Abstract
Background
Clinical trials are an important therapeutic option for patients with cancer. Although financial burden in cancer treatment is well documented, the financial burden associated with clinical trials is not well understood.
Patients and Methods
We conducted a survey regarding economic burden and financial toxicity in patients with cancer enrolled in phase I clinical trials for >1 month. Financial toxicity score was assessed using the Comprehensive Score for Financial Toxicity survey. Patients also reported monthly out‐of‐pocket (OOP) costs.
Results
Two hundred and thirteen patients completed the survey (72% non‐Hispanic White; 45% with annual income ≤$60,000; 50% lived >300 miles from the clinic; 37% required air travel). Forty‐eight percent of patients had monthly OOP costs of at least $1,000. Fifty‐five percent and 64% of patients reported unanticipated medical and nonmedical expenses, respectively. Worse financial toxicity was associated with yearly household income <$60,000 (odds ratio [OR]: 2.7; p = .008), having unanticipated medical costs (OR: 3.2; p = .024), and living >100 miles away from the clinical trial hospital (OR: 2.3; p = .043). Non‐White or Hispanic patients (OR: 2.5; p = .011) and patients who were unemployed or not working outside the home (OR: 2.5; p = .016) were more likely to report high unanticipated medical costs.
Conclusion
Among patients with cancer participating in clinical trials, economic burden is high, and most of patients’ OOP costs were nonmedical costs. Financial toxicity is disproportionally higher in patients with lower income and those who travel farther, and unexpected medical costs were more common among non‐White or Hispanic patients. OOP costs can be substantial and are often unexpected for patients.
Implications for Practice
The financial burden of cancer treatment is well documented, but there are limited data regarding the financial burden associated with cancer clinical trials. This study surveyed 213 patients enrolled in early‐phase clinical trials. Monthly out‐of‐pocket costs were at least $1000 for nearly half of patients. Worse financial toxicity was associated with income <$60,000 and living farther away from the hospital. Racial/ethnic minorities had higher rates of unanticipated medical costs. These data help to quantify the high financial burden for patients and may reveal a cause of disparities in clinical trial enrollment for underrepresented populations.
Keywords: Economic burden of disease, Clinical trials as topic, Oncology, Medical, Clinical trial, Phase I, Out‐of‐pocket costs
Short abstract
This article assesses the economic burden exhibited by patients with cancer enrolled in early‐phase clinical trials by examining out‐of‐pocket medical and nonmedical costs and patient‐reported financial toxicity.
Introduction
Clinical trials are critically important to the development of new therapies for cancer. However, only 3%–5% of all adult patients with cancer participate in clinical trials [1]. Although many factors contribute to the low participation rate, such as eligibility requirements and access to trials, financial concerns also play a role in patients’ decision‐making [2, 3, 4]. Furthermore, patients with low socioeconomic status have been shown to be underrepresented in clinical trials [5].
Financial burden in patients undergoing cancer treatment is well described [6] and is associated with worse quality of life [7, 8]. The price of cancer drugs is increasing at a rate higher than inflation, and with increasing insurance deductibles, premiums, and various forms of cost‐sharing, patients are forced to shoulder an increasing percentage of the costs of these treatments [9]. The financial implications of cancer treatment can be dramatic and even catastrophic; patients with cancer are more than twice as likely to declare bankruptcy as those without cancer [10]. In addition, declaring bankruptcy after a cancer diagnosis is a risk factor for mortality [11].
Patients enrolled in clinical trials are subject to not only the same costs that are associated with routine cancer care but also the additional costs associated with the requirements of clinical trial participation, including more frequent clinic visits, additional tests, frequent travel and possible lodging costs, and loss of work. Furthermore, the burden of travel may be higher for patients enrolled in early‐phase clinical trials [12]. Some data exist regarding the financial toxicity of patients enrolled in clinical trials [13] and the importance of this topic has been highlighted [14, 15], yet many questions remain about the economic burden specific to patients with cancer, especially those enrolled in early‐phase clinical trials. We used the terms “economic burden,” “financial burden,” and “financial toxicity” interchangeably.
In the present study, we aimed to assess the economic burden exhibited by patients with cancer enrolled in early‐phase clinical trials by examining out‐of‐pocket medical and nonmedical costs and patient‐reported financial toxicity.
Subjects, Materials, and Methods
Participants
Patients were recruited between October 2018 and January 2020 during their visit to the Clinical Center for Targeted Therapy, a clinic that specializes in early‐phase clinical trials. Eligible patients were those who were enrolled in a clinical trial for more than 1 month, aged 18 years or older, and able to read and understand English. We limited enrollment to patients who had been in a clinical trial for more than 1 month in order to select those with an understanding of average monthly out‐of‐pocket costs. Patients were excluded if they had any physical or mental conditions, such as visual, cognitive, or mental impairments, that would affect their ability to complete the questionnaire.
Study Design
This was a cross‐sectional study using an electronic survey tool on a tablet. The survey was only available in English. At the time that patients checked in for their appointment, the administrative staff identified patients who had been in a trial for more than 1 month and asked if they would be interested in participating in a survey about the costs of their cancer care. No identifiable patient data were collected. The University of Texas MD Anderson Cancer Center Institutional Review Board approved the study, which complied with Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws.
Study Questionnaire
The survey asked questions about patients’ demographics, cancer diagnosis, and socioeconomic factors. Patients were asked to report out‐of‐pocket medical costs associated with their clinical trial, along with nonmedical costs of transportation, lodging, and food related to their clinical trial visits to the hospital. Patients reported costs by selecting a range of costs per month for each major category that could potentially contribute to the cost of participating in their current clinical trial. For example, the possible response options for the medical costs of co‐pays for tests and diagnostic procedures for the patient's current clinical trial were <$200/month, $200–$500/month, $501–$1000/month, $1,001–$2,000/month, $2,001–$4,000/month, or >$4,000/month. Patients were also asked if they received full or partial expense reimbursement for travel associated with their clinical trial and the source of the reimbursement.
The survey also included the Comprehensive Score for Financial Toxicity (COST) measure, a validated instrument to measure financial toxicity as a patient‐reported outcome in patients with cancer [16]. The COST instrument included in our survey was the version published as a Functional Assessment of Chronic Illness Therapy questionnaire and was used with permission from the authors, who requested that we use the latest version (version 2). The possible range of financial toxicity scores (FTSs) calculated by the COST instrument was 0–44, with lower FTS indicating worse financial toxicity. The COST instrument was validated in patients with advanced cancer, which were similar to our study population. In that validation study, the COST instrument displayed excellent internal consistency (Cronbach alpha = 0.92), superior 7‐day test‐retest reliability (intraclass correlation coefficient = 0.80), and significant convergent validity (Pearson correlation between the COST FTS and psychological distress was 0.26 [p < .001], and between COST FTS and household income, 0.28 [p < .001]) [16]. Internal consistency of the COST instrument in the present study sample of patients in phase I clinical trials was also excellent (Cronbach alpha = 0.89).
Other survey questions regarding demographics, duration of time in current early‐phase clinical trial, and how patients paid for their current treatment were based on the experience of the study investigators regarding developing surveys for patients in early‐phase clinical trials [17] and extant literature on financial toxicity [2, 18]. An initial pilot group was asked for feedback to ensure appropriate usability. Survey data were entered directly into REDCap.
Statistical Analysis
Analyses were performed using IBM SPSS version 24 (IBM, Armonk, NY). Descriptive statistics were used to summarize patient demographics, disease characteristics, out‐of‐pocket medical and nonmedical costs, expectations of cost, and financial toxicity. The FTS was computed from responses to items in the COST instrument. We quantified monthly medical and nonmedical costs using the lower limit of each selected range and reporting that patients spent at least this amount.
We applied multivariable logistic regression to determine factors associated with risks of financial toxicity and much higher than anticipated medical costs during their clinical trial. For the logistic regression model predicting risk of financial toxicity, the FTS was dichotomized at the median to create a binary outcome variable, in which worse financial toxicity was indicated by having an FTS less than or equal to the median FTS of the study sample. For the model predicting the risk of much higher than anticipated medical costs, patients who indicated that their actual medical costs were “much more” than expected were considered to have much higher than anticipated medical costs. Patients who selected any other response (“slightly more,” “equal,” “slightly less,” or “much less”) were considered to not have much higher than anticipated medical costs. For each multivariable model, independent variables that were significant in the univariable analyses were included in the multivariable model and tested for significance. Variables that were tested in univariable analyses for possible inclusion in the multivariable models included age < 60 years, gender, non‐White or Hispanic race, annual household income of ≤$60,000, participation in one or more phase I clinical trials prior to the current trial, length of time since diagnosis, living >100 miles away from the treating hospital, and being unemployed or not working outside the home. In all analyses, p < .05 was considered statistically significant.
Results
Cohort Characteristics
Between October 2018 and January 2020, 342 patients were approached; 270 had been in their clinical trial for more than a month, and 235 of them agreed to participate (Fig. 1). Patients were included in our analysis if they completed the survey, resulting in an effective sample size of 213. Patient characteristics are summarized in Table 1. The median age was 59 years, 59% were female, 72% were non‐Hispanic White, 54% had employer‐sponsored insurance, and 37% had Medicare.
Figure 1.
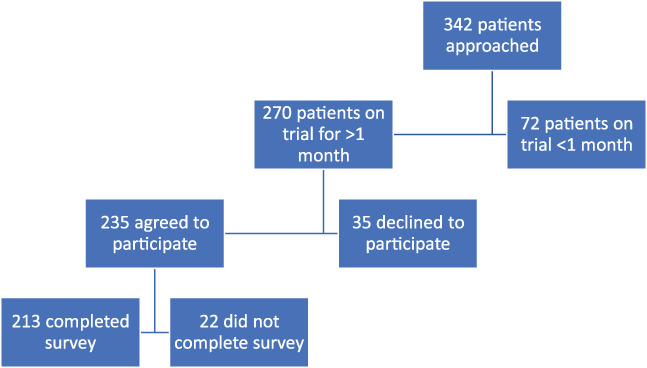
Patient recruitment flow chart. Patients were excluded if they had not been on their trial for more than 1 month or if they did not agree to participate. Twenty‐two patients did not fill out substantial portions of the survey.
Table 1.
Patient characteristics
| Characteristic | Patients (n = 213), n (%) |
|---|---|
| Median age, yr | 59 |
| Gender, female | 125 (59) |
| Race/ethnicity | |
| Non‐Hispanic White | 153 (72) |
| Hispanic | 27 (13) |
| Black | 18 (8) |
| Asian | 6 (3) |
| Other | 4 (2) |
| No response | 5 (2) |
| Type of cancer a | |
| Brain | 5 (2) |
| Breast | 20 (9) |
| Cervical | 7 (3) |
| Colorectal | 27 (13) |
| Head and neck | 16 (8) |
| Liver | 11 (5) |
| Kidney | 9 (4) |
| Lung | 27 (13) |
| Melanoma | 10 (5) |
| Ovarian | 20 (9) |
| Pancreatic | 9 (4) |
| Prostate | 10 (5) |
| Sarcoma | 17 (8) |
| Thyroid | 18 (8) |
| Other | 39 (18) |
| Yearly household income | |
| <$30,000 | 40 (19) |
| $30,001–$60,000 | 53 (25) |
| $60,001–$100,000 | 64 (30) |
| $100,001–$200,000 | 35 (16) |
| $200,001–$400,000 | 11 (5) |
| >$600,000 | 3 (1) |
| No response | 7 (3) |
| Current employment status | |
| Unemployed | 32 (15) |
| Employed, on medical leave | 12 (6) |
| Employed, part time | 13 (6) |
| Employed, full time | 59 (28) |
| Retired | 78 (37) |
| Not working outside the home | 19 (9) |
| Insurance type a | |
| Medicare | 79 (37) |
| Employer‐provided | 114 (54) |
| Medicaid | 5 (2) |
| Personally purchased | 23 (11) |
| VA | 8 (4) |
| Personally purchased supplemental | 18 (8) |
| Other | 3 (1) |
| No response | 5 (2) |
| Length of time in current trial | |
| <1 month | 5 (2) |
| 1 month–2 months | 87 (41) |
| 3 month–4 months | 39 (18) |
| 5 months–6 months | 20 (9) |
| >6 months | 60 (28) |
| Unknown | 2 (1) |
| Form of anticancer medications for current trial | |
| Pills, capsules, or tablets taken by mouth | 77 (36) |
| Intravenous infusion | 91 (43) |
| Both | 42 (20) |
| Unknown | 3 (1) |
| Prior phase I clinical trials | |
| 0 | 134 (63) |
| 1 | 39 (18) |
| 2 | 25 (12) |
| ≥3 | 14 (7) |
| Unknown | 1 (0) |
Some patients selected more than one option, so numbers add up to greater than 100%.
Abbreviation: VA, Veterans Administration.
Travel and payment information is summarized in Table 2. Half of patients lived more than 300 miles away from the clinic, and 27% of patients lived 101–300 miles away from the clinic. Thirty‐seven percent traveled by airplane, 62% traveled by car only, and 1% traveled by bus.
Table 2.
Travel and payment information
| Reported information | Patients (n = 213), n (%) |
|---|---|
| Miles traveled for cancer care | |
| 0–25 | 13 (6) |
| 26–50 | 19 (9) |
| 51–100 | 17 (8) |
| 101–300 | 58 (27) |
| >300 | 106 (50) |
| Median number of accompanying people to clinic visits | 1 |
| Method of travel a | |
| Car (only) | 131 (62) |
| Airplane | 79 (37) |
| Bus | 3 (1) |
| Taxi/ride‐share | 12 (6) |
| Other | 1 (0) |
| Reimbursement for trial‐related expenses | |
| Fully reimbursed | 12 (6) |
| Partially reimbursed | 50 (23) |
| None | 149 (70) |
| Unknown | 2 (1) |
| Method of reimbursement (n = 62) | |
| Insurance | 5 (2) |
| Study sponsor | 39 (18) |
| Other | 18 (8) |
| How current cancer treatment is paid for a | |
| Savings account | 112 (53) |
| Borrow from family/friends | 21 (10) |
| Personal fundraiser | 20 (9) |
| Withdrawal from retirement account | 39 (18) |
| Work more hours/additional jobs | 15 (7) |
| Other | 61 (29) |
| Unknown | 4 (2) |
Some patients selected more than one option, so numbers add up to greater than 100%.
Out‐of‐Pocket Costs
Overall, 48% of patients had monthly total out‐of‐pocket costs of at least $1,000. Fifty‐one percent of patients had monthly out‐of‐pocket nonmedical costs of at least $600, and 51% of patients had monthly out‐of‐pocket medical costs of at least $200. Fourteen percent of patients had monthly total out‐of‐pocket costs of at least $2,500; 21% of patients had monthly out‐of‐pocket nonmedical costs of at least $1,500; 14% of patients had monthly out‐of‐pocket medical costs of at least $700. Overall, 88% of patients had fuel expenses, 80% had parking expenses, 73% had hotel expenses, 46% had rental car/ride‐share/taxi expenses, and 40% had flight expenses. Nonmedical and medical expenses are detailed in Figure 2.
Figure 2.
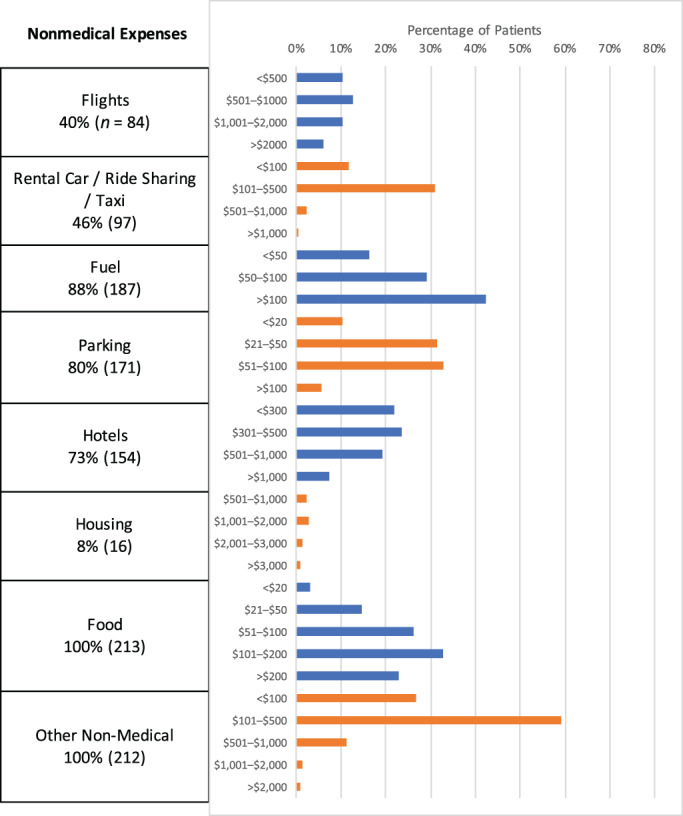
Out‐of‐pocket expenses by category. Percentages represent the percentage of all patients who answered each question. The absolute number of patients is listed in parentheses. Blue and orange colors are used to separate responses from different survey items to improve visual clarity and do not represent distinct patient subgroups.
Overall, patients used a combination of sources to pay for treatment (Table 2). Fifty‐three percent of patients used savings to pay for treatment, 10% borrowed money from friends or family, 9% had a personal fundraiser, 7% worked extra hours or an additional job, and 18% withdrew money from retirement accounts to pay for treatment, including 14 patients (7%) who were younger than 59.5 years.
Financial Burden
The median FTS, calculated from the COST tool, was 20, with an interquartile range of 12. Seventy‐one percent of patients had grade 1 financial toxicity or higher, defined as an FTS of less than 26 [19]. Figure 3 shows a forest plot of financial toxicity variables. Financial toxicity was not associated with length of time since initial cancer diagnosis (p > .05). Also, there were no significant differences in FTS by duration of time a patient had spent in their current early‐phase clinical trial (e.g., mean FTS was 19.6 in patients who had spent <3 months in their current early‐phase clinical trial and 21.0 in those who had spent ≥3 months in their current trial, p = .285).
Figure 3.
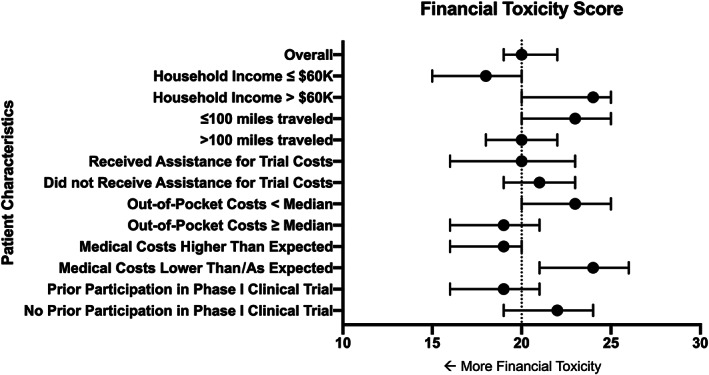
Forest plot of financial toxicity variables.
In the multivariable analysis, worse financial toxicity was associated with an annual household income of ≤$60,000 (odds ratio [OR] 2.7; p = .008), having much higher than anticipated medical costs (OR 3.2; p = .024), participation in one or more phase I clinical trials prior to the current trial (OR 2.2; p = .028), and living >100 miles away from MD Anderson (OR 2.3; p = .043).
Patients were asked to describe how particular statements on the COST survey instrument applied to them in the past 7 days. Thirty‐seven percent of patients were “not at all” sure that they had enough money in savings, retirement, or assets to cover the costs of their treatment. Half of patients stated that they worried “quite a bit” or “very much” about financial issues that they would experience in the future due to their illness or treatment. When patients rated the extent to which the costs of their cancer care had been a financial burden to them, 35% indicated “moderate financial burden,” 33% “significant financial burden,” and 7% “catastrophic financial burden.” Over 60% of patients agreed “quite a bit” or “very much” with the statement “I feel I have no choice about the amount of money I spend on care.” Over half of the early‐phase clinical trial patients indicated that they were “not at all,” “a little bit,” or “somewhat” able to meet their monthly expenses.
Reimbursement of Clinical Trial Expenses
In total, 62 patients (29%) received partial or full reimbursement of trial‐related travel expenses. Among patients who lived more than 100 miles away from the clinic, 34% of patients received partial or full reimbursement for travel expenses related to the clinical trial. When comparing patients who received partial or full reimbursement with those who did not, there were no differences in mean FTS (p = .891). Both groups had the same median rating of “moderate” for “the extent to which the costs of cancer care have been a financial burden to you.” Overall, 95% of patients receiving reimbursement felt no pressure to participate in their clinical trial compared with 94.4% of patients not receiving reimbursement (p = .825).
Unanticipated Costs
Patients often found that their actual costs exceeded their expectations during their clinical trial. Overall, 55% of patients reported unanticipated medical costs and 64% reported unanticipated nonmedical costs. Figure 4 shows a forest plot of expected versus actual nonmedical costs. Results from multivariable logistic regression showed that non‐White or Hispanic patients (OR 2.5; p = .011) and patients who were unemployed or not working outside the home (OR 2.5, p = .016) were more likely to report much higher than anticipated medical costs during their clinical trial.
Figure 4.
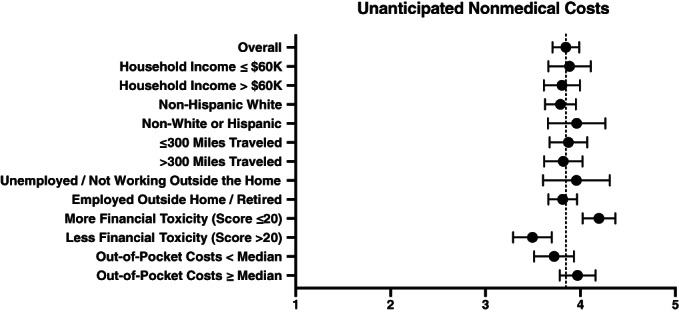
Forest plot of unanticipated nonmedical costs.
Discussion
To the best of our knowledge, the present study is the first in‐depth examination of patient‐reported financial toxicity and out‐of‐pocket costs for patients participating in early‐phase clinical trials. We found that this group of patients experienced substantial economic burden, high out‐of‐pocket costs for both medical and nonmedical expenses, and significant financial toxicity. As most of these out‐of‐pocket expenses were not reimbursable, patients had to rely on many different sources to pay for their trial‐related expenses.
Although several studies have highlighted the issue of financial toxicity during the course of cancer treatment [6, 20], ours is the first and most comprehensive to assess economic burden for patients enrolled in early‐phase clinical trials. To date, there is limited evidence regarding financial toxicity for patients enrolled in clinical trials, with only one prior study examining the role of an equity intervention for patients with cost concerns [13].
We found that financial toxicity was higher for patients with income ≤$60,000 and for patients who traveled farther to receive their care. These data build on existing knowledge that both of these groups are underrepresented in clinical trials [5], suggesting that both groups have barriers to entry into clinical trials and additional financial concerns after enrollment. For early‐phase clinical trials, a lack of racial and ethnic diversity in a patient population may result in subtherapeutic dosing that affects clinical efficacy or a failure to detect additional toxicities that may be experienced by some groups of patients due to differences in pharmacogenomics [21]. This may have the impact of limiting the generalizability of knowledge gained from early‐phase clinical trials [22]. To this end, the American Society of Clinical Oncology issued a policy statement with several recommendations for overcoming financial barriers that may otherwise prevent participation in clinical trials [23]. The present research is responsive to some of these recommendations—for example, our research provided estimates of patient‐reported out‐of‐pocket costs and evidence suggesting that financial support for clinical trial–related out‐of‐pocket costs (such as for travel) may not result in undue influence regarding trial participation.
We also assessed patients’ expectations of costs and whether their actual costs exceeded what was anticipated, and most patients reported that medical and nonmedical costs were higher than anticipated. Non‐White or Hispanic patients were more likely to have unanticipated medical costs. This finding echoes prior research showing that unexpected financial burden is associated with patients who are non‐White or Hispanic, have low income, and incur high out‐of‐pocket costs [24] and raises the possibility that there may be demographic associations with patients’ likelihood of anticipating costs. Efforts to improve financial literacy may be important for these patients.
We measured financial burden using the COST instrument, which calculates an FTS. The median value in our study was 20, indicating worse financial toxicity than that seen in prior studies of patients enrolled in clinical trials, in which the median FTS was 24, and in insured patients with multiple myeloma, in which the median FTS was 23 [13, 25]. This finding is consistent with a prior study reporting that the prevalence of high financial burden (indicated by COST FTS ≤24) was more than twofold higher among phase I clinical trial patients (67%) than among oncology patients treated with curative‐intent surgery (30%) [26].
Patients used a variety of means to pay for their clinical trial–related expenses. Notably, several patients withdrew money from retirement accounts, which, for patients younger than 59.5 years, may result in early withdrawal penalties [27]. These penalties may perpetuate the financial burden experienced by patients by further reducing their available assets. Some patients may qualify for a medical exception to these penalties.
Our study showed that nonmedical expenses drive high costs for patients in early‐phase clinical trials, with patients spending at least $600 per month for nonmedical expenses such as travel, accommodations, parking, and food. These nonmedical expenses represented most out‐of‐pocket costs that patients faced while participating in their clinical trial. Although health insurance aids in reducing patient‐borne out‐of‐pocket medical costs, it typically does not cover nonmedical expenses, which were significant for many patients, with one in five patients spending at least $1,500 per month on nonmedical expenses.
The notion of payments to research participants has previously raised ethical concerns that these payments would inappropriately affect participant decision‐making [28]. However, the U.S. Food and Drug Administration recently updated its information sheet regarding payment and reimbursement to research subjects to clarify that reimbursement for travel expenses and associated costs does not present problems with undue influence [29]. Some states, such as Texas and California, are making policies to encourage reimbursement for clinical trial–related expenses [14, 30]. Finally, a framework has been proposed to guide investigators on how to use payments and reimbursement for research participants, emphasizing reimbursement for out‐of‐pocket costs, compensation for time and burdens, and recruitment incentives [31]. These changes and proposals may encourage investigators and sponsors to more carefully consider the role of payments to research participants. Because over half of the patients in our study had unanticipated costs and 75% of patients exhibited at least moderate financial burden, we believe this is an important consideration in addressing the significant economic burden facing patients enrolled in early‐phase clinical trials.
Some patients in our study had their clinical trial–related expenses reimbursed. Most of these reimbursements were from the study sponsor, although some patients were reimbursed by their insurance plan or other means. We did not find a significant association between the receipt of reimbursement for clinical trial–related expenses and patients’ financial well‐being, but this finding is tentative given the small number of patients in our study who received assistance. In addition, the role of the amount reimbursed remains unknown. It is possible that partial reimbursement only represented a small fraction of expenses and was therefore not enough to make a difference to a patient's financial well‐being, or that expense reimbursement for this patient population was “too little, too late.” One prior study that evaluated a reimbursement intervention for some clinical trial–related expenses did not find improvement in patients’ financial well‐being over time [13]. Moving forward, more research is needed to better understand how to relieve financial toxicity for patients enrolled in clinical trials. Future research should explore potential mechanisms to reimburse out‐of‐pocket expenses for patients in clinical trials. Attention should be paid to whether reimbursement can serve to increase diversity in a clinical trial population.
The COVID‐19 pandemic has dramatically reshaped certain aspects of health care, accelerating the growth of telemedicine [32] and necessitating the loosening of requirements for clinical trials, such as allowing laboratory studies to be performed at local facilities and investigational drugs to be shipped to local health care providers [33]. Some of these changes may persist after the pandemic has resolved, and such changes will have downstream effects in health care delivery and clinical research. If patients are permitted to remain at home and get laboratory studies done and investigational drugs shipped locally, one would expect a significant reduction in nonmedical out‐of‐pocket costs such as travel, parking, and accommodations. These changes may reduce barriers to clinical trial participation and expand access.
Our findings are subject to limitations. The study population was from a single academic medical center, which introduces referral bias, may limit the generalizability of the results to the general population, and underrepresents patients who are uninsured or underinsured. A high number of patients who travel long distances or seek out clinical trials has the potential to skew results. In addition, our study did not assess patients who may have been deterred from clinical trial enrollment owing to concerns about financial toxicity. Finally, although measuring expenses provided real‐world estimates of costs, these values may be inflated if patients electively chose to buy more expensive services (e.g., a luxury hotel rather than a budget hotel).
Conclusion
Patients enrolled in early‐phase oncology clinical trials are faced with substantial out‐of‐pocket costs, economic burden, and financial toxicity. These out‐of‐pocket costs are often unanticipated for patients. Most of patients’ out‐of‐pocket costs in our study were nonmedical. Patients with low income are more likely to experience worse financial toxicity. Compensation may be appropriate for some patients in clinical trials, particularly reimbursement for out‐of‐pocket expenses, and further research should focus on whether this can lead to increased access to clinical trials and a reduction in disparities in clinical trial participation.
Author Contributions
Conception/design: Ryan W. Huey, Goldy C. George, Ya‐Chen Tina Shih, David S. Hong
Provision of study material or patients: Siqing Fu, Filip Janku, Daniel D. Karp, Aung Naing, Sarina Piha‐Paul, Vivek Subbiah, Apostolia M. Tsimberidou, Shubham Pant, Timothy A. Yap, Jordi Rodon, Funda Meric‐Bernstam, David S. Hong
Collection and/or assembly of data: Ryan W. Huey, Penny Phillips, Revenda White
Data analysis and interpretation: Ryan W. Huey, Goldy C. George, Ya‐Chen Tina Shih, David S. Hong
Manuscript writing: Ryan W. Huey, Goldy C. George, Ya‐Chen Tina Shih, David S. Hong
Final approval of manuscript: Ryan W. Huey, Goldy C. George, Penny Phillips, Revenda White, Siqing Fu, Filip Janku, Daniel D. Karp, Aung Naing, Sarina Piha‐Paul, Vivek Subbiah, Apostolia M. Tsimberidou, Shubham Pant, Timothy A. Yap, Jordi Rodon, Funda Meric‐Bernstam, Ya‐Chen Tina Shih, David S. Hong
Disclosures
Filip Janku: Agios, Asana, Astellas, Astex, Bayer, Bicara, BioMed Valley Discoveries, Bioxcel, Bristol‐Myers Squibb, Deciphera, FujiFilm Pharma, Genentech, Ideaya, JS Innopharm, Eli Lilly & Co., Merck, Novartis, Novellus, Plexxikon, Proximagen, Sanofi, Sotio, SpringBank Pharmaceuticals, SQZ Biotechnologies, Synlogic, Synthorx, Symphogen (RF), Asana, Baush Health, Cardiff Oncology, Deciphera, Guardant Health, Ideaya, IFM Therapeutics, Immunomet, Illumina, Jazz Pharmaceuticals, Novartis, PureTech Health, Sotio, Synlogic (C/A), Cardiff Oncology (OI), Bio‐Rad, Biocartis (Other: lab equipment); Shubham Pant: Arcus, Arqule, Bristol‐Myers Squibb, Eli Lilly & Co., Five Prime, GlaxoSmithKline, Holy Stone Healthcare Co., Tyme, Ipsen, Mirati Therapeutics, Novartis, Red Hill Biopharma, Rgenix, Sanofi‐Aventis, Xencor, Astellas, Janssen (RF), Tyme, Inc, 4D Pharma, Xencor, Ipsen, Zymeworks (C/A); Ya‐Chen Tina Shih: Pfizer, AstraZeneca (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank the Editing Services of the Research Medical Library at University of Texas MD Anderson Cancer Center. Dr. Huey acknowledges funding from the Conquer Cancer Foundation. Dr. Shih acknowledges funding from the National Cancer Institute (R01CA225647) and Health Care Services Corporation/Blue Cross Blue Shield of Texas.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Hala T. Borno, Li Zhang, Adam Siegel et al. At What Cost to Clinical Trial Enrollment? A Retrospective Study of Patient Travel Burden in Cancer Clinical Trials. The Oncologist 2018;23:1242–1249.
Implications for Practice: This study is one of the first to measure travel distance for patients in cancer clinical trials using a real‐world GoogleMaps calculator. Out‐of‐pocket expenses such as travel are not typically covered by health care payers; therefore, patients may face considerable cost to attend each study visit. Using a single‐center clinical trials enrollment database, this study found that the burden of travel is highest for patients enrolled in National Institutes of Health‐sponsored trials and phase I studies, as well as for patients living in low‐income areas. Results suggest that a significant proportion of patients enrolled in clinical trials face a substantial travel burden.
References
- 1. Bell JA, Balneaves LG. Cancer patient decision making related to clinical trial participation: An integrative review with implications for patients’ relational autonomy. Support Care Cancer 2015;23:1169–1196. [DOI] [PubMed] [Google Scholar]
- 2. Wong YN, Schluchter MD, Albrecht TL et al. Financial concerns about participation in clinical trials among patients with cancer. J Clin Oncol 2016;34:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unger JM, Cook E, Tai E et al. The role of clinical trial participation in cancer research: Barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book 2016;35:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lara PN Jr, Higdon R, Lim N et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol 2001;19:1728–1733. [DOI] [PubMed] [Google Scholar]
- 5. Sateren WB, Trimble EL, Abrams J et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 2002;20:2109–2117. [DOI] [PubMed] [Google Scholar]
- 6. Zafar SY, Peppercorn JM, Schrag D et al. The financial toxicity of cancer treatment: A pilot study assessing out‐of‐pocket expenses and the insured cancer patient's experience. The Oncologist 2013;18:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fenn KM, Evans SB, McCorkle R et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract 2014;10:332–338. [DOI] [PubMed] [Google Scholar]
- 8. Perrone F, Jommi C, Di Maio M et al. The association of financial difficulties with clinical outcomes in cancer patients: Secondary analysis of 16 academic prospective clinical trials conducted in Italy. Ann Oncol 2016;27:2224–2229. [DOI] [PubMed] [Google Scholar]
- 9. Zafar SY, Newcomer LN, McCarthy J et al. How should we intervene on the financial toxicity of cancer care? One shot, four perspectives. Am Soc Clin Oncol Educ Book 2017;37:35–39. [DOI] [PubMed] [Google Scholar]
- 10. Ramsey S, Blough D, Kirchhoff A et al. Washington state cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramsey SD, Bansal A, Fedorenko CR et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol 2016;34:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borno HT, Zhang L, Siegel A et al. At what cost to clinical trial enrollment? A retrospective study of patient travel burden in cancer clinical trials. The Oncologist 2018;23:1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nipp RD, Lee H, Gorton E et al. Addressing the financial burden of cancer clinical trial participation: Longitudinal effects of an equity intervention. The Oncologist 2019;24:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Largent EA, Lynch HF. Addressing financial barriers to enrollment in clinical trials. JAMA Oncol 2018;4:913–914. [DOI] [PubMed] [Google Scholar]
- 15. Chino F, Zafar SY. Financial toxicity and equitable access to clinical trials. Am Soc Clin Oncol Educ Book 2019;39:11–18. [DOI] [PubMed] [Google Scholar]
- 16. de Souza JA, Yap BJ, Wroblewski K et al. Measuring financial toxicity as a clinically relevant patient‐reported outcome: The validation of the Comprehensive Score for Financial Toxicity (COST). Cancer 2017;123:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George GC, Iwuanyanwu EC, Buford AS et al. Cancer‐related internet use and its association with patient decision making and trust in physicians among patients in an early drug development clinic: A questionnaire‐based cross‐sectional observational study. J Med Internet Res 2019;21:e10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Souza JA, Kung S, O'Connor J et al. Determinants of patient‐centered financial stress in patients with locally advanced head and neck cancer. J Oncol Pract 2017;13:e310–e318. [DOI] [PubMed] [Google Scholar]
- 19. Souza JAD, Aschebrook‐Kilfoy B, Grogan R et al. Grading financial toxicity based upon its impact on health‐related quality of life (HRQol). J Clin Oncol 2016;34(suppl 3):16–16. [Google Scholar]
- 20. Jagsi R, Pottow JA, Griffith KA et al. Long‐term financial burden of breast cancer: Experiences of a diverse cohort of survivors identified through population‐based registries. J Clin Oncol 2014;32:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nazha B, Mishra M, Pentz R et al. Enrollment of racial minorities in clinical trials: Old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book 2019;39:3–10. [DOI] [PubMed] [Google Scholar]
- 22. Huey RW, Hawk E, Offodile AC 2nd. Mind the gap: Precision oncology and its potential to widen disparities. J Oncol Pract 2019;15:301–304. [DOI] [PubMed] [Google Scholar]
- 23. Winkfield KM, Phillips JK, Joffe S et al. Addressing financial barriers to patient participation in clinical trials: ASCO policy statement. J Clin Oncol 2018:JCO1801132. [DOI] [PubMed] [Google Scholar]
- 24. Chino F, Peppercorn JM, Rushing C et al. Out‐of‐pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncol 2017;3:1582–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huntington SF, Weiss BM, Vogl DT et al. Financial toxicity in insured patients with multiple myeloma: A cross‐sectional pilot study. Lancet Haematol 2015;2:e408–416. [DOI] [PubMed] [Google Scholar]
- 26. Allcott N, Dunham L, Levy D et al. Financial burden amongst cancer patients treated with curative intent surgery alone. Am J Surg 2019;218:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Internal Revenue Service . Retirement Topics Tax on Early Distributions. Available at https://www.irs.gov/retirement‐plans/plan‐participant‐employee/retirement‐topics‐tax‐on‐early‐distributions. Accessed September 21, 2020.
- 28. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research . The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Bethesda, MD: Department of Health, Education, and Welfare, 1978. [PubMed]
- 29. Food and Drug Administration . Payment and Reimbursement to Research Subjects. Available at https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/payment‐and‐reimbursement‐research‐subjects. Accessed September 21, 2020.
- 30. Relating to a Cancer Clinical Trial Participation Program , H.B. 3147 Texas Legislature 86(R)(2019).
- 31. Gelinas L, Largent EA, Cohen IG et al. A framework for ethical payment to research participants. N Engl J Med 2018;378:766–771. [DOI] [PubMed] [Google Scholar]
- 32. Wosik J, Fudim M, Cameron B et al. Telehealth transformation: COVID‐19 and the rise of virtual care. J Am Med Inform Assoc 2020;27:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Food and Drug Administration . FDA Guidance on Conduct of Clinical Trials of Medical Products During COVID‐19 Public Health Emergency. Silver Spring, MD: Food and Drug Administration, 2020.


