PURPOSE
To describe long-term outcomes of anti-CD19 chimeric antigen receptor T (CART) cells in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
METHODS
Between January 2013 and June 2016, 42 patients with relapsed or refractory CLL were enrolled in this study and 38 were infused with anti-CD19 CART cells (CART-19). Of these, 28 patients were initially randomly assigned to receive a low (5 × 107) or high (5 × 108) dose of CART-19, and 24 were evaluable for response assessment. After an interim analysis, 10 additional patients received the selected (high) dose and of these, eight were evaluable for response. Patients were followed for a median 31.5 months (range, 2 to 75 months).
RESULTS
At 4 weeks, the complete and overall responses for the 32 evaluable patients were 28% (90% CI, 16% to 44%) and 44% (90% CI, 29% to 60%), respectively. The median overall survival (OS) for all patients was 64 months; there was no statistically significant difference between low- and high-dose groups (P = .84). Regardless of dose, prolonged survival was observed in patients who achieved a CR versus those who did not (P = .035), with median OS not reached in patients with CR versus 64 months in those without CR. The median progression-free survival was 40.2 months in patients with CR and 1 month in those without a CR (P < .0001). Toxicity was comparable in both dose groups.
CONCLUSION
In patients with advanced CLL, a 5 × 108 dose of CART-19 may be more effective than 5 × 107 CART-19 at inducing CR without excessive toxicity. Attainment of a CR after CART-19 infusion, regardless of cell dose, is associated with longer OS and progression-free survival in patients with relapsed CLL.
INTRODUCTION
Despite tremendous progress in development and availability of novel agents for treatment, chronic lymphocytic leukemia (CLL) remains largely incurable and patients with relapsed or refractory (R/R) CLL have a poor prognosis.1 Chimeric antigen receptor (CAR) modified T cells targeting CD19 (CART-19) have shown dramatic activity in some B-cell malignancies, and two agents are now approved by the US Food and Drug Administration for the treatment of patients with acute lymphocytic leukemia up to age 25 years and for adults with advanced non-Hodgkin lymphoma.1-6
Chimeric antigen receptor T (CART) cells have shown dramatic activity in a small number of patients with R/R CLL, with several patients still in remission > 8 years after infusion.7,8 In a prior study, we treated 14 patients who had R/R CLL with a median of 1.6 × 108 (range, 0.14 to 11 × 108) genetically modified cells and observed an overall response rate (ORR) of 57%, including four complete responses (CRs) and four partial responses (PRs).7 In that small cohort, there was no obvious relationship between dose and response or toxicity. To determine an optimal cell dose for future studies, we performed a prospective, randomized, phase II study of two doses of CART-19 in patients with R/R CLL. In the dose-finding stage, we randomly assigned patients to 5 × 107 (low dose [LD]) or 5 × 108 (high dose [HD]) CART-19. After an interim analysis, we tested the preferred dose in 10 more patients.
MATERIALS AND METHODS
Patients
Adults aged 18 years or older with CLL with relapsed or persistent disease after at least two prior treatment regimens were eligible for entry in this study. Patients with p53-related aberrations were eligible if their disease did not achieve complete remission to initial therapy or progressed within 2 years of one prior regimen (Data Supplement).
Study Design and Treatment
Dose.
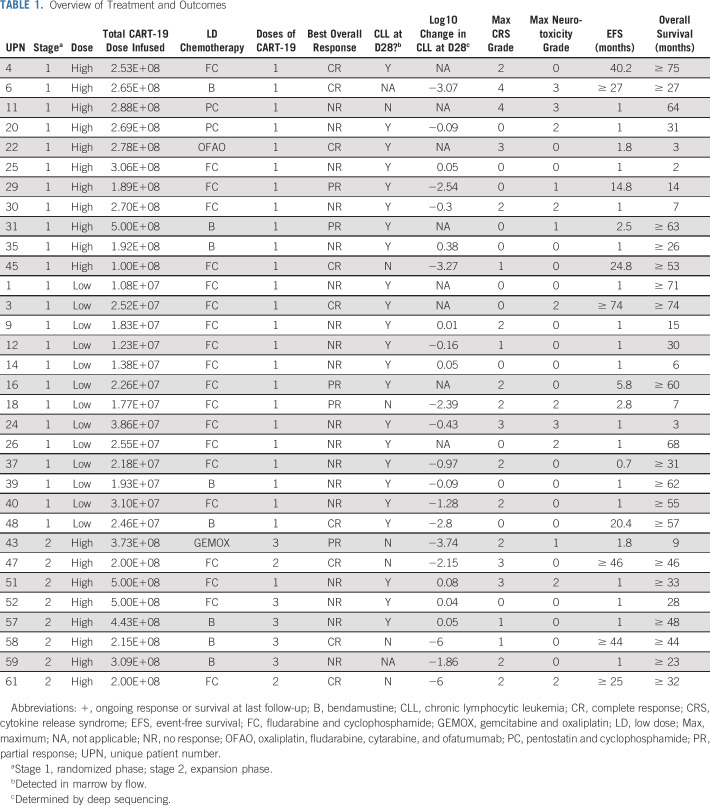
This study was designed to arrive at an optimal dose of CART-19, defined as inducing ≥ 30% CR at 3 months. Patients were enrolled in a dose-finding stage (stage 1) or an expansion stage (stage 2) of the study. In stage 1, patients were randomly assigned to receive CART-19 at either a LD (5 × 107; minimum of 1 × 107) or HD (5 × 108; minimum of 1 × 108); both doses had previously induced CRs in R/R CLL.7 At the end of stage 1 in November 2014, the HD group was chosen for expansion in stage 2 because of a higher CR rate and manageable toxicity profile. At the beginning of stage 2, investigational new drug–compliant manufacturing modifications were instituted. The first patient treated in stage 2 experienced early cytokine release syndrome (CRS) within 12 hours of receiving T cells, and received tocilizumab and steroids within 24 hours of infusion. On the basis of this experience and to improve patient safety, doses of CART-19 in all subsequent patients were administered via split dosing: 10% on day 1, 30% on day 2, and 60% on day 3. A total of eight patients were infused with this fractionated dosing schedule. This design allowed withholding of subsequent doses after early signs of CRS (Table 1).6
TABLE 1.
Overview of Treatment and Outcomes
Lymphodepleting chemotherapy.
Lymphodepleting chemotherapy was recommended but not mandated and included standard doses of commonly accepted regimens for CLL individualized on the basis of prior treatment history (Table 1).
Study end points.
The primary objective was to estimate the efficacy of each CART-19 dose level and determine a preferred cell dose for further study. The primary end point was defined as the proportion of patients with CR by the end of 3 months. Secondary end points included ORR, progression-free survival (PFS), overall survival (OS), toxicity including CRS, manufacturing feasibility, and correlative studies including in vivo T-cell function and persistence. Follow-up is as of July 1, 2019.
Response assessment.
Response was determined on the basis of International Workshop Group CLL response criteria, modified such that patients achieving a CR with incomplete count recovery were categorized as CR.9 Response assessments incorporated independent radiology review.
Study Oversight
The protocol was approved by the Institutional Review Board of the University of Pennsylvania and conducted under a Food and Drug Administration–accepted Investigational New Drug Application. Informed consent was in accordance with the Declaration of Helsinki. This study was registered at ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01747486). Research involving recombinant DNA was conducted under Biosafety Level 2 containment and was approved by the Institutional Biosafety Committee at the University of Pennsylvania, in accordance with the National Institutes of Health Guidelines for Research Involving Recombinant DNA Molecules.
T-Cell Collection, CART-19 Manufacturing, and Product Characteristics
Autologous T cells were collected by leukapheresis with CART cell manufacturing as previously described in stage 1.7 In stage 2, as part of process improvement, manufacturing was changed to incorporate depletion of CD25-expressing cells followed by stimulation and transduction of T cells in media supplemented with IL-7 and IL-15. Clinical-grade CD19 TCR-ζ/4-1BB lentiviral vector was manufactured at the Children’s Hospital of Philadelphia Clinical Vector Core.
Toxicity Grading
Adverse events were graded according to Common Toxicity Criteria, version 4.03, with the exception of CRS, which was assessed according to the Penn grading scale and reassessed using the recently published American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading scale.10-12
Correlative and Exploratory Studies
Sample processing, flow cytometry, cytokine and cytokine receptor analysis, and quantitative polymerase chain reaction (PCR) analyses were performed as previously described.10 For each patient, minimal residual disease was assessed using next-generation sequencing. For each specimen, the total and unique numbers of productive reads were determined, and the frequency of the leukemic clone in each specimen was calculated as previously described.7
Statistical Analyses
The evaluable population was defined as all patients eligible to receive T-cell infusion(s) at the intended dose level and who completed at least 3 months of follow-up after the infusion or who discontinued early due to disease progression, initiation of a new cancer therapy, or death. Patients receiving a lower-than-planned dose level due to manufacturing limitations were not considered evaluable. Evaluable patients receiving a HD from stage 1 and stage 2 were analyzed together.
Descriptive statistics were computed and presented as mean ± standard deviation or median (minimum to maximum range) for continuous variables and number (%) for categorical variables. The ORR was computed as the proportion of patients with CR including incomplete hematologic recovery or partial response during study period, along with exact 90% CIs. Kaplan-Meier curves and median survival times were estimated for OS and progression-free survival (PFS). OS was defined as time from first infusion to death, censored at date of last follow-up. PFS was defined as time from first infusion to date of first documented lack of response, disease progression, or death. Patients without disease progression were censored at date of last follow-up.
T cells expressing the anti-CD19 CAR, measured by flow cytometry, and number of gene-modified T cells identified by quantitative PCR were plotted over time to determine expansion and persistence. Detection of CART-19 above the lower limit of detection (0.1% for percent CD3 CAR+, or 25 copies/µg DNA for quantitative PCR) was used to define persistence. Associations between the levels of expansion and CRS grade (0 to 1 v 2 to 4), best overall response (CR, PR, or no response) were examined by nonparametric Wilcoxon rank-sum, Mann-Whitney, or Kruskal Wallis tests for unpaired data, because of nonnormality of the data.
A two-sided P value < .10 was considered statistically significant. No adjustment for multiple testing was done, because of the preliminary nature of the analyses. Analysis was performed using R, version 3.1.0 1 (R Development Core Team, Vienna, Austria); STATA, version 15.0 (Stata Corp., College Station, TX); or GraphPad Prism, version 7.0 (San Diego, CA).
RESULTS
Patient Characteristics
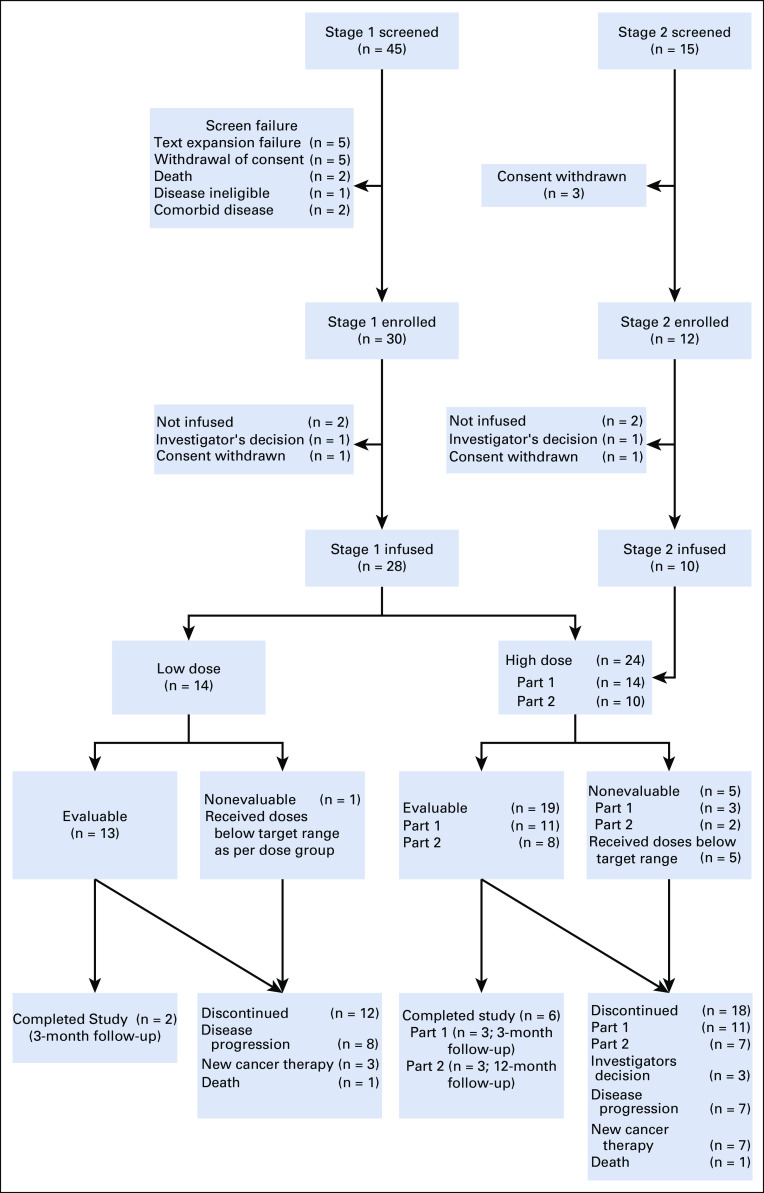
In stage 1, 45 patients were screened, 30 enrolled and randomly assigned to treatment dose, and 28 were infused. In stage 2, 15 patients were screened, 12 enrolled, and 10 infused (Appendix Fig A1, online only). Thus, a total of 38 patients were infused with CART-19. Products for one patient randomly assigned to the LD arm did not meet the target dose; in the HD group (inclusive of stages 1 and 2), five infused patients received doses below the target dose. Therefore, these patients were not considered evaluable for response. Thus, a total of 32 patients (13 in the LD arm and 19 in the HD arm) were evaluable for response. All 38 infused patients were evaluable for toxicity.
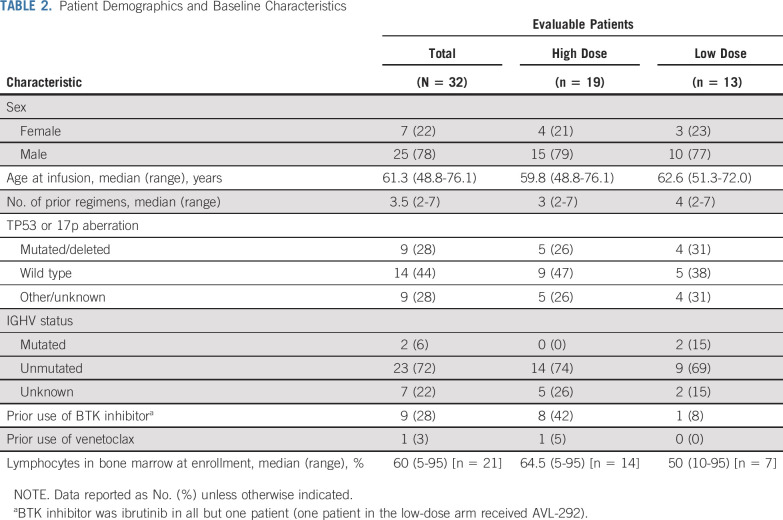
The median age for all evaluable patients was 61.6 years (range, 48.8 to 76.1 years) and patients had received a median of 3.5 (range, 2 to 7) prior therapies (Table 2). Nine patients had received BTK inhibitor and one patient had received venetoclax. A mutation in TP53 or 17p deletion was identified in nine patients (28%), and 23 (72%) had unmutated IGHV. All patients had detectable disease at the time of CART-19 infusion.
TABLE 2.
Patient Demographics and Baseline Characteristics
CART-19 Manufacturing Feasibility and Product Characteristics
Manufactured cell doses below the minimum protocol-specified dose (ie, 1 × 107 or 1 × 108) that were administered to patients were defined as manufacturing failures but could still be infused if the manufactured dose exceeded 2 × 106 CART-19 (n = 6: five in the HD group and one in the LD group). The total CART-19 dose infused in the LD arm was a median of 2.18 × 107 (range, 1.08 × 107 to 3.86 × 107) and in the HD arm was a median of 2.70 × 108 (range, 1.00 × 108 to 5.00 × 108; Table 1).
Characteristics of the apheresis and infused product were previously reported in part and are reproduced in the Data Supplement.13 The median time from apheresis to infusion was 40 days (range, 22 to 244 days) and the median time from completion of lymphodepletion to infusion was 4 days (range, 2 to 11 days).
Response
In stage 1 of the study, 30 patients were randomly assigned to CART-19 dose (n = 15 to the HD arm and 15 to the LD arm); 28 were infused and 24 received the target dose and thus were evaluable for response (Appendix Fig A1). At 3 months, there were four CRs among 11 patients (36%; 90% CI, 14% to 65%) in the HD group and two CRs among 13 patients (15%; 90% CI, 3% to 41%) in the LD group. Although CR in the HD group was not statistically significantly greater than the targeted CR rate of 30%, it was found to be safe and therefore chosen for testing in the expansion cohort (stage 2). In stage 2, 10 patients were infused and eight were evaluable for response. Therefore, a total of 19 patients received CART-19 at the HD level (11 in stage 1 and eight in stage 2).
The ORR to the HD for these 19 patients was 53% (n = 10 of 19; 90% CI, 32% to 73%) including 37% CR (n = 7) and 16% PR (n = 3). Overall response for the 24 patients in the intent-to-treat population assigned to receive the HD was 42% (n = 10), because disease did not respond in any of the five patients assigned to HD but in whom dose was not met. Overall response for the 14 patients in the intent-to-treat population assigned to receive the LD was 29% (n = 4), because disease did not respond in the one patient assigned to LD in whom dose was not met.
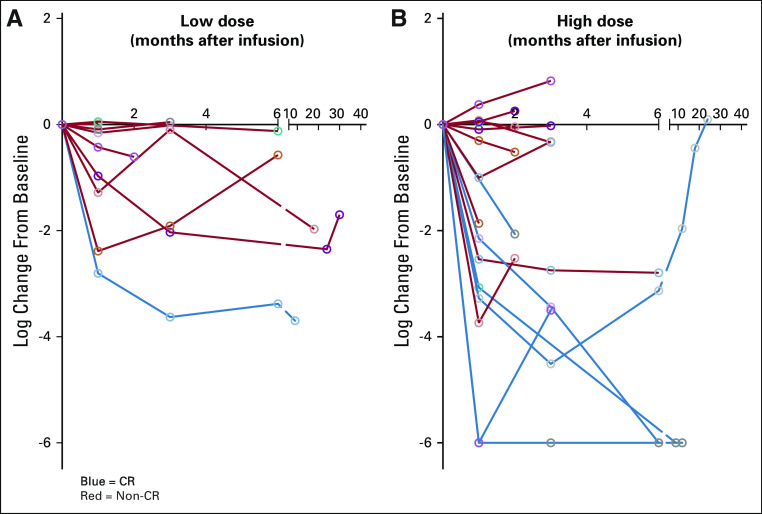
Response outcomes for the treatment cohort are summarized in Table 1. The ORR for all evaluable patients was 44% (n = 14 of 32; 90% CI, 29% to 60%), comprising 28% CR (90% CI, 16% to 44%) and 16% PR (90% CI, 6% to 30%). Depth of response is shown in Table 1 and Appendix Figure A2 (online only).
There was no significant association between response and patient age, number of prior therapies, stage at enrollment, p53-related aberrations, or IGHV mutation status (Data Supplement).
Survival and PFS
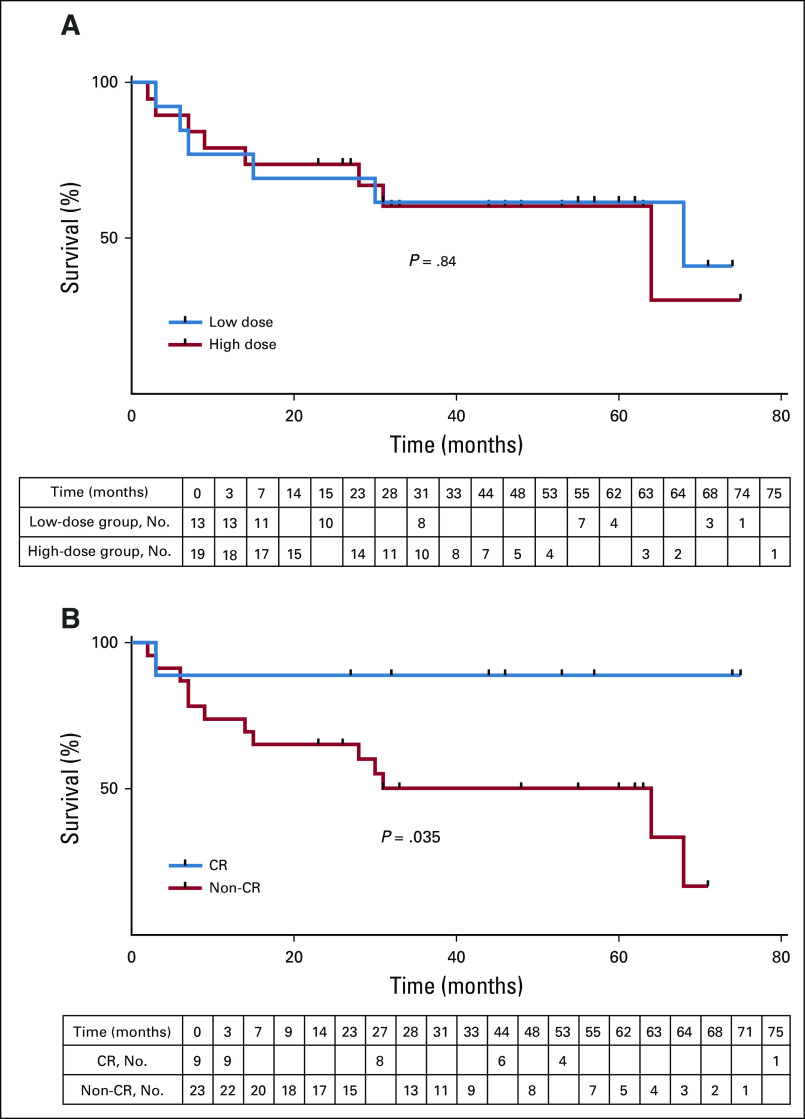
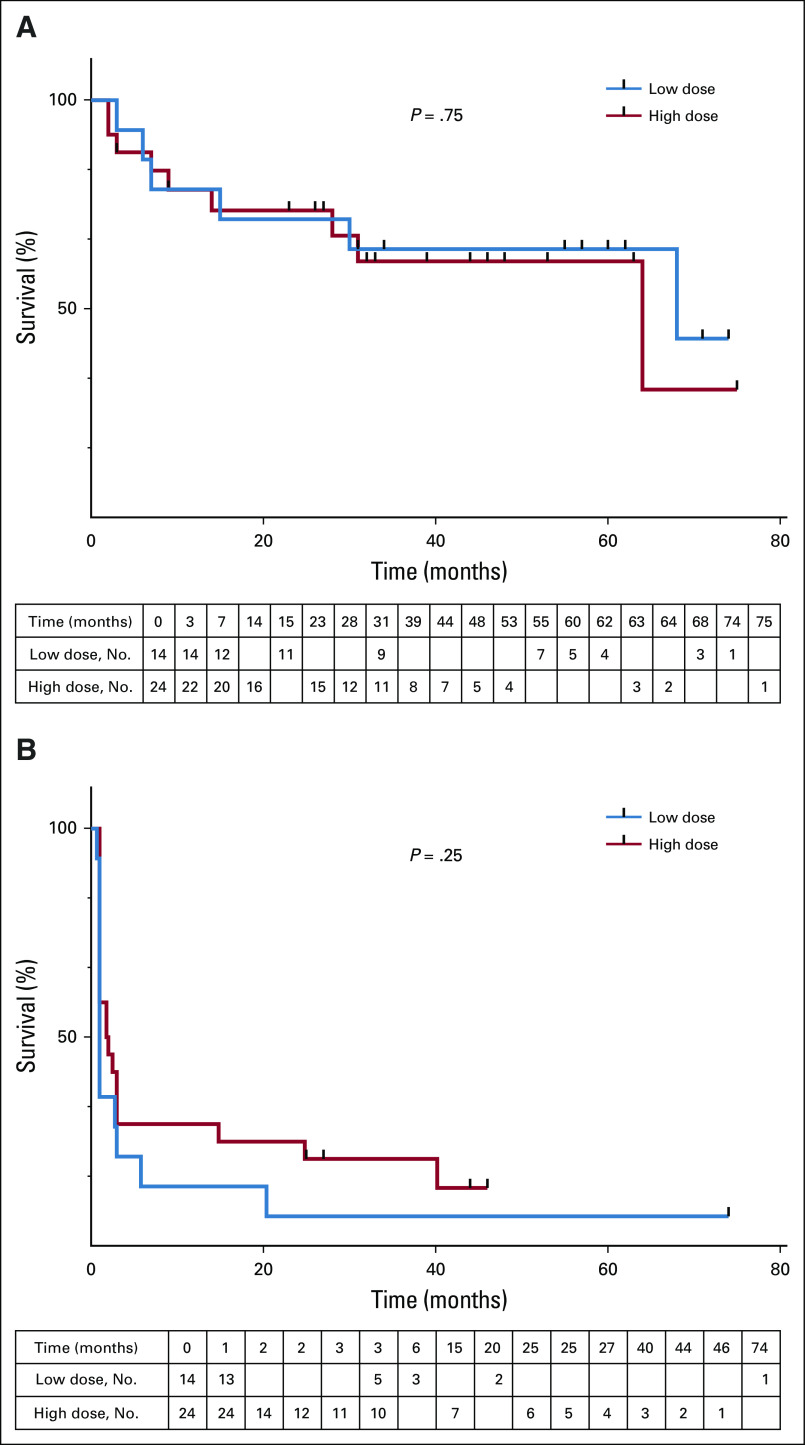
Patients were followed for a median 31.5 months (range, 2 to 75 months). The median OS and PFS for all evaluable patients were 64 months and 1 months, respectively. There was no difference in OS between groups by administered dose (median OS, 64 v 68 months; log-rank P = .84; Fig 1A) or by assigned dose (Appendix Fig A3, online only). The median OS in patients achieving CR, PR, or not responding to CART cells was not reached (14 months and 31 months, respectively). Regardless of dose, OS differed between patients achieving a CR versus those who did not (log-rank P = .035; Fig 1B). OS at 36 months for the LD and HD groups was 62% (90% CI, 36% to 79%) and 60% (38% to 77%). PFS at 36 months for the LD and HD groups was 7.7% (90% CI, 1% to 25%) versus 26% (90% CI, 12% to 44%), respectively. OS at 36 months for the patients with CR and non-CR was 89% (90% CI, 54% to 98%) versus 50% (90% CI, 32% to 66%). Type of lymphodepletion (purine analog with cyclophosphamide v other) was not associated with PFS or OS (data not shown).
FIG 1.
Overall survival. (A) Evaluable patients (n = 32) receiving a high dose (n = 24; red line) or low dose (n = 14; blue line) of CART-19 cells were followed for overall survival. Tick marks represent censored patients (log-rank test P = .84). (B) Evaluable patients who attained a complete response (CR; blue line) or less than a CR (red line) were followed for overall survival. Tick marks represent censored patients (log-rank test P = .035).
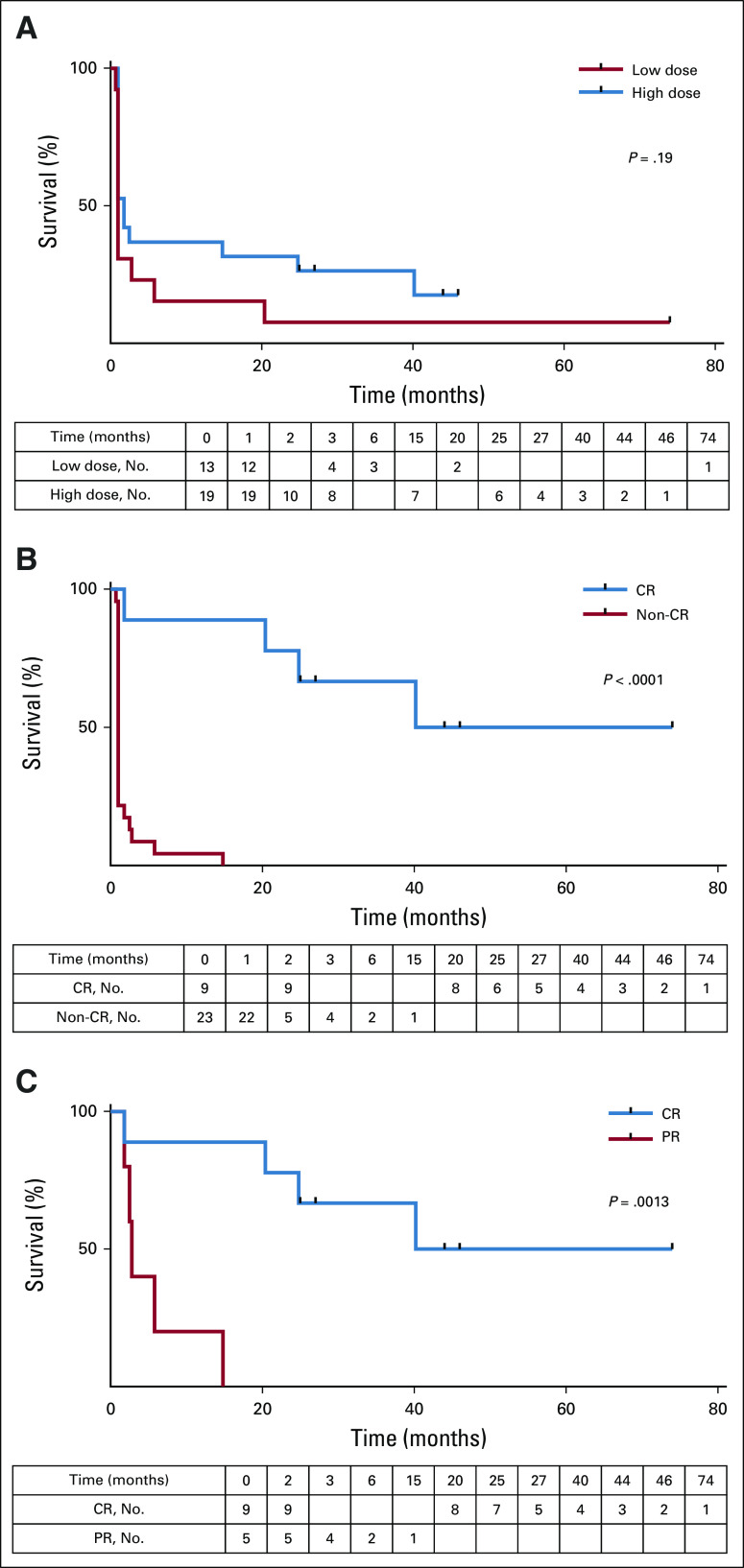
The median PFS for the HD and LD group was 1.8 and 1 month, respectively (log-rank P = .19; Fig 2A; Appendix Fig A3B). In contrast, PFS was significantly better for patients who achieved a CR (log rank P < 0.0001; Fig 2B), with a median PFS of 40.2 months and 1 month, respectively, compared with those who did not achieve a CR. The 36-month PFS was estimated at 67% for patients with CR. None of the patients without CR remained progression-free or alive beyond 36 months. Disease in four of the nine patients achieving a CR relapsed 2, 20, 25, and 40 months after CART-19 infusion. Among those patients who achieved a response, the median PFS was 57.1 months in the CR group and 2.8 months in the PR group (log-rank P = .0013; Fig 2C).
FIG 2.
Progression-free survival (PFS). (A) Patients receiving a high dose (blue line) or low dose (red line) of CART-19 cells were followed for PFS. Patients’ disease was scored as progressing if there was no response at the first evaluation time point (log-rank test, P = .19). (B) Patients whose disease attained a complete response (CR; blue line) or less than a CR (red line) were followed for PFS. Log-rank test P < .0001. (C) Patients with responding disease were divided into complete responders (blue) or partial responders (red). Log-rank test P = .0013.
At last follow-up, 18 patients remain alive (n = 7 or 13 in the LD group and n = 11 of 19 in the HD group; including eight of nine patients who achieved CR and 10 of 23 who did not).
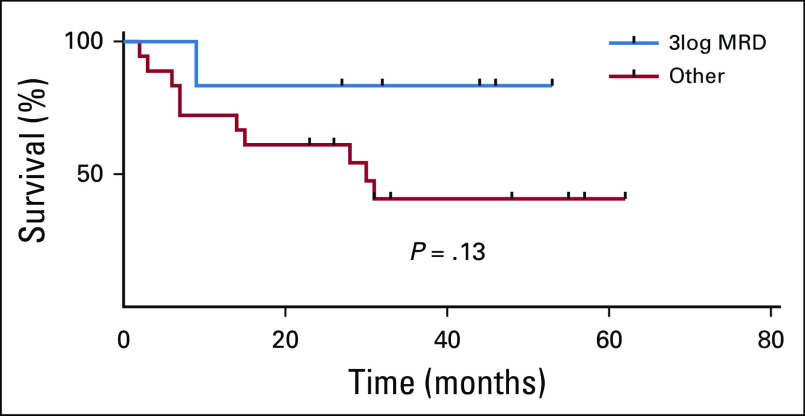
Attainment of a ≥ 3 log reduction in minimal residual disease at day 28 was not significantly associated with overall survival (P = .13; Appendix Fig A4, online only).
Safety
Toxicity after lymphodepleting chemotherapy and CART-19 infusion was similar to previously reported findings (Table 1; Data Supplement).7 The most common treatment-attributable adverse events were CRS (63%) and leukopenia (92%); 53% of patients had grade 3/4 lymphopenia. In the HD (n = 24) and LD (n = 14) groups, the median number of adverse events was 6.5 (range, 1 to 25) and 10.5 (range 2 to 29), respectively (exact Wilcoxon test P = .24). The number of grade 3 to 5 adverse events was also not significantly different between the groups (HD group: median, 2.5, range 0 to 12; v LD group 3.0, range, 0 to 11; P = .39).
There were no treatment-related deaths within the first month of infusion. One patient in the LD group died at approximately 3 months with progressive disease and one patient in the HD group died at 2 months of pneumonia and progressive disease. Of 13 patients in the LD group, four had grade 3 to 4 infections, and three had grade 1 to 2 CRS or fever. Of 19 patients in the HD group, five had grade 3 to 4 infections, one had a second malignancy, and five had grade 3 to 4 CRS.
Cytokine release syndrome. Using the Penn grading scale, of all 38 patients treated, CRS developed in 24 (63%); most were grade 1 or 2 (39%); nine patients (24%) experienced grade 3 (18%) or 4 (5%) CRS. Five patients required intervention with tocilizumab. Of the 18 evaluable patients who received the HD infusion, CRS developed in 13, including seven patients (36%) with grade 1 or 2 CRS and six patients (32%) with grade 3 to 4 CRS. A post hoc analysis was performed to determine the ASTCT consensus grading for CRS. Fifteen events changed: most (n = 14) changed from grade 2 to grade 1; one event changed from grade 3 to grade 2. By ASTCT criteria, the rate of grade 1 to 2 CRS was 48% and the rate of grade 3 to 4 CRS was 11%.
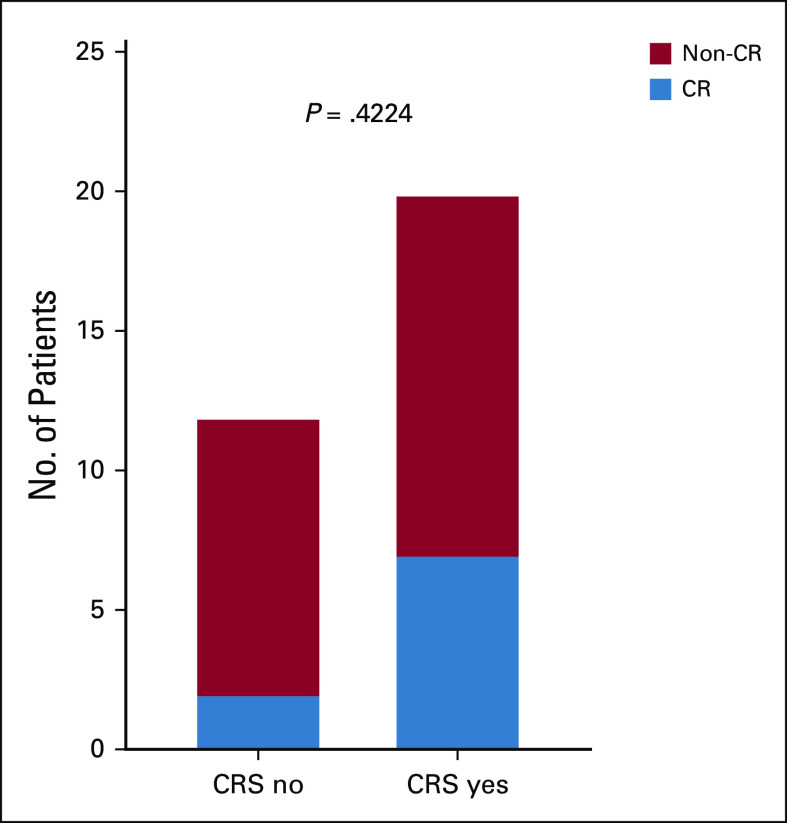
The occurrence of CRS was not significantly associated with achievement of a CR (P = .44; Fig 3). Association of CRS grade with serum cytokines in evaluable patients is shown in Appendix Figure A5 (online only).
FIG 3.
Association of cytokine release syndrome (CRS) with complete response (CR). The number of patients whose disease attained or did not attain a CR was graphed according to development of CRS (Fisher exact two-sided P = .42).
Neurologic toxicity. Grade 3 or higher treatment-attributable neurologic events occurred in three patients (one each for syncope, confusion, and delirium). Otherwise, all neurologic events were grade 1 or 2, with headaches being the most common related event (16%); seizures were not observed (Data Supplement).
CART-19 In Vivo Expansion and Persistence
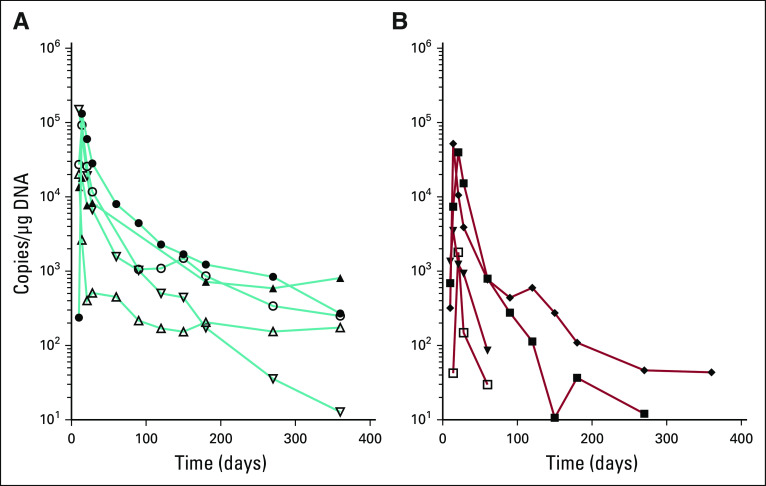
Persistence and quantitation of CART cells was performed by flow cytometry and quantitative PCR (Fig 4; Appendix A6, online only). Expansion was rapid, and low-level persistence was detected in most patients with CR to ≥ 1 year. Notably, CART-19 remained detectable by PCR in most patients at ≥ 3 months, even in patients with PR or no response. The association of peak T-cell expansion with CRS or response indicates higher peak expansion was significantly associated with more severe CRS and with depth of response (all P < .05).
FIG 4.
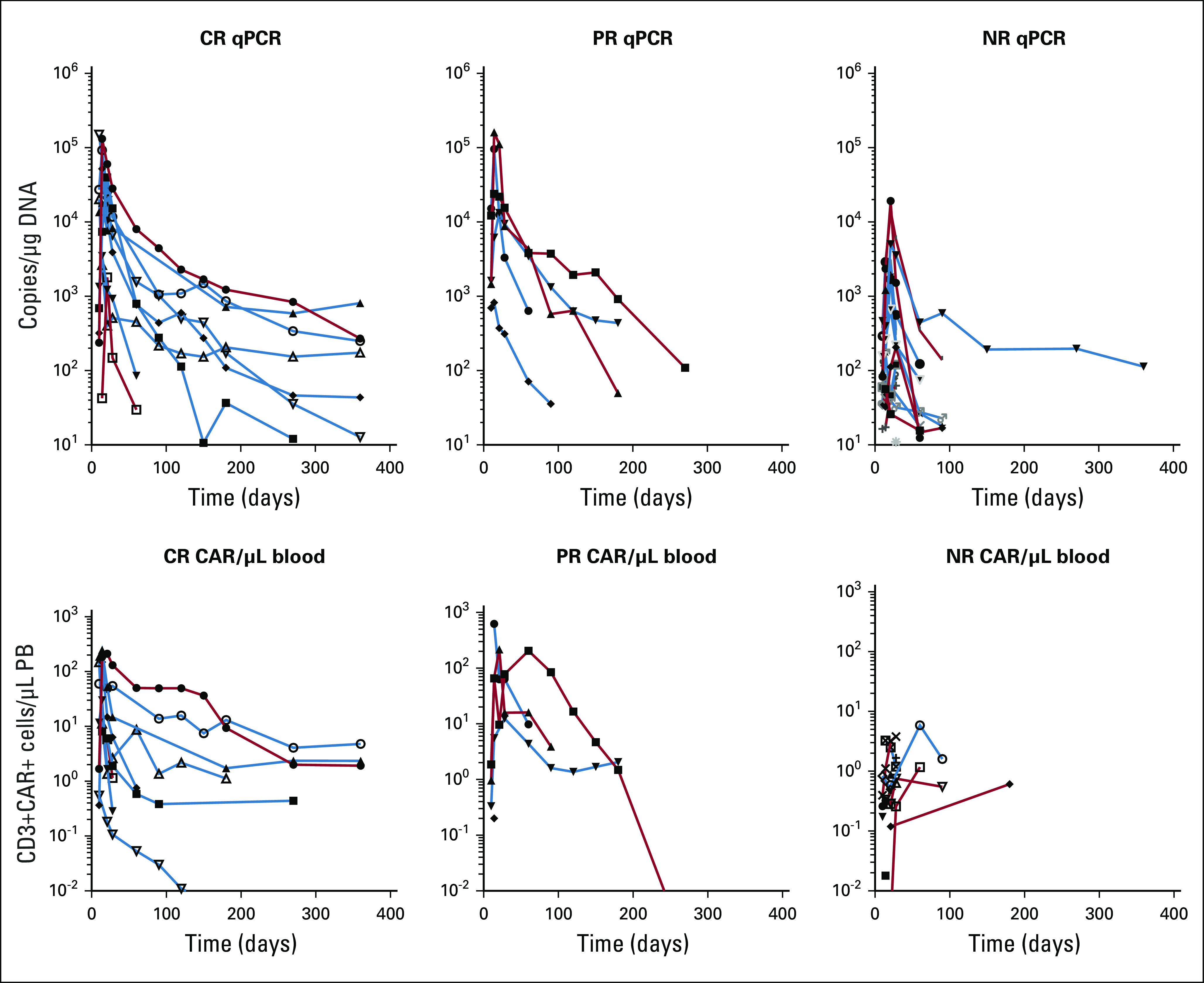
Expansion and persistence of CART-19 cells. CAR T cells were quantified by quantitative polymerase chain reaction (qPCR; top) or flow cytometry (bottom) and charted for patients whose disease achieved a complete response (CR), partial response (PR), or no response (NR). Within each panel, data of patients who received the high dose of CART-19 cells are indicated in blue, and data of those receiving the low dose of CART-19 cells are in red. PB, peripheral blood.
DISCUSSION
In a prior study, 14 patients with R/R CLL received a median dose of 1.6 × 108 CART-19 at a wide range of 0.14 to 11 × 108. The ORR was 57%, including four complete and four PRs without an obvious relationship between dose and response or toxicity.7 Turtle et al8 treated 24 patients with CLL whose disease did not respond to anti-CD20 chemoimmunotherapy as well as ibrutinib. Patients were treated with from 2 × 105 to 2 × 107 transduced CAR T cells/kg as a single dose. The authors noted an ORR of 71% with no dose association with CRS. However, severe neurotoxicity was observed, with one fatal case felt to be potentially dose related, prompting a recommended dose of 2 × 106 transduced cells/kg for that product.
To our knowledge, the study we report here represents the largest cohort of prospectively enrolled patients with CLL to receive CART cells and with the longest follow-up. We sought to determine an association of cell dose with response or toxicity in CLL. Although small numbers limit the statistical significance, the higher target dose (5 × 108 CART-19) using an adaptive split-dosing strategy was safe and possibly more effective than the lower dose.6 Using this strategy, CRS was generally moderate and manageable, and neurotoxicity was negligible.
CART cells can induce CR in 21% to 47% of patients with R/R CLL.7,8,14-16 In the current study, patients receiving what was determined to be an optimal dose of CART-19 at 5 × 108 (actual median dose, 2.70 × 108) achieved a CR rate of 37% (n = 7 of 19 patients). Achieving a CR was highly correlated with long-term PFS.
Thus, strategies to further increase the CR rate to CART cells in CLL are warranted and may be supported by optimization of dose and scheduling. Other important strategies could include prospective selection of patients who are most likely to benefit from CART cells, such as those exhibiting a CD27+PD-1−IL-6R+CD8+ T-cell phenotype, or rationally combining CART cells with small-molecule inhibitors.13,17,18 We conclude that a single course of treatment with CART-19 can induce durable remissions with an acceptable toxicity profile in some patients with advanced CLL.
ACKNOWLEDGMENT
We acknowledge Joan Gilmore, Lester Lledo, and Holly McConville for research coordination and nursing. We thank the Children’s Hospital of Philadelphia Clinical Vector Core.
Appendix
FIG A1.
CONSORT diagram of all patients screened in the trial.
FIG A2.
Minimal residual disease (MRD) kinetics were charted over time in those patients with informative data sets who received the (A) low dose or (B) high dose of CART-19 cells and attained a complete response (CR; blue) or not (red). MRD was quantified using deep sequencing of IGVH (see Methods).
FIG A3.
Graphs of (A) overall survival (OS) and (B) progression-free survival (PFS) for all patients receiving low- or high-dose CART-19 cells, based on their assigned dose.
FIG A4.
Effect of minimal residual disease (MRD) on survival. Overall survival showed a trend toward improvement in patients with a deep remission at day 28 (defined as reduction in MRD > 3log10).
FIG A5.
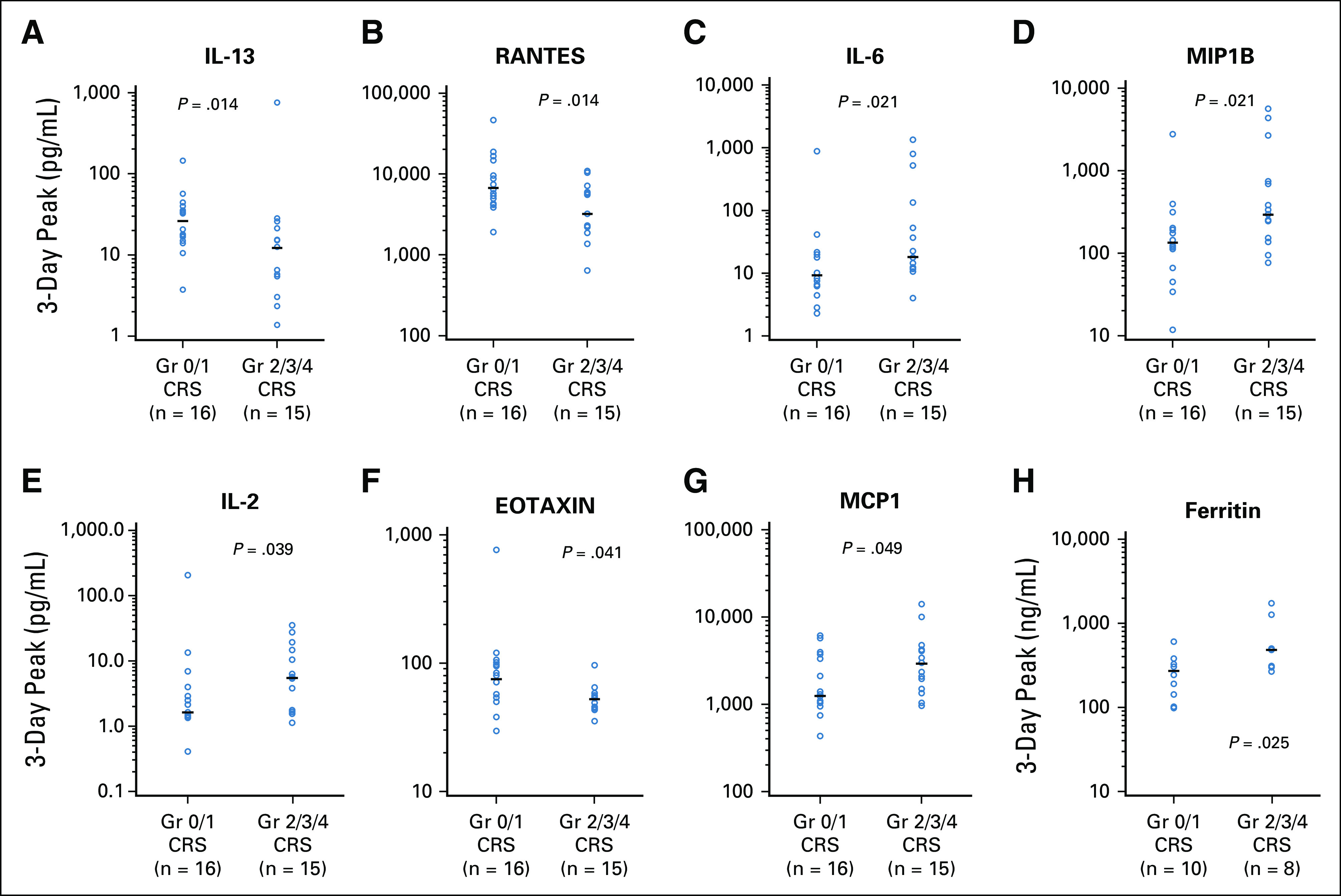
Association of selected cytokines with cytokine release syndrome (CRS) severity. Gr, grade.
FIG A6.
Durability of response by CART-19–cell expansion and persistence. (A) Patients with complete response. (B) Patients with relapsed disease after complete response.
SUPPORT
Supported by the Leukemia & Lymphoma Society and Novartis.
CLINICAL TRIAL INFORMATION
N.V.F. and S.G. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Noelle V. Frey, Saar Gill, Stephen Schuster, Bruce L. Levine, Wei-Ting Hwang, Carl H. June, David L. Porter
Financial support: Carl H. June
Administrative support: Carl H. June
Provision of study material or patients: Saar Gill, Sunita Nasta, Alison Loren, Edward Stadtmauer, Anthony Mato, Bruce L. Levine, Elizabeth Veloso
Collection and assembly of data: Noelle V. Frey, Elizabeth O. Hexner, Stephen Schuster, Sunita Nasta, Bruce L. Levine, Simon F. Lacey, Jan Joseph Melenhorst, Elizabeth Veloso, Avery Gaymon, Edward Pequignot
Data analysis and interpretation: Noelle V. Frey, Saar Gill, Elizabeth O. Hexner, Stephen Schuster, Alison Loren, Jakub Svoboda, Edward Stadtmauer, Daniel J. Landsburg, Anthony Mato, Bruce L. Levine, Elizabeth Veloso, Edward Pequignot, Xinhe Shan, Wei-Ting Hwang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Noelle V. Frey
Consulting or Advisory Role: Novartis, Kite/Gilead
Research Funding: Novartis
Saar Gill
Stock and Other Ownership Interests: Carisma Therapeutics
Honoraria: Fate Therapeutics, Sensei, Aro
Research Funding: Novartis (Inst), Carisma Therapeutics
Patents, Royalties, Other Intellectual Property: Patents for chimeric antigen receptor T cells for acute myeloid leukemia
Elizabeth O. Hexner
Consulting or Advisory Role: Blueprint Medicines, American Board of Internal Medicine Subspecialty Board
Research Funding: Blueprint Medicines (Inst), Tmunity Therapeutics (Inst)
Stephen Schuster
Honoraria: Novartis
Consulting or Advisory Role: Celgene, Nordic Nanovector, Novartis, AbbVie, Acerta Pharma/AstraZeneca, AlloGene, BeiGene, Juno Therapeutics, Loxo Oncology, Tessa Therapeutics
Research Funding: Novartis (Inst), Pharmacyclics (Inst), Adaptive Biotechnologies (Inst), Merck (Inst), Roche (Inst), Celgene (Inst), Juno Therapeutics (Inst), AbbVie (Inst), Incyte (Inst), TG Therapeutics (Inst), DTRM Biopharma (Inst)
Patents, Royalties, Other Intellectual Property: Patent Combination Therapies of CAR and PD-1 Inhibitors (via University of Pennsylvania with royalties to Novartis)
Sunita Nasta
Consulting or Advisory Role: Merck
Research Funding: Millennium, Incyte, Aileron, Debiopharm, Roche, Atara, Rafael Pharmaceuticals, Forty Seven, Pharmacyclics/Janssen
Jakub Svoboda
Consulting or Advisory Role: Seattle Genetics, Bristol-Myers Squibb, AstraZeneca, Pharmacyclics, Imbrium
Research Funding: Celgene (Inst), Seattle Genetics (Inst), Pharmacyclics (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Imbrium
Edward Stadtmauer
Consulting or Advisory Role: Celgene, Takeda, Novartis, Teva, Janssen, Amgen, Sanofi
Daniel J. Landsburg
Consulting or Advisory Role: Celgene, Curis, MorphoSys
Speakers' Bureau: Seattle Genetics
Research Funding: Takeda, Triphase Accelerator (Inst), Curis, Curis (Inst)
Anthony Mato
Consulting or Advisory Role: TG Therapeutics, AbbVie/Genentech, Loxo, Sunesis Pharmaceuticals, Janssen Oncology, Celgene, Pharmacyclics, Verastem, Prime Oncology, Adaptive, Verastem
Research Funding: Regeneron, TG Therapeutics, Sunesis, LOXO, BeiGene, Genentech, AbbVie, Pharmacyclics, Verastem, Adaptive Biotechnologies
Travel, Accommodations, Expenses: TG Therapeutics, Pfizer
Other Relationship: TG Therapeutics, Celgene
Bruce L. Levine
Stock and Other Ownership Interests: Tmunity Therapeutics
Honoraria: Novartis, Terumo, Sysmex, Draper, AstraZeneca
Consulting or Advisory Role: Brammer Bio, Avectas, CRC Oncology, Cure Genetics, Ori Biotech, Vycellix, Incysus, Immuneel, Lilly Asia Ventures
Research Funding: Novartis, Tmunity Therapeutics
Patents, Royalties, Other Intellectual Property: Intellectual property and patents in the field of cell and gene therapy
Travel, Accommodations, Expenses: GE Healthcare, BrammerBio, Avectas, CRC Oncology, Novartis, Terumo
Simon F. Lacey
Consulting or Advisory Role: Gilead Sciences
Research Funding: Novartis (Inst), Tmunity (Inst)
Patents, Royalties, Other Intellectual Property: Patents and intellectual property related to CAR-T technology licensed to Novartis (Inst)
Jan Joseph Melenhorst
Consulting or Advisory Role: Shanghai Unicar Therapy, Simcere of America, IASO Biotherapeutics, Poseida
Speakers' Bureau: Novartis, Johnson & Johnson
Research Funding: Novartis (Inst), Incyte (Inst)
Patents, Royalties, Other Intellectual Property: Patent-related royalties, milestone payments
Edward Pequignot
Patents, Royalties, Other Intellectual Property: As part of University of Pennsylvania's role in the Food and Drug Administration approval of CAR-T therapy, and for my part as an employee of University of Pennsylvania involved in their research in CAR-T, I have received royalties of approximately $300 over the past 2 years.
Wei-Ting Hwang
Research Funding: Janssen (I)
Carl H. June
Stock and Other Ownership Interests: Celldex, Tmunity Therapeutics, Cabaletta, Carisma, Cytosen
Honoraria: Novartis, Pfizer, Johnson & Johnson
Consulting or Advisory Role: Celldex, Viracta Therapeutics, Cabaletta, Carisma, Kiadis Pharma, WIRB-Copernicus Group, Janssen Oncology
Research Funding: Novartis, Tmunity Therapeutics
Patents, Royalties, Other Intellectual Property: Intellectual property rights licensed to Novartis (royalties paid to University of Pennsylvania), intellectual property and patent royalties from Office of Naval Research, Intellectual property rights licensed to Tmunity (Inst)
David L. Porter
Employment: Roche (I)
Stock and Other Ownership Interests: Roche (I)
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Kite Pharma, Incyte, Gerson Lehrman Group, Glenmark, Janssen
Research Funding: Novartis
Patents, Royalties, Other Intellectual Property: Patent/royalty rights
Travel, Accommodations, Expenses: Kite Pharma, Novartis, Janssen
Other Relationship: National Marrow Donor Program, American Board of Internal Medicine
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hallek M, Shanafelt TD, Eichhorst B: Chronic lymphocytic leukaemia. Lancet 391:1524-1537, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Carpenito C, Milone MC, Hassan R, et al. : Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA 106:3360-3365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, et al. : Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531-2544, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. doi: 10.1056/NEJMoa1804980. Bishop MR, Maziarz RT, Waller EK, et al: Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380:45-56, 2019. [DOI] [PubMed]
- 6.Frey N V, Shaw PA, Hexner EO, et al. : Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol 38:415-422, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. doi: 10.1126/scitranslmed.aac5415. Porter DL, Hwang W-T, Frey NV, et al: Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7:303ra139, 2015. [DOI] [PMC free article] [PubMed]
- 8.Turtle CJ, Hay KA, Hanafi L-A, et al. : Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 35:3010-3020, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallek M, Cheson BD, Catovsky D, et al. : Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111:5446-5456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyiadzis MM, Dhodapkar M V, Brentjens RJ, et al. : Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer 6:137, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Santomasso BD, Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625-638, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraietta JA, Lacey SF, Orlando EJ, et al. : Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 24:563-571, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiqi T, Dorritie KA, Soumerai JD, et al: TRANSCEND CLL 004: Minimal residual disease (MRD) negative responses after lisocabtagene maraleucel (Liso-Cel; JCAR017), a CD19-directed CART cell product, in patients (pts) with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL). J Clin Oncol 37:7501, 2019 (suppl 15) [Google Scholar]
- 15.Kochenderfer JN, Dudley ME, Feldman SA, et al. : B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119:2709-2720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenderfer JN, Dudley ME, Kassim SH, et al. : Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33:540-549, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraietta JA, Beckwith KA, Patel PR, et al. : Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127:1117-1127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gill SI, Vides V, Frey N, et al: Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Presented at American Society of Hematology Annual Meeting, San Diego, CA, December 1-4, 2018. [Google Scholar]