Abstract
Lessons Learned
Three‐month adjuvant capecitabine plus oxaliplatin in combination (CAPOX) appeared to reduce recurrence, with mild toxicity in postcurative resection of colorectal cancer liver metastases (CLM).
Recurrence in patients who underwent the 3‐month adjuvant CAPOX after resection of CLM was most commonly at extrahepatic sites.
Background
The role of neoadjuvant and adjuvant chemotherapy in the management of initially resectable colorectal cancer liver metastases (CLM) is still unclear. We evaluated the feasibility of 3‐month adjuvant treatment with capecitabine plus oxaliplatin in combination (CAPOX) for postcurative resection of CLM.
Methods
Patients received one cycle of capecitabine followed by four cycles of CAPOX as adjuvant chemotherapy after curative resection of CLM. Oral capecitabine was given as 1,000 mg/m2 twice daily for 2 weeks in a 3‐week cycle, and CAPOX consisted of oral capecitabine plus oxaliplatin 130 mg/m2 on day 1 in a 3‐week cycle. Primary endpoint was the completion rate of adjuvant chemotherapy. Secondary endpoints included recurrence‐free survival (RFS), overall survival (OS), dose intensity, and safety.
Results
Twenty‐eight patients were enrolled. Median age was 69.5 years, 54% of patients had synchronous metastases, and 29% were bilobar. Mean number of lesions resected was two, and mean size of the largest lesion was 31 mm. Among patients, 20 (71.4%; 95% confidence interval, 53.6%–89.3%) completed the protocol treatment and met its primary endpoint. The most common grade 3 or higher toxicity was neutropenia (29%). Five‐year recurrence‐free survival and overall survival were 65.2% and 87.2%, respectively.
Conclusion
Three‐month adjuvant treatment with CAPOX is tolerable and might be a promising strategy for postcurative resection of CLM.
Keywords: Colorectal liver metastases, Adjuvant chemotherapy, CapeOx, CAPOX, XELOX
Discussion
In patients with technically resectable CLM, both upfront surgery with or without adjuvant chemotherapy and perioperative chemotherapy are considered to be indicated treatment strategies. However, there is no clear evidence for which treatment strategy is preferable or for which drug combination is most effective. In this study, patients were safely treated with 3‐month postoperative adjuvant chemotherapy with CAPOX, and the effect of treatment in suppressing recurrence and prolonging survival was confirmed. To our knowledge, this is the first report of 3‐month adjuvant chemotherapy with CAPOX for postcurative resection of CLM.
We specified that the first cycle of adjuvant treatment should include capecitabine only, after the second cycle of CAPOX, for a total of 3 months. Both capecitabine and oxaliplatin undergo renal excretion, but capecitabine undergoes hepatic metabolism. Liver dysfunction is expected after CLM resection, and additional toxicity must be considered when administering capecitabine, which is metabolized in the liver. In fact, as shown in Figure 1, two (7%) of 28 patients enrolled in this study were unable to continue the second cycle because of gastrointestinal toxicity (diarrhea, nausea) caused by the first cycle of capecitabine alone. Capecitabine dose in the second cycle could be adjusted according to the adverse events observed in the first cycle. The ability to adjust the starting dose of CAPOX likely facilitated the safe continuation of treatment, which in turn likely provided a high treatment completion rate. In our present study, the frequency of neutropenia was 29% for grade 3 and 0% for grade 4, indicating safer treatment and resulting in better outcomes.
Figure 1.
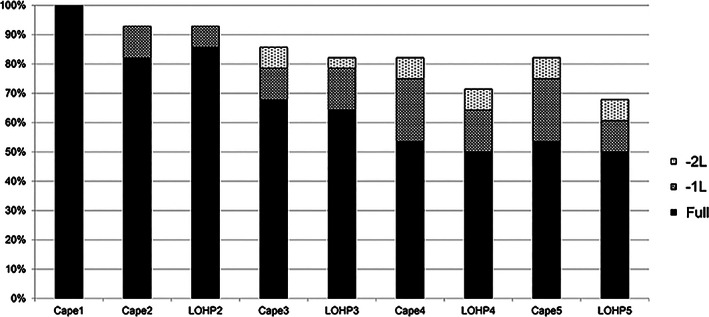
Dose modification and treatment continuation of adjuvant capecitabine plus oxaliplatin in combination (n = 28). Dose administered in each cycle is represented by individual bars. In cycle 1, capecitabine alone is administered (Cape1). In cycle 2, the dose of capecitabine is denoted by Cape2 and the dose of oxaliplatin as LOHP2. The y‐axis shows the percentage of patients receiving the indicated dose (full, –1 dose level, or –2 dose level). Full dose capecitabine was administered at 1,000 mg/m2 twice daily (total 2,000 mg/m2 per day); doses could be reduced to doses 0.8 times starting dose (–1 level) and doses 0.6 times starting dose (–2 level).
Abbreviations: –1L, –1 level; –2L, –2 level; Cape1, capecitabine dose in each case of the first cycle; LOHP2, oxaliplatin dose in each case of the second cycle
Previous studies evaluating perioperative or adjuvant chemotherapy for resectable CLM had a 5‐year disease‐free survival of 33.5% to 49.8% and 5‐year OS of 51.1% to 71.2% [1, 2, 3, 4, 5, 6]. Allowing for the small number of patients in this study, survival appeared promising, with a 5‐year RFS of 65.2% and 5‐year OS of 87.2% (Fig. 2). Adjuvant chemotherapy with 3‐month CAPOX is considered a promising treatment strategy for patients with CLM after curative resection.
Figure 2.
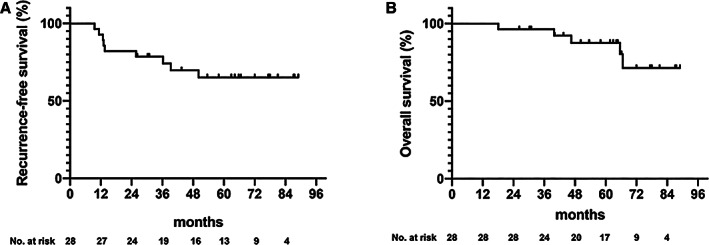
Recurrence‐free survival (A) and overall survival (B).
Trial Information
| Disease | Colorectal cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | None |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Deliverability |
| Secondary Endpoints | Time to progression, overall survival, safety |
| Additional Details of Endpoints or Study Design | The primary endpoint was completion rate of adjuvant chemotherapy. |
| Inclusion criteria: Eligibility criteria were histologically proven colorectal cancer; curatively resected metastatic liver tumors from colorectal cancer; no extrahepatic sites of disease; enrollment within 90 days after hepatic resection; age 20 years or more at the time of registration; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; and adequate organ function, as defined by absolute neutrophil count ≥1,500/mm3, hemoglobin ≥10 g/dL, platelet count ≥10.0 × 104/mm3, total bilirubin ≤1.5 mg/dL, serum transaminases ≤100 U/L, and serum creatinine ≤1.2 mg/dL. | |
| This trial was carried out in accordance with the Helsinki Declaration and Ethical Guidelines for Clinical Studies and was approved by the institutional review boards of all participating institutions. All patients were required to give written informed consent before entering the study. | |
| Study treatment and dose modification: Patients received one course of capecitabine followed by four courses of CAPOX for a total of five courses (15 weeks) as adjuvant chemotherapy after curative resection of CLM. Oral capecitabine was given at 1,000 mg/m2 twice daily for 2 weeks in a 3‐week cycle, whereas CAPOX consisted of oral capecitabine plus a 2‐hour intravenous infusion of oxaliplatin at 130 mg/m2 on day 1 in a 3‐week cycle. The dose was modified for each patient based on hematologic or nonhematologic toxicity. Treatment was delayed if, on the planned day of treatment, the following criteria were present: absolute neutrophil count <1,500/mm3, platelets <75,000/mm3, palmar‐plantar erythrodysesthesia syndrome grade >1, diarrhea grade >1, and other parameters at the attending physician's discretion. If adverse events of a high enough grade occurred during the previous course, the dose was reduced by one level (up to −2 level). Capecitabine doses could be reduced to 1,500 mg/m2 per day (−1 level) and 1,000 mg/m2 per day (−2 level). Oxaliplatin doses could be reduced to 100 mg/m2 (−1 level) and 85 mg/m2 (−2 level). Patients who could not tolerate oxaliplatin could continue to receive capecitabine monotherapy until a maximum total of five courses, disease relapse, or intolerable toxicity. Capecitabine and oxaliplatin could be reduced by two dose levels, but treatment was discontinued if subsequent reduction was indicated. In the event of grade 4 nonhematologic toxicities, treatment was definitively interrupted. Pretreatment evaluation included a medical history; physical examination; complete blood cell count and serum chemistry tests; and chest, abdominal, and pelvic computed tomography scans. Clinical examination and biochemical tests were required before and during each cycle. | |
| All adverse events experienced during the study were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. | |
| Endpoints and statistical analysis: The primary endpoint was completion rate of adjuvant chemotherapy. Based on the treatment completion rate of the EORTC 40983 trial [11], which was conducted in the same subjects as this study, the threshold completion rate of protocol treatment was set to 45% and the expected completion rate was set to 70%. Given a one‐sided α of 0.1 and statistical power of 80%, a minimum of 25 patients was required. Secondary endpoints included RFS, OS, dose intensity, and safety. Dose intensity was calculated as the ratio of the actual to planned dose intensity in milligrams per square meter per week. The survival curve was estimated using the Kaplan‐Meier method. Safety and efficacy analyses were both conducted on an intention‐to‐treat population, defined as all patients enrolled in the study who received at least one dose of chemotherapy. RFS was defined as the time to the earlier of the date at which relapse was diagnosed or the date of death due to any cause, with the day of registration as the base date. OS was determined as the time to date of death due to any cause or last confirmation of survival, with the day of registration as the base date. Statistical data were obtained using SAS software, version 9.4 (SAS Institute Inc., Cary, NC). | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Capecitabine | |
| Generic Name | Capecitabine |
| Trade Name | Xeloda |
| Company Name | Hoffmann‐La Roche, Ltd. |
| Drug Type | Cytotoxic |
| Dose | 1,000 milligrams (mg) per squared meter (m2) |
| Route | Oral (p.o.) |
| Schedule of Administration | 1,000 mg/m2 twice daily for 2 weeks in a 3‐week cycle |
| Oxaliplatin | |
| Generic Name | Oxaliplatin |
| Drug Type | Cytotoxic |
| Drug Class | Platinum compound |
| Dose | 130 milligrams (mg) per squared meter (m2) |
| Route | i.v. |
| Schedule of Administration | 130 mg/m2 on day 1 in a 3‐week cycle |
Patient Characteristics
| Number of Patients, Male | 16 | |
| Number of Patients, Female | 12 | |
| Stage | ||
| Baseline characteristics of resected colorectal liver metastases | n (%) | |
| Synchronicity of liver metastases | ||
| Synchronous | 15 (54) | |
| Metachronous | 13 (46) | |
| Location of the liver metastases | ||
| Unilobar | 20 (71) | |
| Bilobar | 8 (29) | |
| Number of liver metastases | ||
| Mean (range) | 2 (2–7) | |
| 1–3 | 26 (93) | |
| ≥4 | 2 (7) | |
| Maximum tumor size of liver metastasis, mm | ||
| Mean (range) | 31 (2–112) | |
| <50 mm | 23 (82) | |
| ≥50 mm | 5 (18) | |
| Age | Median (range): 69.5 (39–82) years | |
| Performance Status: ECOG | ||
| 0 — 28 | ||
| 1 — 0 | ||
| 2 — 0 | ||
| 3 — 0 | ||
| Unknown — 0 | ||
| Other | From May 2013 to November 2015, 28 patients were enrolled from six institutions. Median age was 69.5 years, 57% of patients were male, and 75% of cases had a left‐sided primary tumor, with 21% in the rectum. Metastases were synchronous in 15 (54%) and metachronous in 13 (46%) of the patients. The metastases were unilobar in 20 patients (71%) and bilobar in 8 patients (29%). The mean number of lesions resected per patient was two (range, one to seven). Mean size of the largest lesion per patient was 31 mm (range, 2–112 mm). Among the patients, seven (25%) had a history of prior oxaliplatin administration before study enrollment. Median time from the date of hepatic resection to the start of adjuvant CAPOX was 48 days (range, 25–77). | |
| Baseline characteristics | n (%) | |
| Primary tumor location | ||
| Left | 21 (75) | |
| Sigmoid | 12 (43) | |
| Rectum | 6 (21) | |
| Descending | 3 (11) | |
| Right | 7 (25) | |
| Transverse | 4 (14) | |
| Cecum | 2 (7) | |
| Ascending | 1 (4) | |
| Hepatectomy | ||
| Segmental | 21 (75) | |
| Partial resection | 4 (14) | |
| Wedge | 2 (7) | |
| Lobectomy | 1 (4) | |
| Residual tumor | ||
| R0 | 25 (89) | |
| RX | 3 (11) | |
| Tumor category of primary | ||
| T1 | 2 (7) | |
| T2 | 2 (7) | |
| T3 | 16 (57) | |
| T4 | 4 (14) | |
| TX | 4 (14) | |
| Lymphatic spread of primary | ||
| N0 | 9 (32) | |
| N1 | 13 (46) | |
| N2 | 5 (18) | |
| NX | 1 (4) | |
| Plasma CEA level at enrollment >ULN (5.0 ng/mL): Yes | 3 (11) | |
| Prior oxaliplatin administration: Yes | 7 (25) |
Abbreviations: CEA, carcinoembryonic antigen; ULN, upper limit of normal.
Primary Assessment Method
| Title | Completion Rate of Adjuvant Chemotherapy |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 28 |
| Number of Patients Evaluable for Toxicity | 28 |
| Number of Patients Evaluated for Efficacy | 28 |
| Outcome Notes | Among the 28 patients, 20 (71.4%: 95% confidence interval, 53.6–89.3%) completed the protocol treatment. Because of toxicity, capecitabine was reduced in 10 cases (36%) and discontinued in 5 cases (19%), whereas oxaliplatin was reduced in 8 cases (29%) and discontinued in 8 cases (29%). The mean relative dose intensity of capecitabine and oxaliplatin was 70.6% (range, 4%–108%) and 74.6 % (range, 0%–100%), respectively. Dose modification and treatment continuation details for each drug are provided in Figure 1. |
Secondary Assessment Method
| Title | Recurrence‐Free Survival, Overall Survival |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 28 |
| Number of Patients Evaluable for Toxicity | 28 |
| Number of Patients Evaluated for Efficacy | 28 |
| Evaluation Method | RECIST version 1.1 |
| Outcome Notes | Survival and recurrence: Figure 2 shows the RFS and OS. With a median follow‐up period of 64.3 months (range, 26.3–87.5 months), a total of nine patients developed disease relapse and four died because of relapse, giving a mean ± SE 5‐year RFS of 65.2% ± 9.55% and 5‐year OS of 87.2%. Median time to recurrence was 11.3 months (range, 9.6–50.1 months). Of the relapsed patients (Table 1), the primary tumors were located in the colon (n = 6) and rectum (n = 3). Residual liver metastasis occurred in two patients (7%), but all recurrences also occurred in extrahepatic sites (n = 9). The most frequent sites of relapse were lung (n = 4) and distant metastases (n = 4). |
Phase II New Arm Adverse Events
| All Cycles Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| White blood cell decreased | 39 | 25 | 36 | 0 | 0 | 0 | 61 |
| Neutrophil count decreased | 43 | 14 | 14 | 29 | 0 | 0 | 57 |
| Anemia | 25 | 61 | 11 | 4 | 0 | 0 | 75 |
| Platelet count decreased | 29 | 46 | 21 | 4 | 0 | 0 | 71 |
| Aspartate aminotransferase increased | 36 | 61 | 4 | 0 | 0 | 0 | 64 |
| Alanine aminotransferase increased | 46 | 50 | 4 | 0 | 0 | 0 | 54 |
| Blood bilirubin increased | 79 | 14 | 4 | 4 | 0 | 0 | 21 |
| Anorexia | 50 | 43 | 4 | 4 | 0 | 0 | 50 |
| Constipation | 68 | 32 | 0 | 0 | 0 | 0 | 32 |
| Diarrhea | 68 | 25 | 4 | 4 | 0 | 0 | 32 |
| Dizziness | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Dysgeusia | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Fatigue | 89 | 7 | 4 | 0 | 0 | 0 | 11 |
| Febrile neutropenia (fever of unknown origin without clinically or microbiologically documented infection; ANC <1.0 × 10e9/L, fever ≥38.5°C) | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 82 | 14 | 4 | 0 | 0 | 0 | 18 |
| Palmar‐plantar erythrodysesthesia syndrome | 43 | 46 | 11 | 0 | 0 | 0 | 57 |
| Mucositis oral | 86 | 11 | 4 | 0 | 0 | 0 | 14 |
| Nausea | 46 | 50 | 4 | 0 | 0 | 0 | 54 |
| Peripheral sensory neuropathy | 79 | 14 | 4 | 4 | 0 | 0 | 21 |
| Skin hyperpigmentation | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Vasculitis | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Vomiting | 89 | 7 | 4 | 0 | 0 | 0 | 11 |
Adverse Events Legend
All 28 patients who received at least one course of treatment were assessed for safety. Common adverse events were oxaliplatin‐related peripheral sensory neuropathy (82%), thrombocytopenia (71%), increased aspartate aminotransferase (64%), leukopenia (61%), neutropenia (57%), and palmar‐plantar erythrodysesthesia syndrome (57%). The most common grade 3 or higher adverse event was neutropenia (29%). No treatment‐related death was observed. Of the patients, one (4%) had a grade 2 oxaliplatin‐related allergy at the third cycle of oxaliplatin administration. This patient had never received prior chemotherapy, including oxaliplatin, before study enrollment. Therefore, of the seven patients who had received prior adjuvant chemotherapy with an oxaliplatin‐based regimen before study enrollment, no one had oxaliplatin‐related allergy.
Abbreviations: ANC, absolute neutrophil count; NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Approximately 25% of patients with colorectal cancer present with liver metastases at the time of the first diagnosis [7]. Hepatic resection is one of the treatment strategies for resectable colorectal cancer liver metastases (CLM) [8]. Complete resection of CLM is the only potential curative treatment. Recurrence mainly occurs within the first 2 years after surgery and is located in the liver in approximately 50% of cases. Among all patients with colorectal cancer and liver metastases, 5‐year survival after resection ranges from 20% to 45% [9, 10, 11, 12]. Combining resection of CLM with neoadjuvant or adjuvant systemic chemotherapy is therefore of major interest in reducing the risk of recurrence and improving long‐term survival.
Adjuvant chemotherapy with fluoropyrimidine plus oxaliplatin doublet combinations, FOLFOX (folinic acid, fluorouracil, and oxaliplatin) or CAPOX (capecitabine plus oxaliplatin), is considered the standard strategy following resection of high‐risk stage II and III colon cancer. Of note, peripheral sensory neuropathy (PSN) is an important dose‐limiting toxicity of oxaliplatin therapy, and a shorter duration of adjuvant FOLFOX or CAPOX therapy would be beneficial for patients if efficacy were not reduced. In their investigation of whether a shorter duration of oxaliplatin‐based adjuvant chemotherapy was as effective as 6 months of the same chemotherapy for resected high‐risk stage II and III colon cancer, the International Duration Evaluation of Adjuvant chemotherapy (IDEA) collaboration reported that adjuvant chemotherapy with 3‐month CAPOX after curative resection for high‐risk stage II and III colon cancer has equivalent efficacy to adjuvant chemotherapy for 6 months, with a significantly lower incidence and severity of adverse events, especially neurotoxicity [13]. Several guidelines recommend 3‐month CAPOX as postadjuvant chemotherapy for high‐risk stage II and III colon cancer [14].
Fluoropyrimidine plus oxaliplatin doublet combination is also recognized as the most promising regimen in CLM in which distant metastases have already developed because of hematogenous spread. However, although both postadjuvant chemotherapy and perioperative chemotherapy for CLM tended to show a favorable effect on progression‐free survival, they did not show an increase in overall survival (OS) [1, 2, 3, 4, 5, 6]. To date, no prospective study has evaluated the efficacy of 3‐month CAPOX as adjuvant chemotherapy after postcurative resection of CLM. Here, we conducted a multi‐institutional, single‐arm, open label, phase II trial to confirm the feasibility of a 3‐month regimen of adjuvant CAPOX for postcurative resection of CLM. Adjuvant chemotherapy with 3‐month CAPOX is considered a promising treatment strategy for patients with CLM after curative resection.
From the results of the IDEA collaboration, despite use of the same fluorouracil plus oxaliplatin doublet combination, there was a difference between FOLFOX and CAPOX in terms of suppression of recurrence in a postoperative adjuvant setting [13]. Furthermore, in the JCOG0603 trial (a randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 (oxaliplatin 85mg/m2, l‐LV 200 mg/m2, 5‐FU bolus 400 mg/m2 and 2400mg/m2 over 48 h) with hepatectomy alone for liver metastasis from colorectal cancer, which had the same background factors as our present study—PS (performance status), synchronicity of liver metastases, site of primary tumor, status of primary lymph node metastases, and number and maximum size of liver metastases), the 6‐month adjuvant FOLFOX arm was shown to be statistically superior to surgery alone in 5‐year disease‐free survival (49.8% in FOLFOX arm and 38.7% in surgery alone; hazard ratio [HR], 0.67; p = .006) but inferior in 5‐year OS (71.2% in FOLFOX arm and 83.1% in surgery alone; HR, 1.25) [8]. One possible reason for the lower postoperative survival in the FOLFOX arm than the surgery alone may be toxicity in JCOG0603. The frequency of chemotherapy‐induced neutropenia in that study was 50% grade 3 or higher and 10% grade 4, with one postoperative death observed in the adjuvant 6‐month FOLFOX arm. On the other hand, our study showed relatively milder bone marrow toxicities (frequency of neutropenia of 29% for grade 3 and 0% for grade 4) and higher survival outcomes (5‐year recurrence‐free survival of 65.2% and 5‐year OS of 87.2%).
One interesting finding of our study was the site of recurrence in patients who underwent adjuvant chemotherapy after resection of CLM. Previously, recurrence after resection of CLM was often considered to occur in the residual liver, but in this study, all cases of recurrence involved extrahepatic metastasis. This was also observed in JCOG0603, in which 66% of recurrent cases in the adjuvant FOLFOX arm were extrahepatic metastases [6]. The very low recurrence of residual liver metastasis in our study suggests that CAPOX as postoperative adjuvant chemotherapy for CLM may suppress the recurrence of metastasis to residual liver. The finding that the extrahepatic recurrence was due to hematogenous metastases reaffirms the importance of a systemic approach to CLM. In addition to our present therapeutic approach, another novel technical approach now under development is the detection of minimum residual disease (MRD) by liquid biopsy [15, 16, 17] . Considering that MRD will in future be more easily detected with liquid biopsy, evidence that 3‐month adjuvant chemotherapy with CAPOX can be adopted as the optimal treatment method with acceptable toxicity will be useful. In Japan, an early intervention clinical trial called the ALTAIR trial (A Randomized, Double‐Blind, Phase III Study Comparing FTD/TPI Therapy Versus Placebo in Patients Who Are Positive for Blood Circulating Tumor DNA After Curative Resection of Colorectal Cancer) is now underway for MRD‐positive postoperative colorectal cancer, including CLM [18].
Several limitations of our present study warrant mention. First, we designed the study to evaluate 3‐month adjuvant chemotherapy with CAPOX as a single‐arm study, and it is therefore difficult to determine the optimal treatment period. Furthermore, the small number of patients hampers evaluation of survival benefit. Second, the study included patients with CLM after curative resection, which may involve a degree of selection bias, and it is not possible to conclude whether perioperative chemotherapy or adjuvant chemotherapy is the better treatment strategy. Finally, we did not assess the enrolled patients' quality of life, and adverse events were evaluated during the protocol treatment period only. The duration of the oxaliplatin‐induced PSN was therefore insufficient.
In conclusion, this study showed that 3‐month adjuvant CAPOX is tolerable and might be a promising therapeutic regimen in postcurative resection of CLM.
Disclosures
Hironaga Satake: Bayer Co., Ltd., Bristol‐Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Bio Pharma Co., Ltd., MSD Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi Co., Ltd., Taiho Pharmaceutical Co.,Ltd., Takeda Co., Ltd. and Yakult Honsha Co., Ltd. (H), Ono Pharmaceutical Co., Ltd., Daiichi Sankyo, Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sanofi (RF); Masahito Kotaka: Chugai Pharmaceutical Co., Ltd., Yakult, Honsya (H); Takeshi Kato: Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly and Company, Bayer Yakuhin, Ltd., SanofiS.A., Yakult, Ono Pharmaceutical Co., (H), Chugai Pharmaceutical Co., Ltd. (RF); Akihito Tsuji: Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan Co., Ltd., Bristol‐Myers Squibb Corporation (H, RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Table
Table 1.
First sites and patterns of recurrence (n = 9)
| Recurrence | Colon (n = 6), n | Rectum (n = 3), n | Total (n = 9), n |
|---|---|---|---|
| First sites of recurrence | |||
| Liver a | 2 | 0 | 2 |
| Lung b | 2 | 2 | 4 |
| Distant lymph nodes a , b | 2 | 2 | 4 |
| Others a , b | 2 | 1 | 3 |
| Patterns of recurrence | |||
| Liver and extrahepatic a | 2 | 0 | 2 |
| Lung and extrahepatic b | 1 | 2 | 3 |
| Lung only | 1 | 0 | 1 |
| Extrahepatic only | 2 | 1 | 3 |
One patient had relapsed liver and distant lymph nodes metastases. One patient had relapsed liver and peritoneal metastases.
Two patients had relapsed lung and distant lymph nodes. One patient had relapsed lung, distant lymph nodes, and bone metastases. One patient had multiple lung metastases.
Acknowledgments
The authors thank the patients and families who participated in this study, as well as Rie Tamaki, Saori Tokuhara, and Mizuho Kono. Editorial assistance and English editing was provided by Guy Harris of DMC Corp.
Footnotes
- ClinicalTrials.gov Identifier: UMIN000011164
- Sponsor: Kobe City Hospital Organization
- Principal Investigator: Hironaga Satake
- IRB Approved: Yes
References
- 1. Hasegawa K, Saiura A, Takayama T et al. Adjuvant oral uracil‐tegafur with leucovorin for colorectal cancer liver metastases: A randomized controlled trial. PLoS One 2016;11:e0162400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitry E, Fields AL, Bleiberg H et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. J Clin Oncol 2008;26:4906–4911. [DOI] [PubMed] [Google Scholar]
- 3. Nordlinger B, Sorbye H, Glimelius B et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup Trial 40983): A randomised controlled trial. Lancet 2008;371:1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nordlinger B, Sorbye H, Glimelius B et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208–1215. [DOI] [PubMed] [Google Scholar]
- 5. Portier G, Elias D, Bouche O et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006;24:4976–4982. [DOI] [PubMed] [Google Scholar]
- 6. Kanemitsu Y, Shimizu Y, Mizusawa J et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone for liver metastasis from colorectal cancer: JCOG0603 study. J Clin Oncol 2020;38(suppl 15):4005a. [DOI] [PubMed] [Google Scholar]
- 7. Garden OJ, Rees M, Poston GJ et al. Guidelines for resection of colorectal cancer liver metastases. Gut 2006;55(suppl 3):iii1–iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanas GP, Taylor A, Primrose JN et al. Survival after liver resection in metastatic colorectal cancer: Review and meta‐analysis of prognostic factors. Clin Epidemiol 2012;4:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fong Y, Fortner J, Sun RL et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg 1999;230:309–318; discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordlinger B, Guiguet M, Vaillant JC et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254–1262. [PubMed] [Google Scholar]
- 11. Scheele J, Altendorf‐Hofmann A, Grube T et al. Resection of colorectal liver metastases. What prognostic factors determine patient selection? [in German]. Chirurg 2001;72:547–560. [DOI] [PubMed] [Google Scholar]
- 12. Fong Y, Cohen AM, Fortner JG et al. Liver resection for colorectal metastases. J Clin Oncol 1997;15:938–946. [DOI] [PubMed] [Google Scholar]
- 13. Grothey A, Sobrero AF, Shields AF et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018;378:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer. Version 4.2020. Plymouth Meeting, PA: National Comprehensive Cancer Center, 2020. [Google Scholar]
- 15. Cohen SJ, Punt CJ, Iannotti N et al. Relationship of circulating tumor cells to tumor response, progression‐free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213–3221. [DOI] [PubMed] [Google Scholar]
- 16. Reinert T, Henriksen TV, Christensen E et al. Analysis of plasma cell‐free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol 2019;5:1124–1131, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tol J, Koopman M, Miller MC et al. Circulating tumour cells early predict progression‐free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol 2010;21:1006–1012. [DOI] [PubMed] [Google Scholar]
- 18. Initial Attack on Latent Metastasis Using TAS‐102 for ct DNA Identified Colorectal Cancer Patients After Curative Resection (ALTAIR) . ClinicalTrials.gov identifier NCT04457297. Bethesda, MD: U.S. National Library of Medicine, July 7, 2020.


