Abstract
Objective
This study aims at uncovering the effects of microRNAs (miRNAs) on the F8 gene and FVIII protein in hemophilia A (HA).
Methods
F8-targeting miRNAs were predicted by TargetScan, miRDB, and starBase. MiRNAs, predicted by at least two of the three databases, were selected for further study, and their expressions in the blood of HA patients without F8 mutations and healthy controls were detected. A dual-luciferase reporter assay was performed to verify the binding between hsa-miR-5581-3p/hsa-miR-542-3p and F8. In addition, the regulation of F8 by hsa-miR-5581-3p/hsa-miR-542-3p was investigated in human umbilical vein endothelial cells (HUVECs) and lymphoblastoid cell line (LCL) that displayed endogenous expression of FVIII. qRT-PCR was used to detect the expressions of miRNAs and F8 gene, and Western blotting was conducted to measure the expression of FVIII protein.
Results
A total of 42 F8-targeting miRNAs were predicted by at least two of the three databases. Among these miRNAs, hsa-miR-5581-3p and hsa-miR-542-3p were highly expressed in the blood of HA patients and have not been reported in previous studies of HA. Both hsa-miR-5581-3p and hsa-miR-542-3p could bind the 3′UTR of F8 mRNA. Upregulation of hsa-miR-5581-3p or hsa-miR-542-3p suppressed the expressions of F8 mRNA and FVIII protein in HUVECs and LCL cells.
Conclusion
Hsa-miR-5581-3p and hsa-miR-542-3p target the F8 gene and suppress the expression of FVIII protein, which may contribute to the development of HA without F8 mutations.
Keywords: Hsa-miR-5581-3p, Hsa-miR-542-3p, F8 gene, FVIII, Hemophilia A
Introduction
Hemophilia is a hereditary bleeding disorder resulting from deficiency or dysfunction of coagulation protein factor VIII (FVIII) and IX (FIX), leading to hemophilia A (HA) and hemophilia B (HB), respectively. The prevalence of HA is about fourfold higher than that of HB, and the prevalence of severe hemophilia is about 37% of all cases.1 Severe hemophilia is manifested by spontaneous muscle or intraarticular hemorrhage, and recurrent joint hemorrhages can result in progressive tissue damage and joint.2 Prophylactic factor replacement is currently the standard treatment option for hemophilia, which is dependent on frequent intravenous injections.3 However, among a list of novel treatment strategies for hemophilia, gene therapy shows a great appeal due to its potential for endogenous production of FVIII or FIX by transferring a functional gene to replace the deficient gene.4
In HA, the deficiency of FVIII is mainly caused by mutations in the F8 gene. Intron 22 and intron 1 inversions are the most frequent F8 variants in HA patients, followed by recurrent mutation at nucleotide 6046 (c.6046C>T) in the F8 gene.5 Missense mutations in this region (c.6046C>T/p.R2016W) damage secretion and activity of FVIII and also impede the correct pre-mRNA splicing, which further impairs FVIII activity.6 Despite clinically reportable DNA variants in 98.1% of HA patients,7 a small portion of HA patients is not affected by F8 variants, which encourages us to find the mechanism for mutation-independent FVIII deficiency.
MicroRNAs (miRNAs) are the most-investigated class of small non-coding RNAs (ncRNAs) for their ubiquitous regulation in the human genome. MiRNAs mainly regulate gene expression in cell cytoplasm in a sequence-specific manner by recruiting an RNA-induced silencing complex to their target RNAs.8 This miRNA-target interaction results in mRNA degradation or translational repression according to the degree of complementarity.9 Numerous functional studies have proved a causal link between miRNA dysregulation and human diseases, particularly cancers.
Rosset et al. firstly found a single nucleotide polymorphism in the 3′UTR of F8 (c.8728A>G, rs1050705) contributed to the complementarity between F8 3′UTR and the seed sequences of miR-26a-5p and miR-26b-5p, which further modified the FVIII residual clotting activity in HA patients.10 A previous study reported that miR-1246 was upregulated in the whole blood of HA patients, and this ncRNA could suppress F8 expression by binding to the non-coding region of F8 mRNA.11 Jankowska et al. identified miR-30c-5p and miR-374b-5p as direct mediators of F8 gene and FVIII in HA patients with normal F8 genotypes.12 They also found that murine miRNAs, including miR-208a, miR-351 and miR-125a, could target the 3′UTR of F8 mRNA in mouse models and mammalian cells.13 The above evidence strongly supports the involvement of miRNAs in the pathogenesis of HA.
The author applied three online databases to predict the F8-binding miRNAs and detected their expressions in the blood of HA patients without F8 gene mutations. Among the upregulated F8-binding miRNAs, hsa-miR-5581-3p and hsa-miR-542-3p were selected for further investigations since they were not reported in previous studies of HA. The function of these two miRNAs was experimentally verified.
Materials and methods
Prediction of F8-targeting miRNAs
F8-targeting miRNAs were predicted by TargetScan (http://www.targetscan.org/vert_72/), miRDB (http://www.mirdb.org/) and starBase (http://starbase.sysu.edu.cn/index.php). MiRNAs predicted by at least two of the three databases were selected for further investigations.
Blood samples
Blood samples were taken from two HA patients (one mild and one moderate) without mutations in the F8 gene and three healthy controls. The participants were enrolled at the Hematology Department of Henan Provincial People’s Hospital. Tubes containing sodium citrate anticoagulant were used for the blood collection. The blood samples were mixed with RNAlater Solution (Qiagen, Valencia, CA, USA) and stored at -20°C until RNA isolation. Mutations in intron 1/22 and all exons of the F8 gene were analyzed by next-generation sequencing and determined by comparison with the reference (NG_011403.1). Both the two HA patients had low FVIII activity (4.7 IU/dL and 19 IU/dL), but the RNA sequencing showed no mutations in the exon and intron of the F8 gene in these two patients. Moreover, the level of vWF:Ag and the activity of other coagulation factors, including FII, FV, FVII, FIX, and FX, remained unchanged in these two HA patients. Mutations of the vWF, LMAN1, and MCFD2 genes were excluded. The collection and use of the blood samples obtained informed consent from all the participants and approval of the ethics committee of Henan Provincial People’s Hospital.
qRT-PCR
A GenElute™ Plasma/Serum RNA Purification Mini Kit (Sigma-Aldrich, St. Louis, MO, USA) and TRIzol reagent (Invitrogen, Carlsbad, CA, USA) were used for total RNA extraction from blood samples and cells, respectively. Extracted RNA was reverse-transcribed using a reverse transcription kit (TaKaRa, Tokyo, Japan) based on the user’s manual. Gene expression was detected by LightCycler 480 (Roche Diagnostics, Indianapolis, IN, USA) under the conditions provided by the SYBR Green Mix (Roche Diagnostics). The thermal cycling consisted of 10 s at 95°C, 45 cycles of 5 s at 95°C, 10 s at 60°C and 10 s at 72°C, and a final extension for 5 min at 72°C. PCR test of each sample was duplicated three times. U6 and GAPDH were used as the internal references of miRNA and mRNA, respectively. Data were analyzed using the 2−ΔΔCt method. ΔΔCt = (Ct target gene-Ct reference gene) experimental group-(Ct target gene-Ct reference gene) control group. Primers used for PCR amplification and their sequences are presented in Table 1.
Table 1.
Primer sequences.
| Name of primer | Sequences |
|---|---|
| miR-5581-3p-F | CCTTGAAGATCCTCCG |
| miR-5581-3p-R | TGGTGTCGTGGAGTCG |
| miR-542-3p-F | AAAGTCAATAGTTAGA |
| miR-542-3p-R | TGGTGTCGTGGAGTCG |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
| F8-F | TGTGTGGTTTTGGTGTCCGT |
| F8-R | GTTGCCCACATAGGAGGCAT |
| GAPDH-F | GTGGCTGGCTCAGAAAAAGG |
| GAPDH-R | GGGGAGATTCAGTGTGGTGG |
Note: F, forward; R, reverse.
Dual-luciferase reporter assay
Wild and mutant sequences of the binding sites of F8 (wt-F8 and mut-F8) were designed and synthesized according to the online prediction. The sequence contained the 3′UTR fragment complementary to the target miRNA. Wt-F8 or mut-F8 was inserted into pGL3-Promoter vector and cotransfected with 50 nM hsa-miR-5581-3p mimic, hsa-miR-542-3p mimic or mimic NC together with Renilla luciferase reporter vector pRL-TK into HEK293T cells. The cells were added with 100 μl of lysis buffer and placed on a shaker for 20 min at room temperature. Suspension of the cell lysates (50 μl) was added with luciferase reaction solution (50 μl, Promega, Madison, WI, USA) before Firefly luciferase activity was measured. The suspension was then mixed with 50 μl of Stop&Glo reagent (Promega) to measure Renilla luciferase activity (internal reference). The ratio of firefly luciferase activity to Renilla luciferase activity was the relative luciferase activity.
Cell culture
Human umbilical vein endothelial cells (HUVECs) and lymphoblastoid cell line (LCL) that displayed endogenous expression of FVIII, and human embryonic kidney cells (HEK293T) were acquired from the American Type Culture Collection (ATCC, Manassas, Virginia, USA). HUVECs were cultured in an endothelial basal medium (EBM-2, PromoCell, Heidelberg, Germany) containing the Supplement Mix (PromoCell). HEK293T cells were cultured in DMEM (Gibco, Grand Island, NY, USA) and LCL cells were in RPMI medium (Gibco). The media for HEK293T and LCL cells were supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin. The cells were cultured at 37°C with 5% CO2.
Cell transfection
HUVECs and LCL cells were transfected with hsa-miR-5581-3p mimic, hsa-miR-542-3p mimic, hsa-miR-5581-3p inhibitor, hsa-miR-542-3p inhibitor or relevant negative controls (NC) (50 nM, GenePharma, Shanghai, China) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). The cells were transfected in a 6-well plate containing serum-free medium, and each well was seeded with 2 × 105 cells. Cells with over 70% transfection efficiency were selected 24 h after the transfection for the following experiments.
Western blotting
Cells were treated with RIPA lysis buffer (Beyotime, Shanghai, China) for protein extraction. Protein concentration was measured using a BCA kit (Beyotime). Protein samples were added into reducing SDS-PAGE loading buffer and boiled for 3 min. The proteins were electrophoresed at 80 V for 30 min. After bromophenol blue entered the separation gel, the electrophoresis was shifted to 120 V and maintained for 1 ~ 2 h. The proteins were transferred to a membrane in an ice bath. The protein transfer was carried out for 60 min with a current of 300 mA. The membrane was immersed in blocking buffer at room temperature for 60 min or at 4°C overnight. Primary antibodies against GAPDH (ab8245, 1:5000, Abcam, Cambridge, MA, USA) and FVIII (ab171825, 1:1000, Abcam) were incubated with the membrane on a shaker for 1 h at room temperature, followed by incubation of secondary antibody for 1 h at room temperature. The membrane was added with a color-developing solution and subjected to protein expression analysis. Recombinant FVIII protein antibody (ab158403, Abcam) was used as a positive control to detect FVIII protein. The bands were quantified by Image J software (version 1.46, National Institutes of Health).
Statistical analysis
GraphPad Prism 7 was used for statistical analysis. All data were expressed as mean ± standard deviation. T-test and One-way analysis of variance were used for two-group and multigroup comparisons, respectively. Tukey’s test was conducted for post hoc multiple comparisons. Each experiment was repeated three times. P < 0.05 was considered statistically significant.
Results
F8-targeting miRNAs
TargetScan, miRDB, and starBase predicted miRNAs that targeted the F8 gene. Among these F8-targeting miRNAs, hsa-miR-5581-3p was predicted by all the three databases, and 41 miRNAs were predicted by two of the three databases (Figure 1A). qRT-PCR was used to detect the expressions of these 42 miRNAs (Supplementary Table 1) in the blood of two HA patients without F8 mutations and three healthy controls. The experiment results showed higher expressions of hsa-miR-5581-3p, hsa-miR-30c-3p, hsa-miR-374b-3p, hsa-miR-542-3p and hsa-miR-6803-3p in HA patients than in healthy controls (Figure 1B, P < 0.05). Since hsa-miR-5581-3p and hsa-miR-542-3p have not been reported in previous studies of hemophilia without F8 mutations, these two miRNAs were selected for further investigations in our study.
Figure 1.
F8-targeting miRNAs. Note: (A) TargetScan, miRDB and starBase predicted miRNAs that targeted the F8 gene; (B) qRT-PCR was used to detect the expressions of F8-targeting miRNAs in HA patients and healthy controls. The data are presented as mean ± standard deviation. Each experiment was repeated three times. * P < 0.05, ** P < 0.01; HA, hemophilia A.
F8 is a target gene of hsa-miR-5581-3p and hsa-miR-542-3p
The binding sites between F8 and hsa-miR-5581-3p/hsa-miR-542-3p and the designed mutations are presented in Figure 2A. A dual-luciferase reporter assay was performed to verify the binding between F8 and hsa-miR-5581-3p/hsa-miR-542-3p. HEK293T cells were transfected with hsa-miR-5581-3p mimic, hsa-miR-542-3p mimic or mimic NC together with luciferase reporter plasmids inserted with wt-F8 or mut-F8. Transfection of wt-F8 + hsa-miR-5581-3p mimic or hsa-miR-542-3p mimic reduced the luciferase activity in the HEK293T cells (Figure 2B–C, P < 0.05), while the luciferase activity in HEK293T cells transfected with mut-F8 + hsa-miR-5581-3p/hsa-miR-542-3p mimic remained unchanged. These results validated the prediction that F8 was targeted by hsa-miR-5581-3p and hsa-miR-542-3p.
Figure 2.
F8 is a target gene of hsa-miR-5581-3p and hsa-miR-542-3p. Note: (A) The binding sites between F8 and hsa-miR-5581-3p/hsa-miR-542-3p and the designed mutations; dual-luciferase reporter assay was performed to verify the binding between F8 and hsa-miR-5581-3p (B) or hsa-miR-542-3p (C) in HEK293T cells. The data are presented as mean ± standard deviation. Each experiment was repeated three times. * P < 0.05, ** P < 0.01.
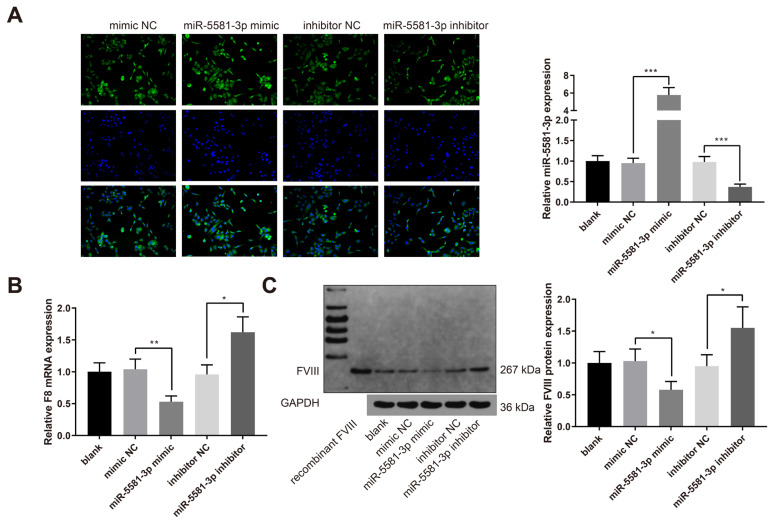
Hsa-miR-5581-3p suppresses the expressions of F8 gene and FVIII protein
HUVECs exhibiting endogenous expression of FVIII were transfected with mimic NC, hsa-miR-5581-3p mimic, inhibitor NC or hsa-miR-5581-3p inhibitor. The results of qRT-PCR experiments showed that hsa-miR-5581-3p was upregulated in the miR-5581-3p mimic group while downregulated in the miR-5581-3p inhibitor group (Figure 3A, P < 0.001, vs. the mimic NC or inhibitor NC group). The expression of F8 mRNA was decreased in the miR-5581-3p mimic group while increased in the miR-5581-3p inhibitor group (Figure 3B, P < 0.05, vs. the mimic NC or inhibitor NC group). Western blot analysis showed that the expression of FVIII protein was also decreased in the miR-5581-3p mimic group while increased in the miR-5581-3p inhibitor group (Figure 3C, P < 0.05, vs. the mimic NC or inhibitor NC group). Taken together, hsa-miR-5581-3p suppresses the expressions of F8 gene and FVIII protein in HUVECs.
Figure 3.
Hsa-miR-5581-3p suppresses the expressions of F8 gene and FVIII protein in HUVECs. Note: HUVECs were transfected with mimic NC, hsa-miR-5581-3p mimic, inhibitor NC or hsa-miR-5581-3p inhibitor. qRT-PCR was used to detect the expressions of miR-5581-3p (A) and F8 mRNA (B); (C) Western blotting was conducted to measure the expression of FVIII protein. The data are presented as mean ± standard deviation. Each experiment was repeated three times. * P < 0.05, ** P < 0.01, *** P < 0.001; HUVEC, human umbilical vein endothelial cells.
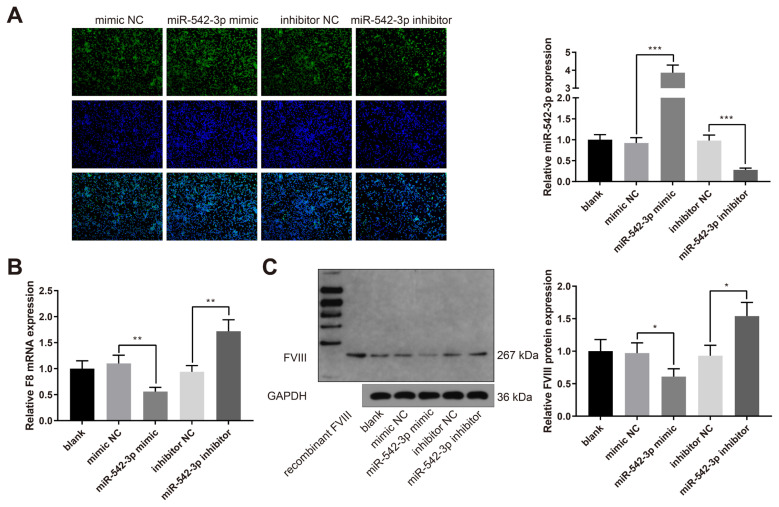
Hsa-miR-542-3p inhibits the expressions of F8 gene and FVIII protein
LCL cells were transfected with mimic NC, hsa-miR-542-3p mimic, inhibitor NC, or hsa-miR-542-3p inhibitor. The qRT-PCR experiments indicated that hsa-miR-542-3p was upregulated in the miR-542-3p mimic group while downregulated in the miR-542-3p inhibitor group (Figure 4A, P < 0.001, vs. the mimic NC or inhibitor NC group). The expression of F8 mRNA was decreased in the miR-542-3p mimic group while increased in the miR-542-3p inhibitor group (Figure 4B, P < 0.01, vs. the mimic NC or inhibitor NC group). Western blot analysis showed that the expression of FVIII protein was also decreased in the miR-542-3p mimic group while increased in the miR-542-3p inhibitor group (Figure 4C, P < 0.05, vs. the mimic NC or inhibitor NC group). Taken together, hsa-miR-542-3p suppresses the expressions of F8 gene and FVIII protein in LCL cells.
Figure 4.
Hsa-miR-542-3p inhibits the expressions of F8 gene and FVIII protein in LCL cells. Note: LCL cells were transfected with mimic NC, hsa-miR-542-3p mimic, inhibitor NC or hsa-miR-542-3p inhibitor. qRT-PCR was used to detect the expressions of miR-542-3p (A) and F8 mRNA (B); (C) Western blotting was conducted to measure the expression of FVIII protein. The data are presented as mean ± standard deviation. Each experiment was repeated three times. * P < 0.05, ** P < 0.01, *** P < 0.001; LCL, lymphoblastoid cell line.
Discussion
Numerous causative DNA variants of the F8 and F9 genes are reported to impact bleeding severity and inhibitor development in hemophilia patients.14 However, no causal mutations in the F8 gene are detected in a small group of HA patients. Mutations in the 3′UTR of F8 affect the splicing and expression of mRNA, which leads to reduced FVIII level and a mild HA.15 MiRNAs inhibit their targets through base-pairing interactions with the 3′UTR of target mRNAs, resulting in translation inhibition or mRNA degradation.16 In this study, hsa-miR-5581-3p and hsa-miR-542-3p were found to interact with the F8 gene and regulate the expression of FVIII.
A total of three databases, including TargetScan, miRDB, and starBase, were utilized to predict miRNAs that target the 3′UTR of F8. At least two databases predicted 42 miRNAs. Subsequently, their expressions in the blood of HA patients with normal F8 genotypes were quantified. Hsa-miR-5581-3p, hsa-miR-30c-3p, hsa-miR-374b-3p, hsa-miR-542-3p, and hsa-miR-6803-3p were highly expressed in HA patients. Hsa-miR-5581-3p and hsa-miR-542-3p were selected for further investigations since they were not reported in previous studies of HA. In addition, luciferase reporter plasmids were used to validate the binding sites between hsa-miR-5581-3p/hsa-miR-542-3p and F8. The experiment results suggest that F8 is a target of hsa-miR-5581-3p and hsa-miR-542-3p. Furthermore, the function of these two miRNAs was investigated in HUVECs and LCL that displayed endogenous expression of FVIII.
Hsa-miR-542-3p suppressed the expressions of F8 mRNA and FVIII in LCL cells. MiR-542-3p is a versatile ncRNA that has been largely investigated. MiR-542-3p acted as a tumor suppressor in many cancers such as colorectal cancer,17 epithelial ovarian cancer,18 hepatocellular carcinoma,19 and breast cancer.20 MiR-542-3p could also reduce chemoresistance in tumor cells and improve the antitumor efficacy of chemotherapy.21 MiR-542-3p was upregulated in activated hepatic stellate cells and promoted fibrosis via inhibiting BMP-7.22 In kidney, it contributed to fibrosis by targeting AGO1.23 Through inhibition of BMP-7, miR-542-3p also suppressed the osteogenic differentiation of vascular smooth muscle cells in aging rats.24 A study by Zhang et al. demonstrated that miR-542-3p facilitated bone formation by promoting SFRP1-mediated osteogenic differentiation in mesenchymal stem cells.25 This study firstly proved the involvement of hsa-miR-542-3p in HA without F8 mutations.
Overexpression of the-miR-5581-3p decreased the expressions of F8 mRNA and FVIII in HUVECs, whereas its inhibition increased their expressions. MiR-5581-3p was the only one F8-targeting miRNA predicted by all the three databases; however, it has been seldom reported for its function. Available data showed that overexpression of miR-5581-3p promoted cellular activities in hepatocellular carcinoma by inhibiting cardiolipin synthase 1.26 This study supplemented the knowledge regarding the physiological function of hsa-miR-5581-3p.
Conclusions
Hsa-miR-542-3p and hsa-miR-5581-3p promote HA by targeting F8 mRNA and inhibiting the expression of FVIII. Not all HA cases are caused by mutations in the F8 gene, and the pathogenic mechanisms for atypical HA are worthy of investigation. Emerging evidence has shown that miRNAs can target the 3′UTR of F8 gene and therefore mediate the expression of FVIII. This study first identifies the involvement of two novel targets—hsa-miR-542-3p and hsa-miR-5581-3p in the regulation of FVIII protein, and provides a theoretical basis for targeting miRNAs in the treatment of HA without F8 mutations.
Supplementary Information
Supplementary table 1.
The 42 F8-targeting miRNAs predicted by at least two of the bioinformatics tools.
| miR-1183 |
| miR-1244 |
| miR-1270 |
| miR-1286 |
| miR-1305 |
| miR-30a-3p |
| miR-30c-3p |
| miR-30e-3p |
| miR-3151-3p |
| miR-3188 |
| miR-3202 |
| miR-33a-5p |
| miR-33b-5p |
| miR-3682-3p |
| miR-374b-3p |
| miR-4264 |
| miR-4288 |
| miR-432-3p |
| miR-4510 |
| miR-4522 |
| miR-4533 |
| miR-4749-3p |
| miR-4768 |
| miR-4772-5p |
| miR-4774-3p |
| miR-4803 |
| miR-511-3p |
| miR-532-5p |
| miR-542-3p |
| miR-5571-5p |
| miR-5581-3p |
| miR-5680 |
| miR-5695 |
| miR-585-5p |
| miR-6127 |
| miR-6129 |
| miR-6130 |
| miR-6133 |
| miR-620 |
| miR-660-3p |
| miR-6801-5p |
| miR-6803 |
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Iorio A, Stonebraker JS, Chambost H, Makris M, Coffin D, Herr C, et al. Establishing the Prevalence and Prevalence at Birth of Hemophilia in Males: A Meta-analytic Approach Using National Registries. Ann Intern Med. 2019;171:540–546. doi: 10.7326/M19-1208. [DOI] [PubMed] [Google Scholar]
- 2.Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187–197. doi: 10.1016/S0140-6736(15)01123-X. [DOI] [PubMed] [Google Scholar]
- 3.Miesbach W, Schwable J, Muller MM, Seifried E. Treatment Options in Hemophilia. Dtsch Arztebl Int. 2019;116:791–798. doi: 10.3238/arztebl.2019.0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2019;2019:1–8. doi: 10.1182/hematology.2019000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garagiola I, Seregni S, Mortarino M, Mancuso ME, Fasulo MR, Notarangelo LD, et al. A recurrent F8 mutation (c.6046C>T) causing hemophilia A in 8% of northern Italian patients: evidence for a founder effect. Mol Genet Genomic Med. 2016;4:152–159. doi: 10.1002/mgg3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donadon I, McVey JH, Garagiola I, Branchini A, Mortarino M, Peyvandi F, et al. Clustered F8 missense mutations cause hemophilia A by combined alteration of splicing and protein biosynthesis and activity. Haematologica. 2018;103:344–350. doi: 10.3324/haematol.2017.178327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnsen JM, Fletcher SN, Huston H, Roberge S, Martin BK, Kircher M, et al. Novel approach to genetic analysis and results in 3000 hemophilia patients enrolled in the My Life, Our Future initiative. Blood Adv. 2017;1:824–834. doi: 10.1182/bloodadvances.2016002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosset C, Vieira IA, Salzano FM, Bandinelli E. A germline variant affects putative miRNA-binding sites at the F8 3′UTR and acts as a potential haemophilia A phenotype modifier in Southern Brazilian patients. Haemophilia. 2016;22:e327–329. doi: 10.1111/hae.12953. [DOI] [PubMed] [Google Scholar]
- 11.Sarachana T, Dahiya N, Simhadri VL, Pandey GS, Saini S, Guelcher C, et al. Small ncRNA Expression-Profiling of Blood from Hemophilia A Patients Identifies miR-1246 as a Potential Regulator of Factor 8 Gene. PLoS One. 2015;10:e0132433. doi: 10.1371/journal.pone.0132433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankowska KI, McGill J, Pezeshkpoor B, Oldenburg J, Atreya CD, Sauna ZE. Clinical manifestation of hemophilia A in the absence of mutations in the F8 gene that encodes FVIII: role of microRNAs. Transfusion. 2020;60:401–413. doi: 10.1111/trf.15605. [DOI] [PubMed] [Google Scholar]
- 13.Jankowska KI, Chattopadhyay M, Sauna ZE, Atreya CD. A Foundational Study for Normal F8-Containing Mouse Models for the miRNA Regulation of Hemophilia A: Identification and Analysis of Mouse miRNAs that Downregulate the Murine F8. Gene Int J Mol Sci. 2020:21. doi: 10.3390/ijms21165621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konkle BA, Johnsen JM, Wheeler M, Watson C, Skinner M, Pierce GF, et al. Genotypes, phenotypes and whole genome sequence: Approaches from the My Life Our Future haemophilia project. Haemophilia. 2018;24(Suppl 6):87–94. doi: 10.1111/hae.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pezeshkpoor B, Berkemeier AC, Czogalla KJ, Oldenburg J, El-Maarri O. Evidence of pathogenicity of a mutation in 3′ untranslated region causing mild haemophilia A. Haemophilia. 2016;22:598–603. doi: 10.1111/hae.12923. [DOI] [PubMed] [Google Scholar]
- 16.Simonson B, Das S. MicroRNA Therapeutics: the Next Magic Bullet? Mini Rev Med Chem. 2015;15:467–474. doi: 10.2174/1389557515666150324123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan L, Yuan P, Yuan H, Wang Z, Run Z, Chen G, et al. miR-542-3p inhibits colorectal cancer cell proliferation, migration and invasion by targeting OTUB1. Am J Cancer Res. 2017;7:159–172. [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Shao W, Feng H. MiR-542-3p, a microRNA targeting CDK14, suppresses cell proliferation, invasiveness, and tumorigenesis of epithelial ovarian cancer. Biomed Pharmacother. 2019;110:850–856. doi: 10.1016/j.biopha.2018.11.104. [DOI] [PubMed] [Google Scholar]
- 19.Tao J, Liu Z, Wang Y, Wang L, Yao B, Li Q, et al. MiR-542-3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting UBE3C. Biomed Pharmacother. 2017;93:420–428. doi: 10.1016/j.biopha.2017.06.070. [DOI] [PubMed] [Google Scholar]
- 20.Wu HX, Wang GM, Lu X, Zhang L. miR-542-3p targets sphingosine-1-phosphate receptor 1 and regulates cell proliferation and invasion of breast cancer cells. Eur Rev Med Pharmacol Sci. 2017;21:108–114. [PubMed] [Google Scholar]
- 21.Lyu H, Wang S, Huang J, Wang B, He Z, Liu B. Survivin-targeting miR-542-3p overcomes HER3 signaling-induced chemoresistance and enhances the antitumor activity of paclitaxel against HER2-overexpressing breast cancer. Cancer Lett. 2018;420:97–108. doi: 10.1016/j.canlet.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji F, Wang K, Zhang Y, Mao XL, Huang Q, Wang J, et al. MiR-542-3p controls hepatic stellate cell activation and fibrosis via targeting BMP-7. J Cell Biochem. 2019;120:4573–4581. doi: 10.1002/jcb.27746. [DOI] [PubMed] [Google Scholar]
- 23.Tao L, Liu X, Da W, Tao Z, Zhu Y. Pycnogenol achieves neuroprotective effects in rats with spinal cord injury by stabilizing the mitochondrial membrane potential. Neurol Res. 2020;42:597–604. doi: 10.1080/01616412.2020.1773610. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Wang H, Yang S, Qian D. Downregulation of miR-542-3p promotes osteogenic transition of vascular smooth muscle cells in the aging rat by targeting BMP7. Hum Genomics. 2019;13:67. doi: 10.1186/s40246-019-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zhu Y, Zhang C, Liu J, Sun T, Li D, et al. miR-542-3p prevents ovariectomy-induced osteoporosis in rats via targeting SFRP1. J Cell Physiol. 2018;233:6798–6806. doi: 10.1002/jcp.26430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin J, Liu Q, Chen C, Liu W. Small regulatory polypeptide of amino acid response negatively relates to poor prognosis and controls hepatocellular carcinoma progression via regulating microRNA-5581-3p/human cardiolipin synthase 1. J Cell Physiol. 2019;234:17589–17599. doi: 10.1002/jcp.28383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1.
The 42 F8-targeting miRNAs predicted by at least two of the bioinformatics tools.
| miR-1183 |
| miR-1244 |
| miR-1270 |
| miR-1286 |
| miR-1305 |
| miR-30a-3p |
| miR-30c-3p |
| miR-30e-3p |
| miR-3151-3p |
| miR-3188 |
| miR-3202 |
| miR-33a-5p |
| miR-33b-5p |
| miR-3682-3p |
| miR-374b-3p |
| miR-4264 |
| miR-4288 |
| miR-432-3p |
| miR-4510 |
| miR-4522 |
| miR-4533 |
| miR-4749-3p |
| miR-4768 |
| miR-4772-5p |
| miR-4774-3p |
| miR-4803 |
| miR-511-3p |
| miR-532-5p |
| miR-542-3p |
| miR-5571-5p |
| miR-5581-3p |
| miR-5680 |
| miR-5695 |
| miR-585-5p |
| miR-6127 |
| miR-6129 |
| miR-6130 |
| miR-6133 |
| miR-620 |
| miR-660-3p |
| miR-6801-5p |
| miR-6803 |