Abstract
Myxofibrosarcoma (MFS) is a well‐recognized histotype of soft tissue sarcomas that generally presents with localized disease. Herein, we describe the case of a patient with metastatic MFS who experienced durable response to sixth‐line therapy with temozolomide. Upon further progression, his tumor was notable for a high tumor mutational burden, and he was subsequently treated with seventh‐line immunotherapy, atezolizumab, achieving a second durable response. This case highlights the role of immunotherapy after administration of alkylating agents. Review of the literature indicates that recurrent tumors treated with alkylating agents often experience hypermutation as a means of developing resistance and that checkpoint inhibitors are subsequently effective in these tumors.
Key Points
To the authors' knowledge, this is the first report of a patient with myxofibrosarcoma with high tumor mutational burden after administration of temozolomide monotherapy.
Hypermutation may be a resistance mechanism for patients with soft tissue sarcoma who develop resistance to alkylating agents.
Checkpoint inhibition may be effective therapy in patients with soft tissue sarcoma with high tumor mutational burden as a consequence of alternate systemic therapy resistance.
Keywords: Soft tissue sarcoma, Temozolomide, Immunotherapy, Antineoplastic drug resistance
Short abstract
This case report highlights the role of immunotherapy after administration of alkylating agents, reporting the first known case of a patient with myxofibrosarcoma who developed high tumor mutational burden after temozolomide monotherapy.
Introduction
Myxofibrosarcoma (MFS) is a relatively common histotype of sarcoma, comprising 5% to 10% of all soft tissue sarcomas (STS) [1]. It is typically diagnosed in the elderly and often presents as an enlarging mass in the extremities [1]. This disease is locally aggressive with a high rate of recurrence and overall 5‐year survival of 61% to 77% [1]. For individuals with metastatic disease, 5‐year survival may be as low as 25% [2]. In local disease, surgical resection and radiotherapy is the standard of care [1]. For those with metastatic disease, anthracycline‐based regimens are considered first‐line therapy [3]; however, there is an emerging literature exploring alternative agents including immunotherapies [4, 5]. Here, we present a case of a patient with MFS that demonstrated a durable response to temozolomide (TMZ) and, on progression, was found to have a high tumor mutational burden (TMB) with subsequent durable response to atezolizumab.
Patient Story
The patient is a 63‐year‐old man who presented in 2013 with a T3N0M0G2 stage IIIB, 10.9 × 6.2 × 5.8 cm posterior left thigh mass. He underwent 50 Gy of neoadjuvant radiation to the thigh, followed by an R0 surgical resection. Pathology was consistent with a grade 2 MFS without lymphovascular invasion.
The patient was monitored with serial computed tomography (CT) imaging for 18 months, at which point he was found to have metastatic recurrence in the right middle lobe of his lung. Positron emission tomography scan confirmed oligometastatic disease. He underwent a right middle lobe wedge resection, and pathology was consistent with metastatic MFS. The mass was 1.4 cm in greatest dimension, with an R2 visceral pleural margin. Recurrence was found 6 months later, with a 3.2 cm mass along the surgical staple line.
At this time, following tumor board recommendations, he was determined not to be a candidate for further local therapy. After discussion and shared decision‐making, he was started on a clinical trial (NCT02326025) of doxorubicin followed by olaratumab. Progression of pulmonary disease was noted after two cycles, and he was subsequently treated with multiple lines of experimental and standard treatment regimens to which he was primarily refractory (Figs. 1 and 2; Table 1).
Figure 1.
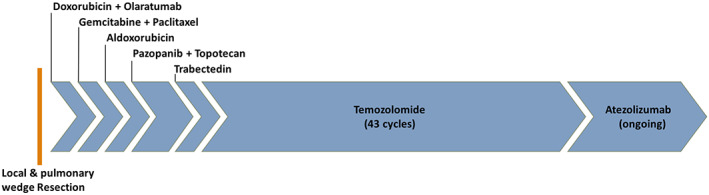
Treatment course. Sizing proportional to time on each treatment.
Figure 2.
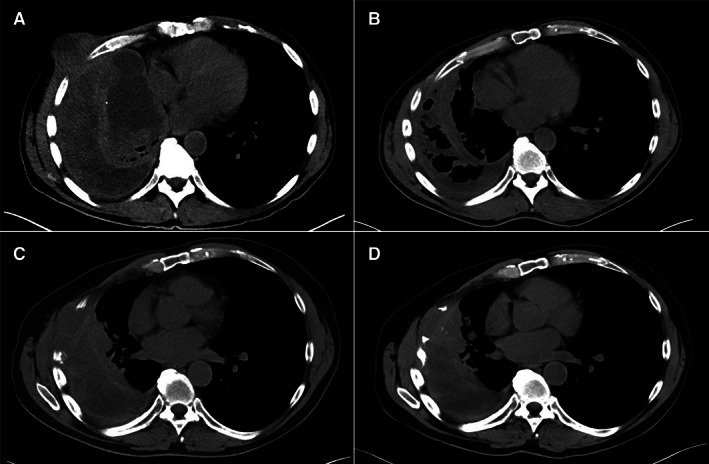
Progression of large metastatic pulmonary myxofibrosarcoma mass on computed tomography scans without contrast. (A): Depicting progression of disease after two cycles of trabectedin with 11.4 × 9.7 cm mass extending through the chest wall. (B): After two cycles of temozolomide (TMZ) with marked decrease in soft tissue component of lesion. (C): Recurrence of disease after 43 cycles of TMZ with a 7.6 × 1.8 × 4.4 cm mass resulting in destruction of adjacent ribs. (D): Stable to slightly decreased size of pulmonary mass after 16 cycles of atezolizumab.
Table 1.
Summary of seven lines of treatment and a description of the radiologic features of the dominant right metastatic lung mass throughout treatment
| Therapy | Clinical trial (if applicable) | Cycles until progression | PFS ratio a | Radiologic features of dominant right lung mass | |
|---|---|---|---|---|---|
| Treatment response | Mass at progression | ||||
| Doxorubicin followed by olaratumab | NCT02326025 | 2 | — | No response | 4.0 cm nodule in largest dimension |
| Gemcitabine/docetaxel | — | 2 | 0.9 | No response | 6.2 cm soft tissue mass in largest dimension |
| Aldoxorubicin | NCT02049905 | 2 | 1.0 | No response | 8.0 cm soft tissue mass in largest dimension with extension into the chest wall |
| Pazopanib and topotecan | NCT02357810 | 7 | 5.1 | 8.1 × 7.6 cm cystic mass (previously solid); solid component decreased to 5.3 × 3.6 cm | 11.0 × 8.5 cm cystic mass with new destruction of anterior ribs and bronchopleural fistula |
| Trabectedin | — | 2 | 0.2 | No response | 11.4 × 9.7 cm cystic mass with increase in solid component extending through the chest wall |
| Temozolomide | — | 43 | 29.2 | Complete resolution of metastatic disease by cycle 9 with residual architectural changes and stable mediastinal lymphadenopathy | 7.6 × 1.8 × 4.4 cm soft tissue mass along surgical staple line |
| Atezolizumab | NCT02091141 | Ongoing with 22 previous cycles | — | Stable 5.0 × 1.1 cm soft tissue mass | n/a |
Progression‐free survival ratio indicating the time to progression on current treatment relative to time to progression on previous treatment.
Abbreviation: PFS, progression‐free survival.
After failure of five prior systemic therapies, he was started on TMZ (150 mg/m2 on days 1–5, with 28‐day cycles) with decrease in the dominant right pulmonary mass from 9.7 × 11.4 cm to 3.5 × 4.8 cm after two cycles (Fig. 2). After eight cycles he had complete resolution of his pulmonary lesions with stable pulmonary effusion and stable axillary and mediastinal lymphadenopathy. He continued TMZ with minimal side effects for 43 cycles, or 38 months, before experiencing recurrence of pulmonary disease. On CT of his chest he was found to have a new 7.6 × 1.8 × 4.4 cm mass, resulting in destruction of the adjacent ribs. The lesion was biopsied and sent for next‐generation sequencing analysis with Foundation One (Foundation Medicine Inc, Cambridge, MA) and Guardant360 (Guardant Health, Redwood City, CA). Results demonstrated tumor molecular burden with 889 mutations per megabase (Mb), but microsatellite stable, with no dominant, targetable somatic alterations. Sequencing data were also notable for a negative O6 methylguanine‐DNA methyltransferase (MGMT), which may contribute to the accumulation of mutations. Based on these results he qualified for, and was enrolled in, clinical trial (NCT02091141) and started on atezolizumab, which is ongoing with stable disease status after 22 cycles (Figs. 1 and 2; Table 1).
Use of TMZ in STS
TMZ is an oral imidazotetrazine prodrug approved for use in the treatment of high‐grade gliomas and metastatic melanoma, with few studies evaluating its efficacy in patients with STS [6].
With respect to STS, a 2003 phase II trial of 26 patients with metastatic STS treated with TMZ demonstrated a progression‐free survival (PFS) of 2.0 months, although patients with leiomyosarcoma had a PFS of 3.9 months [7]. A 2005 double arm phase II trial including 45 patients with STS treated with TMZ, demonstrated a 15.5% partial response rate, which was also primarily confined to individuals with leiomyosarcoma who had a partial response rate of 46% [8]. Neither of these studies enrolled patients with MFS. Lastly, to explore whether TMZ may have additional benefit if paired with targeted therapy, a small study of 14 patients with metastatic hemangiopericytoma and malignant solitary fibrous tumor treated with TMZ and bevacizumab found a PFS of 9.7 months [9].
Use of Checkpoint Inhibitors for STS
Immunotherapies that target checkpoint signaling have shown promise in sarcoma treatment [4, 5, 10]. In a phase II trial of nivolumab with or without ipilimumab in 85 patients with advanced sarcoma, 16% of those who received nivolumab and ipilimumab had an objective response, and overall survival was 10.7 months in the monotherapy group and 14.3 months for the combination therapy [10]. Another phase II study of 20 patients with locally advanced or metastatic sarcoma who received talimogene laherparepvec plus pembrolizumab found that 35% of patients had an objective response at 32 weeks, and the PFS was 17.1 weeks [11].
The effectiveness of checkpoint inhibitors in sarcoma may be partially explained by evidence that sarcomas express PD‐1/PD‐L1, with expression ranging from 0% to 33% across all sarcomas and up to 18% in MFS [12]. There remains, however, a need to identify biomarkers to determine who will respond to these therapies [4]. Of the 35% of patients who had a complete response to talimogene laherparepvec plus pembrolizumab, only one patient expressed PD‐L1 before enrolling in the trial [11].
One promising marker of response to PD‐1/PD‐L1 inhibitors, regardless of cancer type or PD‐1/PD‐L1 expression, is the TMB, or the number of genomic mutations identified per coding area [13]. Moreover, using immunotherapy in patients with an elevated TMB has been shown to incur an overall survival benefit [14]. There is significant variability in TMB among different cancer types, ranging from less than one to thousands of mutations [15]. Our patient had an elevated TMB of 889 mutations/Mb; sarcomas typically have a low/intermediate TMB, with a median TMB for STS of 2.5 mutations/Mb [15].
Combination of TMZ and Atezolizumab
Current research, primarily in the setting of gliomas, suggests a role for the combination of alkylating agents and immunotherapy. There is emerging evidence that tumor genetic hypermutation, a form of tumor resistance to alkylator therapy such as TMZ, results in a tumor microenvironment conducive to a good response to checkpoint inhibition [16, 17].
The therapeutic effect of TMZ comes from the alkylation/methylation of guanine at the O6 location, which results in a mismatch pairing of the methylated/alkylated guanine with thiamine. The resulting mismatch activates the mismatch repair (MMR) pathway which removes the mispaired thiamine, only to have it replaced by another mispaired thiamine; a process that continues to repeat, ultimately resulting in a futile repair cycle and cell death [16, 18]. One pathway of tumor resistance to TMZ is acquired mutations leading to dysfunction of the MMR pathway, which inhibits the cell's ability to repair base mismatches and leads to the accumulation of genomic mutations [16, 19, 20]. A large majority of patients with post–alkylator therapy hypermutation have MMR pathway deficiencies [21, 22]. An additional mechanism of tumor escape involves the upregulation of MGMT, which actively repairs TMZ induced genetic alterations [16].
At the time of recurrence after treatment with TMZ, our patient's tumor pathology demonstrated MGMT methylation, or nonfunctional MGMT, suggesting that hypermutation was the mechanism of escape. This is an emerging area of interest, as 16% to 47% of recurrent TMZ treated gliomas exhibited hypermutation [19, 23]. One limitation to concluding that TMZ was driving hypermutation in our patient is that TMB data are not available prior to treatment with TMZ. It is possible that our patient had an elevated TMB at diagnosis or that another treatment regimen contributed to the elevated mutation burden.
Given the evidence that a high mutation burden portends a good response to checkpoint inhibitors, tumors that develop resistance to TMZ through hypermutation may be good candidates for PD‐1/PD‐L1 inhibitors. Current trials assessing these therapies have had mixed results [19, 24, 25]. A randomized trial of 35 patients with recurrent glioblastoma found that pembrolizumab, a PD‐1 inhibitor, conferred a 486‐day overall survival benefit when given in the neoadjuvant setting [24]. A recently published randomized trial of bevacizumab versus nivolumab in recurrent glioblastoma found no survival benefit; however, TMB was not assessed, and there was a trend toward improvement in overall survival for those with PD‐L1 > 1%. Another recent study retrospectively looking at hypermutated gliomas also found no difference in median overall survival among patients with and without an elevated TMB treated with PD‐1/PD‐L1 inhibitors [19, 25]. Although initial data for gliomas remain inconclusive, the durable response to immunotherapy seen in our patient may indicate a targetable resistance pathway for patients previously responsive to alkylating agents.
Patient Update
As of the submission of this article, our patient has received cycle 22 of atezolizumab with the most recent CT demonstrating stable disease.
Author Contributions
Conception/design: Brian C. Schulte, Victoria Villaflor, Mark Agulnik
Provision of study material or patients: Susan Abbinanti, John P. Hayes, Mark Agulnik
Collection and/or assembly of data: Brian C. Schulte, Susan Abbinanti, John P. Hayes, Mark Agulnik
Data analysis and interpretation: Jason P. Lambden, Max F. Kelsten, Brian C. Schulte, Victoria Villaflor, Mark Agulnik
Manuscript writing: Jason P. Lambden, Max F. Kelsten, Brian C. Schulte, Susan Abbinanti, John P. Hayes, Victoria Villaflor, Mark Agulnik
Final approval of manuscript: Jason P. Lambden, Max F. Kelsten, Brian C. Schulte, Susan Abbinanti, John P. Hayes, Victoria Villaflor, Mark Agulnik
Disclosures
Victoria Villaflor: Takeda (RF), AstraZeneca, Bristol‐Myers Squibb, Genentech (C/A); Mark Agulnik: Eli Lilly & Co., Adaptimmune, Regeneron, AstraZeneca (C/A), Bristol‐Myers Squibb, Bayer (other—speaker's bureau). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Gregory M. Cote, Jie He, Edwin Choy. Next‐Generation Sequencing for Patients with Sarcoma: A Single Center Experience. The Oncologist 2017;22:234–242.
Implications for Practice: The sarcomas are a heterogenous family of over 50 different mesenchymal tumors. Current practice for metastatic disease involves systemic chemotherapy or nonspecific kinase inhibitors such as pazopanib. Sarcomas typically lack the classic kinase alterations seen in many carcinomas. The role of next‐generation sequencing in sarcoma clinical practice remains undefined.
References
- 1. Roland CL, Wang WL, Lazar AJ et al. Myxofibrosarcoma. Surg Oncol Clin N Am 2016;25:775–788. [DOI] [PubMed] [Google Scholar]
- 2. Tsuchie H, Kaya M, Nagasawa H et al. Distant metastasis in patients with myxofibrosarcoma. Ups J Med Sci 2017;122:190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan CW, Merimsky O, Agulnik M et al. Picasso III: A phase III, placebo‐controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol 2016;34:3898–3905. [DOI] [PubMed] [Google Scholar]
- 4. Sambri A, De Paolis M, Spinnato P et al. The biology of myxofibrosarcoma: State of the art and future perspectives. Oncol Res Treat 2020;43:305–312. [DOI] [PubMed] [Google Scholar]
- 5. Uehara T, Fujiwara T, Takeda K et al. Immunotherapy for bone and soft tissue sarcomas. Biomed Res Int 2015;2015:820813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danson SJ, Middleton MR. Temozolomide: A novel oral alkylating agent. Expert Rev Anticanc 2001;1:13–19. [DOI] [PubMed] [Google Scholar]
- 7. Talbot SM, Keohan ML, Hesdorffer M et al. A phase II trial of temozolomide in patients with unresectable or metastatic soft tissue sarcoma. Cancer 2003;98:1942–1946. [DOI] [PubMed] [Google Scholar]
- 8. Garcia del Muro X, Lopez‐Pousa A, Martin J et al. A phase II trial of temozolomide as a 6‐week, continuous, oral schedule in patients with advanced soft tissue sarcoma: A study by the Spanish Group for Research on Sarcomas. Cancer 2005;104:1706–1712. [DOI] [PubMed] [Google Scholar]
- 9. Park MS, Patel SR, Ludwig JA et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer 2011;117:4939–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Angelo SP, Mahoney MR, Van Tine BA et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open‐label, non‐comparative, randomised, phase 2 trials. Lancet Oncol 2018;19:416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly CM, Antonescu CR, Bowler T et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: A phase 2 clinical trial. JAMA Oncol 2020;6:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vargas AC, Maclean FM, Sioson L et al. Prevalence of PD‐L1 expression in matched recurrent and/or metastatic sarcoma samples and in a range of selected sarcomas subtypes. PLoS One 2020;15:e0222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodman AM, Kato S, Bazhenova L et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chalmers ZR, Connelly CF, Fabrizio D et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daniel P, Sabri S, Chaddad A et al. Temozolomide induced hypermutation in glioma: Evolutionary mechanisms and therapeutic opportunities. Front Oncol 2019;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daubon T, Hemadou A, Romero Garmendia I et al. Glioblastoma immune landscape and the potential of new immunotherapies. Front Immunol 2020;11:585616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Margison GP, Santibáñez Koref MF, Povey AC. Mechanisms of carcinogenicity/chemotherapy by O 6‐methylguanine. Mutagenesis 2002;17:483–487. [DOI] [PubMed] [Google Scholar]
- 19. Touat M, Li YY, Boynton AN et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020;580:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hotchkiss KM, Sampson JH. Temozolomide treatment outcomes and immunotherapy efficacy in brain tumor. J Neurooncol 2021;151:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson BE, Mazor T, Hong C et al. Mutational analysis reveals the origin and therapy‐driven evolution of recurrent glioma. Science 2014;343:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J, Cazzato E, Ladewig E et al. Clonal evolution of glioblastoma under therapy. Nat Genet 2016;48:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barthel FP, Johnson KC, Varn FS et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature 2019;576:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cloughesy TF, Mochizuki AY, Orpilla JR et al. Neoadjuvant anti‐PD‐1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019;25:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reardon DA, Brandes AA, Omuro A et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol 2020;6:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]


