Abstract
The Japanese Ministry of Health, Labour and Welfare approved a drug called borofalan (10B), a treatment system, and a dose calculation program for boron neutron capture therapy (BNCT) in March 2020. The application pertaining to the products submitted to the Pharmaceuticals and Medical Devices Agency was supported by a Japanese, open‐label, uncontrolled trial (Study 002) in patients with unresectable, locally recurrent head and neck squamous cell carcinoma after chemoradiotherapy or radiotherapy, or in those with unresectable locally advanced or locally recurrent (LA/LR) head and neck nonsquamous cell carcinoma. The drug was administered as a single intravenous dose using infusion rates of 200 mg/kg per hour for the first 2 hours after the start of administration and 100 mg/kg per hour during irradiation. Neutron irradiation was performed using the devices at a single dose of 12 Gy‐equivalent for oral, pharyngeal, or laryngeal mucosa for up to 60 minutes from 2 hours after the start of drug administration. The primary endpoint was the overall response rate (ORR). The results of Study 002 showed that the ORR based on an assessment of the Independent Central Review Committee per RECIST version 1.1 was 71.4% (90% confidence interval [CI], 51.3%–86.8%). The lower limit of the 90% CI exceeded the prespecified threshold for ORR. When BNCT is applied to patients with unresectable LA/LR head and neck cancer, precautions should be taken, and patients should be monitored for possible onset of dysphagia, brain abscess, skin disorder, crystal urine, cataract, and/or carotid hemorrhage.
Implications for Practice
Borofalan (10B), a treatment system and a dose calculation program for boron neutron capture therapy (BNCT), demonstrated significant efficacy in an open‐label, uncontrolled trial in which overall response rate was the primary endpoint for patients with unresectable locally advanced or locally recurrent head and neck cancer. Although no information about survival benefits was obtained, BNCT will become an effective treatment option that is expected to manage local lesions that are intractable with any standard therapy. In addition, BNCT is expected to maintain quality of life of the intended patient population, on account of its high tumor selectivity and low invasiveness.
Keywords: Boron neutron capture therapy, Head and neck carcinoma, SAKIGAKE Designation System, Pharmaceuticals and Medical Devices Agency
Short abstract
This article summarizes the regulatory review of data leading to the approval of borofalan in Japan.
Introduction
Oral, pharyngeal, and laryngeal cancers have been reported to account for 86.9% of all cases of head and neck cancer, and oral/pharyngeal cancer and laryngeal cancer (excluding carcinoma in situ) affect 21,601 and 5,285 individuals, respectively, in Japan [1, 2]. Drug therapies such as those with nivolumab (Genetic Recombination) and cetuximab (Genetic Recombination) are available to treat patients with unresectable locally advanced or locally recurrent (LA/LR) head and neck cancer. However, the overall response rate (ORR) of nivolumab reported from the CheckMate 141 study is as low as 13.3%, and the treatment effect of the existing therapies is limited [3]. The Japanese Ministry of Health, Labour and Welfare (MHLW) approved STEBORONINE 9,000 mg/300 mL (borofalan (10B), Stella Pharma Corporation, Osaka, Japan) for infusion, the boron neutron capture therapy (BNCT) treatment system NeuCure (Sumitomo Heavy Industries, Ltd., Tokyo, Japan), and the BNCT dose‐calculation program NeuCure Dose Engine (Sumitomo Heavy Industries, Ltd.) as a drug with a new active ingredient and new medical devices used for BNCT to treat patients with unresectable LA/LR head and neck carcinoma in March 2020 (approval date for the drug: March 25, 2020; approval date for the devices: March 11, 2020) [4, 5, 6]. This report provides an outline of the issues discussed in the review of the products, to which the SAKIGAKE Designation System was applied (Designation Numbers: Drug_SAKIGAKE [2017 Drug] No.4 and Device_SAKIGAKE [2016 Device] No.2). The SAKIGAKE Designation System was established in 2015 by MHLW to promote research and development aiming at early practical application of innovative pharmaceutical products, medical devices, and regenerative medicines that are initially developed in Japan [7].
BNCT is expected to achieve cell‐killing effects attributable to the heavy charged particles, that is, alpha particles (helium [4He]) and recoil lithium (7Li) nuclei, generated by the nuclear reaction between the 10B (stable isotope) atoms and the thermal neutrons in the patient. The BNCT drug borofalan (10B) is prepared by increasing the proportion of 10B atoms among all the boron atoms in 4‐borono‐l‐phenylalanine, a derivative of the essential amino acid phenylalanine (Fig. 1). Borofalan (10B) is excreted mainly in urine as the unchanged drug in both rats and humans. Results from nonclinical studies indicate that the tumor accumulation of the drug (tumor concentration of boron/blood concentration of boron) can be up to three. The BNCT treatment system NeuCure is a neutron irradiation system designed for BNCT, which consists of a cyclotron (proton accelerator), a proton beam transporter, a neutron irradiation system, and other associated apparatuses. The transported proton beam is irradiated to a beryllium (9Be) target, generating neutrons with energy of up to 28 MeV. This energy is released when a nuclear reaction occurs because of the collision of protons with the beryllium nuclei. The generated neutrons are irradiated from the device's collimator after being decelerated to an epithermal energy suitable for BNCT irradiation (0.5 eV–40 keV) by a moderator. After being further decelerated because of elastic scattering with internal hydrogen nuclei, the irradiated epithermal neutrons become thermal neutrons that are likely to cause a nuclear reaction with 10B. The thermal neutrons decrease to 50% of the maximal amount at a depth of approximately 6 cm. The dose calculation for BNCT is performed with the BNCT dose‐calculation program NeuCure Dose Engine (Fig. 2).
Figure 1.
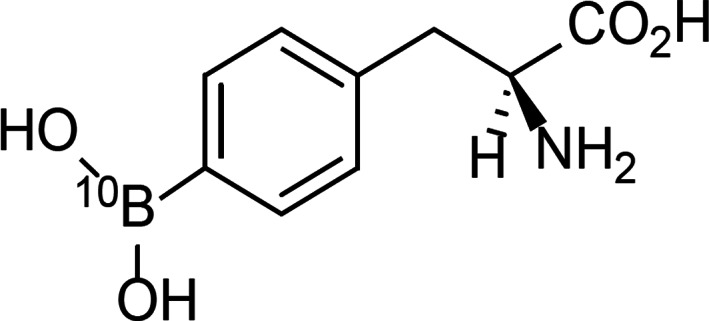
Chemical structure of borofalan (10B). The chemical name of borofalan (10B) is as follows: (S)‐2‐Amino‐3‐[4‐(10B)dihydroxyboranylphenyl]propanoic acid.
Figure 2.
Overview of BNCT with STEBORONINE 9,000 mg/300 mL for infusion, the BNCT treatment system NeuCure, and the BNCT dose‐calculation program NeuCure Dose Engine.
Abbreviations: BNCT, boron neutron capture therapy; CT, computed tomography.
The phase I clinical trial (Study 001) of the BNCT drug borofalan (10B), the BNCT treatment system NeuCure, and the BNCT dose‐calculation program NeuCure Dose Engine (the products/BNCT) was initiated in March 2014 in Japan. In this study, patients with unresectable, locally recurrent head and neck squamous cell carcinoma (HNSCC) or unresectable, LA/LR head and neck nonsquamous cell carcinoma (HNNSCC) were enrolled. The phase II clinical trial (Study 002) of the products was initiated in June 2016 in Japan and included patients with unresectable, locally recurrent HNSCC after chemoradiotherapy (CRT) or radiotherapy (RT) or those with unresectable LA/LR HNNSCC. Data from these two studies were submitted to support the application. Reviewing the data focusing primarily on those from Study 002 as the key study to evaluate the efficacy and safety of the products/BNCT, the Pharmaceuticals and Medical Devices Agency (PMDA) concluded that for the intended patient population, BNCT can be positioned as an effective treatment option that is expected to manage local lesions that are intractable with any standard therapy and maintain the patients’ quality of life (QOL), on account of its high tumor selectivity and low invasiveness. In the following sections, the regulatory review conducted with the efficacy data from Study 002 and the safety data from Studies 001 and 002 will be summarized.
Materials and Methods
To evaluate the safety and tolerability of the products/BNCT in unresectable LA/LR head and neck cancer, an open‐label, uncontrolled trial (Study 001) was conducted in patients with HNSCC or HNNSCC at two sites in Japan. In Study 001, patients who met both of the following criteria were enrolled: (a) HNSCC with prior RT at ≥40 and ≤ 75 Gy to the target lesion, or HNNSCC with prior RT at ≤75 Gy to the target lesion; and (b) the last irradiation to the target lesion was performed 180 or more days before enrollment. Borofalan (10B) was administered as a single intravenous dose at an infusion rate of 200 mg/kg per hour for the first 2 hours after the start of administration and then at 100 mg/kg hours during neutron irradiation. Neutron irradiation was performed using the BNCT devices at a single dose of 10 Gy‐Eq. (low dose) or 12 Gy‐equivalent (high dose) for the oral, pharyngeal, or laryngeal mucosa for up to 60 minutes from 2 hours after the start of drug administration.
To evaluate the efficacy and safety of the products/BNCT in unresectable LA/LR head and neck cancer, an open‐label uncontrolled trial (Study 002) was conducted in patients with HNSCC or HNNSCC at two sites in Japan. In Study 002, patients with HNSCC were considered eligible if they met one of the following criteria: the patient had received CRT with a platinum‐based chemotherapy regimen or the patient had received RT after induction chemotherapy with a platinum‐based chemotherapy regimen; or the patient had local recurrence within 6 months after the completion of RT alone, CRT without any platinum‐based chemotherapy regimen, or CRT with cetuximab. Although the target patients with HNSCC were defined as those with unresectable locally recurrent lesions after CRT or RT, patients with locally recurrent lesions who achieved partial response or stability after CRT or RT but required additional treatment for the residual lesions were also enrolled. The eligibility criteria for patients with HNNSCC in Study 002 included existence of unresectable LA/LR lesions, regardless of a history of RT or chemotherapy. Administration of a combination of antitumor treatments (e.g., CRT with cisplatin or cetuximab, or RT) was prohibited during Study 002.
Borofalan (10B) was administered as a single intravenous dose at an infusion rate of 200 mg/kg per hour for the first 2 hours after the start of administration and subsequently at 100 mg/kg per hour during neutron irradiation. Neutron irradiation was performed using the BNCT devices at a single dose of 12 Gy‐Eq. for the oral, pharyngeal, or laryngeal mucosa for up to 60 minutes from 2 hours after the start of drug administration.
The primary endpoint of Study 002 was determined to be ORR because an improvement in QOL through a conservation of functions such as phonation, swallowing, mastication, and respiration is considered as clinically significant if response is achieved in patients with unresectable LA/LR head and neck cancer. The threshold for ORR was determined to be 20% in reference to clinical study data from the EXTREME study. The EXTREME study was a non‐Japanese, phase III clinical trial conducted to evaluate the efficacy and safety of cetuximab/5‐fluorouracil (5‐FU)/platinum‐based chemotherapy versus 5‐FU/platinum‐based chemotherapy in patients with HNSCC, recurrent or with distant metastasis, who were previously not treated with chemotherapy [8].
Results
In Study 001, nine subjects with recurrent head and neck carcinoma were enrolled. Six and three subjects were allocated in the low‐ (10 Gy‐Eq.) and high‐dose (12 Gy‐Eq.) group, respectively. All of the nine subjects received the products/BNCT and were included in the safety analysis sets.
In Study 002, eight subjects with squamous cell carcinoma and 13 subjects with nonsquamous cell carcinoma were enrolled. All of the 21 subjects received the products/BNCT. Both the efficacy and safety analysis sets included all 21 subjects. Results for the primary endpoint, ORR, based on an assessment of the Independent Central Review Committee (ICRC) per RECIST version 1.1 are shown in Table 1. The lower limit of the 90% confidence interval (CI) exceeded the prespecified threshold for ORR (20.0%). The results for ORR by tissue type were not much different between squamous cell carcinoma and nonsquamous cell carcinoma.
Table 1.
Overall response rate in the overall population and in subgroups based on histology in Study 002
| Overall response | All patients (n = 21), n (%) | SCC (n = 8), n (%) | NSCC (n = 13), n (%) |
|---|---|---|---|
| Best overall response a | |||
| CR | 5 (23.8) | 4 (50.0) | 1 (7.7) |
| PR | 10 (47.6) | 2 (25.0) | 8 (61.5) |
| SD | 5 (23.8) | 1 (12.5) | 4 (30.8) |
| PD | 0 | 0 | 0 |
| NE | 1 (4.8) | 1 (12.5) | 0 |
| Number of subjects with overall response (CR + PR) (ORR [90% CI b]), % | 15 (71.4 [51.3–86.8]) | 6 (75.0 [40.0–95.4]) | 9 (69.2 [42.7–88.7]) |
RECIST version 1.
The Clopper‐Pearson interval.
Abbreviations: CI, confidence interval; CR, complete response; NE, not evaluable; NSCC, nonsquamous cell carcinoma; ORR, overall response rate; PD, progressive disease; PR, partial response; SCC, squamous cell carcinoma; SD, stable disease.
The safety results from Studies 002 and 001 are summarized in Table 2. In Study 002, adverse events (AEs) of any grade reported in ≥20% of subjects included alopecia (n = 19; 90.5%), amylase increased (n = 18; 85.7%), nausea (n = 17; 81.0%), dysgeusia (n = 15; 71.4%), parotitis and decreased appetite (n = 14; 66.7% each), stomatitis (n = 13; 61.9%), vomiting (n = 10; 47.6%), malaise, thirst and radiation skin injury (n = 9; 42.9% each), conjunctivitis and sialoadenitis (n = 7; 33.3% each), blood prolactin abnormal and blood prolactin increased (n = 6; 28.6% each), and constipation (n = 5; 23.8%). AEs of grade 3 or higher reported in any subject included amylase increased (n = 16; 76.2%), and lymphopenia, lymphocyte count decreased, stomatitis, brain abscess, and radiation skin injury (n = 1; 4.8% each). Serious adverse events (SAEs) reported in any subject included brain abscess (n = 1; 4.8%), for which a causal relationship to study treatment could not be ruled out. No AEs leading to death or to treatment discontinuation were reported.
Table 2.
Summary of the clinical safety data in Study 002 and Study 001
| Adverse event type | Study 002 (n = 21), n (%) | Study 001 (n = 9), n (%) |
|---|---|---|
| All adverse events | 21 (100) | 9 (100) |
| Adverse events of grade 3 or higher | 18 (85.7) | 3 (33.3) |
| Adverse events leading to death | 0 | 0 |
| Serious adverse events | 1 (4.8) | 1 (11.1) |
| Adverse events leading to treatment discontinuation | 0 | 0 |
In Study 001, a dose‐limiting toxicity, grade 3 dysphagia was observed in one of six patients of the low‐dose group, whereas no dose‐limiting toxicity was observed in the high‐dose group. Therefore, the recommended dose of the products/BNCT was determined to be 12 Gy‐Eq. AEs of grade 3 or higher reported in any subject included lymphocyte count decreased (n = 3; 33.3%), and anemia, dysphagia, upper gastrointestinal hemorrhage, hypercalcemia, hypoalbuminemia, pharyngeal hemorrhage, pharyngeal inflammation, laryngeal inflammation, and hypertension (n = 1; 11.1% each). SAEs reported in any subject included dysphagia (n = 1; 11.1%), for which a causal relationship to study treatment could not be ruled out. No AEs leading to death or to treatment discontinuation were reported.
Discussion
In Study 002, the primary endpoint, ORR, based on an assessment of the ICRC per RECIST version 1.1, was 71.4% (90% CI, 51.3%–86.8%). The lower limit of the 90% CI showed a response above the prespecified threshold response rate. In patients with unresectable LA/LR head and neck cancer, local lesions may lead to conditions, such as dysphagia, malnutrition, airway narrowing, aspiration, and fistula formation, which result in a significant worsening of the patients’ QOL. Local management of the lesions has a clinical significance, consequently, the PMDA concluded that the products/BNCT has been shown to have efficacy. The efficacy evaluation in Study 002, however, has limitations because the study was conducted using an open‐label, uncontrolled design and no long‐term results were obtained.
In Study 002, the efficacy evaluation of the products/BNCT was primarily based on the results with reference to the ORR, and no information about survival benefits was obtained. Thus, it should be appropriate to advise that the standard therapies such as CRT, if feasible, should be performed in preference to the products/BNCT. In addition, it is necessary to include an advisory in the “precaution concerning indication” section of the package insert that the efficacy or safety of the products/BNCT as adjuvant therapy have not been established, because no clinical data have been obtained regarding the products/BNCT as adjuvant therapy.
Based on the safety results from Studies 001 and 002, AEs requiring particular notice include dysphagia, brain abscess, skin disorder, crystal urine, cataract, and carotid hemorrhage. Although caution should be exercised regarding these AEs when applying the products/BNCT, the PMDA concluded that the safety profile of the products/BNCT is tolerable if appropriate measures including observation and management of AEs are taken by a physician who has adequate knowledge and experience of cancer chemotherapy.
In Study 002, patients who had received their last RT irradiation to the lesion site <90 days prior were excluded, because the effectiveness assessment of the RT may still be indefinite and the accumulated dose from RT may affect the safety of the products/BNCT for those patients. Although these clinical safety data of the products/BNCT have not been obtained in this patient group, the PMDA suggests that it is acceptable to administer the products/BNCT to patients who did not respond to RT and experienced no AE of grade 3 or higher, based on the following considerations: (a) Whether or not to repeat RT is determined by the clinical symptoms of organs in the radiation field as well as the accumulated dose and tolerance dose of organs in the radiation field. (b) The accumulated dose in the normal tissue after RT cannot be accurately measured.
In Study 002, patients who had already received irradiation to the lesion site at a total dose of ≥75 Gy were excluded because the dose‐received exceeded the standard dose. No efficacy or safety data of the products/BNCT were obtained for this patient group. However, the PMDA considers that the products/BNCT in these patients is considered to be acceptable because of the same reason as described above, given that clinical symptoms of organs within the radiation field are carefully monitored.
The incidence of mucositis in patients receiving RT for head and neck cancer was 80%, and the incidence of grade 3 or 4 of mucositis was 39% [9]. In Study 001, the recommended radiation dose for the products/BNCT was 12 Gy‐Eq. based on the tolerable dose for mucous membranes. By considering these points, in Study 002, patients who have no mucous membrane within the range of 1.0–5.0 cm from the skin surface along the radiation axis were excluded to properly assess the occurrence of AEs in mucous membranes. No efficacy or safety data of the products/BNCT have been obtained in this patient group, but the PMDA considers that whether or not to administer the products/BNCT to these patients can be determined based on the accumulated and tolerable doses in the normal tissues other than mucous membranes, given that mucous membranes of those patients will receive a dose of <12 Gy‐Eq.
Because the available safety information regarding the use of the products in Japanese patients is very limited, the PMDA concluded that postmarketing surveillance should be conducted in all patients receiving the drug to accumulate safety information in a rapid and unbiased way, and that the safety information obtained needs to be promptly provided to the medical staff in clinical practice. The Safety Specification for this all‐case surveillance should include dysphagia, brain abscess, severe skin disorder, crystalluria, cataract, carotid hemorrhage, and late effects. The target sample size and follow‐up period should be 150 patients and 3 years, respectively, based on the feasibility and the incidence of events included in the Safety Specification occurred in relevant studies including Studies 001 and 002.
The products/BNCT is a combination of a boron drug and neutron beam. This is a unique therapy because effectiveness on tumors and the extent of unintended exposure of normal tissues to a neutron beam depend on the extent of 10B accumulation in the tumor, unlike conventional radiation therapies such as x‐ray and proton radiation. In addition, this therapy requires expert knowledge regarding treatment planning, quality control of the devices, and radiation safety management of the device including radioactivation. Given these factors, the PMDA concluded that it should be performed by eligible medical staff (i.e., physicians and medical physics experts) with adequate knowledge, skill, and experience at a medical institution well‐prepared to manage the therapy.
Because the safety information pertaining to BNCT is very limited not only in Japan but in other countries. Therefore, the PMDA concluded that the safety and effectiveness information should be collected from all patients who are treated using the devices by conducting use‐results surveys after marketing, and if necessary, additional measures for risk minimization should be taken. The PMDA also concluded that it is reasonable to conduct the use‐results surveys of the devices in coordination with the postmarketing surveys of the drug, and that it is appropriate to conduct the use‐results surveys with the same content and period as the postmarketing surveys of the drug. In addition, the PMDA indicated that necessary measures should be taken to assure that the radiation exposure of health care professionals is minimized based on the perspective of “dose limit” recommended by the International Commission on Radiological Protection, because radioactivation of the device and surrounding building is considered to be higher for this therapy than for conventional radiation therapy equipment such as linear accelerators [10, 11].
Conclusion
Based on the above results of the PMDA's review, the application was deliberated at the Second Committee on New Drugs and the Committee on Medical Devices and In‐vitro Diagnostics of the Pharmaceutical Affairs and Food Sanitation Council, MHLW, and the products/BNCT were approved as an option for local treatment of patients with unresectable LA/LR head and neck cancer who cannot be treated with any standard therapy such as CRT.
The investigators of Study 002 noted that the focus of Study 002 was to assess locoregional control and safety of BNCT within the first 3 months of its implementation to achieve early approval of BNCT, thus limiting the evaluation of efficacy and safety beyond 3 months [12]. The long‐term safety results obtained from the all‐case surveillance of the products/BNCT should be provided to the concerned medical personnel as soon as they are available. In addition to the primary endpoint (ORR), Study 002 also included secondary endpoints to evaluate tumor response, such as the duration of response and change in 18F‐fluorodeoxyglucose accumulation; however, the small number of subjects and the short evaluation period limit the clinical interpretation. Further clinical studies are needed to establish the products/BNCT as a standard treatment for patients with unresectable LA/LR head and neck cancer.
Author Contributions
Conception/design: Akihiro Ishiguro, Tetsuo Nakabayashi, Hiroyuki Arai, Hiroshi Suzuki
Collection and/or assembly of data: Hitoshi Kanno, Hironori Nagata, Satoshi Tsuzuranuki, Shintaro Nakano, Takahiro Nonaka, Koushin Kiyohara, Toshinari Kimura, Akihiko Sugawara, Yuzuru Okazaki, Shinichi Takae, Hiroyuki Arai
Data analysis and interpretation: Hitoshi Kanno, Hironori Nagata, Satoshi Tsuzuranuki, Shintaro Nakano, Takahiro Nonaka, Koushin Kiyohara, Toshinari Kimura, Akihiko Sugawara, Yuzuru Okazaki, Shinichi Takae, Hiroyuki Arai
Manuscript writing: Akihiro Ishiguro, Tetsuo Nakabayashi, Hiroyuki Arai, Hiroshi Suzuki
Final approval of manuscript: Hitoshi Kanno, Hironori Nagata, Akihiro Ishiguro, Satoshi Tsuzuranuki, Shintaro Nakano, Takahiro Nonaka, Koushin Kiyohara, Toshinari Kimura, Akihiko Sugawara, Yuzuru Okazaki, Shinichi Takae, Tetsuo Nakabayashi, Hiroyuki Arai, Hiroshi Suzuki
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Cancer Registry Committee of the Japan Society for Head and Neck Cancer . Report of Head and Neck Cancer Registry of Japan: Clinical Statistics of Registered Patients, 2016. Japan Society for Head and Neck Cancer, 2019. Available at http://www.jshnc.umin.ne.jp/pdf/2016syourei_houkoku.pdf. Accessed August 6, 2020.
- 2. Cancer and Disease Control Division of the Ministry of Health, Labour and Welfare . Cancer Incidence of Japan 2016 [in Japanese]. Available at https://www.mhlw.go.jp/content/10900000/000553552.pdf. Accessed August 6, 2020.
- 3. Ferris R, Blumenschein G, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pharmaceutical Division, Safety and Environmental Health Bureau of the Ministry of Health, Labour and Welfare . Report on the Deliberation Results: Steboronine 9000 mg/300 mL for Infusion. March 3, 2020. Available at https://www.pmda.go.jp/files/000237990.pdf. Accessed January 10, 2021.
- 5. Medical Device Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau of the Ministry of Health, Labour and Welfare . Report on the Deliberation Results: NeuCure BNCT Dose Engine. February 19, 2020. Available at https://www.pmda.go.jp/files/000237993.pdf. Accessed January 10, 2021.
- 6. Medical Device Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau of the Ministry of Health, Labour and Welfare . Report on the Deliberation Results: NeuCure BNCT System. February 19, 2020. Available at https://www.pmda.go.jp/files/000237994.pdf. Accessed January 10, 2021.
- 7. Ministry of Health, Labour and Welfare . Strategy of SAKIGAKE. Available at https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/140729-01.html. Accessed January 10, 2021.
- 8. Vermorken J, Mesia R, Rivera F et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–1127. [DOI] [PubMed] [Google Scholar]
- 9. Trotti A, Bellm L, Epstein J et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol 2003;66:253–262. [DOI] [PubMed] [Google Scholar]
- 10. International Commission on Radiological Protection . 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ottawa, Ontario, Canada: International Commission on Radiological Protection, 1991. Available at https://www.icrp.org/publication.asp?id=ICRP%20Publication%2060. Accessed August 6, 2020.
- 11. International Commission on Radiological Protection . Radiological Protection and Safety in Medicine. ICRP Publication 73. Ottawa, Ontario, Canada: International Commission on Radiological Protection, 1996. Available at https://www.icrp.org/publication.asp?id=ICRP%20Publication%2073. Accessed August 6, 2020.
- 12. Hirose K, Konno A, Hiratsuka J et al. Boron neutron capture therapy using cyclotron‐based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): An open‐label phase II trial. Radiother Oncol. 2021;155:182–187. [DOI] [PubMed] [Google Scholar]


