Abstract
Background
Abemaciclib is a CDK4/6 inhibitor used to treat hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer. The prognostic value of patient‐reported outcomes (PROs) has been minimally explored for treatment outcomes with CDK4/6 inhibitors. The performance of PROs compared with Eastern Cooperative Oncology Group performance status (ECOG‐PS) is unknown.
Materials and Methods
This study pooled data from single‐arm trial, MONARCH 1, and randomized trials, MONARCH 2 and 3. In total, 900 patients initiated abemaciclib and 384 comparator therapy. Pretreatment PRO association with progression‐free survival (PFS) was modeled using Cox proportional hazards regression. Prediction performance was assessed via the C‐statistic (c). PROs were recorded via the European Organisation for Research and Treatment of Cancer QLQ‐C30.
Results
Patient‐reported physical function, pain, role function, fatigue, and appetite loss were associated with PFS on univariable and adjusted analysis (p < .05). Physical function (c = 0.55) was most predictive, superior to ECOG‐PS (c = 0.54), with multivariable analysis indicating both provide independent information (p < .02). In the pooled randomized arms of MONARCH 2 and 3, the PFS treatment benefit (hazard ratio [95% confidence interval]) of abemaciclib (vs. comparators) was 0.75 (0.57–1.0) for low physical function, compared with 0.48 (0.40–0.59) for intermediate/high (p[interaction] = .01).
Conclusion
PROs were identified as prognostic factors for PFS in patients initiating abemaciclib, with patient‐reported physical function containing independent predictive information beyond ECOG‐PS. Low physical function was associated with a decrease in the magnitude of PFS benefit from abemaciclib. PROs should be explored as prognostic, predictive, and stratification factors for clinical use and research trials of CDK4/6 inhibitors.
Implications for Practice
For the first time, pretreatment patient‐reported outcomes have been shown to be independent prognostic markers for progression‐free survival (PFS) in patients diagnosed with hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2−) advanced breast cancer treated with abemaciclib. Importantly, patients with low physical function had a smaller PFS benefit from abemaciclib (vs. comparator) than patients with intermediate/high physical function. The present study demonstrates patient‐reported outcomes as a simple, effective, inexpensive, and independent prognostic marker for patients with HR+/HER2− advanced breast cancer treated with abemaciclib.
Keywords: Abemaciclib, Patient‐reported outcome measures, Progression‐free survival, Advanced breast cancer, Prognosis
Short abstract
Describing a cohort of patients with HR+/HER2− advanced breast cancer treated with abemaciclib, this article evaluates the prognostic association between pretreatment patient‐reported outcomes and progression‐free survival, the prediction performance of patient‐reported outcomes compared with ECOG performance status, and the effect of patient‐reported outcomes on the treatment benefit of abemaciclib versus comparator therapy.
Introduction
Patient‐reported outcomes (PROs) are structured self‐reported measures encompassing physical, emotional, social, cognitive, disease, and quality of life status [1]. In cancer clinical trials, PROs are an important secondary outcome for establishing the effectiveness of treatments [2, 3, 4, 5]. More recently, a range of PROs have been found to be prognostic of cancer outcomes [6, 7, 8, 9, 10, 11, 12, 13, 14, 15], potentially even more so than common clinicopathological data or established metrics such as the physician‐assessed Eastern Cooperative Oncology Group performance status (ECOG‐PS) [10, 16, 17]. However, it is still unclear whether the prognostic importance differs significantly between treatments and cancers and whether PROs can predict treatment efficacy in addition to prognosis [7, 8, 10]. Limitations of the broader research in this area include small sample sizes and haphazard approaches to evaluating the broad range of PROs commonly collected [18].
Abemaciclib is a CDK4/6 inhibitor—a treatment option for hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2−) advanced breast cancer (ABC). Abemaciclib in combination with a nonsteroidal aromatase inhibitor (NSAI) (first line) or fulvestrant (second line) has demonstrated significant ability to improve progression‐free survival (PFS) in participants compared with an NSAI or fulvestrant alone [19, 20, 21, 22, 23].
However, there has been minimal research investigating prognostic factors for patients with HR+/HER2− ABC initiating CDK4/6 inhibitors, limiting the ability to provide realistic expectations for this increasingly used emerging medicine [24, 25]. Di Leo et al. [26] previously identified liver metastases, progesterone receptor–negative tumors, and high histological grade tumors as important factors for prognosis and/or treatment benefit in patients with HR+/HER2− ABC initiating abemaciclib.
More general to ABC, pretreatment PROs have been shown to be prognostic of PFS in patients initiating chemotherapy options [27, 28]. PROs have also shown to be associated with PFS in other cancer types [6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. However, as the importance of prognostic variables can differ vastly between received treatments and cancer types, there is a need to evaluate PROs for CDK 4/6 inhibitors specifically. To date, there has been no research on the prognostic significance of PROs for CDK 4/6 inhibitor therapies. In addition, the potential for PROs to guide treatment decisions on CDK 4/6 inhibitors have not yet to be evaluated.
In a cohort of patients with HR+/HER2− ABC treated with abemaciclib, this study aimed to evaluate (a) the prognostic association between pretreatment PROs and PFS, (b) the prediction performance of PROs compared with ECOG‐PS, and (c) the effect of PROs on the treatment benefit of abemaciclib versus comparator therapy.
Materials and Methods
Patient Population
This study was a secondary analysis of individual‐participant data from clinical trials: MONARCH 1 [19] (phase II trial NCT02102490), MONARCH 2 [21, 22] (phase III trial NCT02107703), and MONARCH 3 [20, 23] (phase III trial NCT02246621). Briefly, inclusion criteria of all trials included patients with HR+/HER2− advanced/metastatic breast cancer, an ECOG‐PS of 0 or 1, and postmenopausal status [19, 20, 21, 22, 23]. MONARCH 1 included patients who had disease progression with prior endocrine therapy and at least two chemotherapy regimens in the metastatic setting [19]. In MONARCH 1, patients initiated abemaciclib at 200 mg oral twice daily [19]. MONARCH 2 included participants randomized, 2:1, to abemaciclib (150 mg oral twice daily) plus fulvestrant or placebo plus fulvestrant, following early disease relapse with neoadjuvant or adjuvant endocrine therapy or progression on first‐line endocrine therapy [21, 22]. MONARCH 3 included participants with no prior systemic therapy in the advanced setting who were randomized, 2:1, to abemaciclib (oral 150 mg twice daily) plus NSAI (daily anastrozole or letrozole) or placebo plus NSAI [20, 23].
Predictor and Outcome Definitions
The primary assessed outcome was PFS. Overall survival (OS) was assessed as a secondary outcome. PFS was measured from randomization to the time of disease progression or death from any cause, with disease progression investigator‐assessed as per RECIST version 1.1 [19, 20, 21, 22, 23, 29]. OS was defined as the time from randomization to date of death from any cause [19, 20, 21, 22, 23].
Pretreatment PROs were recorded in all three studies via the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30 (version 3.0) questionnaire [1]. The QLQ‐C30 incorporates multi‐item and single‐item measures that are linearly transformed to standardize scores to a range between 0 and 100 [30]. These include five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, nausea, and vomiting), a global health status, and six single items assessing symptoms commonly reported by patients with cancer (dyspnea, loss of appetite, insomnia, constipation and diarrhea, and financial difficulties). Single‐item raw scores were captured on a four‐point scale ranging from 1, “Not at all,” to 4, “Very much” [30]. For functional scale measures, higher scores represent a better level of function from a patient perspective. For symptom scales, higher scores represent a worse level of symptoms.
Statistical Analysis
The association between pretreatment PROs and PFS was assessed via Cox proportional hazards regression. All analyses were stratified by study and treatment arm. Complete case analyses were conducted, as there were minimal missing data. Akaike information criterion and visual checks were used to evaluate potential nonlinear associations between PROs and PFS. Prediction performance was assessed using the C‐statistic (c). Upon standard guidelines recommending ≥10 events for every degree of freedom, the study was well powered to evaluate available PROs. For identified PRO‐PFS associations, a sensitivity analysis of the PRO association with OS was conducted. All values of p < .05 were considered statistically significant. Analyses were performed using R version 3.4.3.
Analyses adjusted for known prognostic factors (previously identified pretreatment ECOG‐PS, bone‐only disease, liver metastases, progesterone receptor status, and histological tumor grade) were conducted to evaluate the independence of PROs [26]. Exploratory multivariable analysis of the prognostic performance of PROs compared with ECOG‐PS was conducted, assessed via c.
Using the intention‐to‐treat arms of MONARCH 2 and 3, the heterogeneity in PFS benefit of abemaciclib versus the comparator therapy arms was assessed using a PRO‐by‐treatment interaction term in a Cox proportional regression model.
Kaplan‐Meier method was used for plotting the association between PRO values and survival outcomes. For plotting, PRO scores were grouped into three levels (low, intermediate, high) based on the EORTC QLQ‐C30 Tables of Reference Values for “Breast Cancer: recurrent/metastatic” [31]. “Low” represents PRO scores within the lower 25th percentile of the reference group, “intermediate” within the middle 50th percentiles, and “high” within the upper 25th percentile.
Results
Patient Population
In the pooled cohort of patients with HR+/HER2− ABC, 900 participants were treated with abemaciclib and 384 were in the placebo, comparator therapy arms. Supplemental online Table 1 presents baseline patient characteristics by study. Differences in distributions by study were observed with respect to histological tumor grade, presence of liver tumor site, and bone‐only disease. Except for patient‐reported emotional function, the distributions of pretreatment PRO values were similar between study cohorts (supplemental online Table 2). Median follow‐up was 22 (95% confidence interval [CI], 21–23) months in the pooled sample.
Prognostic Performance of PROs for Abemaciclib Treatment
In the pooled abemaciclib‐treated cohort from MONARCH 1, 2, and 3, the association between pretreatment PROs and PFS was optimally described by a linear association. On univariable and adjusted analysis, a significant association between PFS and patient‐reported physical function, pain, role function, fatigue, and appetite loss were demonstrated in patients who were treated with abemaciclib (p < .05; supplemental online Table 3). Of these, physical function (c = 0.55), pain (c = 0.54), and role function (c = 0.54) were the most predictive (supplemental online Table 3). No heterogeneity in the prognostic performance of physical function, pain, or role function was observed between studies (supplemental online Table 4). In the pooled comparator arms (i.e., placebo arms) of MONARCH 2 and 3, no significant association between PFS and patient‐reported physical function, pain, or role function was observed (supplemental online Table 5).
Figure 1 presents Kaplan‐Meier estimates of PFS by patient‐reported physical function, pain, and role function according to EORTC reference value groups [30] for patients who initiated abemaciclib. The probability of a PFS event occurring within the first 12 months of abemaciclib therapy was, respectively, observed to range from 54% to 65% for low to high physical function, from 57% to 62% for low to high pain, and from 54% to 62% for low to high role function (Fig. 1).
Figure 1.
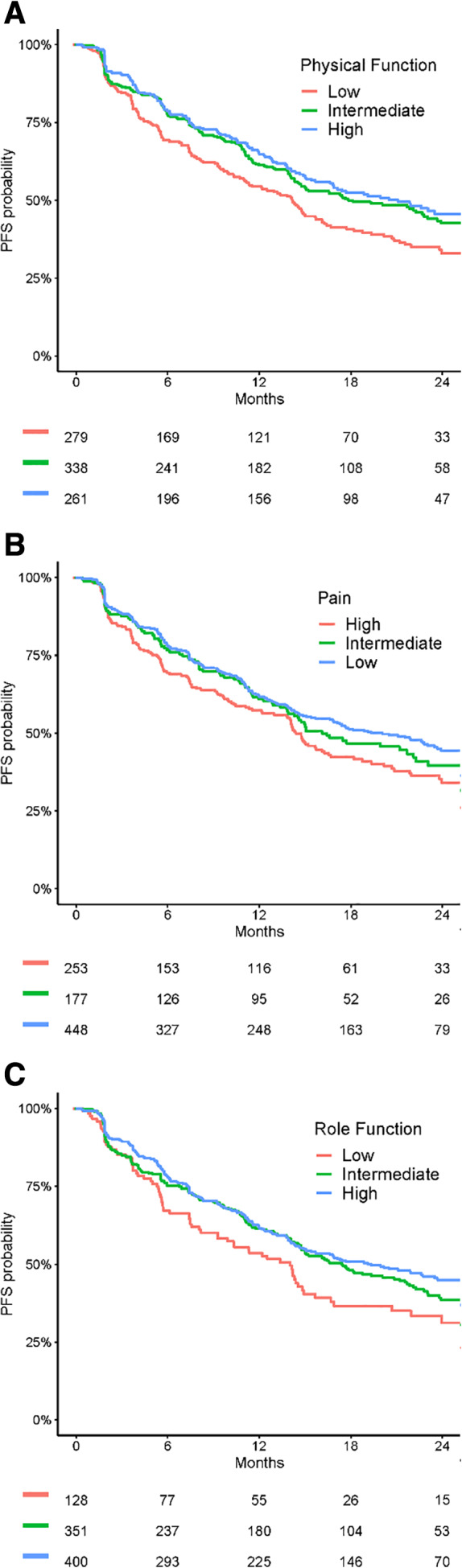
Kaplan‐Meier estimates of PFS by patient‐reported physical function (A), pain (B), and role function (C) for patients treated with abemaciclib. Abbreviation: PFS, progression‐free survival.
Sensitivity analysis indicated that on univariable and adjusted analysis, patient‐reported physical function, pain, and role function were also significantly associated with OS (p < .02; supplemental online Table 6).
Comparison of Patient‐Reported Physical Function with ECOG‐PS
The PFS predictive performance (c) of patient‐reported physical function (low vs. intermediate/high) in patients treated with abemaciclib was 0.55. Comparably, ECOG‐PS (1+ vs. 0) demonstrated a predictive performance of 0.54 (Table 1). Although ECOG‐PS was related with physical function (supplemental online Table 7), multivariable analysis indicates that both variables provide independent prognostic information (Table 1).
Table 1.
Associations between patient‐reported physical function and ECOG‐PS with progression‐free survival for patients treated with abemaciclib
| Predictors | Univariable | Multivariable a | ||||||
|---|---|---|---|---|---|---|---|---|
| n | m% (95% CI) | HR (95% CI) | p value | c | n | HR (95% CI) | p value | |
| Physical function | <.001 | 0.55 | .01 | |||||
| Intermediate or high b | 599 | 63 (59–67) | 1 | 597 | 1 | |||
| Low c | 279 | 54 (49–61) | 1.41 (1.16–1.72) | 279 | 1.31 (1.07–1.60) | |||
| ECOG‐PS | .001 | 0.54 | .01 | |||||
| 0 | 528 | 63 (59–68) | 1 | 514 | 1 | |||
| 1+ | 370 | 56 (51–61) | 1.35 (1.12–1.63) | 362 | 1.29 (1.06–1.57) | |||
Model includes both pretreatment physical function and ECOG‐PS.
Intermediate/high physical function ≥73.3.
Low physical function <73.3.
Abbreviations: CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; m%, probability of progression‐free survival within first 12 months.
Exemplifying the discordance between physician defined ECOG‐PS and patient‐reported physical function, of the 528 abemaciclib‐treated patients assigned an ECOG‐PS of 0 (fully active, without restriction), 19% (n = 100) self‐reported the worst reportable score for physical function, 9% (49) reported the worst score for strenuous activities, 6% (31) reported the worst score for taking a long walk, and 1% (4) reported the worst score for needing to stay in bed or a chair all day (supplemental online Table 8).
Treatment Benefit of Abemaciclib
In the pooled, randomized arms of MONARCH 2 and 3, the relative PFS benefit (hazard ratio [95% CI]) of abemaciclib (vs. comparator arms) was 0.75 (0.57–1.0) for patients reporting low physical function, compared with 0.48 (0.40–0.59) for intermediate/high physical function (p[interaction] = .01). Figure 2 presents Kaplan‐Meier estimates of the PFS benefit of abemaciclib (vs. comparator) in the randomized arms of MONARCH 2 and 3, subgrouped by low and intermediate/high physical function. In MONARCH 2, abemaciclib (vs. comparator therapy) was observed to increase the 12‐month probability of PFS by 14% (52% vs. 38%) for the low physical function cohort, compared with 22% (64% vs. 42%) for intermediate/high physical function cohort (supplemental online Table 9). In MONARCH 3, abemaciclib (vs. comparator therapy) was observed to increase the 12‐month probability of PFS by 4% (69% vs. 65%) for the low physical function cohort, compared with 22% (75% vs. 53%) for intermediate/high physical function cohort (supplemental online Table 10). The above results indicate that low physical function was associated with a decrease in the magnitude of PFS benefit from abemaciclib, with the impact most pronounced in MONARCH 3.
Figure 2.
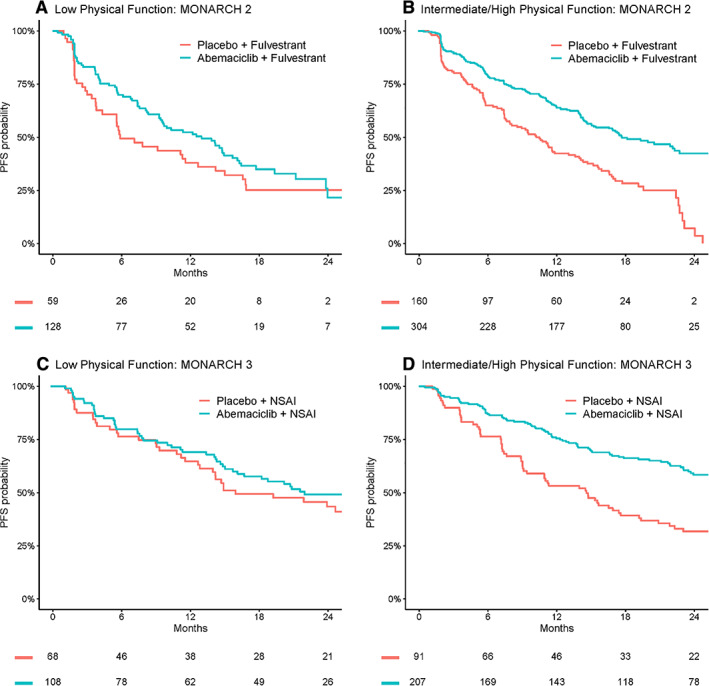
Kaplan‐Meier comparisons of PFS by pretreatment patient‐reported physical function for the randomized arms of MONARCH 2 and MONARCH 3 (abemaciclib vs. comparator). Abbreviations: NSAI, nonsteroidal aromatase inhibitor; PFS, progression‐free survival.
In the pooled, randomized arms of MONARCH 2 and 3, the relative PFS benefit (hazard ratio [95% CI]) of abemaciclib (vs. comparator arms) was 0.60 (0.47–0.78) for patients assigned an ECOG‐PS of 1+, compared with 0.51 (0.41–0.62) for an ECOG‐PS score of 0 (p[interaction] = .3). Supplemental online Figure 1 presents Kaplan‐Meier estimates of the PFS benefit of abemaciclib (vs. comparator) in the randomized arms of MONARCH 2 and 3, subgrouped by ECOG‐PS score.
Discussion
For the first time, pretreatment PROs have been shown to be independent prognostic markers for PFS in patients diagnosed with HR+/HER2− ABC treated with abemaciclib, with patient‐reported physical function most predictive in the assessed cohort. Furthermore, in the pooled randomized arms of MONARCH 2 and 3, patients with low physical function had a smaller PFS benefit from abemaciclib (vs. comparator) than patients with intermediate/high physical function.
In this study, PROs, including physical function, pain, role function, fatigue, and appetite loss, were identified as significantly and independently associated with PFS. Patient‐reported physical function was the most predictive. These results are consistent with previous reports in ABC where PROs were generally prognostic, with physical function shown to be of particular value [27, 32, 33]. Nonetheless, this is the first study to specifically focus on patients with HR+/HER2− ABC treated with a CDK 4/6 inhibitor, and variability in the prognostic value of specific PRO measures has been observed between cancer types and subgroups previously [6, 7, 12].
Although PROs are commonly used as trial outcomes, their use in routine practice is limited. Emerging evidence indicates their use in routine clinical care can result in enhancement of quality of life, patient satisfaction, and survival outcomes from anticancer treatments [34, 35]. Preliminary evidence also suggests PROs may improve a clinician's ability to predict clinical outcomes [36], potentially even more so than common clinicopathological data [10, 16, 17]. However, a lack of a nuanced understanding of the variability in the importance of PROs between cancer medicines and cancer types is limiting the ability to apply PROs in clinical practice [7, 18, 36, 37]. The present study extends existing research by (a) focusing on an unexplored ABC subtype treated with an emerging treatment option, (b) evaluating a range of collected PRO measures (as opposed to an arbitrary selection), and (c) evaluating the independence of PROs from other known prognostic markers. The present study demonstrates PROs as a simple, effective, inexpensive, and independent prognostic marker for HR+/HER2− ABC treated with abemaciclib, which is a patient cohort with a scarcity of validated prognostic markers. Ultimately, it will be of interest for future research to consider incorporating PROs in the development of clinical prediction models and tools, which may be able to provide patients with realistic expectations of treatment outcomes and support shared decision‐making [6]. At present this has been minimally explored [8, 24, 26, 38, 39], particularly at a cancer subtype and treatment level.
ECOG‐PS is a well‐established, physician‐assessed report of patient functioning, and it is commonly used to stratify randomized control trials in ABC [40, 41, 42, 43]. Despite correlation between patient‐reported physical function and ECOG‐PS, multivariable analysis revealed both measures contained independent prognostic information. This independence was likely driven by observed differences in patient and physician reports of physical function, highlighting that the considerations of the patient and clinician do not always align. This study's findings are similar to those of Hopkins et al. [10], where ECOG‐PS and patient‐reported physical function were demonstrated as independent prognostic markers for a cohort of patients with lung cancer treated with the immune checkpoint inhibitor atezolizumab. This consistency in findings suggests that appreciation of both ECOG‐PS and patient‐reported physical function could allow a more comprehensive prediction of likely outcomes. Furthermore, it could be considered whether pretreatment patient‐reported physical function has value as a clinical trial stratification factor to better ensure standardization between treatment arms [44].
A key finding of this study was the significant reduction of PFS benefit from abemaciclib (vs. comparator therapy) in patients reporting low pretreatment physical function. This indicates physical function may be an important predictor of a subgroup of patients likely to achieve less benefit from abemaciclib ± fulvestrant/NSAI, compared with fulvestrant/NSAI alone. There is a need to evaluate whether patient‐reported physical function predicts treatment benefit of other CDK4/6 inhibitor therapies, as well as CDK4/6 inhibitors used at different lines of therapy. Of note, patients enrolled in MONARCH 3 had the most pronounced treatment benefit heterogeneity across patient‐reported physical function. This observation will need to be evaluated in other existing CDK 4/6 inhibitors to confirm whether there is a difference within the drug class and between combination approaches with fulvestrant or NSAIs.
Evidence‐based medicine is backboned by randomized clinical trials, which are stringently regulated. The result is that the data used in this post hoc analysis are high‐quality, particularly the PFS and PRO data, which are not often collected in routine clinical care. Nonetheless, the inclusion criteria of trials can limit generalizability. For example, no participants within the pooled data had ECOG‐PS greater than 2, and this exclusion criterion likely affects the distribution of PRO values also. Thus, future research should aim to confirm the identified associations within real‐world populations with broader ECOG‐PS and PRO distributions. It is acknowledged that this study does not reveal causative relationships between PROs and PFS; rather, the research is hypothesis generating and focuses on associations from a relatively inexpensive, easy‐to‐collect marker. A significant strength of the study was the robustness of the patient‐reported physical function association with PFS, which was observed to decrease in predictive performance only minimally when grouped according to the EORTC references ranges [31]. It is hypothesized that using the EORTC references ranges will facilitate the use of PROs within clinical practice—albeit to our knowledge this is the first time the prognostic performance of PROs has been presented in this manner.
Conclusion
Pretreatment PROs were identified as significant prognostic factors for PFS in patients with HR+/HER2− ABC treated with abemaciclib. Patient‐reported physical function was most predictive of PFS, and it contained predictive information independent of and superior to known clinical prognostic markers including ECOG‐PS. Most interestingly, in patients reporting low pretreatment physical function, a decrease in the magnitude of PFS benefit from abemaciclib was observed. This study highlights significant potential for patient‐reported physical function as simple and inexpensive prognostic factor and as a predictive marker that informs on likely magnitude of treatment benefit from CDK4/6 inhibitors for HR+/HER2− ABC.
Author Contributions
Conception/design: Sarah Badaoui, Ganessan Kichenadasse, Andrew Rowland, Michael J. Sorich, Ashley M. Hopkins
Provision of study material or patients: Michael J. Sorich, Ashley M. Hopkins
Collection and/or assembly of data: Sarah Badaoui, Michael J. Sorich, Ashley M. Hopkins
Data analysis and interpretation: Sarah Badaoui, Ganessan Kichenadasse, Andrew Rowland, Michael J. Sorich, Ashley M. Hopkins
Manuscript writing: Sarah Badaoui, Ganessan Kichenadasse, Andrew Rowland, Michael J. Sorich, Ashley M. Hopkins
Final approval of manuscript: Sarah Badaoui, Ganessan Kichenadasse, Andrew Rowland, Michael J. Sorich, Ashley M. Hopkins
Disclosures
Andrew Rowland: Pfizer (RF); Michael J. Sorich: Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1: Kaplan Meier comparisons of PFS by pre‐treatment ECOG‐PS for the randomised arms of MONARCH 2 and MONARCH 3 (abemaciclib versus comparator).
Appendix S1: Supporting information
Acknowledgments
The authors would like to acknowledge the contributions of Professor Cheri Ostroff and Mr. Mark Haseloff, who had an important role in the design, analysis, and communication of this research. Mark and Cheri are vital members of our research team, and we are deeply greatly for the contributions they make as part of our consumer advisory committee. M.J.S and A.R are supported by Beat Cancer Research Fellowships from Cancer Council South Australia. A.M.H is supported by a Postdoctoral Fellowship from the National Breast Cancer Foundation, Australia (PF‐17‐007).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 2. Vodicka E, Kim K, Devine E et al. Inclusion of patient‐reported outcome measures in registered clinical trials: Evidence from ClinicalTrials.gov (2007–2013). Contemp Clin Trials 2015;43:1–9. [DOI] [PubMed] [Google Scholar]
- 3. Sanders C, Egger M, Donovan J et al. Reporting on quality of life in randomised controlled trials: Bibliographic study. BMJ 1998;317:1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaufman PA, Toi M, Neven P et al. Health‐related quality of life in MONARCH 2: Abemaciclib plus fulvestrant in hormone receptor‐positive, HER2‐negative advanced breast cancer after endocrine therapy. The Oncologist 2020;25:e243–e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goetz MP, Martin M, Tokunaga E et al. Health‐related quality of life in MONARCH 3: Abemaciclib plus an aromatase inhibitor as initial therapy in HR+, HER2‐ advanced breast cancer. The Oncologist 2020;25:e1346–e1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mierzynska J, Piccinin C, Pe M et al. Prognostic value of patient‐reported outcomes from international randomised clinical trials on cancer: A systematic review. Lancet Oncol 2019;20:e685–e698. [DOI] [PubMed] [Google Scholar]
- 7. Gotay CC, Kawamoto CT, Bottomley A et al. The prognostic significance of patient‐reported outcomes in cancer clinical trials. J Clin Oncol 2008;26:1355–1363. [DOI] [PubMed] [Google Scholar]
- 8. Quinten C, Coens C, Mauer M et al. Baseline quality of life as a prognostic indicator of survival: A meta‐analysis of individual patient data from EORTC clinical trials. Lancet Oncology 2009;10:865–871. [DOI] [PubMed] [Google Scholar]
- 9. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopkins AM, Wagner J, Kichenadasse G et al. Patient‐reported outcomes as a prognostic marker of survival in patients with advanced non‐small cell lung cancer treated with immunotherapy. Int J Cancer 2020;147:3085–3089. [DOI] [PubMed] [Google Scholar]
- 11. Rutherford C, Campbell R, White K et al. Patient‐reported outcomes as predictors of survival in patients with bowel cancer: A systematic review. Qual Life Res 2019;28:2871–2887. [DOI] [PubMed] [Google Scholar]
- 12. Patel SB, Akerley WL, Ose D et al. Prognostic value of patient‐reported outcomes (PROs) in advanced cancer. J Clin Oncol 2018;36(suppl 30):8a. [Google Scholar]
- 13. Reck M, Thatcher N, Smit EF et al. Baseline quality of life and performance status as prognostic factors in patients with extensive‐stage disease small cell lung cancer treated with pemetrexed plus carboplatin vs. etoposide plus carboplatin. Lung Cancer 2012;78:276–281. [DOI] [PubMed] [Google Scholar]
- 14. Qi Y, Schild SE, Mandrekar SJ et al. Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non‐small cell lung cancer. J Thorac Oncol 2009;4:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrmann E, Gerss J, Bierer S et al. Pre‐treatment global quality of health predicts progression free survival in metastatic kidney cancer patients treated with sorafenib or sunitinib. J Cancer Res Clin Oncol 2009;135:61–67. doi: 10.1007/s00432-008-0438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quinten C, Maringwa J, Gotay CC et al. Patient self‐reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst 2011;103:1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basch E, Bennett A, Pietanza MC. Use of patient‐reported outcomes to improve the predictive accuracy of clinician‐reported adverse events. J Natl Cancer Inst 2011;103:1808–1810. [DOI] [PubMed] [Google Scholar]
- 18. Coens C, Pe M, Dueck AC et al. International standards for the analysis of quality‐of‐life and patient‐reported outcome endpoints in cancer randomised controlled trials: Recommendations of the SISAQOL Consortium. Lancet Oncol 2020;21:e83–e96. [DOI] [PubMed] [Google Scholar]
- 19. Dickler MN, Tolaney SM, Rugo HS et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(‐) metastatic breast cancer. Clin Cancer Res 2007;23:5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goetz MP, Toi M, Campone M et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–3646. [DOI] [PubMed] [Google Scholar]
- 21. Sledge GW Jr, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2‐ advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 22. Sledge GW Jr, Toi M, Neven P et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor‐positive, ERBB2‐negative breast cancer that progressed on endocrine therapy‐MONARCH 2: A randomized clinical trial. JAMA Oncol 2019;6:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnston S, Martin M, Di Leo A et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Modi ND, Sorich MJ, Rowland A et al. A literature review of treatment‐specific clinical prediction models in patients with breast cancer. Crit Rev Oncol Hematol 2020;148:102908. [DOI] [PubMed] [Google Scholar]
- 25. Vogenberg FR Predictive and prognostic models: Implications for healthcare decision‐making in a modern recession. Am Health Drug Benefits 2009;2:218–222. [PMC free article] [PubMed] [Google Scholar]
- 26. Di Leo A, O'Shaughnessy J, Sledge GW Jr et al. Prognostic characteristics in hormone receptor‐positive advanced breast cancer and characterization of abemaciclib efficacy. NPJ Breast Cancer 2018;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coates A, Gebski V, Signorini D et al. Prognostic value of quality‐of‐life scores during chemotherapy for advanced breast cancer. Australian New Zealand Breast Cancer Trials Group. J Clin Oncol 1992;10:1833–1838. [DOI] [PubMed] [Google Scholar]
- 28. Svensson H, Hatschek T, Johansson H et al. Health‐related quality of life as prognostic factor for response, progression‐free survival, and survival in women with metastatic breast cancer. Med Oncol 2012;29:432–438. [DOI] [PubMed] [Google Scholar]
- 29. Schwartz LH, Litière S, de Vries E et al. RECIST 1.1‐Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fayers P, Aaronson NK, Bjordal K et al. EORTC QLQ–C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer, 2001. [Google Scholar]
- 31. Scott NW, Fayers P, Aaronson NK et al. EORTC QLQ‐C30 Reference Values Manual. Brussels: European Organisation for Research and Treatment of Cancer, 2008. [Google Scholar]
- 32. Staren ED, Gupta D, Braun DP The prognostic role of quality of life assessment in breast cancer. Breast J 2011;17:571–578. [DOI] [PubMed] [Google Scholar]
- 33. Smyth EN, Shen W, Bowman L et al. Patient‐reported pain and other quality of life domains as prognostic factors for survival in a phase III clinical trial of patients with advanced breast cancer. Health Qual Life Outcomes 2016;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotronoulas G, Kearney N, Maguire R et al. What is the value of the routine use of patient‐reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32:1480–1501. doi: 10.1200/jco.2013.53.5948 [DOI] [PubMed] [Google Scholar]
- 35. Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. doi: 10.1001/jama.2017.7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seow H, Tanuseputro P, Barbera L et al. Development and validation of a prognostic survival model with patient‐reported outcomes for patients with cancer. JAMA Netw Open 2020;3:e201768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nelson EC, Eftimovska E, Lind C et al. Patient reported outcome measures in practice. BMJ 2015;350:g7818. [DOI] [PubMed] [Google Scholar]
- 38. Hopkins AM, Rowland A, McKinnon RA et al. Predictors of long‐term disease control and survival for HER2‐positive advanced breast cancer patients treated with pertuzumab, trastuzumab and docetaxel. Front Oncol 2019;9:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hopkins AM, Rowland A, Logan JM et al. Primary predictors of survival outcomes for HER2‐positive advanced breast cancer patients initiating ado‐trastuzumab emtansine. Breast 2019;46:90–94. [DOI] [PubMed] [Google Scholar]
- 40. Sorich MJ, Rowland A, Karapetis CS et al. Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for NSCLC: Pooled analysis of clinical trials. J Thorac Oncol 2019;14:1440–1446. [DOI] [PubMed] [Google Scholar]
- 41. Mezquita L, Auclin E, Ferrara R et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol 2018;4:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kazandjian D, Gong Y, Keegan P et al. Prognostic value of the lung immune prognostic index for patients treated for metastatic non‐small cell lung cancer. JAMA Oncol 2019;5:1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kerrigan K, Patel SB, Haaland B et al. Prognostic significance of patient‐reported outcomes in cancer. JCO Oncol Pract 2020;16:e313–e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Atkinson TM, Andreotti CF, Roberts KE et al. The level of association between functional performance status measures and patient‐reported outcomes in cancer patients: A systematic review. Support Care Cancer 2015;23:3645–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1: Kaplan Meier comparisons of PFS by pre‐treatment ECOG‐PS for the randomised arms of MONARCH 2 and MONARCH 3 (abemaciclib versus comparator).
Appendix S1: Supporting information


