AE. Episodes of grade 3 and grade 4 RT‐related adverse events including lymphopenia, esophagitis, laryngitis, and leukopenia did not interrupt RT. These results indicate that the combination therapy did not increase RT‐related toxicity compared with RT alone and that adverse events associated with the combination therapy were less severe than those associated with CCRT [1, 3].
We found that tumor microenvironment markers were associated with survival. High tumor PD‐L1 (≥1%) expression at baseline was correlated with better OS. Interestingly, whole CD8+ or CD4+ T‐cell population in tumor tissues was not associated with survival, but their subpopulations (PD‐1+CD8+, PD‐L1+CD4+, and PD‐1+CD4+ T cells) were associated with OS/PFS. Our findings in peripheral blood supported the important role of CD4+, CD8+, and CD4+ regulatory T (Treg) cells in antitumor response. The association between OS/PFS and T‐cell subsets of Ki67+CD8+, interferon‐γ+CD8+, Ki67+Treg, and cytotoxic T‐lymphocyte–associated protein 4–positive CD4+ T cells in peripheral blood suggest that the T‐cell status and function, such as proliferation, activation, or exhaustion, deserve more studies in future.
This first study of RT plus camrelizumab as first‐line treatment showed promising efficacy and a manageable safety profile in patients with locally advanced ESCC.
Trial Information
| Disease | Esophageal cancer |
| Stage of Disease/Treatment | Primary |
| Prior Therapy | None |
| Type of Study | Phase I/Ib |
| Primary Endpoints | Safety, toxicity |
| Secondary Endpoints | Efficacy, biomarker associations |
| Additional Details of Endpoints or Study Design | |
| The main inclusion criteria were age ≥18 years; newly diagnosed, locally advanced ESCC (T3–4N0M0 or T1–4N+M0, stage II–IVa according to the 8th [2017] edition of the American Joint Committee on Cancer staging system); no prior antitumor treatment; ineligible for or declined concurrent chemoradiotherapy; Eastern Cooperative Oncology Group score 0 or 1; adequate liver function; and normal blood cell counts. The main exclusion criteria were diagnosis of immunodeficiency, ongoing systemic immunosuppressive therapy, active autoimmune disease, human immunodeficiency virus, and clinically significant concurrent cancer. | |
| Cervical, thoracic, and above‐abdomen computed tomography scans and upper gastrointestinal radiography were obtained every 3 months after treatment until disease progression. All patients underwent endoscopic ultrasonography, the standard clinical practice for potential tumor biopsy, after receiving 40 Gy of radiation [20 ] (i.e., at the end of 4 weeks of RT) in order to confirm the pathological response rates and laboratory tests. | |
| The primary endpoints were safety and feasibility. Treatment‐related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. We estimated that the risk of grade 5 adverse events would occur at a rate of about 5% according to the reported adverse events in concurrent chemoradiotherapy. | |
| The secondary endpoints were radiologic and pathologic response, scored according to RECIST version 1.1 by individual clinicians. Outcome measures were objective response rate 4 weeks after the end of RT and PFS and OS. Locoregional recurrence–free survival (LRFS) and distant metastasis–free survival (DMFS) rates were also assessed in this study. We expected that median OS, being a benchmark, would exceed 14 months according to median OS of 12 months with RT alone. | |
| The exploratory endpoints included biomarkers that associated with OS and PFS. Pairs of tumor tissue biopsy samples and EDTA‐anticoagulant–treated peripheral blood specimens were to be collected before treatment (i.e., baseline) and during treatment (after the delivery of 40 Gy RT). Immunohistochemistry and six‐color immunofluorescence were used to investigate PD‐L1 expression and identify tumor‐infiltrating lymphocytes in tumor tissues. Multiple‐color flow cytometry was applied to investigate markers in peripheral T‐cell populations. | |
| Among enrolled and eligible patients, the truncated sequential probability test was used to evaluate objective response rate. Statistical tests included two‐sided Fisher's exact tests and two‐sided Mann‐Whitney U tests. The Kaplan‐Meier method was used to estimate PFS, OS, LRFS, and DMFS. The best cutoff of Kaplan‐Meier survival analysis was calculated by the Youden index of the receiver operating characteristic curve. Differences in survival and recurrence rates were compared with log‐rank tests for all markers. SPSS (version 21.0; STATA, College Station, TX) or R version 3.2.2 packages were used for all analyses. All reported p values were two‐sided, and the significance level was set at .05. | |
| Investigator's Analysis | Drug tolerable, hints of efficacy |
Drug Information: Camrelizumab
| Generic Name | Camrelizumab |
| Drug Type | Antibody |
| Drug Class | Immune therapy |
| Dose | 200 milligrams (mg) per flat dose |
| Route | i.v. |
| Schedule of Administration | SHR‐1210 was infused intravenously at a fixed dose of 200 mg once every 2 weeks from the beginning of radiotherapy for up to 32 weeks (i.e., for 16 cycles). Radiotherapy was delivered as RapidArc (volumetric arc) intensity‐modulated RT with a simultaneous integrated boost. The radiotherapy was given according to Chinese treatment guidelines for esophageal carcinoma and was prescribed to cover 95% of the planning gross tumor volume (PGTV), given at 2.0 Gy per fraction, five fractions per week, to a total of 60 Gy over 6 weeks. The dose prescribed to cover 95% of the planning target volume (PTV) was 1.8 Gy per fraction, five fractions per week, for a total of 54 Gy over 6 weeks. Target volumes were as described previously. Briefly, gross tumor volume (GTV) was determined based on the results of upper gastrointestinal radiography, esophageal endoscopy, and chest computed tomography. If lymphatic metastasis was present in the mediastinum, supraclavicular region, or abdominal cavity, GTV in involved lymph nodes was delineated. The clinical target volume (CTV) was delineated as GTV and plus 3‐cm margins in the vertical direction, which covered the corresponding lymphatic drainage areas, and 0.6‐cm margins in the anteroposterior and transverse directions, which did not exceed the anatomic boundary. PGTV and PTV was defined as GTV or CTV plus 5‐mm margins, individually. No additional adjuvant or induction chemotherapy was performed. |
Patient Characteristics
| Number of Patients, Male | 12 |
| Number of Patients, Female | 7 |
| Stage |
Stage II: 1 Stage III: 11 Stage IV: 7 |
| Age | Median (range): 64 (46–74) years |
| Number of Prior Systemic Therapies | 0 |
| Performance Status: ECOG |
0 –– 10 1 –– 9 2 –– 0 3 –– 0 Unknown –– 0 |
| Other |
Smoking status: Never: 8; Former or current: 11 Drinking status: Never: 9; Former or current: 10 Location: Cervical segment: 1; Upper thoracic segment: 5; Middle thoracic segment: 11; Inferior thoracic segment: 2 |
| Cancer Types or Histologic Subtypes | Esophageal squamous cell carcinoma, 19 |
Primary Assessment Method
| Title | Safety and Feasibility |
| Number of Patients Screened | 26 |
| Number of Patients Enrolled | 19 |
| Number of Patients Evaluable for Toxicity | 19 |
| Number of patients Evaluated for Efficacy | 19 |
| Evaluation Method | NCI, CTCAE, version 4.0 |
| Response Assessment CR | n = 2 (10.5%) |
| Response Assessment PR | n = 12 (63.2%) |
| Response Assessment SD | n = 2 (10.5%) |
| Response Assessment PD | n = 3 (15.8%) |
| Response Assessment OTHER | n = 0 (0%) |
| (Median) Duration Assessments PFS | 11.7 months, CI: 0–30.3 |
| (Median) Duration Assessments OS | 16.7 months, CI: 5.9–27.9 |
Secondary Assessment Method
| Title | Objective Response Rate, OS, PFS |
| Number of Patients Screened | 26 |
| Number of Patients Enrolled | 19 |
| Number of Patients Evaluable for Toxicity | 19 |
| Number of Patients Evaluated for Efficacy | 19 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 2 (10.5%) |
| Response Assessment PR | n = 12 (63.2%) |
| Response Assessment SD | n = 2 (10.5%) |
| Response Assessment PD | n = 3 (15.8%) |
| Response Assessment OTHER | n = 0 (0%) |
| (Median) Duration Assessments PFS | 11.7 days, CI: 0–30.3 |
| (Median) Duration Assessments OS | 16.7 days, CI: 5.9–27.9 |
| Outcome Notes | |
| Evaluation of objective response rate (with RECIST 1.1) showed 2 (10%) complete responses, 12 (63%) partial responses, 2 (10%) stable disease, and 3 (16%) progressive disease. The median OS time was 16.7 months (95% CI, 5.9–27.9); OS rates were 63.2% at 12 months and 31.6% at 24 months (Figure 1B ). The median PFS time was 11.7 months (95% CI, 0–30.3); PFS rates were 47.4% at 12 months and 35.5% at 24 months. | |
Adverse Events
| All Cycles Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| Skin and subcutaneous tissue disorders | 11 | 79 | 11 | 0 | 0 | 0 | 89 |
| Lung infection | 89 | 5 | 5 | 0 | 0 | 0 | 11 |
| Pneumonitis | 89 | 0 | 5 | 5 | 0 | 0 | 11 |
| Esophagitis | 53 | 32 | 16 | 0 | 0 | 0 | 47 |
| Laryngitis | 95 | 0 | 0 | 5 | 0 | 0 | 5 |
| Constipation | 74 | 16 | 11 | 0 | 0 | 0 | 26 |
| Blurred vision | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Proteinuria | 63 | 37 | 0 | 0 | 0 | 0 | 37 |
| Blood and lymphatic system disorders ‐ Leukopenia | 68 | 16 | 11 | 5 | 0 | 0 | 32 |
| Blood and lymphatic system disorders ‐ Neutrophilic granulopenia | 68 | 0 | 32 | 0 | 0 | 0 | 32 |
| Blood and lymphatic system disorders ‐ Lymphopenia | 53 | 0 | 11 | 32 | 5 | 0 | 47 |
| Anemia | 95 | 5 | 0 | 0 | 0 | 0 | 5 |
| Alanine aminotransferase increased | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Hyperglycemia | 74 | 26 | 0 | 0 | 0 | 0 | 26 |
| Fatigue | 95 | 0 | 5 | 0 | 0 | 0 | 5 |
| Cough | 95 | 0 | 0 | 5 | 0 | 0 | 5 |
| Respiratory, thoracic and mediastinal disorders | 84 | 16 | 0 | 0 | 0 | 0 | 16 |
| Hypothyroidism | 84 | 16 | 0 | 0 | 0 | 0 | 16 |
Treatment‐related adverse events occurring in all cycles.
All patients experienced some form of treatment‐related AEs (Table 2), but most were grade 1–2, and no grade 5 events were reported. The most common type of toxicity was cutaneous capillary hemangioma (15 [79%] with grade 1 and 2 [10%] with grade 3), which was the immune‐related AE; all cases were managed with local therapy or observation. Nine patients (47%) experienced grade 3 adverse events: six with lymphopenia (which was not treated), one with radiation pneumonitis, one with radiation laryngitis (treated with dexamethasone), and one with leukopenia (treated with granulocyte‐macrophage colony‐stimulating factor). The patient with radiation laryngitis had cervical esophageal cancer. One patient (5%) experienced grade 4 lymphopenia, which was not treated. Three patients experienced grade 1 hypothyroidism. Other organ‐specific immune‐related AEs, such as thyroiditis, stomatitis, and colitis, were not found.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Radiation pneumonitis | 3 | Possible |
| Radiation laryngitis | 3 | Unrelated |
Serious treatment‐related adverse events occurred in all cycles.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Drug tolerable, hints of efficacy |
In the current study, we report that radiotherapy (RT) plus camrelizumab, used as first‐line therapy, has promising antitumor activity and a manageable toxicity profile for patients with locally advanced esophageal squamous cell carcinoma (ESCC). We further found associations between a variety of immune function–related biomarkers and overall survival (OS) and progression‐free survival (PFS) (Fig. 2).
Figure 2.

Consortium diagram and treatment schedule. (A): Consortium diagram. (B): Clinical treatment schedule. Abbreviations: CT, chemotherapy; IF, immunofluorescence; PD‐1, programmed cell death protein 1; Q2W, every 2 weeks; RT, radiotherapy; TME, tumor microenvironment.
Although concurrent radiotherapy (CCRT) is considered standard therapy for locally advanced ESCC, local recurrence rates after such therapy are as high as 50%, and median OS times are only about 18.1–19 months [1, 2, 3]. For patients who refuse or were ineligible for CCRT, RT alone is the treatment of choice; however, 5‐year OS rates after RT alone are <10% after two‐dimensional RT and 20%–30% for three‐dimensional conformal RT [1, 4, 5]. Recent clinical trials indicated that using anti–programmed cell death protein 1 (PD‐1) antibodies (nivolumab, pembrolizumab, or camrelizumab) as second‐line or consolidation therapy achieved objective clinical response rates of 22%–33% and a median PFS time of 1.8–3.6 months in patients with esophageal cancer [6, 7, 8, 9, 10]. In the current study of 19 enrolled patients, the combination of RT and camrelizumab resulted in median OS and PFS times of 16.7 months and 11.7 months in patients with newly diagnosed locally advanced ESCC who could not tolerate or declined CCRT (Table 1, 2, 3; Fig. 1). These durations are comparable to those after CCRT [1, 2, 3]. Indeed, clinical and preclinical studies of animal models have shown synergistic antitumor effects from adding RT to immunotherapy for advanced solid tumors [11, 12, 13, 14]. Our findings support these studies.
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Age at enrollment, years | |
| Mean | 62.6 |
| Median (range) | 64 (46–74) |
| Sex | |
| Female | 7 (37) |
| Male | 12 (63) |
| ECOG performance status score | |
| 0 | 10 (56) |
| 1 | 9 (47) |
| Smoking status | |
| Never | 8 (42) |
| Former or current | 11 (58) |
| Drinking status | |
| Never | 9 (47) |
| Former or current | 10 (56) |
| Location | |
| Cervical segment | 1 (5) |
| Upper thoracic segment | 5 (26) |
| Middle thoracic segment | 11 (58) |
| Inferior thoracic segment | 2 (11) |
| AJCC8 disease stage | |
| II | 1 (5) |
| III | 11 (58) |
| IV | 7 (37) |
Abbreviations: AJCC8, 8th edition of the American Joint Committee on Cancer Staging Manual (2017); ECOG, Eastern Cooperative Oncology Group.
Table 2.
Patient and treatment characteristics
| ID | Age at Dx, yr | Sex | Hx of smoking | No. pack‐years | Hx of drinking | ECOG score | Pre‐treatment disease stage a | Pre‐treatment disease stage b | Reason for no chemoRT | No. of SHR‐1210 cycles | Pathology findings after 40 Gy | Response after 40 Gy | Respond at end of RT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | F | No | 0 | No | 1 | III (T3N1M0) | III (T3N1M0) | Hypertension, age c | 16 | 5% residual TCs | PR | PR |
| 2 | 71 | F | No | 0 | No | 1 | III (T3N1M0) | III (T3N1M0) | Hypertension, diabetes, age | 11 (PD) | 5% residual TCs | SD | PR |
| 3 | 46 | F | No | 0 | No | 0 | III (T3N1M0) | IVA (T3N2M0) | Achalasia cardia | 16 | 0% residual TCs | PR | CR |
| 4 | 49 | M | No | 0 | Yes | 0 | III (T3N1M0) | III (T3N1M0) | Refused | 9 (PD) | 40% residual TCs | PR | PD |
| 5 | 69 | F | Yes | 25 | No | 1 | IIA (T3N0M0) | III (T3N0M0) | Vegetarian diet, Hx syphilis | 16 | 1% residual TCs | PR | PR |
| 6 | 62 | M | No | 0 | Yes | 0 | III (T4N1M0) | IVA (T4N1M0) | Hepatitis B | 16 | 0% residual TCs | SD | PD |
| 7 | 56 | M | Yes | 38 | Yes | 0 | III (T3N1M0) | III (T3N1M0) | Hypertension | 16 | 10% residual TCs | PR | PR |
| 8 | 61 | F | No | 0 | No | 1 | IV (T3N1M1) | III (T3N1M0) | Pyramidal fracture | 16 | <10% residual TCs | PR | PR |
| 9 | 66 | M | Yes | 40 | Yes | 0 | IIB (T2N1M0) | III (T2N1M0) | Hypertension | 16 | 0% residual TCs | PR | PR |
| 10 d | |||||||||||||
| 11 | 72 | M | Yes | 40 | Yes | 1 | III (T3N1M0) | III (T3N1M0) | Hepatitis B, age | 16 | 1% residual TCs | PR | PR |
| 12 | 71 | M | Yes | 75 | Yes | 0 | IV (T3N1M1b) | IVA (T3N2M0) | Hypertension, age | 14 (PD) | 5% residual TCs | PR | PR |
| 13 | 58 | M | Yes | 10 | No | 1 | III (T3N1M0) | IVA (T3N2M0) | Paraplegia, hepatitis C | 7 (anorexia) | >50% residual TCs | SD | SD |
| 14 | 65 | F | No | 0 | No | 0 | III (T3N1M0) | III (T3N1M0) | Refused | 16 | 0% residual TCs | SD | SD |
| 15 | 64 | F | No | 0 | No | 0 | III (T3NIM0) | IVA (T3N2M0) | Hypertension, diabetes, hyperlipemia | 16 | 5% residual TCs | SD | PR |
| 16 | 59 | M | Yes | 30 | Yes | 1 | III (T3NIM0) | IVA (T3N2M0) | Hypertension, coronary disease | 10 (PD) | >10% residual TCs | PR | PD |
| 17 | 50 | M | Yes | 30 | Yes | 1 | IV (T4N1M1b) | IVA (T4N2M0) | Refused | 16 | 5% residual TCs | PR | PR |
| 18 | 74 | M | Yes | 50 | Yes | 1 | IIB (T1N1M0) | IIA (T1N1M0) | Gastric ulcer, emphysema, age | 14 (refused treatment) | 0% residual TCs | SD | CR |
| 19 | 72 | M | Yes | 14 | Yes | 0 | IIA (T3N0M0) | III (T3N0M0) | Hypertension, age | 16 | 1% residual TCs | SD | PR |
| 20 | 54 | M | Yes | 1 | Yes | 1 | III (T3NIM0) | III (T3NIM0) | Hypertension, cerebral infarction | 16 | 0% residual TCs | PR | PR |
Histopathologic diagnosis was squamous cell carcinoma in all cases. Radiation dose was 60 Gy in all cases except for patient 20, who received 52 Gy because of grade 2 radiation pneumonitis. SHR‐1210 was given in 200‐mg doses for the indicated number of cycles.
Staged per American Joint Committee on Cancer (AJCC) 6th (2002) edition.
Staged per AJCC 8th (2017) edition.
More than 72 years.
Excluded upon discovery of second primary cancer in bladder after 20 Gy RT and two cycles of SHR‐1210.
Abbreviations: chemoRT, chemoradiotherapy; CR, complete response; Dx, diagnosis; ECOG, Eastern Cooperative Oncology Group; F, female; Hx, history; ID, identifier; M, male; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease; TC, tumor cell.
Table 3.
Treatment failures
| Patient ID | Type of failure | Treatment for failure | Vital status | Cause of death |
|---|---|---|---|---|
| 1 | Local recurrence | Docetaxel + cisplatin | Dead | Progressive disease |
| 2 | Distant metastasis in liver | Surgery and chemotherapy | Dead | Progressive disease |
| 4 | Local recurrence | Docetaxel + cisplatin | Dead | Progressive disease |
| 5 | Local and regional lymph node recurrence | Best supportive care | Dead | Progressive disease |
| 6 | Distant metastasis in liver | Radiofrequency ablation, docetaxel + cisplatin, maintenance pembrolizumab | Alive | — |
| 9 | Local recurrence | Best supportive care | Dead | Progressive disease |
| 11 | Distant metastasis in bone | Best supportive care | Dead | Progressive disease |
| 12 | Regional lymph node recurrence | Docetaxel + cisplatin | Dead | Progressive disease |
| 13 | Local recurrence and esophageal fistula | Stent + best supportive care | Dead | Progressive disease |
| 14 | Local recurrence | Best supportive care | Dead | Progressive disease |
| 16 | Local recurrence and esophageal fistula | Stent + best supportive care | Dead | Massive hemorrhage |
| 17 | Local recurrence | Best supportive care | Dead | Progressive disease |
| 18 | Regional lymph node recurrence | Best supportive care | Dead | Progressive disease |
We found that the toxicity profile after RT plus camrelizumab was similar to previous reports of either modality given alone (Table 4). The grade 3 and grade 4 RT‐related adverse events in the current study consisted of lymphopenia, esophagitis, laryngitis, and leukopenia; these were managed without the need to interrupt RT. These results indicate the combination therapy did not increase RT‐related toxicity compared with RT alone and that the adverse events of the combination therapy less severe than those associated with CCRT [1, 3]. Although we could not distinguish RT and immune‐related pneumonitis on computed tomography scan, the incidence of pneumonitis in the present study (5% in grade 3) was not higher than that when camrelizumab was applied alone (6.7% in grade 3) [7]. The most common immune‐related adverse event (AE) in our study was grade 1 and 2 cutaneous capillary hemangioma (17 [89%] patients). Cutaneous capillary hemangioma has been reported in other clinical trials of camrelizumab [7, 15, 16] but has not been reported after nivolumab or pembrolizumab; its mechanism, which may be specific to camrelizumab, is under investigation [17]. Three patients experienced grade 1 hypothyroidism. Other organ‐specific immune‐related AEs, such as thyroiditis, stomatitis, and colitis, were not found. These results indicated that the incidence of immune‐related AEs did not increase when RT was combined with anti–PD‐1 antibody.
Table 4.
Treatment‐related adverse events
| Adverse events | Grade 1,n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Total, n (%) |
|---|---|---|---|---|---|
| Reactive capillary hemangiomas | 15 (79) | 2 (10) | 0 (0) | 0 (0) | 17 (90) |
| Pulmonary infection | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 2 (10) |
| Radiation pneumonitis | 0 (0) | 1 (5) | 1 (5) | 0 (0) | 2 (10) |
| Radiation esophagitis | 6 (32) | 3 (16) | 0 (0) | 0 (0) | 9 (47) |
| Radiation laryngitis | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 1 (5) |
| Constipation | 3 (16) | 2 (10) | 0 (0) | 0 (0) | 5 (26) |
| Decreased appetite | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 2 (10) |
| Blurred vision | 2 (10) | 0 (0) | 0 (0) | 0 (0) | 2 (10) |
| Skin albinism | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 2 (10) |
| Proteinuria | 7 (37) | 0 (0) | 0 (0) | 0 (0) | 7 (37) |
| Leukopenia | 3 (16) | 2 (10) | 1 (5) | 0 (0) | 6 (32) |
| Neutrophilic granulopenia | 0 (0) | 6 (32) | 0 (0) | 0 (0) | 6 (32) |
| Lymphopenia | 0 (0) | 2 (10) | 6 (32) | 1 (5) | 9 (47) |
| Anemia | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 1 (5) |
| Liver disorder | 2 (10) | 0 (0) | 0 (0) | 0 (0) | 2 (10) |
| Albuminosis | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 2 (10) |
| Hyperglycemia | 0 (0) | 5 (26) | 0 (0) | 0 (0) | 5 (26) |
| Fatigue | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (5) |
| Cough | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 1 (5) |
| Hemoptysis | 3 (16) | 0 (0) | 0 (0) | 0 (0) | 3 (16) |
| Hypothyroidism | 3 (16) | 0 (0) | 0 (0) | 0 (0) | 3 (16) |
We did observe some evidence of clinical benefit from RT plus camrelizumab in a few patients, suggesting that identifying those patients who are likely to benefit would be helpful. With tumor tissue samples from 17 patients at baseline and 16 patients during treatment assessed by multiplex immunofluorescence assay (Fig. 3), we found T‐cell subsets in tumor tissues were disrupted by RT combined with camrelizumab treatment (Fig. 4). Programmed cell death ligand 1 (PD‐L1) expression, density of PD‐1+CD8+ cells, ratio of PD‐L1+/CD4+ T cells in baseline tumors, and density of PD‐1+CD4+ T cells in both baseline and on‐treatment tumors were associated with patient survival (Fig. 5). We conclude that PD‐L1+CD4+ tumor‐infiltrating lymphocytes (TILs) (mostly consisting of regulatory T [Treg] cells) and PD‐1+CD8+ TILs contributed to the inhibitory immune microenvironment in ESCC. Infiltration of TILs has previously been linked with a positive survival benefit in esophageal cancer [18]. However, a recent study revealed patients with higher‐grade renal cell carcinoma had high levels of exhausted PD‐1 and T cell immunoglobulin and mucin domain‐containing protein 3 (TIM3) double‐positive CD8+ TILs [19]. The PD‐1+CD8+ TILs were probably bystander TILs that recognized unrelated tumor antigens [20] and thus could not function as tumor‐specific killers even though PD‐1/PD‐L1 signaling was inhibited. On the contrary, our result indicated that PD‐1+CD4+ TILs probably generated antitumor activity, which was consistent with a recent study from triple negative breast cancer that showed PD‐1+CD4+ TILs were associated with better survival [21]. It has been reported that CD4+ T cells enhance the killer function of CD8+ T cells mediated by interleukin‐21 and adapting antigen presentation [22]. RT convert tumors into “in situ vaccines” [23], and RT plus PD‐1 antibody promoted the release of tumor antigens [24]. These results suggested that the combination of RT and PD‐1 antibody could result in a synergistic antitumor effect by both priming and reactivating immune response.
Figure 3.
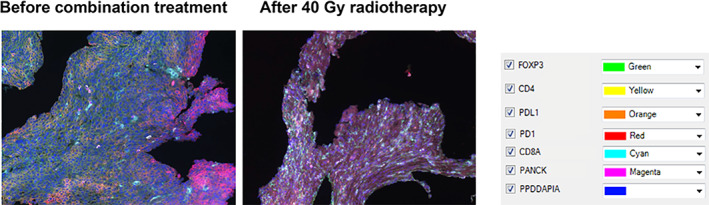
Multiplex staining for tumor‐infiltrating T cells in esophageal squamous cell carcinoma tissue sections. Representative images of a patient (patient 3) who had a major pathological response. The PD‐1 and PD‐L1 expression during treatment decreased compared with that before treatment, whereas CD4+ and CD8+ T cells accumulated during treatment. Abbreviations: FOXP3, forkhead box P3; PANCK, pan Cytokeratin; PD1, programmed cell death protein 1; PDL1, programmed cell death ligand 1; PPDDAPIA, DAPI.
Figure 4.
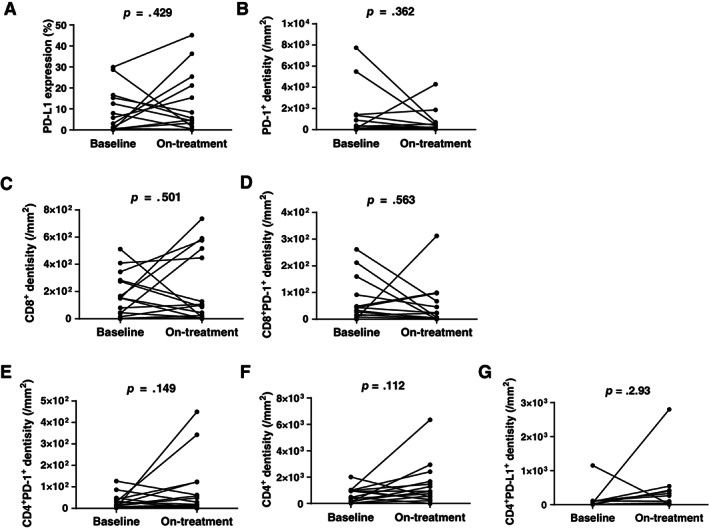
Dynamics of tumor‐infiltrating T cells at baseline and during treatment. Pairs of biopsy tumor tissues that were collected before treatment (at baseline) and during treatment after 40 Gy radiotherapy were stained using a multiplex immunofluorescence method. Paired t test was used to evaluate the differences in multiple T‐cell subsets between at baseline and on‐treatment timepoint. p < .05, significant difference. Abbreviations: PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death ligand 1.
Figure 5.

Intratumoral T cells were associated with clinical outcomes. Kaplan‐Meier curves for overall survival and progression‐free survival of patients in tumor tissue programmed cell death ligand 1 (PD‐L1) expression (A), programmed cell death protein 1 (PD‐1)+CD8+ T cells (B), PD‐L1+CD4+ T cells (C), and PD‐1+CD4+ T cells (D) at baseline and PD‐1+CD4+ T cells (E) during treatment. Log‐rank p values are reported from an unadjusted analysis. The cutoff values were determined by calculated by the Youden index of the receiver operating characteristic curve. Abbreviations: BL, baseline; CI, confidence interval; OS, overall survival; PFS, progression‐free survival; RT, radiotherapy (during treatment).
With 18 pairs of peripheral blood T cells at baseline and during treatment evaluated by flow cytometric analysis, we found the ratios of CD3+/lymphocytes, CD8+/CD3+, CD4+/CD3+, and CD4+Foxp3+ Treg/CD3+ cells were not significantly affected by RT plus camrelizumab, although the total lymphocytes decreased (Fig. 6). The decreased expression of PD‐1 on T cells after combination treatment indicated regulatory effect of anti–PD‐1 antibody (Fig. 6). Notably, we found the CD4+ and CD8+ T cells in the peripheral blood conversely associated with survival regardless of the time they were measured (baseline vs. during treatment) (Table 5; Fig. 7). Furthermore, we found the specific T‐cell subsets, such as Ki67+CD8+, interferon‐γ+CD8+, Ki67+Treg, and cytotoxic T‐lymphocyte–associated protein 4–positive CD4+ T cells, also closely associated with patient survival (Table 5; Fig. 7).
Figure 6.

Dynamics of peripheral blood T cells at baseline and during treatment. Pairs of EDTA‐anticoagulant peripheral blood specimens were collected at baseline and during treatment after 40 Gy radiotherapy. Flow cytometry was used to identify T‐cell subsets and PD‐1/PD‐L1 expression. Paired t test was used to evaluate the differences in T‐cell subpopulations between at baseline and during treatment timepoint. p < .05, significant difference. Abbreviations: Lym, lymphocyte; PBMC, peripheral blood mononuclear cell; PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death ligand 1; Treg, regulatory T cell.
Table 5.
Peripheral T cells were associated with clinical outcomes
| T‐cell subsets | Baseline | During treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | |||||
| p value a | HR b (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | |
| CD4+/CD3+ | .007 | 0.17 (0.06–0.51) | .004 | 0.16 (0.05–0.47) | — c | .014 | 0.28 (0.80–1.00) | |
| CD8+/CD3+ | .004 | 9.95 (3.34–29.65) | .001 | 7.73 (2.56–23.28) | — | .002 | 4.49 (1.17–17.21) | |
| Ki67+/CD8+ | .011 | 0.25 (0.08–0.79) | .002 | 0.20 (0.06–0.64) | .038 | 0.24 (0.08–0.71) | .003 | 0.09 (0.03–0.28) |
| IFN‐γ+/CD8+ | — | .010 | 3.5 (0.90–14.22) | .034 | 0.31 (0.10–0.93) | — | ||
| Treg d /lymphocytes | .002 | 0.20 (0.03–1.46) | .019 | 0.29 (0.05–1.59) | — | — | ||
| Ki67+/Treg | — | — | <.001 | 6.07 (0.94–39.30) | .019 | 3.31 (0.75–14.64) | ||
| CTLA4+/CD4+ | .010 | 3.66 (1.03–13.01) | — | — | — | |||
From log‐rank test.
Hazard in high group/hazard in low group.
p ≥ .05.
Treg was defined as CD4+CD25+Foxp3+ T cell.
Abbreviations: —, ≥0.05; CI, confidence interval; CTLA4, cytotoxic T‐lymphocyte–associated protein 4; HR, hazard ratio; IFN‐γ, interferon γ; OS, overall survival; PFS, progression‐free survival; Treg, regulatory T cell.
Figure 7.
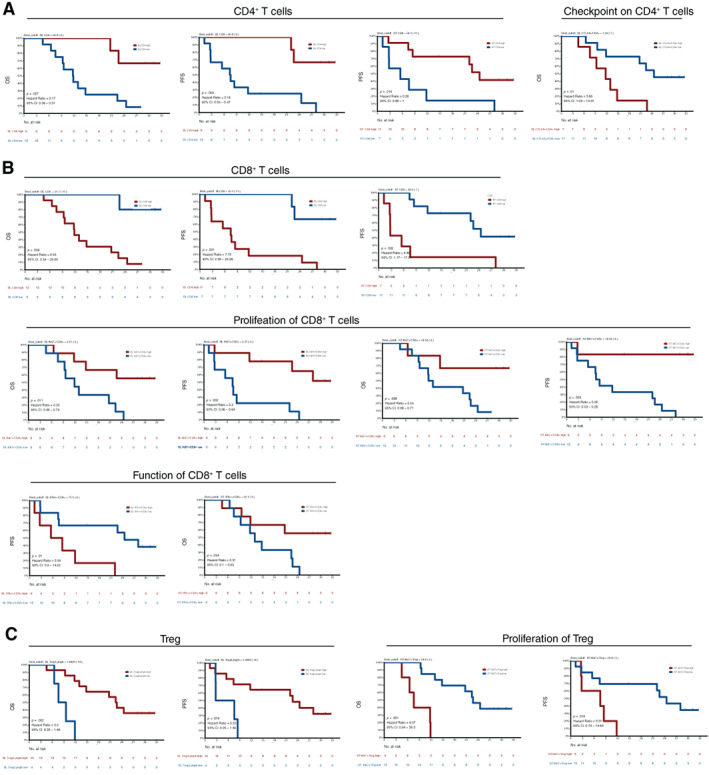
Kaplan‐Meier estimates of overall survival and progression‐free survival in patients in peripheral blood T‐cell subsets predicting prognosis over time. (A): CD4+ T cells and their subsets at baseline or during treatment were associated with OS and PFS. (B): CD8+ T cells and their subsets at baseline or during treatment were associated with OS and PFS. (C): CD4+CD25+Foxp3+ Treg cells and their subsets during treatment were associated with OS and PFS. The cutoff values were determined by calculated by the Youden index of the receiver operating characteristic curve. Log‐rank p values are reported from an unadjusted analysis. Abbreviations: BL, baseline; CI, confidence interval; CTLA4, cytotoxic T‐lymphocyte–associated protein 4; IFN‐r, Interferon‐gamma; Lymph, lymphocyte; OS, overall survival; PFS, progression‐free survival; RT, radiotherapy (during treatment); Treg, regulatory T cell.
These findings from tumor microenvironment and systemic immune status not only provide candidates for predictive biomarkers but also indicate that the specific status and function of T‐cell subsets, such as proliferation, activation, or exhaustion, might play more important roles in antitumor immune response compared with “bulk” T cells and need to be emphasized in further studies.
Conclusively, this study of RT combined with camrelizumab showed promising efficacy and a manageable safety profile in patients with locally advanced ESCC. Our results further revealed several potential immune biomarkers in tumor tissues and peripheral blood. However, this study was limited by the small number of patients included. We have launched a single‐arm phase I study (NCT03671265) to further explore the response to CCRT combined with camrelizumab as first‐line therapy for locally advanced ESCC. Based on the preliminary result, a phase III, randomized, double‐blind, placebo‐controlled study of camrelizumab versus placebo in combination CCRT in these patients (NCT04426955) was carried out this year.
Disclosures
The authors indicated no financial relationships.
Figures and Tables
Acknowledgments
This work was supported by the National Nature Science Foundation of China (grants 81872462 and 81972772) and the Clinical Trial Supporting Foundation of Tianjin Medical University Cancer Institute & Hospital (grant C1707). We thank Jiangsu HengRui Medicine Co., China, for kindly providing the anti–PD‐1 antibody camrelizumab. We thank Zhongxing Liao, M.D., Ph.D., and Christine Wogan, University of Texas MD Anderson Cancer Center, Houston, TX, for their careful and precise revision of the manuscript.
Footnotes
- ClinicalTrials.gov Identifier: NCT03222440
- Sponsor: Tianjin Medical University Cancer Institute & Hospital
- Principal Investigators: Ping Wang, Qingsong Pang
- IRB Approved: Yes
Contributor Information
Ping Wang, Email: pangqingsong@tjmuch.com, Email: wangping@tjmuch.com.
Qingsong Pang, Email: pangqingsong@tjmuch.com.
References
- 1. Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced esophageal cancer: Long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation therapy oncology group. JAMA 1999;281:1623–1627. [DOI] [PubMed] [Google Scholar]
- 2. Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (Radiation Therapy Oncology Group 94‐05) phase III trial of combined‐modality therapy for esophageal cancer: High‐dose versus standard‐dose radiation therapy. J Clin Oncol 2002;20:1167–1174. [DOI] [PubMed] [Google Scholar]
- 3. Suntharalingam M, Winter K, Ilson D et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: The NRG Oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol 2017;3:1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okawa T, Kita M, Tanaka M et al. Results of radiotherapy for inoperable locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys 1989;17:49–54. [DOI] [PubMed] [Google Scholar]
- 5. Wu KL, Chen GY, Xu ZY et al. Three‐dimensional conformal radiation therapy for squamous cell carcinoma of the esophagus: A prospective phase I/II study. Radiother Oncol 2009;93:454–457. [DOI] [PubMed] [Google Scholar]
- 6. Doi T, Piha‐Paul SA, Jalal SI et al. Safety and antitumor activity of the anti‐programmed death‐1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol 2018;36:61–67. [DOI] [PubMed] [Google Scholar]
- 7. Huang J, Xu B, Mo H et al. Safety, activity, and biomarkers of SHR‐1210, an anti‐PD‐1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res 2018;24:1296–1304. [DOI] [PubMed] [Google Scholar]
- 8. Kudo T, Hamamoto Y, Kato K et al. Nivolumab treatment for oesophageal squamous‐cell carcinoma: An open‐label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631–639. [DOI] [PubMed] [Google Scholar]
- 9. Shah MA, Kojima T, Hochhauser D et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE‐180 study. JAMA Oncol 2019;5:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang J, Xu J, Chen Y et al. Camrelizumab versus investigator's choice of chemotherapy as second‐line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open‐label, phase 3 study. Lancet Oncol 2020;21:832–842. [DOI] [PubMed] [Google Scholar]
- 11. Antonia SJ, Villegas A, Daniel D et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 12. Antonia SJ, Villegas A, Daniel D et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 13. Luke JJ, Lemons JM, Karrison TG et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rech AJ, Dada H, Kotzin JJ et al. Radiotherapy and CD40 activation separately augment immunity to checkpoint blockade in cancer. Cancer Res 2018;78:4282–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang W, Yang Y, Ma Y et al. Camrelizumab (SHR‐1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single‐arm, phase 1 trials. Lancet Oncol 2018;19:1338–1350. [DOI] [PubMed] [Google Scholar]
- 16. Mo H, Huang J, Xu J et al. Safety, anti‐tumour activity, and pharmacokinetics of fixed‐dose SHR‐1210, an anti‐PD‐1 antibody in advanced solid tumours: A dose‐escalation, phase 1 study. Br J Cancer 2018;119:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teng Y, Guo R, Sun J et al. Reactive capillary hemangiomas induced by camrelizumab (SHR‐1210), an anti‐PD‐1 agent. Acta Oncol 2019;58:388–389. [DOI] [PubMed] [Google Scholar]
- 18. Okadome K, Baba Y, Yagi T et al. Prognostic nutritional index, tumor‐infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg 2020;271:693–700. [DOI] [PubMed] [Google Scholar]
- 19. Kawashima A, Kanazawa T, Kidani Y et al. Tumour grade significantly correlates with total dysfunction of tumour tissue‐infiltrating lymphocytes in renal cell carcinoma. Sci Rep 2020;10:6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simoni Y, Becht E, Fehlings M et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018;557:575–579. [DOI] [PubMed] [Google Scholar]
- 21. He TF, Yost SE, Frankel PH et al. Multi‐panel immunofluorescence analysis of tumor infiltrating lymphocytes in triple negative breast cancer: Evolution of tumor immune profiles and patient prognosis. PLoS One 2020;15:e0229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zander R, Schauder D, Xin G et al. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity 2019;51:1028–1042.e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015;1:1325–1332. [DOI] [PubMed] [Google Scholar]
- 24. Twyman‐Saint Victor C, Rech AJ, Maity A et al. Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


