Abstract
Infection with hepatitis B virus (HBV) remains an important public health problem with a high burden worldwide. The Saudi Association for the Study of Liver diseases and Transplantation formed a working group to develop HBV practice guidelines in Saudi Arabia. The methodology used to develop these guidelines was based on reviewing the available evidence, local data, and major international practice guidelines on the management of HBV. The aim of these guidelines is to assist healthcare providers in the management of HBV in Saudi Arabia. These updated guidelines summarize the latest local studies performed on HBV epidemiology, major changes in the prevalence of this virus, and advances in disease management.
Keywords: Antiviral, epidemiology, guidelines, HBV, hepatitis B virus, liver disease, treatment
INTRODUCTION
Infection with hepatitis B virus (HBV) remains an important public health problem with a high burden worldwide.[1,2] The Saudi Association for the Study of Liver diseases and Transplantation (SASLT) formed a working group in 2012 to develop HBV practice guidelines in Saudi Arabia, which were published in 2014.[3]
The present document aims to update these guidelines with the most current information for the optimal management of HBV infection. The evidence and recommendations in these guidelines follow the same grading system that was used in the 2014 guidelines [Table 1].
Table 1.
Grading of recommendations
| Grade | Recommendation |
|---|---|
| A | Recommendation based on high-quality evidence: at least one high-quality randomized controlled trial or at least one high-quality meta-analysis. |
| B | Recommendation based on medium-quality evidence: high-quality cohort study, case-control study or systematic review. |
| C | Recommendation based on weak evidence: case series or case report. |
| D | Recommendation based only on expert opinion. |
These guidelines aim to assist healthcare professionals in the management of HBV in Saudi Arabia. The present update summarizes the latest studies performed on HBV epidemiology in the country, major changes in the prevalence of this virus, and advances in the treatment of the disease.
These guidelines were produced based on formal review and analysis of the current literature, recently published international guidelines and the authors' experience in chronic hepatitis B (CHB) management. Considering that this manuscript is an updated version of the SASLT guidelines, references published after 2012 have been privileged. Older supportive references can be found in the previous version published in 2014. Additionally, it is important to bear in mind that this is an update of our 2014 guidelines, and consultation of the older version is necessary for complete management of HBV.
EPIDEMIOLOGY
HBV chronically infects approximately 292 million people globally and is a leading cause of cirrhosis, hepatocellular carcinoma (HCC), and liver-related death (LRD).[4] A vaccine was developed in 1981, and most countries in the world have infant vaccination programs in place. This, along with a number of public health efforts have been charged with combating HBV, by primarily focusing on infection prevention. While these efforts have achieved significant reductions in prevalence among younger age cohorts over the last three decades in many parts of the world, there remains a sizable older population who were infected before the emergence of large-scale vaccination programs. This sizable infected population remains at-risk for continued progression to cirrhosis, HCC and death, contributing heavily to the global burden of disease, including in Saudi Arabia.[5]
HBV was once considered hyper-endemic in Saudi Arabia. Historical community-based epidemiological studies in children from the 1980s showed a hepatitis B surface antigen (HBsAg) seroprevalence of around 7%.[6] However, the universal vaccination program that started in 1990 aimed at immunizing all Saudi-children prior to school entry. This led to substantial declines in seroprevalence, verifying long-term vaccine protection marked by the complete absence of HBsAg or anti-hepatitis B core antigen detection in children, studied in randomly selected children and adolescents from high, moderate, and low endemicity regions.[7,8] Results from other non-affiliated independent studies supported these findings. For example, among 74,662 Saudis undergoing premarital screening in 2008, 1.3% tested HBsAg+, showing substantially reduced prevalence rates as compared to the pre-vaccination era.[9] Similarly, blood donor statistics from the late 1990s/early 2000s suggest prevalence rates between 1.5% and 2.6% within the adult population.[10,11,12] No published accounts exist on the uptake of HBV antiviral therapy in the last trimester of pregnancy or the administration of HBIG to children of infected mothers in Saudi Arabia.
HBV infection in this region occurs primarily as a result of perinatal infection, horizontal transmission between family members and transmission from injections.[13] Although robust screening programs for viral hepatitis exist in Saudi Arabia, particularly for pre-marital couples, military and pre-employment recruits, expatriates/immigrants, and blood donors, these screening measures generally target the younger population thereby excluding the older populations where the infection is more prevalent. No national HBV prevalence study exists among all ages in the general population of Saudi Arabia to date, although there are several prevalence estimates within subpopulations. Premarital screening studies have reported HBsAg seropositivity in the range of 1.3% to 1.7%; however, such screenings likely over-sample younger Saudis.[9,14,15] The reduction in prevalence resulting from these measures can be seen in a study comparing two cohorts of couples attending in vitro fertilization clinics sampled 10 years apart, reporting a 4.7% prevalence in data collected from 2002 to 2005 and a 1.7% prevalence in data collected from 2012 to 2015.[16]
Although there are no large-scale epidemiological studies that accurately estimate the epidemiology or burden of CHB in the country, it is believed that the burden of disease is high based on clinical observation and death from chronic HBV complications, mainly cirrhosis, liver failure, and HCC.[5,17] Recently, a modeling analysis estimated the current prevalence of HBV in Saudi Arabia at 1.7%,[18] in line with recent studies and estimates,[9,14,15] and represents the most accurate, validated and up-to-date assessment of HBV epidemiology within the country. The model estimated that in 2017 there were 574,000 (366,000 – 650,000) HBsAg + cases in Saudi Arabia, a prevalence rate of 1.7%. HBsAg prevalence is projected to drop to 1.2% in 2030, estimating 485,000 (311,000–550,000) chronic infections. Among the total infected population, 77% of cases were estimated to be between 35 and 60 years of age. Furthermore, the model also estimated 490 incident cases of decompensated cirrhosis, 1,500 incident cases of HCC and 1,740 liver-related deaths in 2017. HBsAg prevalence was estimated to be <0.1% among infants and 0.1% among 5-year-olds in 2017.[18]
NATURAL HISTORY OF HBV
The natural history of CHB infection has been divided into five phases which consider the presence of HBeAG, HBV DNA levels, alanine aminotransferase (ALT) values and the eventual presence or absence of liver inflammation.[1,3] Understanding these phases is fundamental to the evaluation and management of CHB, and critical in the assessment of patient's status and in guiding decisions regarding candidacy for treatment and treatment endpoints.[3] The new nomenclature is based on the description of the two main characteristics of chronicity: infection and hepatitis [Table 2] and considers HBV markers (HBsAg, HBeAg/Anti-HBe, and HBV DNA) as well as liver disease markers, namely biochemical parameters (ALT) and fibrosis markers (non-invasive markers or liver biopsy in selected cases).[1]
Table 2.
Natural history and assessment of patients with chronic HBV infection based upon HBV and liver disease markers[1,3]
| Phases | HBsAg/HBeAg | HBV DNA | ALT | Liver inflammation | Old terminology and Observations |
|---|---|---|---|---|---|
| PHASE 1Chronic HBV infection | High/Positive | High (>107 IU/mL) | Normal | None/minimal | “Immune tolerant” This phase is more frequent and more prolonged in subjects infected prenatally or in the first years of life. Patients are highly contagious. |
| PHASE 2 Chronic hepatitis B |
High-Intermediate/Positive | Lower (104-107 IU/mL) | Increased | Moderate/severe | “Immune reactive HBeAg positive“ This phase may last for several weeks to years. It may occur after several years of immune tolerance and is more frequently in subjects infected during adulthood. |
| PHASE 3 Chronic HBV infection |
Low/Negative | Low or undetectable (<2,000 IU/mL)a | Normal | None | “Inactive carrier” This state confers a favorable long-term outcome with low risk of cirrhosis or HCC. |
| PHASE 4 Chronic hepatitis B |
Intermediate/Negative | Fluctuating levels (>2,000 IU/mL) | Fluctuating levels/Elevated* | Moderate/severe | “HBeAg negative chronic hepatitis” Characterized by periodic reactivations. It is sometimes difficult to distinguish true inactive HBV carriers (good prognosis) from patients with active HBeAg-negative CHB (have active liver disease with a high risk of progression to advanced hepatic fibrosis, cirrhosis, and HCC) |
| PHASE 5 Resolved HBV infection |
Negative/Negative | Undetectable (<10 IU/mL) | Normal | None (HCC risk if cirrhosis has developed before HBsAg loss) | “HBsAg negative/anti-HBc positive” Reduced risk of cirrhosis, decompensation, and HCC. Immunosuppression may reactivate HBV in these patients. |
aHBV DNA levels can be between 2,000 and 20,000 IU/ml in some patients without signs of chronic hepatitis. *Persistently or intermittently
Although this categorization is useful in HBV treatment management, in a significant number of patients a single determination of HBV replication markers combined with disease activity markers does not allow an immediate classification to one of these phases. Some patients fall into this “grey area” where management needs to be individualized.[1]
Factors that affect progression to cirrhosis and HCC
The host's immune response dictates the risk of progression to cirrhosis and HCC in these patients. The 5-year cumulative incidence of cirrhosis varies from 8% to 20% in untreated CHB patients and, among those with cirrhosis, the 5-year cumulative risk of hepatic decompensation is 20%. The annual risk of HCC in patients with cirrhosis has been reported to vary between 2-5%.[1,19]
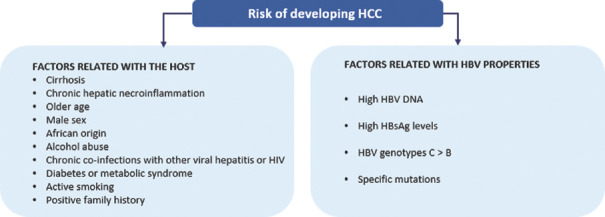
The main concern for diagnosed CHB patients is HCC. The risk of developing HCC depends on factors related with the host and/or to HBV properties[20] [Figure 1]. These factors appear to affect progression to cirrhosis in untreated CHB patients.[1]
Figure 1.
Factors that affect the risk of developing HCC in diagnosed CHB patients
Several risk scores for HCC in CHB patients have been developed and validated in Asian untreated CHB patients, such as GAG-HCC, CU-HCC, and REACH-B.[21] However, they do not seem to offer good predictability in studies that include Caucasian CHB patients.[22,23,24] The recently developed and validated new score, PAGE-B, seems to offer good predictability for HCC during the first 5 years of therapy with entecavir or tenofovir in Caucasian, mostly European, CHB patients and can be easily applied in clinical settings once it is based on available parameters such as platelets, age and gender.[25] The PAGE-B score has been shown to be a good predictor of HCC development even in untreated CHB patients.[1,26]
HBV DIAGNOSIS
HBV screening
The impact of vaccination has been profound in reducing the burden of HBV in Saudi Arabia, particularly in children and young adults, but the prevalence remains high in the older population. Seroprevalence studies have suggested a high prevalence percentage in the older population, making them an important screening target.[7,8,27,28]
Detection of HBsAg is the most commonly used test to diagnose chronic HBV infection,[29] while hepatitis B surface antibody (anti-HBs), and hepatitis B core antibody (anti-HBc) are used to identify patients who have been exposed to HBV or have been previously vaccinated. The anti-HBs test will reveal if a patient is protected against HBV.[30] Although the anti-HBc test assesses if a person has been previously exposed to HBV, it does not indicate by itself whether either immunity or chronic infection has developed as a result of this exposure.[31,32]
The HBV core gene encodes HBeAg, which is secreted from infected hepatocytes and is a marker of active virus replication. However, acquisition of precore and/or basal core promoter mutations can prevent or reduce HBeAg production, leading to an HBeAg-negative phenotype of patients with high levels of viral replication.[33] The presence of anti-HBe usually indicates HBeAg seroconversion and has generally been considered an endpoint for HBV therapy in HBeAg-positive patients.
In Saudi Arabia, the various screening programs in the country supplemented the vaccination program and controlled the burden of chronic HBV infection. Screening for HBV infection also fulfills the main principles of screening that include: being simple and safe; reducing mortality and morbidity; leading to earlier intervention and hence improving outcome; cost-effectiveness; and linking infected patients to appropriate health care.[34]
Although the universal childhood vaccination program in Saudi Arabia is strictly enforced, high-risk populations should continue to be screened for hepatitis B [Table 3]. The mandatory premarital screening program in Saudi Arabia is a positive step in providing an estimate of both disease prevalence in the general population and future potential disease burden.[9,15] Seropositive patients are referred to the general hepatology clinic where they undergo further assessment and are then linked to a long-term follow-up evaluation. Identifying patients in the early stages of infection helps in avoiding long-term negative outcome, which is an important screening principle. Based on the overall prevalence of HBV in Saudi Arabia a mass screening program is not required. However, targeting older patients (>40 years) may be cost-effective given the higher prevalence of infection in this population subgroup.
Table 3.
High-risk individuals who should be screened for chronic hepatitis B virus infection
| Expatriate individuals as part of their pre-employment assessment |
| Healthcare workers |
| Household contacts of HBV carriers |
| Sexual contacts of HBV carriers or individuals with high risk sexual behavior |
| Dialysis patients |
| History of intravenous drugs |
| Individuals with abnormal liver enzymes |
| Pregnant women |
| Inmates |
| Individuals needing immunosuppressive or cancer chemotherapy |
| HCV- or HIV-infected individuals |
| Blood or organ donors |
HBV PREVENTION
Patients with chronic HBV infection should be advised regarding lifestyle modifications to minimize the risk of disease transmission.[5] Measures include vaccinating all household members, avoid sharing injection equipment including glucometers and razors with others. Patients should also be educated not to donate blood and make sure they cover skin cuts and wounds carefully, and thoroughly clean blood spills with appropriate disinfectants.[35] For individuals negative for HBsAg and anti-HBs and who have not been vaccinated, hepatitis B vaccination is recommended, particularly for higher risk individuals, including patients with established liver disease and healthcare providers.[36,37] Post-vaccination testing of anti-HBs status is recommended in newborns of HBV-infected mothers or infants with family members with CHB, healthcare workers (HCWs), dialysis patients, workers in dialysis units and operation rooms, immunocompromised individuals and those undergoing chemotherapy, and sexual partners of patients with chronic HBV infection. A booster injection is advised in these groups when the anti-HBs titer falls below 10 mIU/mL.[38,39,40,41,42] Individuals exposed to contaminated blood or body fluids should receive hepatitis B immunoglobulin and the hepatitis B vaccine as soon as possible.[43] If anti-HBc-positive donor organs are used for HBV seronegative recipients, antiviral therapy should be administered to prevent de novo HBV infection.[44]
Optimization of body weight and treatment of metabolic complications, including diabetes and dyslipidemia, are recommended to prevent concurrent development of metabolic syndrome and fatty liver disease, as it has been shown that fatty liver disease is associated with fibrosis progression independent of viral factors.[45,46,47,48] Similarly, abstinence from alcohol is recommended in patients with chronic HBV infection.[49] Smoking chronic HBV patients are at a higher risk of HCC development particularly in the presence of concurrent metabolic syndrome.[50,51] Individuals with CHB should be immunized against hepatitis A if not immune for this condition.[52]
Mother-to-child transmission is one of the most important routes of HBV infection.[53] Details on antiviral treatment during pregnancy will be discussed in the special population section of the guidelines.
HBV MANAGEMENT
Pretreatment assessment
Assessment for chronic HBV infection should combine the patient's complete history, a physical examination, as well as liver disease markers (ALT and fibrosis) and markers for HBV infection (HBsAg, HBeAg/anti-HBe, HBV DNA). Prevention of transmission to others must be considered and therefore the sexual partners and first-degree relatives of these patients should be advised to test for HBV serological markers (HBsAg, anti-HBs, anti-HBc) and vaccinated if negative for these markers.[1,3,36] Recommendations for pretreatment assessment of HBV are illustrated in Table 4.
Table 4.
Pretreatment assessment of HBV (adapted from[1])
| Relevance for HBV management | Pretreatment assessment |
|---|---|
| Assessment of severity of liver disease to identify patients for treatment and HCC surveillance | Physical examination Biochemical parameters (aspartate aminotransferase [AST] and ALT, gamma-glutamyl transpeptidase [GGT], alkaline phosphatase, bilirubin, and serum albumin and gamma globulins, full blood count and prothrombin time) Abdominal hepatic ultrasound Liver biopsy Non-invasive tests (liver stiffness measurements and serum biomarkers of liver fibrosis or cirrhosis) |
| Determination of the phase of chronic HBV infection | HBeAg and anti-HBe detection |
| Establishing diagnosis, the phase of the infection, the decision to treat and subsequent monitoring of patients | HBV DNA serum levels |
| In HBeAg-negative chronic HBV infection and in patients to be treated with interferon-alfa (IFNα) | Serum HBsAg quantification |
| Determining other causes of chronic liver disease | Hepatitis D Hepatitis C HIV Hepatitis A (patients with negative anti-HAV should be advised to be vaccinated against HAV) |
Goal and endpoint of therapy
The main goal of treatment for chronic HBV infection is to improve survival and quality of life by preventing disease progression to cirrhosis- and liver-related complications, namely HCC development.[1,36] Additional goals are to prevent mother-to-child transmission, hepatitis B reactivation and prevention/treatment of associated extrahepatic manifestations. The likelihood of achieving these goals depends upon the timing of therapy and on the stage of disease and patient's age when the treatment is started. A further goal of treatment can be regression of fibrosis or cirrhosis in patients with advanced fibrosis or cirrhosis.
In patients with HBV-induced HCC the use of nucleos (t) ide analogue (NA) therapy is done mainly to suppress HBV replication, and to prevent recurrence of HCC after curative therapies. Stabilization of the HBV-induced liver disease can be considered a prerequisite for the safe and effective application of HCC treatment.[1]
For patients with acute hepatitis B the main goal of therapy is to prevent the risk of acute or subacute liver failure. Additional goals may consider improving the quality of life by reducing disease-associated symptoms and lowering the risk of chronicity.[1]
The recommendations for endpoints of chronic HBV therapy are shown in Table 5.
Table 5.
Endpoints of HBV treatment
| Recommendations | Grade of recommendation |
|---|---|
| Main endpoint Induction of long-term suppression of HBV DNA |
A |
| Valuable endpoint Induction of HBeAg loss (± anti-HBe seroconversion) in HBeAg-positive patients with chronic hepatitis B |
B |
| Additional endpoint ALT normalization (biochemical response)a |
B |
| Optimal endpoint HBsAg loss (± anti-HBs seroconversion)b |
B |
aAchieved in most patients with long-term suppression of HBV replication. bIndicates profound suppression of HBV replication and viral protein expression
Treatment indications
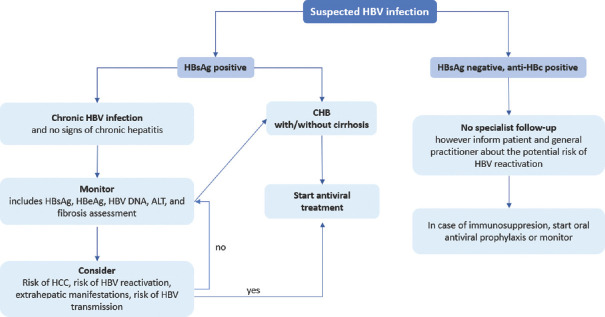
Indications for treatment are in general the same for HBeAg-positive and HBeAg-negative patients, and this is based mainly upon the combination of serum HBV DNA levels, serum ALT levels and severity of disease[1] [Figure 2].
Figure 2.
Algorithm for the management of HBV infection
Non-cirrhotic patients should be considered for treatment if they have HBV DNA levels >2,000 IU/mL, serum ALT >~40 IU/L and severity of liver disease assessed by liver biopsy showing at least moderate necroinflammation and/or at least moderate fibrosis. Patients with HBV DNA greater than 20,000 IU/mL and ALT greater than 2x ULN can begin treatment without a liver biopsy. Patients with HBV DNA >2,000 IU/mL and at least moderate fibrosis may initiate treatment even if ALT levels are normal. In patients unwilling or unable to undergo liver biopsy, non-invasive markers of fibrosis may instead be used to decide on treatment indications. Treatment indications should also take into account patient's age, health status, risk of HBV transmission, family history of HCC or cirrhosis and extrahepatic manifestations [Figure 2].[1]
Recommendations for initiation of treatment
All patients with chronic hepatitis B (HBV DNA > 2,000 IU/mL, ALT > ULN), regardless of HBeAg status, and/or at least moderate liver necroinflammation or fibrosis (Grade A)
Patients with cirrhosis (compensated or decompensated), with any detectable HBV DNA level and regardless of ALT levels (Grade A)
Patients with HBV DNA > 20,000 IU/mL and ALT > 2xULN, regardless of the degree of fibrosis (Grade B)
Patients with HBeAg-positive chronic HBV infection (persistently normal ALT and high HBV DNA levels) may be treated if they are > 30 years, regardless of the severity of liver histological lesions (Grade D)
Patients with chronic HBV infection (HBV DNA > 2,000 IU/mL, ALT > ULN), regardless of HBeAg status, and a family history of HCC or cirrhosis and extrahepatic manifestations (Grade D)
Monitoring of therapy of patients currently not treated
Patients not candidate for antiviral therapy should be periodically assessed to determine whether an indication for treatment has developed. Serum ALT and HBV DNA levels as well as fibrosis severity by non-invasive markers should be regularly evaluated. HBeAg-positive untreated patients should be tested for ALT every 3 months, HBV DNA determinants every 6–12 months and assessment for liver fibrosis every 12 months. HBeAg-negative patients with HBV DNA <2,000 IU/ml should have ALT determinations every 6–12 months and periodical HBV DNA and liver fibrosis assessments, every year. Determination of quantitative HBsAg levels can help in the decision for follow-up frequency in these patients.[54] However, two studies with Saudi patients have shown that correlation of qHBsAg levels with liver fibrosis was poor in these patients, wherein qHBsAg levels demonstrated a poor association with histological findings. Overall, these studies have shown that predictability based on qHBsAg levels of 1000 IU was not supportive of fibrosis.[55,56]
For HBeAg-negative patients with HBV DNA ≥2,000 IU/ml, ALT should be determined at least every 3 months for the first year and every 6 months thereafter. Assessments of HBV DNA and liver fibrosis by non-invasive methods every year for at least 3 years should be considered. If they do not fulfill any treatment indication within the first 3 years of follow-up, they should be followed for life, similar to all patients in this phase.[57]
Recommendations for monitoring of therapy of patients currently not treated
Patients with HBeAg-positive chronic HBV infection who are younger than 30 years should be followed at least every 3-6 months (Grade B)
Patients with HBeAg-negative chronic HBV infection and serum HBV DNA <2,000 IU/ml should be followed every 6-12 months (Grade B)
Patients with HBeAg-negative chronic HBV infection and serum HBV DNA ≥2,000 IU/ml should be followed every 3 months for the first year and thereafter every 6 months (Grade D)
Treatment of CHB
Overall, the available NA therapies for HBV can be categorized into medications that have low barrier for resistance, including Lamivudine (LAM), Telbivudine (TBV) and Adefovir (ADV),[58] and medications that have high barrier for resistance, which include Entecavir (ETV), Tenofovir Disoproxil Fumarate (TDF) and Tenofovir Alafenamide (TAF).[59,60,61]
LAM is one of the first medications used to treat CHB. However, the use of LAM is associated with an unacceptably high rate of resistance, reaching 49% at 3 years and 70% in 5 years.[58] ADV had a better resistance profile compared to LAM, with 11% and 29% resistance rate in 3 and 5 years, respectively.[58] But this rate is now considered too high when compared with the relatively new generation of nucleot(s)ide analogues.
ETV is a potent guanosine analogue. First evaluated vs LAM, it showed an excellent efficacy and safety profile.[62] The virological response rate (defined as negative HBV DNA by PCR) approaches 100% over 5 years of its use, in both HBeAg-negative and HBeAg-positive patients.[59] ETV has a high barrier for resistance, where after 5 years of its use, only 1% had emergence of resistance.[63]
TDF is a prodrug of tenofovir which is a nucleotide inhibitor that acts on both hepatitis B and HIV.[58] Similar to ETV, TDF has an excellent efficacy profile. A retrospective cohort study of treatment-naive CHB patients who were treated with TDF or ETV showed that both were effective in achieving complete viral response and had a favorable safety profile.[64] After seven years of treatment with TDF, 99.3% of patients achieved and maintained virological response.[58] Treatment with TDF did not result in detectable resistance after 8 years of treatment.[65]
Due to the side-effect profile, difficulty of use, and the presence of better alternatives, the use of “conventional interferon” is not recommended, and peglated interferon should be used instead.[1,36] Since the release of the last guidelines in 2014, the concept of futility “endpoint” or the “stopping rule” when treating hepatitis B with peglated interferon has been introduced.[1,36,66]
The importance of pretreatment predictors of response has been highlighted previously. The new evidence of stopping treatment with interferon is of great importance due to its side-effect profile, and the excellent predictive value for those parameters on treatment outcomes, which are dependent on serum HBsAg levels.[67] In HBeAg-positive patients, the lack of decline in qHBsAg levels (in genotypes A and D) or maintaining qHBsAg levels to more than 20,000 (in genotypes B and C) is a strong predictor of failure of treatment, with a negative predictive value of 92 to 100% for HBeAg seroconversion.[67] In HBeAg-negative patients, the data is not as robust as is with HBeAg-positive patients,[53] with most of the evidence on genotype D.[68,69] Overall, patients who have no decline in qHBsAg levels and have no decrease in HBV DNA level by 2-log, will have very low chance of sustained treatment response, defined by HBV DNA level less than 2000 IU/mL after the end of treatment.[54]
TAF is another NA that acts by inhibiting reverse transcription of the pregenomic RNA to HBV DNA. Compared with TDF, it has higher plasma stability and thus less accumulation in bone and kidney tissue. In both HBeAg-positive and HBeAg-negative patients, TAF achieved non-inferiority to TDF in terms of efficacy of suppressing the viral load.[70,71] For HBeAg-positive patients, 873 were assigned randomly to either TAF or TDF in a 2:1 design. Sixty four percent of the patients achieved virological response in the TAF group compared to 67% in TDF group, after 48 weeks of treatment, which met the definition of non-inferiority.[70] The results from HBeAg-negative study comparing TAF to TDF were similar. A total of 426 patients randomized in a 2:1 distribution between TAF and TDF, the virological response was 94% and 93% respectively between the two groups at 48 weeks.[71] Long-term study on the same cohort was used for the aforementioned two trials of TAF approval. Patients were analyzed after 96 weeks from starting treatment. In the HBeAg-positive patients group the response rate was similar between TAF and TDF (73% and 75% respectively). This was also demonstrated in the HBeAg-negative group, with TAF and TDF having an insignificant difference in virological response (90% and 91%, respectively).
Recent evidence has suggested that sustained decrease in glomerular filtration rate and bone density was observed in patients receiving TDF.[72] In several head-to-head trials between TAF and TDF, TAF was superior in term of renal and bone density safety compared with TDF.[70,71] The difference in bone density was observed in both spine and hip. When compared to TDF, TAF was associated with a significantly smaller decrease in bone density, with a mean change of -0.10% in TAF group versus -1.72% in TDF group.[70] The difference between TAF and TDF was also observed on the glomerular filtration rate. TAF was associated with a significantly smaller drop in eGFR -1.8 mL/min vs -4.8 mL/min for TDF.
The significant difference in terms of renal and bone health was reaffirmed by switching from TDF to TAF with a demonstrable improvement in bone density and estimated glomerular filtration rate (eGFR). After patients were on TDF for a median of 222 weeks, they were switched to TAF, and within 48 weeks the patients showed a significant improvement in both bone mineral density and GFR.[73] It is thus recommended by international guidelines to avoid/switch people who have high-risk factors for bone disease or renal disease from TDF to either TAF or ETV.[1,36]
Several new and more potent NAs are currently under different clinical phases of development.[74] However, novel therapeutic drugs are needed to eradicate all forms of replicating HBV either through targeting different steps of HBV life cycle or modulating the host immune system. Entry is the first step for HBV infecting hepatocytes. Therefore, entry inhibitors have been proposed as promising agents for protecting uninfected hepatocytes. Currently, new drugs targeting viral entry receptors are being developed and evaluated.[75] Eliminating HBV cccDNA within the hepatocyte is another therapeutic option that is also being investigated.[76] RNA interference-mediated inhibition of gene expression and protein production has become a tool in antiviral gene therapy and has been evaluated in HBV infected animal models.[77] Interrupting HBV life cycle through targeted core assembly modulators or the various stages of cellular replication is also being investigated with promising potentials in the near future.[78] Inhibition of HBsAg release may help to restore the HBV-specific T-cell-mediated immune response. Recent clinical studies have demonstrated that treatment with HBsAg release inhibitors leads to the loss of HBsAg in the majority of CHB patients.[79,80]
Host immune responses play important roles in the control of chronic HBV infection. Based on the immunopathogenesis of HBV infection, modulating innate or adaptive immunity, or both in combination with other direct antiviral drugs to control HBV infection, may provide new strategies for CHB treatment. Currently, multiple agents including therapeutic vaccines are under investigation.[81,82]
Recommendations
The treatment of choice is the long-term administration of a potent NA with a high barrier to resistance, regardless of the severity of liver disease (Grade A)
Preferred regimens are ETV, TDF and TAF as monotherapies (Grade A)
LAM, ADV and TBV are not recommended in the treatment of CHB (Grade A)
TREATMENT OF HBV IN SPECIAL POPULATIONS
HBV-HCV coinfection
Early studies, after the introduction of direct antiviral therapy (DAA) for the treatment of HCV, reported HBV reactivation after initiation of DAA.[83] Based on these studies, the US Food and Drug Administration issued a warning about the risk of HBV reactivation. However, subsequent studies showed that the risk of reactivation is very low for patients with chronic or past infection with HBV.[84] Patients who meet the criteria for HBV treatment should be treated concurrently or before initiation of DAA. HBV DNA and ALT should be monitored every four to eight weeks while on DAA and three months after completion of therapy. Patients with positive HBsAg who do not meet the criteria for HBV treatment should be monitored closely while on DAA. We recommend ALT level monitoring every four weeks while on DAA for patients who are HBsAg-negative but HBcAb-positive. If ALT starts to rise, HBsAg and HBV DNA must be obtained to determine the need to start HBV treatment.
Recommendations
Treatment of HCV through DAAs may lead to reactivation of HBV. Patients who meet the criteria for HBV treatment should be treated concurrently or before initiation of DAA (Grade A)
HBV DNA and ALT should be monitored every four to eight weeks while on DAA and three months after completion of therapy (Grade D)
ALT level should be monitored every four weeks while on DAA for patients who are HBsAg-negative but HBcAb-positive. If ALT starts to rise, HBsAg and HBV DNA must be obtained to determine the need to start HBV treatment (Grade D).
HBV-HDV coinfection
We refer the reader to our previous guidance on the management of HBV co-infection with hepatitis delta virus (HDV).[3] There are several ongoing studies for targeted therapy. However, based on the currently available literature no new recommendations can be made on the management of HBV-HDV co-infection.
HBV-HIV coinfection
Patients with HBV-HIV coinfection are at increased risk of rapid fibrosis progression, development of HCC, and liver-related mortality.[85] The prevalence of HBV in patients with HIV coinfection in Saudi Arabia is 3%, which is much higher than the general population.[86] All patients with HBV-HIV coinfection should receive antiretroviral therapy (ART).[87] Patients must be followed closely after initiation of ART, given the risk of immune reconstitution syndrome, which may lead to HBV flare. The regimen must include tenofovir with either formulation TDF or TAF. TAF has a better safety profile and is preferred over TDF unless the patient has CrCl < 15 mL/minute. Emtricitabine and LAM should be included in the ART regimen.[88]
Recommendations
All HIV-positive patients with HBV co-infection should start ART irrespective of CD4 cell count (Grade A)
HBV-HIV co-infected patients should be treated with TDF- or TAF-based ART regimen (Grade A)
Immunocompromised patients
Hepatitis B flare during chemotherapy treatment or treatment with other immunosuppressive agents is potentially life threatening. The risk is very high, particularly with the use of CD20 depleting agents.[89] Therefore, all patients undergoing immunosuppressive treatment or chemotherapy, even short-term courses, should be screened for HBsAg, anti-HBc, and anti-HBs (and HBV DNA, if HBsAg is already positive).[90] We recommend prophylaxis for all patients with positive HBsAg before initiating chemotherapy or other immunosuppressive agents. For HBsAg-negative and anti-HBc positive patients, we recommend HBV prophylaxis if they are candidates for anti CD20 or are undergoing stem cell transplantation. HBV prophylaxis should be continued for at least six months after completion of immunosuppressive treatment and twelve months if patients were taking anti CD20.
We recommend starting HBV prophylaxis for HBsAg or anti-HBc positive patients undergoing treatment with tumor necrosis factor (TNF) inhibitors. The risk of HBV reactivation is moderate with anti-TNF therapy. However, most patients will require another immunosuppressant agent. Therefore, we recommend HBV prophylaxis for all HBsAg or anti-HBc positive patients who are going to receive anti-TNF therapy.
HBV reactivation has been reported in some patients who received immunotherapy such as anti-programmed cell death (PD) -1 and anti-programmed cell death-ligand 1 (PD-L1) therapy. Therefore, we recommend HBV prophylaxis for all patients who are HBsAg or anti-HBc positive before initiation of such therapies.[91]
Recommendations
Prophylaxis for all patients with positive HBsAg should be done before initiating chemotherapy or other immunosuppressive agents (Grade A)
HBsAg-negative/anti-HBc-positive patients, should undergo HBV prophylaxis if they are candidates for anti CD20 or are undergoing stem cell transplantation. HBV prophylaxis should continue for at least six months after completion of immunosuppressive treatment and for twelve months if taking anti CD20 (Grade D).
HBV and pregnancy
The most effective way to prevent mother-to-child transmission is to detect HBV early in pregnancy. Therefore, all pregnant women must be screened for HBV during the first trimester. HBV vaccine is safe in pregnancy and should be given to pregnant women who are not immune to HBV and have no evidence of chronic HBV infection.[36] Pregnant women should be treated if they meet the standard indication of therapy. We recommend HBV treatment if HBV DNA is greater than 100,000 IU/mL in the late second trimester (between 24-28 weeks of gestation). TDF is the drug of choice during pregnancy. However, more recently, a multi-center experience from China reported no mother-to-child transmission or developmental anomalies in 71 infants born to mothers who received TAF during the last trimester of pregnancy. In addition, no severe adverse events were reported in either mothers or infants.[92] LAM is a less favorable option but can be considered if TDF or TAF are not available. If the patient is receiving ETV, ADV, or interferon during pregnancy, we recommend switching to TDF or TAF. The mode of delivery should be based on obstetric indications and not solely on HBV infection. Breastfeeding is not a contraindication, provided immunoprophylaxis was given at birth.[93]
Recommendations
All pregnant women must be screened for HBV during the first trimester (Grade A)
All pregnant women with HBV DNA greater than 100,000 IU/mL in the late second trimester (between 24-28 weeks of gestation) should start antiviral prophylaxis with TDF, or TAF as an alternative (Grade D)
Switch to TDF or TAF is recommended if the patient is receiving ETV, ADV, or interferon during pregnancy (Grade D)
Breastfeeding is not contraindicated in HBsAg-positive untreated women or on TDF-based treatment or prophylaxis (Grade B)
HBV INTERNATIONAL REGULATIONS
International guidelines for the management of HBV-infected HCWs attempt to ethically balance the risk of disease transmission to the patient with the right of the infected HCW to perform their work in a safe manner and without loss of the right to confidentiality about their own health condition. Although there are some differences among guidelines, it is important to note that none of them prohibits the practice of invasive procedures by an HBV-infected HCW.[94]
Financial support and sponsorship
Nil.
Conflicts of interest
Drs. Abaalkhail, Khathlan, and Sanai have served as consultants and speakers for Bristol Myers Squibb and Gilead. Dr. Sanai has received grant support from Bristol Myers Squibb, Gilead and Roche Pharmaceuticals. Others have nothing to disclose..
Acknowledgements
The authors would like to thank to Alexandra Sanfins, PhD and Ana Rolo, PhD for providing medical writing support and assistance with the manuscript.
REFERENCES
- 1.Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 3.Abaalkhail F, Elsiesy H, AlOmair A, Alghamdi MY, Alalwan A, AlMasri N, et al. SASLT practice guidelines for the management of hepatitis B virus. Saudi J Gastroenterol. 2014;20:5–25. doi: 10.4103/1319-3767.126311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 5.Sanai FM, Alghamdi H, Alswat KA, Babatin MA, Ismail MH, Alhamoudi WK, et al. Greater prevalence of comorbidities with increasing age: Cross-sectional analysis of chronic hepatitis B patients in Saudi Arabia. Saudi J Gastroenterol. 2019;25:194–200. doi: 10.4103/sjg.SJG_447_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Faleh FZ. Hepatitis B infection in Saudi Arabia. Ann Saudi Med. 1988;8:474–80. [Google Scholar]
- 7.Al-Faleh FZ, Al-Jeffri M, Ramia S, Al-Rashed R, Arif M, Rezeig M, et al. Seroepidemiology of hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J Infect. 1999;38:167–70. doi: 10.1016/s0163-4453(99)90245-1. [DOI] [PubMed] [Google Scholar]
- 8.Al-Faleh FZ, Ayoola EA, Arif M, Ramia S, Al-Rashed R, Al-Jeffry M, et al. Seroepidemiology of hepatitis B virus infection in Saudi Arabian children: A baseline survey for mass vaccination against hepatitis B. J Infect. 1992;24:197–206. doi: 10.1016/0163-4453(92)93006-c. [DOI] [PubMed] [Google Scholar]
- 9.Alswaidi FM, O'Brien SJ. Is there a need to include HIV, HBV and HCV viruses in the Saudi premarital screening program on the basis of their prevalence and transmission risk factors? J Epidemiol Community Health. 2010;64:989–97. doi: 10.1136/jech.2009.093302. [DOI] [PubMed] [Google Scholar]
- 10.El-Hazmi M. Prevalence of HBV, HCV, HIV-1, 2 and HTLV-I/II infections among blood donors in a teaching hospital in the Central region of Saudi Arabia. Saudi Med J. 2004;25:26–33. [PubMed] [Google Scholar]
- 11.Sallam T, El-Bingawi H, Alzahrani K, Alzahrani B, Alzahrani A. Prevalence of hepatitis B and hepatitis C viral infections and impact of control program among blood donors in Al-Baha region, Saudi Arabia. Saudi J Health Sci. 2020;9:56–60. [Google Scholar]
- 12.Alzahrani FM, Muzaheed, Shaikh SS, Alomar AI, Acharya S, Elhadi N. Prevalence of Hepatitis B Virus (HBV) among blood donors in eastern Saudi Arabia: Results from a five-year retrospective study of HBV seromarkers. Ann Lab Med. 2018;39:81–5. doi: 10.3343/alm.2019.39.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis DP, Favero MS, Maynard JE. Transmission of hepatitis B virus. Semin Liver Dis. 1981;1:27–32. doi: 10.1055/s-2008-1063927. [DOI] [PubMed] [Google Scholar]
- 14.Antiretrovir JA. Current Prevalence of HBV and HCV Seropositivity: The initiative for attentiveness and deterrence of viral hepatitis in the Qassim Region of Saudi Arabia. J Antivir Antiretrovir. 2012;4:75. [Google Scholar]
- 15.Abdullah SM. Prevalence of hepatitis B and C virus infection and their co-relation with hematological and hepatic parameters in subjects undergoing premarital screening in the Jazan Region, Kingdom of Saudi Arabia. Pakistan J Med Sci. 2018;34:316–21. doi: 10.12669/pjms.342.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albadran A, Hibshi A, Saeed B, Coskun S, Awartani KA. Hepatitis B and C virus prevalence in couples attending an in vitro fertilization clinic in a tertiary care hospital in Saudi Arabia: Comparison with ten years earlier. Ann Saudi Med. 2017;37:272–5. doi: 10.5144/0256-4947.2017.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esmat G. A review of chronic hepatitis B epidemiology and management issues in selected countries in the Middle East. J Viral Hepat. 2012;19:9–22. doi: 10.1111/j.1365-2893.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 18.Sanai FM, Alghamdi M, Dugan E, Alalwan A, Al-Hamoudi W, Abaalkhail F, et al. A tool to measure the economic impact of Hepatitis B elimination: A case study in Saudi Arabia. J Infect Public Health. 2020;13:1715–23. doi: 10.1016/j.jiph.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis. Liver Int. 2016;36:1239–51. doi: 10.1111/liv.13142. [DOI] [PubMed] [Google Scholar]
- 20.Varbobitis I, Papatheodoridis GV. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin Mol Hepatol. 2016;22:319–26. doi: 10.3350/cmh.2016.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papatheodoridis GV, Chan HLY, Hansen BE, Janssen HLA, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–67. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, et al. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: Limited role for risk scores in Caucasians. Gut. 2015;64:1289–95. doi: 10.1136/gutjnl-2014-307023. [DOI] [PubMed] [Google Scholar]
- 23.Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, Arends P, et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol. 2015;62:363–70. doi: 10.1016/j.jhep.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 24.Sangiovanni A, Colombo M. Surveillance for hepatocellular carcinoma in patients with advanced liver fibrosis. Saudi J Gastroenterol. 2021;27:64–72. doi: 10.4103/sjg.sjg_636_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–6. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer W, van der Meer A, Boonstra A, Plompen E, Pas S, de Knegt R. The PAGE-B score accurately predicts clinical outcome and outperforms other biomarkers over 15 years of follow-up in a diverse cohort of chronic hepatitis B patients. Hepatology. 2015;62(Suppl):93A–207A. [Google Scholar]
- 27.AlFaleh F, AlShehri S, AlAnsari S, AlJeffri M, AlMazrou Y, Shaffi A, et al. Long-term protection of hepatitis B vaccine 18 years after vaccination. J Infect. 2008;57:404–9. doi: 10.1016/j.jinf.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: Are we off the hook. Saudi J Gastroenterol. 2012;18:349–57. doi: 10.4103/1319-3767.103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol Hepatol. 2008;2:553–62. doi: 10.1586/17474124.2.4.553. [DOI] [PubMed] [Google Scholar]
- 30.Coffin CS, Zhou K, Terrault NA. New and old biomarkers for diagnosis and management of chronic Hepatitis B virus infection. Gastroenterology. 2019;156:355–68.e3. doi: 10.1053/j.gastro.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grob P, Jilg W, Bornhak H, Gerken G, Gerlick W, Günther S, et al. Serological pattern “anti-HBc alone”: Report on a workshop. J Med Virol. 2000;62:450–5. doi: 10.1002/1096-9071(200012)62:4<450::aid-jmv9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 32.Lok AS, Lai CL, Wu PC. Prevalence of isolated antibody to hepatitis B core antigen in an area endemic for hepatitis B virus infection: Implications in hepatitis B vaccination programs. Hepatology. 1988;8:766–70. doi: 10.1002/hep.1840080411. [DOI] [PubMed] [Google Scholar]
- 33.Nie H, Evans AA, London WT, Block TM, Ren XD. Quantitative dynamics of hepatitis B basal core promoter and precore mutants before and after HBeAg seroconversion. J Hepatol. 2012;56:795–802. doi: 10.1016/j.jhep.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall K. Max Wilson and the Principles and Practice of Screening for Disease. Int J Neonatal Screen. 2020;6:15. doi: 10.3390/ijns6010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aljumah A, Babatin M, Hashim A, Abaalkhail F, Bassil N, Safwat M, et al. Hepatitis B care pathway in Saudi Arabia: Current situation, gaps and actions. Saudi J Gastroenterol. 2019;25:73–80. doi: 10.4103/sjg.SJG_421_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terrault NA, Lok ASF, Mcmahon BJ, Chang K-M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology. 2018;67:1560–99. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25:93–159. doi: 10.3350/cmh.2019.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DK, Bridges CB, Harriman KH. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older — United states, 2016. Morb Mortal Wkly Rep. 2016;65:88–9. doi: 10.15585/mmwr.mm6504a5. [DOI] [PubMed] [Google Scholar]
- 39.FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B vaccination: A completed schedule enough to control HBV lifelong?. Milan, Italy, 17.18 November 2011. Vaccine. 2013;31:584–90. doi: 10.1016/j.vaccine.2012.10.101. [DOI] [PubMed] [Google Scholar]
- 40.Pinkbook | Hepatitis B | Epidemiology of Vaccine Preventable Diseases | CDC [Internet] [cited 2020 Jul 21]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html .
- 41.Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27:e1942. doi: 10.1002/rmv.1942. doi: 10.1002/rmv. 1942. [DOI] [PubMed] [Google Scholar]
- 42.2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host | Clinical Infectious Diseases | Oxford Academic [Internet] [cited 2020 Jul 21]. Available from: https://academic.oup.com/cid/article/58/3/e44/336537 .
- 43.Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis [Internet] [cited 2020 Jul 21]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm . [PubMed]
- 44.Ouseph R, Eng M, Ravindra K, Brock GN, Buell JF, Marvin MR. Review of the use of hepatitis B core antibody-positive kidney donors. Transplant Rev (Orlando) 2010;24:167–71. doi: 10.1016/j.trre.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Wong GLH, Chan HLY, Yu Z, Chan AWH, Choi PCL, Chim AML, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B-A prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883–93. doi: 10.1111/apt.12658. [DOI] [PubMed] [Google Scholar]
- 46.Chan AWH, Wong GLH, Chan HY, Tong JHM, Yu YH, Choi PCL, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667–76. doi: 10.1111/jgh.13536. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Sinn DH, Gwak GY, Kang W, Paik YH, Choi MS, et al. Insulin resistance and the risk of hepatocellular carcinoma in chronic hepatitis B patients. J Gastroenterol Hepatol. 2017;32:1100–6. doi: 10.1111/jgh.13647. [DOI] [PubMed] [Google Scholar]
- 48.Kim NH, Cho YK, Kim BI, Kim HJ. Effect of metabolic syndrome on the clinical outcomes of chronic Hepatitis B patients with nucleos(t) ide analogues treatment. Dig Dis Sci. 2018;63:2792–9. doi: 10.1007/s10620-018-5165-6. [DOI] [PubMed] [Google Scholar]
- 49.Villa E, Barchi T, Grisendi A, Bellentani S, Rubbiani L, Ferretti I, et al. Susceptibility of chronic symptomless HBsAg carriers to ethanol-induced hepatic damage. Lancet. 1982;320:1243–4. doi: 10.1016/s0140-6736(82)90104-0. [DOI] [PubMed] [Google Scholar]
- 50.Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette Smoking, Alcohol Drinking, Hepatitis B, and Risk for Hepatocellular Carcinoma in Korea. [cited 2020 Jul 21]. Available from: https://academic.oup.com/jnci/article.abstract/96/24/1851/2521104 . [DOI] [PubMed]
- 51.Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic Hepatitis B: A large cohort study. Gastroenterology. 2017;153:1006–17.e5. doi: 10.1053/j.gastro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Prevention of Hepatitis A Through Active or Passive Immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) [Internet] [cited 2020 Jul 21]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm .
- 53.Beasley RP, Chin-Yun Lee G, Roan CH, Hwang LY, Lan CC, Huang FY, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis b immune globulin and hepatitis b vaccine. Lancet. 1983;322:1099–102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- 54.Cornberg M, Wong VWS, Locarnini S, Brunetto M, Janssen HLA, Chan HLY. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66:398–411. doi: 10.1016/j.jhep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Alghamdi A, Aref N, El-Hazmi M, Al-Hamoudi W, Alswat K, Helmy A, et al. Correlation between Hepatitis B surface antigen titers and HBV DNA levels. Saudi J Gastroenterol. 2013;19:252–7. doi: 10.4103/1319-3767.121035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed F, Bajaifar M, Ahmed M, Alalwan A, Sanai F, Albeladi K, et al. Quantitative HBsAg levels do not identify hepatic fibrosis in HBeAg-negative chronic hepatitis B patients. Saudi J Gastroenterol. 2019;25:286–92. doi: 10.4103/sjg.SJG_80_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: A systematic review. J Hepatol. 2012;57:196–202. doi: 10.1016/j.jhep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 58.Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1457–64. doi: 10.1007/s10620-014-3486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lampertico P, Soffredini R, Viganò M, Minola E, Cologni G, Rizzi M, et al. 755 5-year entecavir treatment in nuc-naïve, field-practice patients with chronic hepatitis B showed excellent viral suppression and safety profile but no prevention of HCC in cirrhotics. J Hepatol. 2013;58:S306–7. [Google Scholar]
- 60.Cathcart AL, Lik-Yuen Chan H, Bhardwaj N, Liu Y, Marcellin P, Pan CQ, et al. No resistance to tenofovir alafenamide detected through 96 weeks of treatment in patients with chronic hepatitis B infection. Antimicrob Agents Chemother. 2018;62:e01064–18. doi: 10.1128/AAC.01064-18. doi: 10.1128/AAC.01064-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lok ASF, Mcmahon BJ, Brown RS, Wong JB, Ahmed AT, Farah W, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 62.Chang TT, Gish RG, De Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 63.Chang TT, Lai CL, Yoon SK, Lee SS, Coelho HSM, Carrilho FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–30. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 64.Yu HM, Kwon SY, Kim J, Chung HA, Kwon SW, Jeong TG, et al. Virologic response and safety of tenofovir versus entecavir in treatment-naïve chronic Hepatitis B patients. Saudi J Gastroenterol. 2015;21:146–51. doi: 10.4103/1319-3767.157558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, Kitrinos KM, Marcellin P, Gane EJ. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+and HBeAg- patients with chronic hepatitis B after 8 years of treatment. J Viral Hepat. 2017;24:68–74. doi: 10.1111/jvh.12613. [DOI] [PubMed] [Google Scholar]
- 66.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, et al. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872–80. doi: 10.1002/hep.26436. [DOI] [PubMed] [Google Scholar]
- 68.Rijckborst V, Hansen BE, Ferenci P, Brunetto MR, Tabak F, Cakaloglu Y, et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol. 2012;56:1006–11. doi: 10.1016/j.jhep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Goulis I, Karatapanis S, Akriviadis E, Deutsch M, Dalekos GN, Raptopoulou-Gigi M, et al. On-treatment prediction of sustained response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B patients. Liver Int. 2015;35:1540–8. doi: 10.1111/liv.12725. [DOI] [PubMed] [Google Scholar]
- 70.Chan HLY, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185–95. doi: 10.1016/S2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 71.Buti M, Gane E, Seto WK, Chan HLY, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 72.Lampertico P, Chan HLY, Janssen HLA, Strasser SI, Schindler R, Berg T. Review article: Long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44:16–34. doi: 10.1111/apt.13659. [DOI] [PubMed] [Google Scholar]
- 73.Lampertico P, Buti M, Fung S, Ahn SH, Chuang WL, Tak WY, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: a randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol Hepatol. 2020;5:441–53. doi: 10.1016/S2468-1253(19)30421-2. [DOI] [PubMed] [Google Scholar]
- 74.Lai CL, Ahn SH, Lee KS, Um SH, Cho M, Yoon SK, et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut. 2014;63:996–1004. doi: 10.1136/gutjnl-2013-305138. [DOI] [PubMed] [Google Scholar]
- 75.Blank A, Markert C, Hohmann N, Carls A, Mikus G, Lehr T, et al. First-in-human application of he novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol. 2016;65:483–9. doi: 10.1016/j.jhep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Hu J, Cai X, Huang Y, Zhou X, Tu Z, et al. APOBEC3B edits HBV DNA and inhibits HBV replication during reverse transcription. Antiviral Res. 2018;149:16–25. doi: 10.1016/j.antiviral.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017;9:eaan0241. doi: 10.1126/scitranslmed.aan0241. doi: 10.1126/scitranslmed.aan0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lam AM, Espiritu C, Vogel R, Ren S, Lau V, Kelly M, et al. Preclinical characterization of NVR 3-778, a first-in-class capsid assembly modulator against hepatitis B virus. Antimicrob Agents Chemother. 2018;63:e01734–18. doi: 10.1128/AAC.01734-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): A non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877–89. doi: 10.1016/S2468-1253(17)30288-1. [DOI] [PubMed] [Google Scholar]
- 80.Usman Z, Mijočević H, Karimzadeh H, Däumer M, Al-Mathab M, Bazinet M, et al. Kinetics of hepatitis B surface antigen quasispecies during REP 2139-Ca therapy in HBeAg-positive chronic HBV infection. J Viral Hepat. 2019;26:1454–64. doi: 10.1111/jvh.13180. [DOI] [PubMed] [Google Scholar]
- 81.Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55:762–9. doi: 10.1016/j.jhep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 82.Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10:e1004210. doi: 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: A review of cases reported to the U.S. Food and drug administration adverse event reporting system. Ann Intern Med. 2017;166:792–8. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 84.Belperio PS, Shahoumian TA, Mole LA, Backus LI. Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology. 2017;66:27–36. doi: 10.1002/hep.29135. [DOI] [PubMed] [Google Scholar]
- 85.Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 86.Alhuraiji A, Alaraj A, Alghamdi S, Alrbiaan A, Alrajhi AA. Viral hepatitis B and C in HIV-infected patients in Saudi Arabia. Ann Saudi Med. 2014;34:207–10. doi: 10.5144/0256-4947.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Günthard HF, Saag MS, Benson CA, Del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in Adults: 2016 recommendations of the international antiviral society-USA Panel. JAMA. 2016;316:191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gibert CL. Treatment Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents: An Update. Fed Pract. 2016;33(Suppl 3):31S–36S. [PMC free article] [PubMed] [Google Scholar]
- 89.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–44.e3. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 90.Shih CA, Chen WC, Yu HC, Cheng JS, Lai KH, Hsu JT, et al. Risk of severe acute exacerbation of chronic HBV infection cancer patients who underwent chemotherapy and did not receive anti-viral prophylaxis. PLoS One. 2015;10:e0132426. doi: 10.1371/journal.pone.0132426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7:322. doi: 10.1186/s40425-019-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dionne-Odom J, Tita ATN, Silverman NS. #38: Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6–14. doi: 10.1016/j.ajog.2015.09.100. [DOI] [PubMed] [Google Scholar]
- 93.Ding Y, Cao L, Zhu L, Huang Y, Lin C, Wang Y, et al. Efficacy and safety of tenofovir alafenamide fumarate for preventing mother-to-child transmission of hepatitis B virus: A national cohort study. Aliment Pharmacol Ther. 2020;52:1377–1386. doi: 10.1111/apt.16043. [DOI] [PubMed] [Google Scholar]
- 94.Lewis JD, Enfield KB, Sifri CD. Hepatitis B in healthcare workers: Transmission events and guidance for management. World J Hepatol. 2015;7:488–97. doi: 10.4254/wjh.v7.i3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]