PURPOSE
Preoperative chemoradiotherapy may improve the radical resection rate for resectable or borderline resectable pancreatic cancer, but the overall benefit is unproven.
PATIENTS AND METHODS
In this randomized phase III trial in 16 centers, patients with resectable or borderline resectable pancreatic cancer were randomly assigned to receive preoperative chemoradiotherapy, which consisted of 3 courses of gemcitabine, the second combined with 15 × 2.4 Gy radiotherapy, followed by surgery and 4 courses of adjuvant gemcitabine or to immediate surgery and 6 courses of adjuvant gemcitabine. The primary end point was overall survival by intention to treat.
RESULTS
Between April 2013 and July 2017, 246 eligible patients were randomly assigned; 119 were assigned to preoperative chemoradiotherapy and 127 to immediate surgery. Median overall survival by intention to treat was 16.0 months with preoperative chemoradiotherapy and 14.3 months with immediate surgery (hazard ratio, 0.78; 95% CI, 0.58 to 1.05; P = .096). The resection rate was 61% and 72% (P = .058). The R0 resection rate was 71% (51 of 72) in patients who received preoperative chemoradiotherapy and 40% (37 of 92) in patients assigned to immediate surgery (P < .001). Preoperative chemoradiotherapy was associated with significantly better disease-free survival and locoregional failure-free interval as well as with significantly lower rates of pathologic lymph nodes, perineural invasion, and venous invasion. Survival analysis of patients who underwent tumor resection and started adjuvant chemotherapy showed improved survival with preoperative chemoradiotherapy (35.2 v 19.8 months; P = .029). The proportion of patients who suffered serious adverse events was 52% versus 41% (P = .096).
CONCLUSION
Preoperative chemoradiotherapy for resectable or borderline resectable pancreatic cancer did not show a significant overall survival benefit. Although the outcomes of the secondary end points and predefined subgroup analyses suggest an advantage of the neoadjuvant approach, additional evidence is required.
INTRODUCTION
Approximately 20% of patients with pancreatic ductal adenocarcinoma (PDAC) have resectable or borderline resectable disease. Standard treatment is resection followed by adjuvant chemotherapy.1,2 Only approximately half of the patients who undergo tumor resection actually receive adjuvant chemotherapy.3-5 Furthermore, approximately half of the resections are microscopically incomplete (R1)6,7; one quarter of patients will develop disease recurrence within 6 months.8
Preoperative (neoadjuvant) chemoradiotherapy in patients with resectable or borderline resectable PDAC has not yet been proven superior, although it is standard of care for many other cancers.9,10 Preoperative chemoradiotherapy, by inducing downstaging of the tumor, might increase the R0 resection rate. R0 resection rate is an important prognostic factor, diminishing local and distant recurrence rates, hence improving survival. In addition, compliance with preoperative chemoradiotherapy is better compared with postoperative chemotherapy. Potential disadvantages of preoperative chemoradiotherapy are more complicated surgery by radiation toxicity and fewer resections because of early tumor progression. Whether the latter is a disadvantage in the long run is not completely certain.
Most of the studies that advocate preoperative chemoradiotherapy are nonrandomized studies with selection bias by reporting survival after resection rather than by intention to treat (ITT). Interpretation and comparison of these studies are difficult, if not impossible. Surgical radicality after preoperative chemoradiotherapy has never been studied in a multicenter randomized trial. A recent meta-analysis of the effect of preoperative chemo(radio)therapy in resectable or borderline resectable PDAC showed a prolonged overall survival (OS) when compared with immediate surgery (18.8 v 14.8 months).7 This evidence, however, is weak because most studies were observational. Apart from ours, 11 trials are reported, 4 of which are phase III trials. Three trials were completed, of which 2 reported results (1 in abstract only)11,12; 1 was prematurely closed because of positive results at interim analysis,13 and 2 were prematurely closed because of poor accrual.14,15 Four are active or recruiting, and 1 is of unknown status16-20 (Data Supplement, online only). The Dutch Pancreatic Cancer Group (DPCG) initiated the PREOPANC trial with the aim to investigate whether preoperative chemoradiotherapy provides better OS than immediate surgery in patients with resectable or borderline resectable PDAC.
PATIENTS AND METHODS
Patients and Study Design
This randomized phase III study was performed in 16 high-volume pancreatic surgery centers from the DPCG. The protocol was centrally approved by the Erasmus MC ethics committee (MEC-2012-249; December 11, 2012).
Eligible patients had pathologically confirmed resectable or borderline resectable PDAC, without distant metastases (M0), according to the Union for International Cancer Control classification (TNM 7th edition).21 A multiphase computed tomography (CT) scan of the abdomen, including noncontrast enhanced, arterial, venous, and portal contrast phase axial scans, were required within 4 weeks before randomization. Tumor size, location, and relation to the celiac axis, superior mesenteric artery (SMA) and superior mesenteric vein (SMV), common hepatic artery, and portal vein were reported. A tumor without arterial involvement and with venous involvement < 90° was considered resectable; a tumor with arterial involvement < 90° and/or venous involvement between 90° and 270° without occlusion was considered borderline resectable. Other inclusion criteria were a WHO performance status of ≤ 1 and adequate hematologic, renal, and hepatic function. Exclusion criteria were cT1 tumor (< 2 cm, without vascular involvement), history of malignancy within 5 years, and previous radiotherapy or chemotherapy that precluded treatment.22 Eligible patients provided written informed consent and were randomly assigned before biliary drainage, which carried a risk of dropout but optimally reflects clinical practice, wherein immediate surgery is preferably performed before biliary drainage.23
Treatment
Patients were randomly assigned 1:1 to preoperative chemoradiotherapy or immediate surgery. Patients assigned to preoperative chemoradiotherapy underwent a staging laparoscopy to rule out occult metastases. Chemoradiotherapy was to start within 4 weeks after random assignment. Patients with jaundice underwent biliary drainage, preferably with a self-expandable metal stent; bilirubin level had to be < 1.5 times the normal limit before chemotherapy was started. Radiotherapy consisted of 15 fractions of 2.4 Gy in 3 weeks to the pancreatic tumor and suspicious lymph nodes, combined with 1,000 mg/m2 gemcitabine on days 1, 8, and 15 of 4 weeks, preceded and followed by a modified course of gemcitabine (1,000 mg/m2 gemcitabine on days 1 and 8 of 3 weeks).24-26 Within 4 weeks thereafter, CT evaluation was performed. Explorative laparotomy, with subsequent resection, if possible, was conducted between 14 and 18 weeks after random assignment. After resection and confirmation of PDAC, the remaining gemcitabine was administered at 1,000 mg/m2 on days 1, 8, and 15 in 4 courses of 4 weeks.
For patients randomly assigned to immediate surgery, preoperative biliary drainage was recommended for bilirubin levels > 250 μmol/L. Surgery was to be performed within 4 weeks after random assignment; staging laparoscopy was at the surgeon’s discretion. After resection and confirmation of PDAC, patients received 6 courses of adjuvant gemcitabine at 1,000 mg/m2 on days 1, 8, and 15 of 4 weeks.
In both groups, resection was performed according to the consensus statement of the International Study Group on Pancreatic Surgery (ISGPS).27 A classic or a pylorus-preserving pancreatoduodenectomy with locoregional lymph node dissection was performed for pancreatic head tumors. For tumors that involved the pancreatic body or tail, pancreas body and tail resection with splenectomy was performed. Reconstruction after pancreatoduodenectomy was left to the surgeon’s preference. A standardized pathology procedure, on the basis of the Leeds Pathology Protocol, was applied,28 including description of the tumor origin, extension, lymph node metastases, vascular and/or perineural invasion, and resection margins. Margins were considered microscopically positive (R1) if vital tumor was present at ≤ 1 mm from the transection margins (pancreas, bile duct, stomach, and/or duodenum) or the circumferential dissection (the anterior and posterior sides of the pancreas, the SMA, and the SMV).29
All patients underwent follow-up assessment with CT scans and serum cancer antigen 19-9 (CA19.9) at 6, 12, 18, and 24 months after random assignment and yearly thereafter. WHO performance status, weight, disease status (locoregional and distant), death, and cause of death were assessed at follow-up.
End Points
The primary end point was OS, defined as time from random assignment to death as a result of any cause. Secondary end points were disease-free survival (DFS), locoregional failure–free interval (LFFI), distant metastasis–free interval (DMFI), resection rate, R0 resection rate (per protocol), and toxicity of both surgery and pre- and postoperative treatment. In case of missing follow-up data, patients were censored when last known to be alive and disease free. Subgroup analyses were prespecified for resectable and borderline resectable PDAC separately and for patients who underwent resection and started adjuvant chemotherapy. Post hoc, a per-protocol analysis was added, including data of patients who appeared to have no distant metastases and started the intended treatment. In addition, a per-protocol analysis was added that investigated the prognostic value of R0 resection on OS. Postoperative mortality was defined as mortality as a result of any cause within 30 days after resection or during the index hospitalization if > 30 days. Postoperative complications were registered and graded according to ISGPS guidelines. Toxicity was scored according to Common Terminology Criteria for Adverse Events (version 4.0).30
Statistical Analysis
The trial was designed to have 80% power to detect a 6-month difference in median OS by ITT between both treatment groups (17 months with preoperative chemoradiotherapy and 11 months with immediate surgery). At least 176 events were required to detect this (2-sided test; α-level, 0.05; β-level, 0.20). Assuming a 10% dropout rate, 122 patients in each treatment arm were required. Primary analyses were performed by ITT, irrespective of any protocol deviations or violations. The Kaplan-Meier curves for OS, DFS, LFFI, and DMFI (including the hazard ratio [HR] and 95% CI) were compared between the 2 groups with the log-rank test (stratified for resectability [resectable v borderline resectable]). We tested differences between the resectable and borderline resectable groups with the interaction test of hazard rates. Moreover, for the per-protocol analyses and the predefined subgroup analyses, the outcomes were presented as Kaplan-Meier curves and compared with the log-rank test (stratified for resectability). The resection rate, R0 resection rate, and toxicity were quantified by proportions and odds ratios and associated 95% CIs; Fisher’s exact test was used to test for differences. All tests were 2-sided and performed at the 5% significance level. All statistical analyses were performed using version 3.5.2 of the R statistical package (R Foundation for Statistical Computing, Vienna, Austria). This trial was registered with EudraCT (2012-003181-40) and the Netherlands Trial Register (3709).
RESULTS
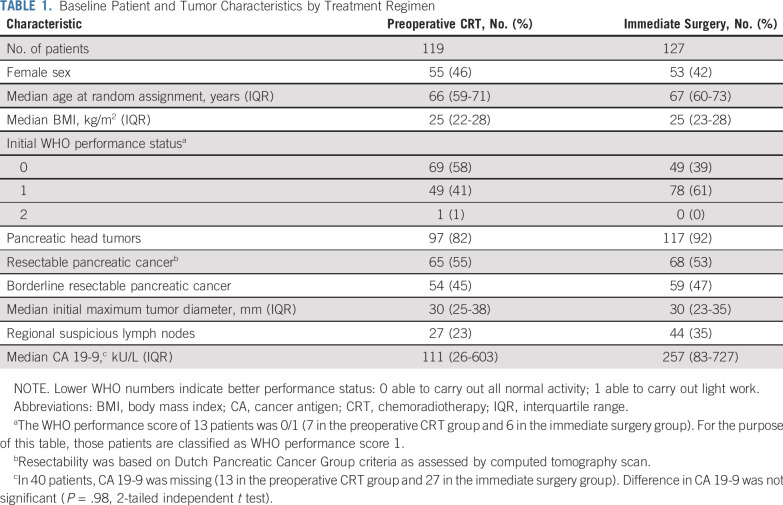
From April 2013 to July 2017, 248 patients from 16 centers were randomly assigned: 120 were assigned to preoperative chemoradiotherapy and 128 to immediate surgery. Two patients were excluded from the analysis for withdrawal of informed consent, which left 119 and 127 patients, respectively, for the ITT analysis (Fig 1). Baseline characteristics were well balanced between both groups (Table 1). Seven patients in the preoperative chemoradiotherapy group did not receive preoperative treatment, 3 of whom had an urgent indication for surgery (Data Supplement).
FIG 1.
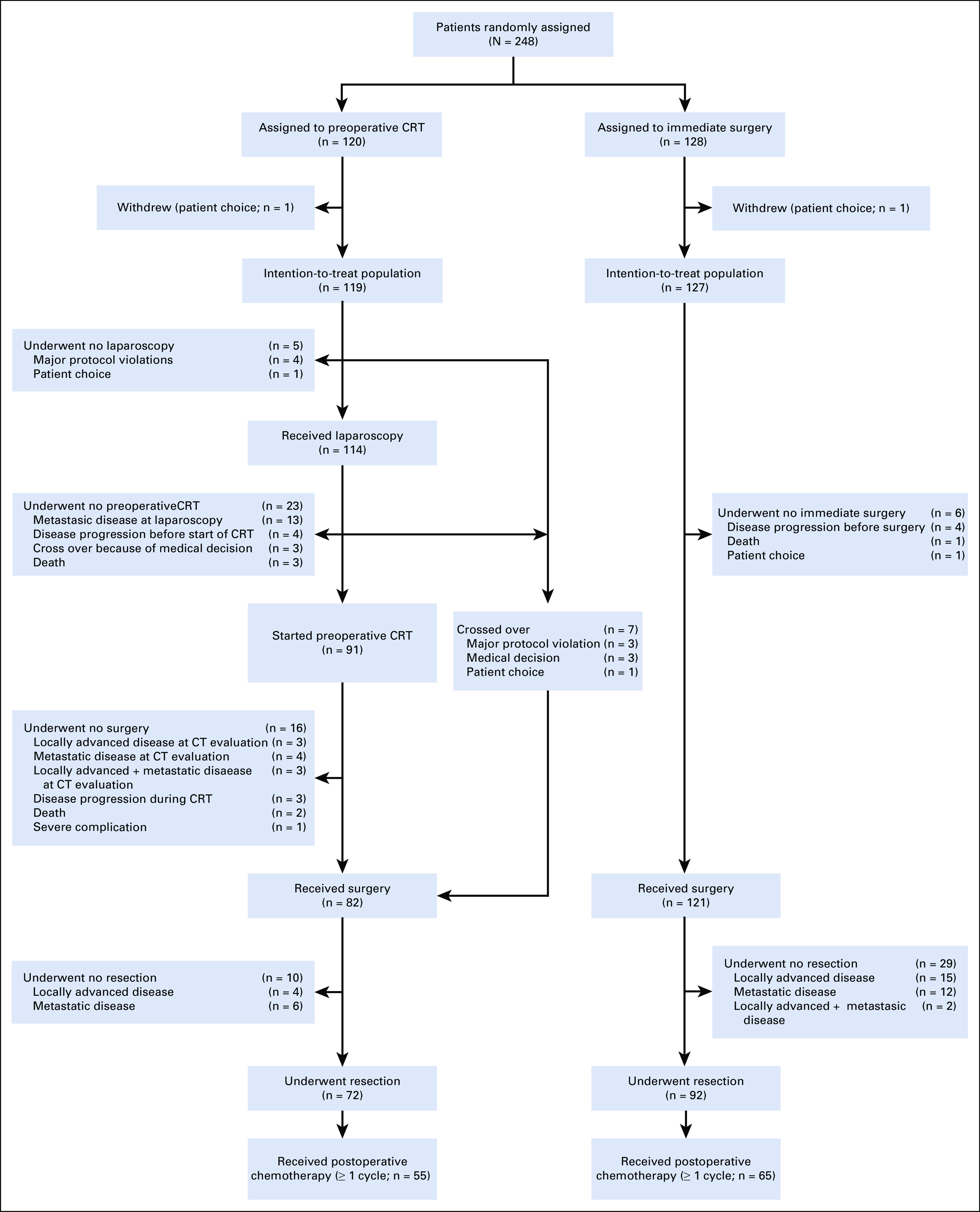
CONSORT diagram. CRT, chemoradiotherapy; CT, computed tomography.
TABLE 1.
Baseline Patient and Tumor Characteristics by Treatment Regimen
In the preoperative chemoradiotherapy group, 5 patients (4%) had no staging laparoscopy; in 13 patients (11%), metastatic disease was found at laparoscopy (Fig 1). After laparoscopy, 91 patients (91 of 119; 76% by ITT) started preoperative chemoradiotherapy, which in 10 patients was postponed because of persistent high bilirubin levels. Eighty-one patients (89%) completed chemoradiotherapy. Reasons for not completing chemoradiotherapy were disease progression (3 patients) and toxicity (5 patients). Two patients died as a result of a myocardial infarction during preoperative treatment. CT evaluation revealed disease progression in 10 patients (Fig 1). Explorative laparotomy was performed in 82 patients (including 7 patients who underwent immediate surgery for several reasons), of whom 72 underwent a resection (72 of 119; 61% by ITT). The R0 resection rate was 71% (51 of 72 patients). Of these 72 patients, 24 (33%) had pathologic lymph nodes, 28 (39%) perineural invasion, and 14 (19%) venous invasion (Data Supplement).
In the immediate surgery group, 6 patients (5%) did not undergo surgery (Fig 1). Staging laparoscopy or laparotomy revealed metastatic disease in 14 patients (12%) and unexpected locally advanced disease in 15 patients (12%). Resection was performed in 92 patients (92 of 127; 72% by ITT). The R0 resection rate was 40% (37 of 92 patients). Of these 92 patients, 72 (78%) had pathologic lymph nodes, 67 (73%) perineural invasion, and 33 (36%) venous invasion (Data Supplement).
The resection rate was not significantly different between the preoperative chemoradiotherapy and the immediate surgery groups (61% v 72%; P = .058). However, the R0 resection rate was higher in patients treated with preoperative chemoradiotherapy (72% v 40%; P < .001), and fewer patients had pathologic lymph nodes (33% v 78%; P < .001), perineural invasion (39% v 73%; P < .001), or venous invasion (19% v 36%; P = .024). Overall, patients with an R0 resection had a better OS than patients with non-R0 resection (HR, 0.47; 95% CI, 0.31 to 0.72; P < .001; Data Supplement).
In 14 patients (14 of 164; 9%), histopathology revealed a different diagnosis than PDAC, not statistically different between both groups (6% v 11%; P = .127; Data Supplement). In the preoperative chemoradiotherapy group, 68 of the 72 patients had PDAC. Fifty-five of those patients (55 of 68; 81%) started adjuvant chemotherapy, of whom 34 (62%) completed their treatment. By ITT, 46% (55 of 119 patients) started adjuvant chemotherapy. In the immediate surgery group, 82 of 92 patients had PDAC, of whom 65 (79%) started adjuvant chemotherapy and 35 completed it (53%). By ITT, 51% (65 of 127 patients) started adjuvant chemotherapy.
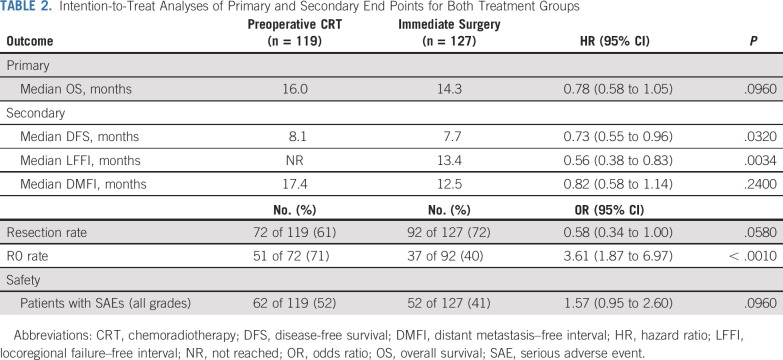
After a median follow-up of 27 months, 180 patients (73%) died: 81 (68%) in the preoperative chemoradiotherapy group and 99 (78%) in the immediate surgery group. The median OS in the preoperative chemoradiotherapy group was 16.0 months (95% CI, 13.0 to 20.9 months) and 14.3 months (95% CI, 12.7 to 17.9 months) in the immediate surgery group (HR, 0.78; 95% CI, 0.58 to 1.05; P = .096). The DFS (HR, 0.73; 95% CI, 0.55 to 0.96; P = .032) and LFFI (HR, 0.56; 95% CI, 0.38 to 0.83; P = .0034) were significantly better in the preoperative chemoradiotherapy group. The DMFI was comparable (HR, 0.82; 95% CI, 0.58 to 1.14; P = .24; Table 2; Fig 2). The per-protocol analysis of 91 patients who started preoperative chemoradiotherapy compared with the 104 patients in the immediate surgery group who underwent exploration and had no distant metastasis showed a median OS of 20.2 v 16.8 months (HR, 0.73; 95% CI, 0.51 to 1.03; P = .073; Data Supplement).
TABLE 2.
Intention-to-Treat Analyses of Primary and Secondary End Points for Both Treatment Groups
FIG 2.
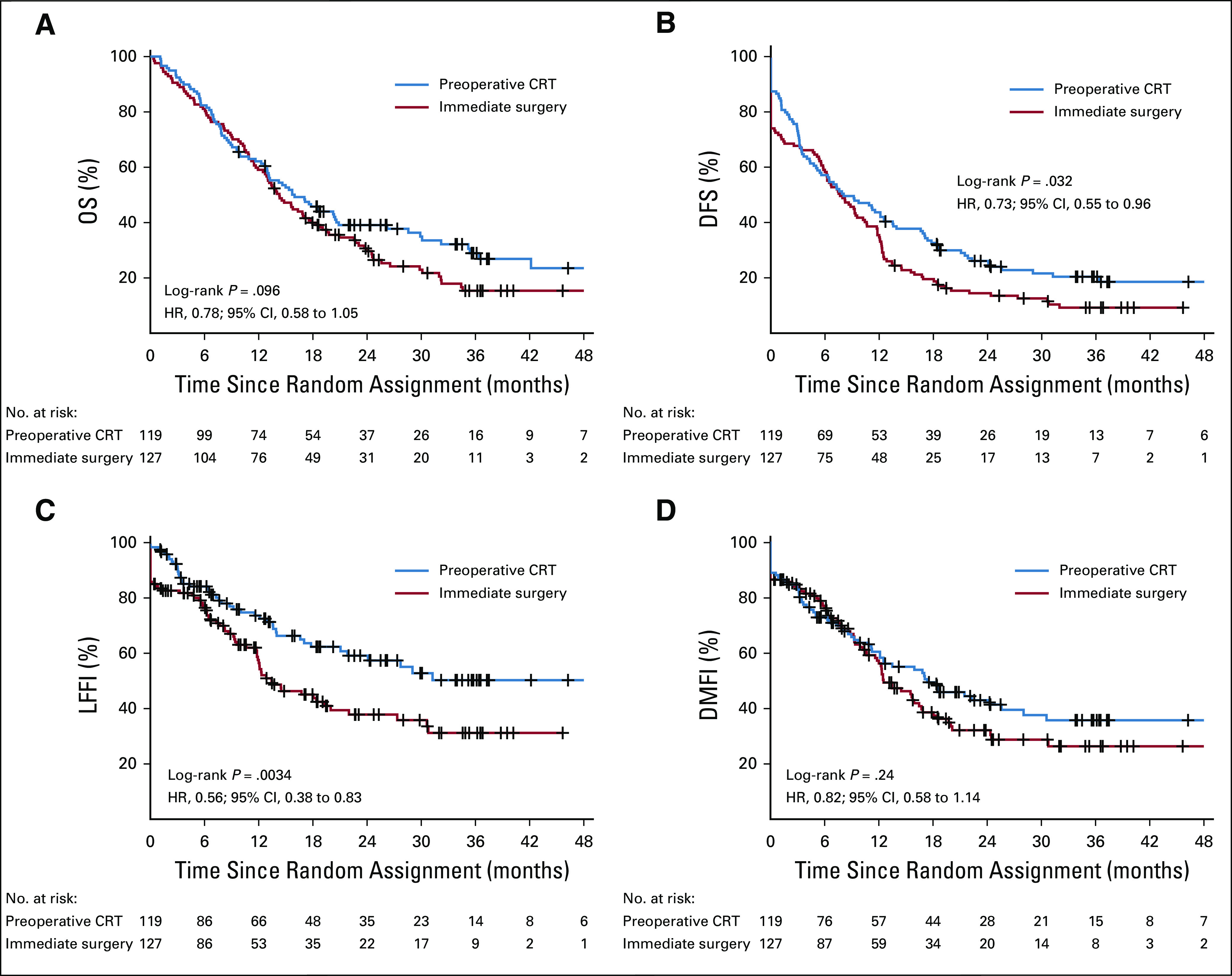
(A) Overall survival (OS), (B) disease-free survival (DFI), (C) locoregional failure–free interval (LFFI), and (D) distant metastasis–free interval (DMFI) in 246 patients randomly assigned to preoperative chemoradiotherapy (CRT; 119 patients) or immediate surgery (127 patients) according to intention-to-treat analysis. Tick marks indicate censored observations. HR, hazard ratio.
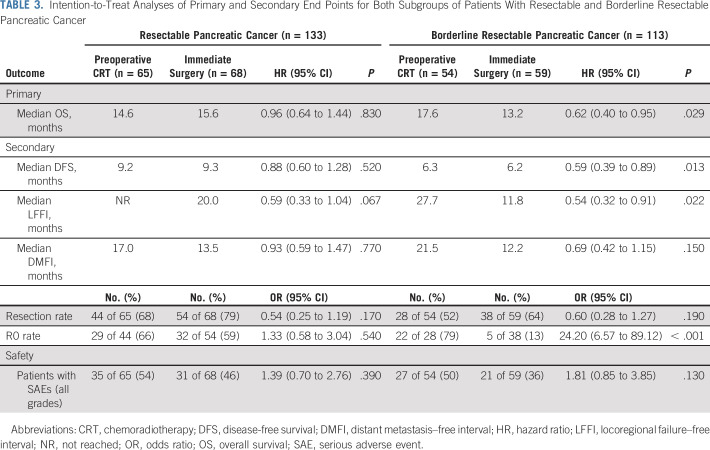
The predefined subgroup of patients with suspected resectable PDAC showed no significant difference in OS, DFS, LFFI, and DMFI (Table 3). The predefined subgroup of patients with suspected borderline resectable PDAC showed a significantly improved OS, DFS, and LFFI for preoperative chemoradiotherapy (Table 3). The interaction test of hazard rates showed no significant difference between these subgroups (P = .14). The predefined subgroup of patients with tumor resection who started adjuvant treatment showed a significantly improved median OS of 35.2 months (95% CI, 26.2 months to not available) in the preoperative chemoradiotherapy group and 19.8 months (95% CI, 16.8 to 32.2 months) in the immediate surgery group (HR, 0.58; 95% CI, 0.35 to 0.95; P = .029) as well as significant differences in DFS, LFFI, and DMFI (Fig 3).
TABLE 3.
Intention-to-Treat Analyses of Primary and Secondary End Points for Both Subgroups of Patients With Resectable and Borderline Resectable Pancreatic Cancer
FIG 3.
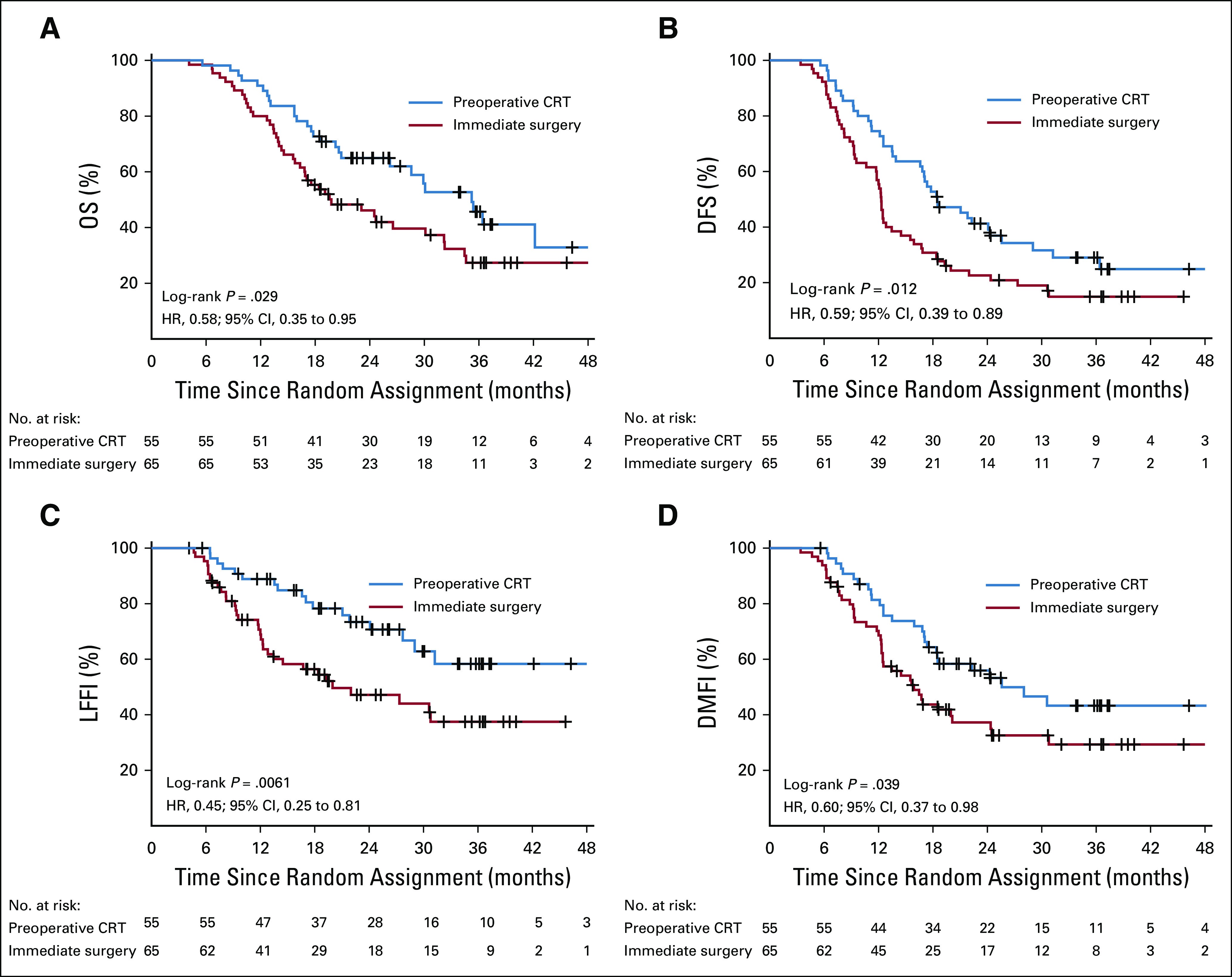
(A) Overall survival (OS), (B) disease-free survival (DFI), (C) locoregional failure–free interval (LFFI), and (D) distant metastasis–free interval (DMFI) in the 120 patients who had a resection of the tumor and started the postoperative chemotherapy and randomly assigned to preoperative chemoradiotherapy (CRT; 55 patients) or immediate surgery (65 patients). Tick marks indicate censored observations. HR, hazard ratio.
With regard to toxicity, 62 patients (52%) in the preoperative chemoradiotherapy group and 52 (41%) in the immediate surgery group experienced at least 1 serious adverse event (P = .096). Grade 5 serious adverse events were observed in 16 patients (7%), 8 in each group. This includes 3 postoperative mortalities in each group (Data Supplement). Two serious adverse events were considered as suspected unexpected serious adverse reactions (Data Supplement). At least 1 postoperative complication occurred in 49 (68%) of 72 patients in the preoperative chemoradiotherapy group and 46 (50%) of 92 patients in the immediate surgery group (P = .026). By ITT, these figures were 41% v 36% (P = .44).
DISCUSSION
To our knowledge, this is the first completed multicenter, randomized trial on preoperative chemoradiotherapy versus immediate surgery in patients with resectable or borderline resectable PDAC, and did not demonstrate an OS benefit in the ITT population (median, 16.0 v 14.3 months; HR, 0.78; P = .096). Nevertheless, the secondary end points DFS, LFFI, R0 resection rate, and pathologic parameters were superior with preoperative chemoradiotherapy. Together with the predefined subgroup analysis of patients undergoing a resection and starting adjuvant chemotherapy, this suggests a clinically relevant benefit of preoperative chemoradiotherapy in patients with resectable or borderline resectable PDAC. We consider this in line with the evidence from nonrandomized and early-terminated randomized trials.6,7,11-15,31
Compliance of intended preoperative chemoradiotherapy (ITT, 76%) was better than that of intended postoperative chemotherapy in the immediate surgery group (ITT, 51%). Preoperative chemoradiotherapy was completed by 81 (89%) of 91 patients; postoperative treatment was completed by 69 of 120 patients (58% in both study arms). In view of the high dropout rate (24%) in the preoperative chemoradiotherapy group, the per-protocol analysis showed a nonsignificant trend in OS in the preoperative chemoradiotherapy group, in line with the results of the primary and secondary end points.
Laparoscopy or laparotomy revealed metastases in 11% in the preoperative chemoradiotherapy group and 12% in the immediate surgery group. Unexpected locally advanced disease was found in 5% in the preoperative chemoradiotherapy group and 12% in the immediate surgery group. A previous study reported unsuccessful laparotomy in up to 25% of patients despite contemporary imaging techniques,32 which corresponds to the 24% in our immediate surgery group. Thirteen patients (14%) showed disease progression during preoperative chemoradiotherapy who might have had tumor progression shortly after surgery if randomly assigned for immediate surgery.
A comparison of our results with those of published trials of adjuvant gemcitabine and capecitabine (ESPAC-4; median OS, 28 months)1 or modified fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX; PRODIGE 24/CCTG PA.6; median OS, 54.4 months)2 is difficult. Adjuvant trials exclude patients with disease progression before surgery, occult metastases, or locally advanced disease detected at exploration as well as patients poorly recovering from surgery. To enable observational comparison with these trials, we analyzed data of the 120 patients who underwent resection and started adjuvant chemotherapy. This subgroup analysis showed a clinically and statistically relevant OS benefit of preoperative chemoradiotherapy over immediate surgery (35.2 v 19.8 months; P = .029).
Preoperative FOLFIRINOX might further improve the outcome and is currently being investigated in the PREOPANC-2 trial (Netherlands Trial Register identifier: NTR7292, 2018-06-19), the NorPACT-1 trial (ClinicalTrials.com identifier: NCT02919787),19 and the PANACHE01-PRODIGE48 trial (ClinicalTrials.com identifier: NCT02959879).20 The addition of radiotherapy to preoperative FOLFIRINOX could be a next step, as preoperative chemoradiotherapy gives higher R0 rates, less lymph node positivity, and local recurrences compared with preoperative chemotherapy only,33 in line with our results. FOLFIRINOX followed by (chemo)radiotherapy for borderline resectable or locally advanced PDAC is feasible, with high R0 resection rates and prolonged median progression-free survival and OS.34-36
A predefined subgroup analysis showed superior OS after preoperative chemoradiotherapy for borderline resectable PDAC and no significant difference for resectable PDAC. This suggests a benefit in borderline resectable disease and a lack of benefit in resectable disease. Indeed, theoretically, the effect of creating R0 resection and other pathologic advantages by preoperative treatment might be greater in borderline resectable disease. However, these differences must be interpreted with caution because the interaction test of hazard rates between both groups was not significant. The benefit of preoperative treatment in borderline resectable PDAC was also observed in an interim analysis after inclusion of 58 patients in a published phase II/III trial.12 On the other hand, the recently presented phase II/III Prep-02/JSAP-05 trial showed a significant benefit of preoperative chemotherapy (gemcitabine and S-1) over immediate surgery for resectable PDAC, with a median OS of 36.7 v 26.6 months.13 Ongoing studies, both in resectable and borderline resectable disease, will clarify whether preoperative treatment works predominantly in borderline resectable disease or in both groups16-20 (Data Supplement). Probably the biologic behavior is more important than the local extent of the tumor’s susceptibility to neoadjuvant therapy.
Some of our findings need further clarification. First, the median OS in the immediate surgery group was better than expected (14 instead of 11 months), which probably resulted in an underpowered study. This might be explained by effective lines of salvage therapies in patients with locoregional or distant failure. Second, 10 patients had persistent jaundice after biliary drainage, which caused a delay of preoperative treatment. These aspects should be taken into account when considering neoadjuvant therapy in patients with suspected PDAC. In addition, 14 patients (6%) had other pathology than PDAC; 9 (4%) had a cholangiocarcinoma, which implies a more favorable prognosis than PDAC.
In conclusion, this national, multicenter, randomized, phase III trial of preoperative chemoradiotherapy versus immediate surgery in resectable or borderline resectable PDAC did not show a significant OS benefit of preoperative chemoradiotherapy. The consistent benefits for most secondary end points and the better compliance with preoperative chemoradiotherapy compared with postoperative adjuvant chemotherapy suggest superiority of the neoadjuvant approach.
ACKNOWLEDGMENT
We thank all patients who participated in the trial and relatives for their support. In addition to the authors, we thank the following coworkers in the PREOPANC trial; A. Bel (Amsterdam UMC, Amsterdam), R.J. Bennink (Amsterdam UMC, Amsterdam), J.C. Beukema (University Medical Center Groningen [UMCG], Groningen), M. Bijlsma (Amsterdam UMC, Amsterdam), T. Bisseling (Radboud UMC, Nijmegen), D. Boerma (St Antonius Hospital, Nieuwegein), T.L. Bollen (St Antonius Hospital, Nieuwegein), K. Bosscha (Jeroen Bosch Hospital, Den Bosch), M. Bruno (Erasmus MC, Rotterdam), A.M. Bruynzeel (Amsterdam UMC, Amsterdam), F. Dijk (Amsterdam UMC, Amsterdam), J. Erdmann (Amsterdam UMC, Amsterdam), G. Franssen (Erasmus MC, Rotterdam), H. van Goor (Radboud UMC, Nijmegen), J. Hagoort (Erasmus MC, Rotterdam), E. van der Harst (Maasstad Hospital, Maasstad), K. Haustermans (UZ Leuven, Leuven), E.M. Hendriksen (Medisch Spectrum Twente [MST], Enschede), K. van der Hoeven (Radboud UMC, Nijmegen), G.A.P. Hospers (UMCG, Groningen), M.C.C.M. Hulshof (Amsterdam UMC, Amsterdam), C. Hurkmans (Catharina Hospital, Eindhoven), M.P. van Intven (UMC Utrecht [UMCU], Utrecht), J.M. Jansen (OLVG, Amsterdam), K.P. de Jong (UMCG, Groningen), G. Kazemier (Amsterdam UMC, Amsterdam), J. Klaasse (UMCG, Groningen), B.M. van der Kolk (Radboud UMC, Nijmegen), M. Koopman (UMCU, Utrecht), M.C.J.C. Legdeur (MST, Enschede), M.S. Liem (MST, Enschede), M. Los (St Antonius Hospital, Nieuwegein), I.Q. Molenaar (UMCU, Utrecht), S.S.K.S. Phoa (Amsterdam UMC, Amsterdam), A.C. Poen (Isala Oncology Center, Zwolle), J.F.M. Pruijt (Jeroen Bosch Hospital, Den Bosch), S. Radema (Radboud UMC, Nijmegen), T. Rozema (Verbeeten Instituut, Tilburg), H. Rutten (Radboud UMC, Nijmegen), H.C. van Santvoort (St Antonius Hospital, Nieuwegein), C.H. Scharloo-Karels (Amsterdam UMC, Amsterdam), G.P. van der Schelling (Amphia Hospital, Breda), T. Seerden (Amphia Hospital, Breda), M.W.T. Tanck (Erasmus MC, Rotterdam), A. J. ten Tije (Amphia Hospital, Breda), R. Timmer (St Antonius Hospital, Nieuwegein), N. G. Venneman (MST, Enschede), and L. Welling (Leiden UMC, Leiden).
PRIOR PRESENTATION
Presented at the 2018 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by the Dutch Cancer Society (KWF) (UVA 2012-5696), which funded the data management (central and local) and onsite monitoring of the trial. The Dutch Cancer Society (KWF) did not influence the design or analysis of the study.
2012-003181-40
Written on behalf of the Dutch Pancreatic Cancer Group.
See accompanying Editorial on page 1757
C.H.v.E. and G.v.T contributed equally to this article.
AUTHOR CONTRIBUTIONS
Conception and design: Olivier R. Busch, Ronald M. van Dam, Ferry A.L.M. Eskens, Sebastiaan Festen, Bas Groot Koerkamp, Marjolein Y.V. Homs, Jeanin E. van Hooft, Gijs A. Patijn, Johanna W. Wilmink, Aeilko H. Zwinderman, Cornelis J. Punt, Casper H. van Eijck, Geertjan van Tienhoven
Administrative support: Eva Versteijne, Janine M. Akkermans-Vogelaar
Provision of study material or patients: Mustafa Suker, Bert A. Bonsing, Jeroen Buijsen, Geert-Jan M. Creemers, Ronald M. van Dam, Ferry A.L.M. Eskens, Bas Groot Koerkamp, Ignace H. de Hingh, Marjolein Y.V. Homs, Jeanin E. van Hooft, Emile D. Kerver, Maurice J. C. van der Sangen, Geertjan van Tienhoven
Collection and assembly of data: Eva Versteijne, Mustafa Suker, Karin Groothuis, Marc G. Besselink, Bert A. Bonsing, Jeroen Buijsen, Geert-Jan M. Creemers, Ronald M. van Dam, Ferry A.L.M. Eskens, Sebastiaan Festen, Jan Willem B. de Groot, Bas Groot Koerkamp, Ignace H. de Hingh, Jeanin E. van Hooft, Emile D. Kerver, Karen J. Neelis, Gabriel M.R.M. Paardekooper, Gijs A. Patijn, Judith de Vos-Geelen, Casper H. van Eijck, Geertjan van Tienhoven
Data analysis and interpretation: Eva Versteijne, Mustafa Suker, Janine M. Akkermans-Vogelaar, Marc G. Besselink, Bert A. Bonsing, Jeroen Buijsen, Olivier R. Busch, Ronald M. van Dam, Ferry A.L.M. Eskens, Jan Willem B. de Groot, Bas Groot Koerkamp, Ignace H. de Hingh, Emile D. Kerver, Saskia A.C. Luelmo, Joost Nuyttens, Gijs A. Patijn, Maurice J.C. van der Sangen, Johanna W. Wilmink, Aeilko H. Zwinderman, Cornelis J. Punt, Casper H. van Eijck, Geertjan van Tienhoven
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ferry A.L.M. Eskens
Consulting or Advisory Role: Merck Serono, Roche, Eisai, Ipsen
Travel, Accommodations, Expenses: Pfizer
Jan Willem B. de Groot
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, Pierre Fabre, MSD Oncology, SERVIER
Bas Groot Koerkamp
Research Funding: Tricumed (Inst)
Ignace H. de Hingh
Research Funding: Roche (Inst), QP&S (Inst), RanD Biotech (Inst)
Marjolein Y.V. Homs
Travel, Accommodations, Expenses: SERVIER
Jeanin E. van Hooft
Honoraria: Cook Medical, Boston Scientific, Medtronic
Consulting or Advisory Role: Boston Scientific, Cook Medical
Saskia A.C. Luelmo
Travel, Accommodations, Expenses: Astellas Pharma
Judith de Vos-Geelen
Research Funding: SERVIER (Inst)
Travel, Accommodations, Expenses: SERVIER
Johanna W. Wilmink
Consulting or Advisory Role: SERVIER, Celgene
Speakers’ Bureau: Medscape
Research Funding: SERVIER, Celgene, Novartis, AstraZeneca (Inst), Pfizer (Inst), EMD Serono (Inst), Roche (Inst), Merck (Inst)
Cornelis J. Punt
Consulting or Advisory Role: Bayer AG (Inst), Nordic Pharma (Inst)
Travel, Accommodations, Expenses: Nordic Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Neoptolemos JP, Palmer DH, Ghaneh P, et al. : Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 389:1011-1024, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Hammel P, Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395-2406, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Bakens MJ, van der Geest LG, van Putten M, et al. : The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: A nationwide population-based analysis. Cancer Med 5:2825-2831, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merkow RP, Bilimoria KY, Tomlinson JS, et al. : Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 260:372-377, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Mayo SC, Gilson MM, Herman JM, et al. : Management of patients with pancreatic adenocarcinoma: National trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 214:33-45, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. : Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med 7:e1000267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versteijne E, Vogel JA, Besselink MG, et al. : Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 105:946-958, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tummers WS, Groen JV, Sibinga Mulder BG, et al. : Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg 106:1055-1065, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. : Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074-2084, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Sauer R, Becker H, Hohenberger W, et al. : Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731-1740, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Reni M, Balzano G, Zanon S, et al. : Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): A randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol 3:413-423, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Jang JY, Han Y, Lee H, et al. : Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: A prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 268:215-222, 2018 [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1093/jjco/hyy190. Unno M, Motoi F, Matsuyama Y, et al: Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol 37, 2019 (suppl 4, abstr 189) [DOI] [PubMed] [Google Scholar]
- 14.Casadei R, Di Marco M, Ricci C, et al. : Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: A single-center prospective, randomized, controlled trial which failed to achieve accrual targets. J Gastrointest Surg 19:1802-1812, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Golcher H, Brunner TB, Witzigmann H, et al. : Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: Results of the first prospective randomized phase II trial. Strahlenther Onkol 191:7-16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettrich TJ, Berger AW, Perkhofer L, et al. : Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer—The NEONAX trial (AIO-PAK-0313), a prospective, randomized, controlled, phase II study of the AIO pancreatic cancer group. BMC Cancer 18:1298, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinrich S, Pestalozzi B, Lesurtel M, et al. : Adjuvant gemcitabine versus neoadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: A randomized multicenter phase III study (NEOPAC study). BMC Cancer 11:346, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hozaeel W, Pauligk C, Homann N, et al: Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX in resectable pancreatic cancer: The NEPAFOX trial. J Clin Oncol 33, 2015 33 (suppl; abstr TPS4152)
- 19.Labori KJ, Lassen K, Hoem D, et al. : Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1))—Study protocol for a national multicentre randomized controlled trial. BMC Surg 17:94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz L, Vernerey D, Bachet JB, et al. : Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy—A multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). BMC Cancer 18:762, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sorbin LH, Gospodarowicz MK, Wittekind C (eds): TNM Classification of Malignant Tumours (ed 7). Chichester, United Kingdom, Wiley, 2009. [Google Scholar]
- 22.Versteijne E, van Eijck CH, Punt CJ, et al. : Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): Study protocol for a multicentre randomized controlled trial. Trials 17:127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. doi: 10.1056/NEJMoa0903230. van der Gaag NA, Rauws EA, van Eijck CH, et al: Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 362:129-137, 2010. [DOI] [PubMed] [Google Scholar]
- 24. doi: 10.1016/j.ijrobp.2010.02.030. Small W Jr, Mulcahy MF, Rademaker A, et al: Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int J Radiat Oncol Biol Phys 80:476-482, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Talamonti MS, Small W, Jr, Mulcahy MF, et al. : A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol 13:150-158, 2006 [DOI] [PubMed] [Google Scholar]
- 26. doi: 10.1200/JCO.2007.13.9014. Small W Jr, Berlin J, Freedman GM, et al: Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: A multicenter phase II trial. J Clin Oncol 26:942-947, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Tol JA, Gouma DJ, Bassi C, et al. : Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 156:591-600, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon KV, Gomez D, Smith AM, et al. : Impact of margin status on survival following pancreatoduodenectomy for cancer: The Leeds Pathology Protocol (LEEPP). HPB 11:18-24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbeke CS, Leitch D, Menon KV, et al. : Redefining the R1 resection in pancreatic cancer. Br J Surg 93:1232-1237, 2006 [DOI] [PubMed] [Google Scholar]
- 30. National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE): Version 4.0. Washington, DC, Department of Health and Human Services, 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 31. doi: 10.1016/j.ijrobp.2008.02.065. Stessin AM, Meyer JE, Sherr DL: Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: An analysis of data from the Surveillance, Epidemiology, and End Results (SEER) registry. Int J Radiat Oncol Biol Phys 72:1128-1133, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Fong ZV, Alvino DML, Fernández-Del Castillo C, et al. : Reappraisal of staging laparoscopy for patients with pancreatic adenocarcinoma: A contemporary analysis of 1001 patients. Ann Surg Oncol 24:3203-3211, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Cloyd JM, Chen HC, Wang X, et al. : Chemotherapy versus chemoradiation as preoperative therapy for resectable pancreatic ductal adenocarcinoma: A propensity score adjusted analysis. Pancreas 48:216-222, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Suker M, Nuyttens JJ, Groot Koerkamp B, et al. : FOLFIRINOX and radiotherapy for locally advanced pancreatic cancer: A cohort study. J Surg Oncol 118:1021-1026, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancini BR, Stein S, Lloyd S, et al. : Chemoradiation after FOLFIRINOX for borderline resectable or locally advanced pancreatic cancer. J Gastrointest Oncol 9:982-988, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. doi: 10.1001/jamaoncol.2018.0329. Murphy JE, Wo JY, Ryan DP, et al: Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: A phase 2 clinical trial. JAMA Oncol 4:963-969, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]