Abstract
Lessons Learned
The combination of carotuximab with axitinib did not provide a benefit over axitinib monotherapy in patients with metastatic clear cell renal cell carcinoma who had previously progressed on one or more vascular endothelial growth factor (VEGF)‐targeted therapies.
Exploratory evaluation of pretreatment circulating biomarkers suggested the combination might benefit patients who have low baseline VEGF levels.
Background
Endoglin is an angiogenic receptor expressed on proliferating tumor vessels and renal cell carcinoma (RCC) stem cells that is implicated as a mechanism of resistance to vascular endothelial growth factor receptor (VEGFR) inhibitors. This study evaluated an antiendoglin monoclonal antibody (carotuximab, TRC105) combined with axitinib in patients with advanced or metastatic clear cell renal cell carcinoma (mccRCC) who had progressed following one or more prior VEGF inhibitors.
Methods
TRAXAR was a multicenter, international randomized 1:1 (stratified by ECOG, 0 vs. 1), phase II study of carotuximab combined with axitinib versus axitinib alone in mccRCC patients who had progressed following one or more prior VEGF inhibitors. The primary endpoint was progression‐free survival (PFS) assessed by independent central review (ICR) per RECIST 1.1
Results
A total of 150 patients were randomized. The combination therapy resulted in shorter median PFS by RECIST 1.1 than axitinib monotherapy (6.7 vs. 11.4 months). The combination was tolerated similarly to axitinib monotherapy, and there were no treatment related deaths. Exploratory evaluation of pretreatment circulating biomarkers suggested the combination might benefit patients who have low baseline VEGF levels.
Conclusion
The combination of carotuximab with axitinib did not demonstrate additional efficacy over single agent axitinib in patients with mccRCC who progressed following one or more prior VEGF inhibitor treatment.
Keywords: Phase II, TRAXAR, Axitinib, Renal cell cancer, Endoglin, TRC105, Carotuximab
Discussion
The analysis of the primary endpoint of PFS, performed following 69 events of progression by ICR or death, did not show any benefit for adding carotuximab to axitinib. This combination resulted in numerically shorter median PFS by RECIST 1.1 compared with axitinib alone (Fig. 1).
Figure 1.
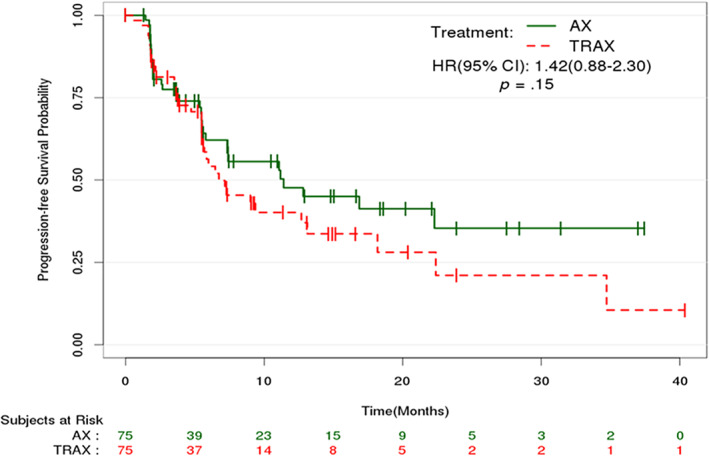
Kaplan‐Meier estimates of progression‐free survival by RECIST 1.1 by independent central review. Abbreviations: AX, axitinib monotherapy; CI, confidence interval; HR, hazard ratio; TRAX, TRC105 + axitinib.
Both drugs could be administered at their recommended single‐agent doses without increasing the toxicities of the individual drugs (Table 1); however, dose escalation to the maximum recommended dose of axitinib was less commonly achieved in the combination arm. Adverse events were rarely a reason for study discontinuation.
We explored whether any pretreatment circulating angiogenic biomarkers could help identifying which patients benefitted most from the combination therapy. Biomarkers were modeled as continuous measures, and each was tested for a prognostic association with PFS using Cox proportional hazards models. Interaction p values (PIntx) for predictive analyses were not subjected to multiple testing correction. We observed that patients with low baseline VEGF levels exhibited longer median PFS in arm TRC105 + axitinib (TRAX) compared with arm axitinib monotherapy (AX) (22.4 vs. 16.9 months, PIntx = .004; Fig. 2). Increased expression of VEGF and VEGFR have previously been associated with clinical responses and improved PFS on sunitinib. Lower VEGF levels might indicate an overall diminished VEGF signaling and perhaps a greater role for drugs targeting angiogenesis through non‐VEGF related mechanisms, such as carotuximab.
Figure 2.
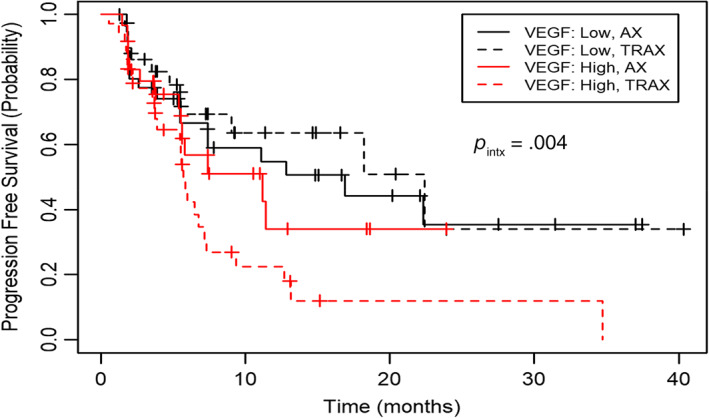
Kaplan‐Meier estimate of progression‐free survival by RECIST 1.1 by independent central review according to baseline median VEGF levels stratified by treatment arm. Abbreviations: AX, axitinib monotherapy; TRAX, TRC105 + axitinib; VEGF, vascular endothelial growth factor.
Lack of PFS benefit in the TRAX arm when compared with the AX arm led to termination of further development of carotuximab. It is critical to establish solid safety and efficacy signals in a phase Ib study prior to committing patients, investigators, and resources on a larger randomized clinical trial. Despite the favorable efficacy of the combination of axitinib and carotuximab found in a phase Ib study with 18 patients with mRCC [1], another randomized clinical trial in which carotuximab was added to bevacizumab in 59 patients with mRCC failed to improve PFS [2]. Much still remains to be learned in how best to use antiangiogenic therapies in mRCC and whether any biomarker can guide selection of patients. Indeed, the observation of improved PFS in the subpopulation of patients with mRCC with lower than median VEGF emphasizes the value of patient preselection using predictive biomarkers to improve clinical outcomes.
Trial Information
| Disease | Renal cell carcinoma – clear cell |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of Study | Phase II, randomized |
| Primary Endpoint | Progression‐free survival |
| Additional Details of Endpoints or Study Design | |
| The analysis of the primary endpoint of PFS compared arm AX and arm TRAX using a log‐rank test stratified by performance status (0 or 1). A hazard ratio of 0.67 was considered to be clinically relevant. Based on 1:1 randomization and the use of a one‐sided log‐rank test at the α = 0.10 level of significance, 115 events were required to have 80% power to detect a hazard ratio of 0.67. The expected PFS of patients treated with axitinib who had progressed following first‐line treatment with a VEGF inhibitor was assumed to be 4.8 months. Based on a planned accrual period of 12 months, and a minimum follow‐up period of 4.3 months, approximately 150 patients were recruited. Statistical analyses were done using R. The trial population for safety included all patients who received a dose of either study drug according to treatment actually received. An interim analysis for futility was planned following 55 events that defined the PFS endpoint but was not done at the recommendation of the data monitoring committee because of the rate of accrual exceeding the rate of expected events defining PFS. | |
| PK/PD data were previously reported in the phase I study: https://pubmed.ncbi.nlm.nih.gov/30190302/ | |
| Investigator's Analysis | Level of activity did not meet planned endpoint |
Drug Information: TRAX Arm
| Axitinib | |
| Generic Name | Axitinib |
| Trade Name | Inlyta |
| Company Name | Pfizer |
| Drug Type | Small molecule |
| Drug Class | VEGFR |
| Dose | 5 mg per flat dose |
| Route | oral (po) |
| Schedule of Administration | 5 mg twice daily (b.i.d.), which was escalated to a maximum dose of 10 mg b.i.d. in the absence of hypertension or other significant toxicity. |
| Carotuximab/TRC105 | |
| Generic Name | Carotuximab/TRC105 |
| Company Name | TRACON Pharmaceuticals Inc. |
| Drug Type | Antibody |
| Drug Class | Endoglin |
| Dose | 10 mg/kg |
| Route | IV |
| Schedule of Administration | Patients randomized to the combination arm received carotuximab 10 mg/kg intravenously (with appropriate premedication) every week, with the initial dose divided over 2 days. Premedication included acetaminophen 650 mg, diphenhydramine 50 mg (or similar H1 receptor antagonist), famotidine 20 mg (or similar H2 receptor antagonist), and methylprednisolone 50 mg. Methylprednisolone was tapered and discontinued within the first weeks of dosing. Dose modifications of carotuximab and axitinib were allowed per patient tolerance. |
Drug Information: AX Arm
| Axitinib | |
| Generic Name | Axitinib |
| Trade Name | Inlyta |
| Company Name | Pfizer |
| Drug Type | Small molecule |
| Drug Class | VEGFR |
| Dose | 5 mg per flat dose |
| Route | oral (po) |
| Schedule of Administration | 5 mg twice daily (b.i.d.), which was escalated to a maximum dose of 10 mg b.i.d. in the absence of hypertension or other significant toxicity. |
Patient Characteristics: TRAX Arm
| Number of Patients, Male | 49 |
| Number of Patients, Female | 26 |
| Stage | metastatic/advanced |
| Age | Median: 63 years |
| Number of Prior Systemic Therapies | Median: 1 or more |
| Performance Status: ECOG |
0 — 39 1 — 36 2 — 0 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes | Clear cell renal cell carcinoma, 75 |
Patient Characteristics: AX Arm
| Number of Patients, Male | 57 |
| Number of Patients, Female | 18 |
| Stage | metastatic/advanced |
| Age | Median: 65 years |
| Number of Prior Systemic Therapies | Median: 1 or more |
| Performance Status: ECOG |
0 — 38 1 — 37 2 — 0 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes | Clear cell renal cell carcinoma 75 |
Primary Assessment Method: TRAX Arm
| Title | PFS |
| Number of Patients Screened | 75 |
| Number of Patients Enrolled | 75 |
| Number of Patients Evaluable for Toxicity | 73 |
| Number of Patients Evaluated for Efficacy | 75 |
| Evaluation Method | RECIST 1.1 |
| (Median) Duration Assessments PFS | 6.7 Months, CI: 5.6–13.1 |
Primary Assessment Method: AX Arm
| Title | PFS |
| Number of Patients Screened | 75 |
| Number of Patients Enrolled | 75 |
| Number of Patients Evaluable for Toxicity | 74 |
| Number of Patients Evaluated for Efficacy | 75 |
| Evaluation Method | RECIST 1.1 |
| (Median) Duration Assessments PFS | 11.4 Months, CI: 5.8–NE |
Adverse Events
Table 1.
Most common (n > 1) and all grade 3 and above AEs by arm
| Safety | TRAX (n = 73), n (%) | AX (n = 74), n (%) | TRAX‐AX, All, % | ||||
|---|---|---|---|---|---|---|---|
| All | Grade 3 | Grade 4 | All | Grade 3 | Grade 4 | ||
| Headache | 48 (65.8) | 5 (6.9) | 0 | 12 (16.2) | 0 | 0 | 49.6 |
| Epistaxis | 47 (64.4) | 0 | 0 | 6 (8.1) | 1 (1.34) | 0 | 56.3 |
| Diarrhea | 44 (60.3) | 10 (13.7) | 0 | 44 (59.5) | 7 (9.5) | 0 | 0.8 |
| Fatigue | 42 (57.5) | 7 (9.6) | 0 | 40 (54.1) | 2 (2.7) | 0 | 3.4 |
| Nausea | 38 (52.1) | 2 (2.7) | 0 | 27 (36.5) | 2 (2.7) | 0 | 15.6 |
| Vomiting | 30 (41.1) | 1 (1.4) | 0 | 15 (20.3) | 1 (1.4) | 0 | 20.8 |
| Hypertension | 26 (35.6) | 11 (15.1) | 0 | 34 (46.0) | 17 (23.0) | 1 (1.4) | ‐10.4 |
| Weight loss | 25 (34.3) | 3 (4.1) | 0 | 17 (23.0) | 1 (1.4) | 0 | 11.3 |
| Gingival bleeding | 25 (34.3) | 0 | 0 | 3 (4.1) | 0 | 0 | 30.2 |
| Decreased appetite | 21 (28.8) | 2 (2.7) | 0 | 25 (33.8) | 1 (1.4) | 0 | ‐5.0 |
| Anemia | 21 (28.8) | 11 (15.1) | 0 | 12 (16.2) | 2 (2.7) | 0 | 12.6 |
| Constipation | 20 (27.4) | 0 | 0 | 13 (17.6) | 0 | 0 | 9.8 |
| Infusion related reaction | 19 (26.0) | 2 (2.7) | 0 | 0 | 0 | 0 | 26.0 |
| Palmar‐plantar erythrodysaesthesia syndrome | 18 (24.7) | 1 (1.4) | 0 | 14 (18.9) | 5 (6.8) | 0 | 5.8 |
| Stomatitis | 18 (24.7) | 2 (2.7) | 0 | 8 (10.8) | 0 | 0 | 13.9 |
| Hypothyroidism | 16 (21.9) | 0 | 0 | 20 (27.0) | 1 (1.4) | 0 | ‐5.1 |
| Back pain | 9 (12.3) | 3 (4.1) | 1 (1.4) | 17 (23.0) | 2 (2.7) | 0 | ‐10.7 |
| proteinuria | 3 (4.1) | 1 (1.4) | 0 | 15 (20.3) | 8 (10.8) | 0 | ‐16.2 |
AEs were coded by using MedDRA dictionary version 14.1. If more than one event was recorded for a patient, the patient was counted only once at the highest grade.
Abbreviations: AE, adverse event; AX, axitinib monotherapy; TRAX, TRC105 + axitinib.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Level of activity did not meet planned endpoint |
Angiogenesis is a complex process that is regulated by multiple pathways [3]. Antiangiogenic drugs such as axitinib, sorafenib, sunitinib, pazopanib, and cabozantinib primarily target the vascular endothelial growth factor (VEGF) pathway and have revolutionized the treatment of renal cell carcinoma (RCC) [4]. Axitinib, a selective VEGF receptor (VEGFR)‐1, ‐2, and ‐3 inhibitor, has been compared with sorafenib in the phase III AXIS trial [5] as a second‐line therapy for patients with RCC after progression on sunitinib or cytokine therapy. Axitinib significantly increased median progression‐free survival (PFS) over sorafenib (6.7 vs. 4.7 months; hazard ratio, 0.67; p = .0001), but without significant improvement in overall survival.
Inhibition of complementary, non‐VEGF driven angiogenic pathways is an alternative strategy that may improve antitumor activity and limit resistance to VEGF inhibitors. Beyond the VEGF axis, angiogenesis depends upon multiple growth factors and stromal elements [3]. Upregulated non‐VEGF pathways may include the interleukin‐6, transforming growth factor (TGF)‐β, PDGF, bFGF, c‐Met, and angiopoietin axes. In particular, the TGF‐β axis, including the soluble bone morphogenic proteins (BMPs) and the endoglin and ALK1 receptors, may provide an escape pathway for tumor angiogenesis. Endoglin (CD105) is a homodimeric TGF‐β coreceptor expressed on proliferating vascular endothelium in solid tumors [4, 6] and is upregulated by hypoxia through the induction of hypoxia inducible factor 1α [4, 7]. Endoglin is essential for normal vascular development [8], and loss of endoglin expression is associated with the Osler‐Weber‐Rendu syndrome, a disease characterized by abnormal blood vessel formation that is associated with improved cancer survival, suggesting that targeting endoglin may have beneficial clinical effects [9, 10]. The introduction of the endoglin heterozygous genotype resensitizes spontaneous tumors to VEGF inhibitors, as does the conditional deletion of the endoglin gene in endothelium [9]. In patients with solid tumors, including RCC, high tumor microvessel density is correlated with poor prognosis [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. Furthermore, endoglin expression has been demonstrated on human renal cancer stem cells isolated from nephrectomy specimens and is associated with a tumor initiating function [24].
Carotuximab (TRC105) is a chimeric immunoglobulin G1 antibody that binds human endoglin with high avidity, competitively inhibits BMP ligand binding required for endothelial signal transduction, induces antibody‐dependent cellular cytotoxicity of proliferating human vascular endothelial cells and endoglin‐expressing tumor cells, and inhibits angiogenesis stimulated by VEGF and fibroblast growth factor [25]. Carotuximab potentiates VEGF inhibitors in preclinical models of angiogenesis and in tumor xenografts, and was well tolerated at 10 mg/kg every week and 15 mg/kg every 2 weeks as a single agent in a phase I trial, where it demonstrated a safety profile that was distinct from that of VEGF inhibitors, including lack of hypertension and proteinuria [26]. These preclinical and early phase I clinical data set the stage for our randomized phase II clinical trial TRAXAR reported here.
Unfortunately, carotuximab did not demonstrate additional activity when combined with axitinib in patients with metastatic clear cell RCC that progressed following prior VEGF inhibitor treatment. These data are consistent with a randomized trial of bevacizumab and carotuximab in patients with all types of RCC histology (which included 22% nonclear cell) that failed to demonstrate a survival advantage over single agent bevacizumab [2].
Carotuximab was administered at doses that achieved pharmacodynamic effects, as telangiectasia and associated epistaxis or gingival bleeding were observed routinely in patients on the combination arm. These reversible signs and symptoms are expected pharmacologic effects of carotuximab binding to the endoglin receptor to interrupt BMP9 binding and resemble the characteristics of Osler‐Weber‐Rendu syndrome. Epistaxis or gingival bleeding are observed routinely in trials of carotuximab given as a single agent or concurrently with VEGF inhibitors and provide confirmation that patients have been administered carotuximab doses required to inhibit BMP binding to endoglin.
Dalantercept, a ligand trap for BMP9, also reported negative data in a trial of clear cell RCC in combination with axitinib, although in this trial only a minority of patients demonstrated signs or symptoms of telangiectasia [27], indicating that most patients may have received a subtherapeutic drug exposure required for pharmacologic inhibition of the endoglin pathway.
Across all patients, no PFS advantage was noted in the TRAX arm when compared with the AX arm. We explored whether any circulating angiogenic biomarkers could help identifying which patients benefitted most from the combination therapy. We observed that patients who had low baseline VEGF levels demonstrated greater benefit from the combination than single agent axitinib, using independent central review–defined PFS by RECIST. Increased baseline expression of VEGF and VEGFR have previously been associated with clinical responses and improved PFS in patients with metastatic urothelial carcinoma on sunitinib [28]. It is therefore reasonable to speculate that lower VEGF levels indicate an overall diminished VEGF signaling, and perhaps a greater role for drugs targeting angiogenesis through non‐VEGF related mechanisms such as carotuximab. Across multiple preclinical models, synergistic effects have been reported using antiendoglin and anti‐VEGF agents to simultaneously block both pathways [29].
In conclusion, the finding of a biomarker selected population that might benefit from the combination of carotuximab and axitinib was not robust enough to merit further clinical development of this therapy. Hopefully the lessons of this trial will guide more effective therapeutic development for patients with advanced RCC that has progressed on VEGF‐targeted therapy.
Disclosures
Toni K. Choueiri: TRACON (RF); Yousef Zakharia: Amgen, Roche Diagnostics, Novartis, Janssen, Eisai, Exelixis, Castle Bioscience, Array, Bayer, Pfizer, Clovis, EMD Serono (C/A), Janssen R&D (data safety monitoring committee), Pfizer, Novartis (H); Andrew B. Nixon: TRACON (RF); Bonne Adams: TRACON Pharmaceuticals, Inc. (E); Charles Theuer: TRACON Pharmaceuticals, Inc. (E); Neeraj Agarwal: Astellas, AstraZeneca, Bayer, Bristol‐Myers Squibb, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Janssen, Merck, Nektar, Novartis, Pfizer, Pharmacyclics, Seattle Genetics (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors express their appreciation to the patients who participated in this investigational study and to the study staff. This study was presented in part at ESMO 2019. This research was supported by TRACON Pharmaceuticals, Inc. San Diego, CA.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT01806064
- Sponsor: TRACON Pharmaceuticals, Inc.
- Principal Investigator: Toni K. Choueiri
- IRB Approved: Yes
References
- 1. Choueiri TK, Michaelson MD, Posadas EM et al. An open label phase Ib dose escalation study of TRC105 (anti‐endoglin antibody) with axitinib in patients with metastatic renal cell carcinoma. The Oncologist 2019;24:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dorff TB, Longmate JA, Pal SK et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer: Bevacizumab and TRC105 in Renal Cancer. Cancer 2017;123:4566–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873–887. [DOI] [PubMed] [Google Scholar]
- 4. Seon BK, Haba A, Matsuno F et al. Endoglin‐targeted cancer therapy. Curr Drug Deliv 2011;8, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 6. Rosen LS, Gordon MS, Robert F et al. Endoglin for targeted cancer treatment. Curr Oncol Rep 2014;16:365. [DOI] [PubMed] [Google Scholar]
- 7. Sánchez‐Elsner T, Botella LM, Velasco B et al. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor‐β pathways. J Biol Chem 2002;277:43799–43808. [DOI] [PubMed] [Google Scholar]
- 8. Li DY, Sorensen LK, Brooke BS et al. Defective angiogenesis in mice lacking endoglin. Science 1999;284:1534–1537. [DOI] [PubMed] [Google Scholar]
- 9. Duarte CW, Murray K, Lucas FL et al. Improved survival outcomes in cancer patients with hereditary hemorrhagic telangiectasia. Cancer Epidemiol Biomarkers Prev 2014;23:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lenato G, Guanti G. Hereditary haemorrhagic telangiectasia (HHT): Genetic and molecular aspects. Curr Pharm Des 2006;12:1173–1193. [DOI] [PubMed] [Google Scholar]
- 11. Kumar S, Ghellal A, Li C et al. Breast carcinoma: Vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res 1999;59:856–861. [PubMed] [Google Scholar]
- 12. Duff SE, Li C, Garland JM et al. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J 2003;17:984–992. [DOI] [PubMed] [Google Scholar]
- 13. Saad R, Elgohary Y, Memari E et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in esophageal adenocarcinoma. Hum Pathol 2005;36:955–961. [DOI] [PubMed] [Google Scholar]
- 14. Yao Y, Kubota T, Takeuchi H et al. Prognostic significance of microvessel density determined by an anti‐CD105/endoglin monoclonal antibody in astrocytic tumors: Comparison with an anti‐CD31 monoclonal antibody. Neuropathology 2005;25:201–206. [DOI] [PubMed] [Google Scholar]
- 15. Ding S, Li C, Lin S et al. Comparative evaluation of microvessel density determined by CD34 or CD105 in benign and malignant gastric lesions. Hum Pathol 2006;37:861–866. [DOI] [PubMed] [Google Scholar]
- 16. Erdem O, Taskiran C, Onan MA et al. CD105 expression is an independent predictor of survival in patients with endometrial cancer. Gynecol Oncol 2006;103:1007–1011. [DOI] [PubMed] [Google Scholar]
- 17. Kyzas PA, Agnantis NJ, Stefanou D. Endoglin (CD105) as a prognostic factor in head and neck squamous cell carcinoma. Virchows Arch 2006;448:768–775. [DOI] [PubMed] [Google Scholar]
- 18. Marioni G, Marino F, Giacomelli L et al. Endoglin expression is associated with poor oncologic outcome in oral and oropharyngeal carcinoma. Acta Otolaryngol 2006;126: 633–639. [DOI] [PubMed] [Google Scholar]
- 19. Taskiran C, Erdem O, Onan A et al. The prognostic value of endoglin (CD105) expression in ovarian carcinoma. Int J Gynecol Cancer 2006;16:1789–1793. [DOI] [PubMed] [Google Scholar]
- 20. Yang L, Lu W, Huang G et al. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer 2006;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El‐Gohary YM, Silverman JF, Olson PR et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol 2007;127:572–579. [DOI] [PubMed] [Google Scholar]
- 22. Rubatt JM, Darcy KM, Hutson A et al. Independent prognostic relevance of microvessel density in advanced epithelial ovarian cancer and associations between CD31, CD105, p53 status, and angiogenic marker expression: A Gynecologic Oncology Group study. Gynecol Oncol 2009;112:469–474. [DOI] [PubMed] [Google Scholar]
- 23. Dallas NA, Samuel S, Xia L et al. Endoglin (CD105): A marker of tumor vasculature and potential target for therapy. Clin Cancer Res 2008;14:1931–1937. [DOI] [PubMed] [Google Scholar]
- 24. Bauman TM, Huang W, Lee MH et al. Neovascularity as a prognostic marker in renal cell carcinoma. Hum Pathol 2016;57:98–105. [DOI] [PubMed] [Google Scholar]
- 25. Nolan‐Stevaux O, Zhong W, Culp S et al. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti‐endoglin antibodies. PLoS One 2012;7:e50920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosen LS, Hurwitz HI, Wong MK et al. A phase I FIRST‐IN‐HUMAN STUDY of TRC105 (anti‐endoglin antibody) in patients with advanced cancer. Clin Cancer Res 2012;18:4820–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voss, M. H. et al. The DART study: Results from the dose‐escalation and expansion cohorts evaluating the combination of dalantercept plus axitinib in advanced renal cell carcinoma. Clin Cancer Res 2017;23:3557–3565. [DOI] [PubMed] [Google Scholar]
- 28. Grivas PD, Daignault S, Tagawa ST et al. Double‐blind, randomized, phase II trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer 2014;120:692–701. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Tian H, Blobe GC et al. Effects of the combination of TRC105 and bevacizumab on endothelial cell biology. Invest New Drugs 2014;32:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]


