Abstract
Lessons Learned
Cediranib and olaparib combination did not result in clinically meaningful activity in patients with metastatic pancreatic ductal adenocarcinoma without known BRCA mutation.
Background
Cediranib, a vascular endothelial growth factor receptor inhibitor, suppresses expression of BRCA1/2 and RAD51 inducing homologous recombination DNA repair deficiency (HRD) in several cancer cell lines and xenograft models [1]. Olaparib provides a clinical benefit in patients with metastatic pancreatic adenocarcinoma (mPDAC) with germline BRCA mutation (gBRCAmt) [2]. We hypothesized that cediranib induces HRD in the absence of gBRCAmt and synergizes with olaparib, resulting in an objective response in patients with mPDAC.
Methods
Patients with mPDAC with at least one prior systemic chemotherapy were enrolled. Patients with known gBRCAmt were excluded. Patients took cediranib 30 mg daily and olaparib 200 mg twice daily, orally. The primary endpoint was objective response (OR) rate.
Results
Nineteen patients received the study drugs. Seven patients came off treatment before the first restaging scan: six because of clinical progression and one because of an adverse event. No OR was observed. Six patients had stable disease (SD) as a best overall response. The median duration of SD was 3.1 months. The median overall survival was 3.4 months. Common treatment‐related adverse events were fatigue, hypertension, and diarrhea.
Conclusion
Cediranib and olaparib combination did not result in clinically meaningful activity in patients with mPDAC without gBRCAmt.
Keywords: Pancreatic ductal adenocarcinoma, Cediranib, Olaparib, BRCA
Discussion
Clinical efficacy of a poly‐(ADP‐ribose) polymerase (PARP) inhibitor in patients with HRD has been well‐documented in pancreatic cancer and other solid tumors [2, 3, 4]. Hypoxia induces downregulation of homologous recombination DNA repair (HR) genes in several cancer cell lines and xenograft models including breast, lung, colon, and prostate cancers [5, 6, 7]. Cediranib, a vascular endothelial growth factor receptor (VEGFR) inhibitor, suppresses expression of BRCA1/2 and RAD51, inducing HR deficiency (HRD) in breast and ovarian cancer cell lines and their xenograft models [1]. The central hypothesis of this study is that cediranib induces tumor hypoxia leading to a HRD phenotype and sensitizes the tumors to a PARP inhibitor, resulting in objective responses and disease control in the absence of a deleterious mutation in BRCA or other HR related genes. To that end, we accrued patients with metastatic solid tumors in four cohorts: pancreatic ductal adenocarcinoma (mPDAC), triple‐negative breast cancer, small cell lung cancer, and non‐small cell lung cancer. Patients with known germline BRCA mutation were excluded. Patients were treated with cediranib 30 mg orally once daily and olaparib 200 mg orally twice daily. The primary objective was to determine the objective response rate in each of the disease cohorts.
We found that the combination of cediranib plus olaparib did not result in any objective response in patients with mPDAC (Fig. 1). Six patients achieved stable disease as best overall response. The median duration of stable disease was only 3.1 months, with the range of 1.2 to 6.8 months. This did not translate into durable disease control. Given the lack of clinically meaningful activity of cediranib and olaparib combination in patients with mPDAC, the accrual to the PDAC cohort was terminated early because of futility.
Figure 1.
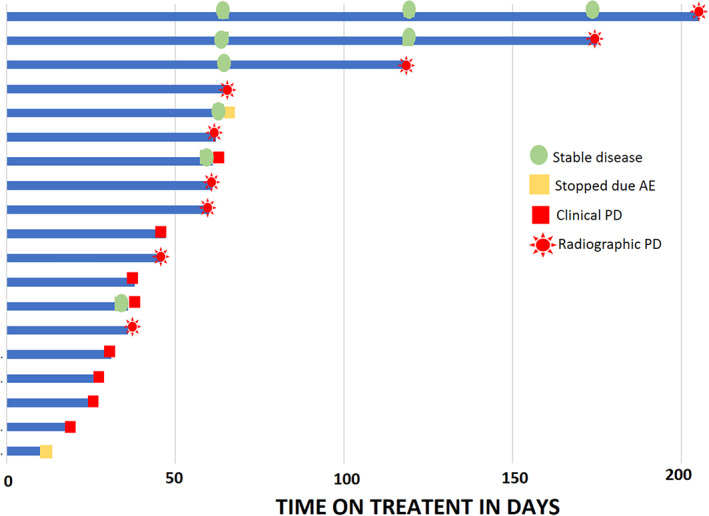
Duration of treatment.
Abbreviations: AE, adverse event; PD, progressive disease
A potential explanation for the lack of activity is that cediranib did not induce HRD phenotype deleterious enough to result in synthetic lethality with olaparib as hypothesized. It should be noted that although the preclinical studies supporting our hypothesis testing were done in several tumor cell lines and xenograft models including lung, colon, breast, prostate and cervical cancers [5, 6, 7], no preclinical studies were performed in pancreatic cancer models.
This regimen is currently under clinical investigation in other solid tumors with tissue and blood‐based correlative studies and will be reported separately.
Trial Information
| Disease | Pancreatic cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Overall response rate |
| Secondary Endpoint | Toxicity |
| Additional Details of Endpoints or Study Design | |
| The primary objective was to assess the objective response rate (ORR) of the combination of cediranib and olaparib in patients with metastatic pancreatic ductal adenocarcinoma. Simon's two‐stage design was used. The null hypothesis that the true ORR is 5% was tested against a one‐sided alternative. In the first stage, 18 response‐evaluable patients were accrued. At least one confirmed objective response was required to proceed to the second stage accrual for a total of 32 patients. The null hypothesis would be rejected if four or more responses are observed in 32 patients. This design yields a type I error rate of 10% and power of 90% when the true ORR is 20%. | |
| Investigator's Analysis | Inactive because results did not meet primary endpoint |
Drug Information
| Generic/Working Name | cediranib |
| Company Name | AstraZeneca |
| Drug Type | Small molecule |
| Drug Class | VEGFR |
| Dose | 30 mgs per flat dose |
| Route | oral (po) |
| Schedule of Administration | once daily |
| Generic/Working Name | olaparib |
| Trade Name | Lynparza |
| Company Name | AstraZeneca |
| Drug Type | Small molecule |
| Drug Class | PARP |
| Dose | 200 mg per flat dose |
| Route | oral (po) |
| Schedule of Administration | twice daily |
Patient Characteristics
| Number of patients, male | 11 |
| Number of patients, female | 8 |
| Stage | IV |
| Age | Median (range): 68 (45–85) years |
| Number of Prior Systemic Therapies | Median (range): 3 (1–5) |
| Performance Status: ECOG |
0 — 7 1 — 12 2 — 0 3 — 0 Unknown — 0 |
| Total Number of Patients | n = 19 |
| Race |
White: 16 (84%) Black or African American: 1 (5%) Asian: 2 (11%) |
| Prior Lines of Therapy |
Median: 3 1: 3 (16%) 2: 6 (32%) ≥3: 10 (53%) |
| Prior Therapies |
FOLFIRINOX: 14 (74%) Gemcitabine‐based regimen: 18 (95%) |
| BRCA 1/2 Mutation Status |
Known or suspected germline BRCA mutation: 0 Unknown: 19 |
Primary Assessment Method
| Title | Clinical activity summary |
| Number of Patients Screened | 24 |
| Number of Patients Enrolled | 24 |
| Number of Patients Evaluable for Toxicity | 19 |
| Number of Patients Evaluated for Efficacy | 18 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | 0 (0%) |
| Response Assessment PR | 0 (0%) |
| Response Assessment SD | 6 (33%) |
| Response Assessment PD | 12 (67%) |
| (Median) Duration Assessments OS | 3.4 months |
| (Median) Duration Assessments Duration of Treatment | 47 days |
Adverse Events (all cycles)
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Fatigue | 26% | 37% | 37% | 0% | 0% | 0% | 74% |
| Hypertension | 53% | 0% | 32% | 16% | 0% | 0% | 47% |
| Diarrhea | 68% | 26% | 5% | 0% | 0% | 0% | 32% |
| Platelet count decreased | 68% | 26% | 5% | 0% | 0% | 0% | 32% |
| Nausea | 79% | 11% | 11% | 0% | 0% | 0% | 21% |
| Anorexia | 79% | 21% | 0% | 0% | 0% | 0% | 21% |
| Aspartate aminotransferase increased | 79% | 11% | 11% | 0% | 0% | 0% | 21% |
| Alkaline phosphatase increased | 79% | 11% | 11% | 0% | 0% | 0% | 21% |
| Vomiting | 84% | 11% | 5% | 0% | 0% | 0% | 16% |
| White blood cell decreased | 84% | 11% | 5% | 0% | 0% | 0% | 16% |
| Lymphocyte count decreased | 84% | 0% | 11% | 5% | 0% | 0% | 16% |
| Dizziness | 84% | 16% | 0% | 0% | 0% | 0% | 16% |
| Alanine aminotransferase increased | 84% | 5% | 11% | 0% | 0% | 0% | 16% |
| Voice alteration | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Anemia | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Constipation | 89% | 5% | 5% | 0% | 0% | 0% | 11% |
| Hyponatremia | 89% | 5% | 0% | 5% | 0% | 0% | 11% |
| Peripheral sensory neuropathy | 89% | 11% | 0% | 0% | 0% | 0% | 11% |
Commonly reported treatment‐related adverse events.
Abbreviation: NC/NA, no change from baseline/no adverse events.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Inactive because results did not meet primary endpoint |
Maintenance olaparib prolongs progression‐free survival (PFS) in patients with a germline BRCA mutation (gBRCAmt) and metastatic pancreatic ductal adenocarcinoma (mPDAC) [2] and has become a standard of care. Although there is no question that the approval of olaparib has provided a hope for patients with mPDAC, the scope of the benefit is limited to the 4% to 7% of the pancreatic cancer patients who carry gBRCAmt [8, 9]. Preclinical studies showed that hypoxia and cediranib downregulate the expression of BRCA1, BRCA2, and RAD51, the key factors of homologous recombination DNA repair (HR), resulting in HR deficiency (HRD) and sensitivity to a PARP inhibitor in several tumor cell lines [1, 5, 6, 7, 10]. Consistent with these preclinical data, cediranib and olaparib has demonstrated superior progression‐free survival and overall survival in women with platinum‐sensitive ovarian cancer compared with olaparib monotherapy regardless of germline BRCA mutation status [11, 12]. The question we asked in this study was if HRD phenotype could be induced in patients without germline BRCA mutation with an hypoxia‐inducing, vascular endothelial growth factor receptor inhibitor, cediranib [13], resulting in a synthetic lethality with a poly‐(ADP‐ribose) polymerase inhibitor, olaparib.
We accrued patients without known mutations in BRCA genes in four disease‐specific cohorts: pancreatic ductal adenocarcinoma (PDAC), non‐small cell lung cancer, small cell lung cancer, and triple‐negative breast cancer. Herein, we report the results from the safety and clinical activity analyses of PDAC cohort.
We found that the combination of cediranib 30 mg once daily plus olaparib 200 mg twice daily did not result in any objective response in patients with metastatic PDAC. Although six (32%) patients achieved stable disease as best overall response, including four patients with regression in the tumor burden, the median duration of disease control was only 3.1 months. This did not translate into durable disease control. Given the lack of clinically meaningful activity of cediranib and olaparib combination in patients with mPDAC without gBRCAmt, the accrual to the PDAC cohort was terminated early because of futility.
There are a few things to comment about the study populations. First, during enrollment of the PDAC cohort, the study intentionally excluded those with known germline BRCA mutation to test our hypothesis of cediranib‐induced HRD phenotype. Although the testing was not required to prove, none of the study patients had known deleterious mutations in BRCA genes prior to enrollment. Second, the study population was heavily pretreated. Eighty‐five percent of the patients had two or more lines of prior systemic therapies. Five (32%) patients had clinical progression prior to the first restaging scan in 2 months. The median overall survival of 3.4 months suggested that the patients were indeed in terminal stage of their disease. Plus, the median duration of drug exposure was less than 2 months, which undermines our confidence in sufficiency of treatment exposure to test the clinical activity of the regimen. Although the adverse event profile was consistent with prior reports, [11, 12, 14] adverse events such as fatigue, anorexia, and diarrhea requiring dose‐interruption, and hypertension requiring close blood pressure monitoring and adjustment of antihypertensives made this regimen less easily tolerated and challenging for some patients (Table 1).
A potential explanation for lack of activity is that cediranib does not induce HRD phenotype in heavily pretreated patients with pancreatic cancer or does not sensitize the tumors to a PARP inhibitor. It should be noted that although the preclinical studies supporting our hypothesis testing were done in several tumor cell lines and xenograft models including lung, colon, breast, prostate, and cervical cancers [5, 6, 7, 10], it was not tested in pancreatic cancer models.
Olaparib remains a treatment option for metastatic patients with PDAC with gBRCAmt as a maintenance therapy after a course of platinum‐based chemotherapy without disease progression. We did not find any sign of clinically meaningful activity with the cediranib and olaparib combination in patients with heavily pretreated mPDAC and without gBRCAmt.
Future studies should focus on the patient population with a limit on prior lines of therapy in which patients can receive sufficient treatment exposure. The hypothesis of cediranib‐induced HRD phenotype remains to be tested in other tumors. The analyses of clinical activities in other tumor cohorts are ongoing. Biomarkers analyses including circulating tumor DNA and angiogenesis markers in mPDAC patients are underway.
Cediranib and olaparib did not result in any clinically meaningful activity in patients with heavily pretreated metastatic pancreatic ductal adenocarcinoma without BRCA mutation.
Disclosures
Dana B. Cardin: Rafael (C/A), Abbvie, EMD Serono, Bristol Myers Squibb, Rafael, Corcept (RF); Patricia M. LoRusso: Five Prime, Takeda, Agenus, IQVIA, TRIGR, Pfizer, Immuno Met, Black Diamond, GlaxoSmithKline, QED Therapeutics, AstraZeneca, EMD Serono, Shattuck, Astellas, Salarius,Silverback, MacroGenics, Kyowa Kirin, Kineta, Zentalis, Molecular Templates,ABL Bio, SK Life Science, STCube, Bayer, I‐Mab (C/A), Abbvie, ADC Therapeutics, ALX Oncology, Astellas, Astex, AstraZeneca, Bayer, Black Diamond, Boehringer Ingelheim, Calico Life Sciences, Corvus, CytomX, Eisai, Eli Lilly, EMD Serono,Five Prime, FLX Bio, F Star Delta, Genentech, Genmab, Incyte, Linnaeus, MedImmune, Merck, Moderna, NextCure, Pfizer, Ribon, Sotio, Stemline, Takeda,Tesaro (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Table
Table 1.
Common (>10%) treatment‐related adverse events
| AE terms | Grade 1 (n = 15), n (%) | Grade 2 (n = 13), n (%) | Grade 3 (n = 8), n (%) | Grade 4 (n = 0), n (%) | Total (n = 19), n (%) |
|---|---|---|---|---|---|
| Fatigue | 7 (37) | 7 (37) | 14 (74) | ||
| Hypertension | 6 (32) | 3 (16) | 9 (47) | ||
| Diarrhea | 5 (26) | 1 (5) | 6 (32) | ||
| Thrombocytopenia | 5 (26) | 1 (5) | 6 (32) | ||
| Nausea | 2 (11) | 2 (11) | 4 (21) | ||
| Anorexia | 4 (21) | 4 (21) | |||
| AST increased | 2 (11) | 2 (11) | 4 (21) | ||
| Alkaline phosphatase increased | 2 (11) | 2 (11) | 4 (21) | ||
| Vomiting | 2 (11) | 1 (5) | 3 (16) | ||
| Leukopenia | 2 (11) | 1 (5) | 3 (16) | ||
| Lymphopenia | 2 (11) | 1 (5) | 3 (16) | ||
| Dizziness | 3 (16) | 3 (16) | |||
| ALT increased | 1 (5) | 2 (11) | 3 (16) | ||
| Hoarseness | 2 (11) | 2 (11) | |||
| Anemia | 2 (11) | 2 (11) | |||
| Constipation | 1 (5) | 1 (5) | 2 (11) | ||
| Hyponatremia | 1 (5) | 1 (5) | 2 (11) | ||
| Peripheral sensory neuropathy | 2 (11) | 2 (11) |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate transaminase.
Acknowledgments
This study was funded by NIH/UM1 Grant Number 5UM1CA186689‐04. We thank all the study participants, their families, and the study personnel of the Experimental Therapeutics Clinical Trials Network.
Disclosures of potential conflicts of interest may be found at the end of this article.
Footnotes
- ClinicalTrials.gov Identifier: NCT02498613
- Sponsor: NCI
- Principal Investigator: Joseph Kim
- IRB Approved: Yes
References
- 1. Kaplan AR, Gueble SE, Liu Y et al. Cediranib suppresses homology‐directed DNA repair through down‐regulation of BRCA1/2 and RAD51. Sci Transl Med 2019;11:eaav4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Golan T, Hammel P, Reni M et al. Maintenance olaparib for germline BRCA‐mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fong PC, Boss DS, Yap TA et al. Inhibition of poly(ADP‐ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–134. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman B, Shapira‐Frommer R, Schmutzler RK et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bindra RS, Schaffer PJ, Meng A et al. Down‐regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol 2004;24:8504–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bindra RS, Gibson SL, Meng A et al. Hypoxia‐induced down‐regulation of BRCA1 expression by E2Fs. Cancer Res 2005;65:11597–11604. [DOI] [PubMed] [Google Scholar]
- 7. Meng AX, Jalali F, Cuddihy A et al. Hypoxia down‐regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother Oncol 2005;76:168–176. [DOI] [PubMed] [Google Scholar]
- 8. Holter S, Borgida A, Dodd A et al. Germline BRCA mutations in a large clinic‐based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 2015;33:3124–3129. [DOI] [PubMed] [Google Scholar]
- 9. Ferrone CR, Levine DA, Tang LH et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol 2009;27:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hegan DC, Lu Y, Stachelek GC et al. Inhibition of poly(ADP‐ribose) polymerase down‐regulates BRCA1 and RAD51 in a pathway mediated by e2f4 and p130. Proc Natl Acad Sci USA 2010;107:2201–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu JF, Barry WT, Birrer M et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum‐sensitive ovarian cancer: A randomised phase 2 study. Lancet Oncol 2014;15:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu JF, Barry WT, Birrer M et al. Overall survival and updated progression‐free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum‐sensitive ovarian cancer. Ann Oncol 2019;30:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burrell JS, Walker‐Samuel S, Baker LC et al. Evaluation of novel combined carbogen USPIO (CUSPIO) imaging biomarkers in assessing the antiangiogenic effects of cediranib (AZD2171) in rat C6 gliomas. Int J Cancer 2012;131:1854–1862. [DOI] [PubMed] [Google Scholar]
- 14. Liu JF, Tolaney SM, Birrer M et al. A phase 1 trial of the poly(ADP‐ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti‐angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple‐negative breast cancer. Eur J Cancer 2013;49:2972–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]


