Abstract
Background:
We aimed to evaluate the therapeutic effect of additional ursodeoxycholic acid (UDCA) with mesalazine, compared to mesalazine alone in patients with ulcerative colitis (UC). The mechanism was evaluated by monitoring the changes of IL-23-IL-17 axis and the intestinal microflora.
Methods:
In this prospective, single center study, patients with UC were randomly assigned to the Mesalazine group (n=20) or the UDCA + Mesalazine group (n=20). Mayo score and Inflammatory Bowel Disease Questionnaire (IBDQ), and fecal samples for 16S rRNA sequencing and blood samples for IL-23 and IL-17 ELISA were collected for analysis.
Results:
Mayo scores and IBDQ score of the UDCA + Mesalazine group were significantly better than those of the Mesalazine group (P = 0.015 and P < 0.001, respectively). At post-treatment week 4, IL-23 and IL-17 levels were significantly lower in the UDCA + Mesalazine group compared to those in the Mesalazine group (both P < 0.038). In patients with UC after treatment, Firmicutes in the UDCA + Mesalazine group was higher than those in the Mesalazine group (P < 0.001). The UDCA + Mesalazine group showed lower percentage of Proteobacteria compared to those in the Mesalazine group (P < 0.001).
Conclusion:
Additional UDCA could provide better therapeutic effects than mesalazine alone, possibly due to the change of IL-23 and IL-17 and the proportional distribution of intestinal microflora.
Keywords: IL-23, IL-17, mesalazine, microflora, ulcerative colitis, ursodeoxycholic acid
INTRODUCTION
Ulcerative colitis (UC) is a chronic non-specific intestinal inflammatory disease, with the main clinical manifestations of UC including diarrhea, mucopurulent hematochezia, and abdominal pain. Although the etiology remains unclear, the pathological changes of intestine are wide and frequently recur, indicating the long disease duration and the probable life-long effect on the quality of life.[1] Some of the conventional drugs used to treat UC are ineffective, so many new biologics targeting the immune mechanism of UC were developed, based on the findings of the intestinal mucosal immune system dysfunction and pathological T cell responses that were considered as the direct cause of UC.[2,3,4] In particular, IL-23/IL-17 axis can regulate pro-inflammatory factors and is positively correlated with the disease activity of UC.[5,6,7] Although these immune-centered treatments are effective, the high prices may limit the use and side effects may not be tolerated by some patients.[8,9] Thus, the need for new therapeutic medications is of utmost importance.
Ursodeoxycholic acid (UDCA) is a secondary bile acid formed by colonic bacteria. UDCA has shown good cytoprotective, anti-inflammatory properties and therapeutic effects in a rat colitis model.[10] In liver diseases, UDCA improved the stability of microbiota in patients with hepatic insufficiency and primary biliary cholangitis.[11,12] In intestinal diseases, UDCA can prevent recurrent infection caused by Clostridium difficile by inhibiting the activity of other secondary bile acids.[13] In addition, UDCA has proved that it can reverse the physiological imbalance within hepatocytes caused by apoptosis.[14]
Previous studies indicated that compared to patients with UC in remission, the ecological imbalance of the intestinal microflora is more pronounced in active phase patients,[15] and further aggravates the intestinal mucosa and the systemic inflammatory response.[16,17,18,19] UDCA as a phospholipase A2 inhibitor, probably can improve the symptoms of UC and have therapeutic effect on UC through restoration of the intestinal mucus phosphatidylcholine content by reducing the impacts from ectophospholipase-containing intestinal microflora, which is considered as one of the important mechanism of pathogenesis of UC.[20] UDCA derivative, UDCA-LPE has shown to improve the mucosal inflammation in a genetic mouse model of UC via changing the pattern of colonic microbiota distribution.[21]
In the present study, we examined the therapeutic effects of additional UDCA, compared to mesalazine only. For investigating the mechanism of UDCA, we also compared the change of IL-23/IL-17 axis and the distribution of intestinal microflora in patients with UC between the UDCA + Mesalazine and the Mesalazine groups.[10,11,12,13,14,22]
METHODS
Participants
The study protocol was approved by the institutional review board of Fuzhou General Hospital (IRB No. 2016-020), and was registered in Chinese Clinical Trial Registry (ChiCTR200038316). All participants signed a written consent. Newly diagnosed patients with UC were enrolled at the Department of Gastroenterology and Outpatient Clinic of Fuzhou General Hospital. Diagnoses were established based on the consensus guideline published by the British Society of Gastroenterology.[23] Disease severity was defined according to the improved Truelove and Witts disease Degree classification combined with endoscopy.[24,25] Inclusion criteria were: (1) age from 18 to 75 years; (2) disease severity-mild to moderate. No UC treatment was prescribed before baseline for all patients. Patients were randomly assigned to the Mesalazine group and the UDCA + Mesalazine group, n = 20 in each group. Mesalazine (Heilongjiang Timehome Pharmaceutical Co., Ltd , China) was prescribed as 1 g, QID based on the British Gastroenterology Society guidelines, and UDCA (Daewoong Pharmaceutical Co., Ltd, Seoul, South Korea) was 200 mg, BID used the suggestion for reflux esophagitis treatment listed in the package insert as reference. Twenty healthy volunteers were also included as an individual group. Patients with underlying disease had to be controlled well by maintaining appropriated medications. Immunosuppressive agents, steroid hormones, non-steroidal anti-inflammatory drugs and other types of aminosalicylic acid were prohibited during the study period. Antibiotics and probiotics were prohibited for 3 months before UC treatment. The demographical and clinical information, fecal and blood samples were collected at baseline, post-treatment 1 week and 4 weeks, for further analysis.
Evaluations of clinical status and quality of life of patients with UC
Colonoscopy examination was performed at baseline, post-treatment 1 and 4 weeks, using a standard colonoscope (Olympus, Tokyo, Japan). Mayo score[25] was evaluated at baseline and post-treatment 4 weeks,[25] Inflammatory Bowel Disease Questionnaire (IBDQ)[26] were evaluated at baseline and post-treatment 4 weeks.[26]
Measurement of IL-17 and IL-23
Serum samples were collected after overnight starvation for the measurement of IL-17 and IL-23 using ELISA kit (Shanghai Xitang Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer's instruction. The absorbance was measured as the optical density at 450 nm using a microplate reader to calculate the concentrations of IL-17 or IL-23 in the samples.
Evaluation of intestinal microflora
Fecal samples were collected at baseline and post-treatment 1 and 4 weeks, stored at -80°C until analysis was performed. Bacterial 16S ribosomal RNA (rRNA) sequencing was performed to identify the intestinal microflora after constructing a sequencing library using MetaVx Library Construction Kit (GENEWIZ, Inc., South Plainfield, NJ, USA). The quality of the library was evaluated by Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA), and next generation sequencing was performed using Illumina MiSeq sequencer (Illumina, San Diego, CA, USA) and aligned with SILVA 128 rRNA databases.
Statistical analysis
Because of the small sample size, all the continuous data were presented by median and interquartile range (IQR), and the corresponding analyses were performed by non-parametric tests. For comparison between the two treatment groups, Mann-Whitney test was performed. For the comparisons of two repeated measurements within group, Wilcoxon signed ranks test was performed. Bonferroni correction was applied in the above analyses to avoid type I error of multiple comparisons. Categorical data (gender) were presented by count and percentage, and the association of gender and treatments were tested with Fisher's exact test. All analyses were performed with IBM SPSS Statistic 25.0. A two-sided P value less than 0.05 indicated statistical significance.
RESULTS
Patient characteristics
The Mesalazine group included 12 males and 8 females, with ages of 22 to 74 years (median of 50.0 years). The UDCA + Mesalazine group comprised 10 male and 10 female patients, with ages of 22 to 64 years (median of 50.5 years). The baseline characteristics including age, gender, Montreal classification, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels were all comparable between the two treatment groups (P > 0.05), [Table 1]. No drug-related adverse event was found during the study period in both treatment groups.
Table 1.
Comparisons for the characteristics of the two treatment groups in patients with UC
| Patients with UC |
Healthy control group (n=20) | |||
|---|---|---|---|---|
| Mesalazine (n=20) | UDCA+Mesalazine (n=20) | |||
| Age (years) | 50.0 (42.0, 57.5) | 50.5 (40.5, 59.0) | 42.5 (30.0, 50.5) | |
| Gender | Male | 12 (60.0%) | 10 (50.0%) | 9 (45.0%) |
| Female | 8 (40.0%) | 10 (50.0%) | 11 (55.0%) | |
| Montreal classification | E1 (proctitis) | 13 (65.0%) | 13 (65.0%) | NA |
| E2 (left-sided colitis) | 7 (35.0%) | 7 (35.0%) | NA | |
| E3 (pancolitis) | 0 | 0 | NA | |
| Baseline CRP (mg/mL) | 8.5 (6.9, 12.2) | 9.0 (8.0, 12.2) | NA | |
| Baseline ESR (mm/hour) | 24.6 (19.3, 27.8) | 23.8 (17.2, 27.1) | NA | |
Continuous data are presented by medians and IQR. Gender is presented by count and percentage. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; UC, ulcerative colitis. NA: not available
Mayo score
To evaluate the efficacy of treatments for patients with UC, Mayo scores were calculated before and after treatment, based on gastrointestinal symptoms, endoscopic findings, and physician evaluation. Mayo scores at baseline of the two treatment groups were comparable. After treatment, Mayo scores were significantly decreased in both groups. In patients of the Mesalazine group, Mayo scores were significantly decreased from medians of 7.0 at baseline to 5.5 and 3.5 at post-treatment weeks 1 and 4, respectively. Mayo score in patients of the UDCA + Mesalazine group were significantly decreased from medians of 8.5 at baseline to 2.0 at post-treatment week 4, and also significantly decreased from medians of 4.0 at post-treatment week 1 to 2.0 at post-treatment week 4. For the comparisons between the two treatment groups, at post-treatment weeks 1 and 4, Mayo scores in patients of the UDCA + Mesalazine group were significantly lower than those in the Mesalazine group (both P = 0.015, medians of 4.0 vs. 5.5 at post-treatment week 1 and medians of 2.0 vs. 3.5 at post-treatment week 4) [Table 2]. Endoscopic Mayo sub-scores were comparable between the two groups at baseline. In the Mesalazine group, there was no significant change of endoscopic Mayo sub-scores from baseline to post-treatment week 4. In the UDCA + Mesalazine group, endoscopic Mayo sub-score at post-treatment week 4 was significantly lower than that at baseline and post-treatment 1 week (medians of 1.0 at post-treatment week 4 vs. 2.0 and 2.0 at baseline and post-treatment week 1, both P < 0.001). Endoscopic Mayo sub-scores of the UDCA + Mesalazine group were significantly lower than those in the Mesalazine group at post-treatment weeks 4 (medians of 1.0 vs. 2.0, P = 0.017) [Table 2].
Table 2.
Change trends of Mayo and IBDQ scores from baseline to post-treatment week 4 in patients with UC of the two treatment groups
| Patients with UC |
P | |||
|---|---|---|---|---|
| Mesalazine (n=20) | UDCA+Mesalazine (n=20) | |||
| Mayo score | Baseline | 7.00 (5.00, 9.00) | 8.50 (7.00, 10.50) | 0.183 |
| Post-treatment week 1 | 5.50 (4.00, 9.00)a | 4.00 (2.50, 6.00) | 0.015* | |
| Post-treatment week 4 | 3.50 (2.00, 6.50)a | 2.00 (1.00, 3.50)ab | 0.045* | |
| Endoscopic Mayo sub-score | Baseline | 2.0 (2.0, 2.0) | 2.0 (2.0, 2.0) | >0.999 |
| Post-treatment week 1 | 2.0 (1.0, 3.0) | 2.0 (2.0, 2.0) | 0.650 | |
| Post-treatment week 4 | 2.0 (1.0, 2.0) | 1.0 (1.0, 2.0)ab | 0.017* | |
| IBDQ score | ||||
| Total | Baseline | 134.0 (122.5, 140.0) | 142.0 (132.5, 151.0) | 0.064 |
| Post-treatment week 4 | 163.5 (149.5, 167.5)a | 182.5 (173.5, 191.5)a | <0.001* | |
| Social ability | Baseline | 19.5 (16.0, 22.0) | 22.5 (19.5, 26.0) | 0.072 |
| Post-treatment week 4 | 25.0 (18.0, 30.0)a | 30.0 (26.0, 32.0)a | 0.014* | |
| Emotional ability | Baseline | 50.5 (40.5, 60.0) | 52.0 (44.0, 62.0) | 0.774 |
| Post-treatment week 4 | 59.0 (52.0, 62.0) | 64.5 (59.0, 76.5)a | 0.009* | |
| Systemic symptoms | Baseline | 21.5 (19.5, 22.5) | 23.5 (20.0, 27.0) | 0.083 |
| Post-treatment week 4 | 25.5 (23.5, 26.5)a | 29.0 (25.5, 32.0)a | 0.012* | |
| Intestinal symptoms | Baseline | 43.5 (38.0, 47.0) | 48.0 (36.5, 52.0) | 0.310 |
| Post-treatment week 4 | 54.5 (46.5, 59.0)a | 57.5 (51.5, 61.5)a | 0.180 | |
| Data are presented by medians and IQR. | ||||
*P<0.05 indicates a statistically significant difference between the two treatment groups. aindicates a statistically significant change compared to baseline within group. bindicates a statistically significant change compared to post-treatment 1 week within group. IBQD, Inflammatory Bowel Disease Questionnaire; UC, ulcerative colitis
IBDQ score
To better clarify the impact of treatment on quality of life in patients with UC, IBDQ scores before and after treatment were measured. At baseline, the scores of social ability, emotional ability, systemic symptoms, intestinal symptoms, and total score were comparable between the two treatment groups. At post-treatment week 4, the results illustrated that the scores of social ability (medians of 30.0 vs. 25.0, P = 0.014), emotional ability (medians of 64.5 vs. 59.0, P = 0.009), and systemic ability (medians of 29.0 vs. 25.5, P = 0.012) were all significantly higher in patients of the UDCA + Mesalazine group, compared to those in the Mesalazine group. Meanwhile, the total IBDQ scores at post-treatment week 4 were significantly higher in the UDCA + Mesalazine group compared to those in the Mesalazine group (182.5 vs. 163,5P < 0.001) [Table 2].
Serum IL-23 and IL-17 levels
At baseline, serum IL-23 and IL-17 levels had no significant difference between the two treatment groups. Serum IL-23 and IL-17 levels were both decreased after treatment. In the Mesalazine group, IL-23 levels at post-treatment week 4 were significantly lower than those at post-treatment 1 week (medians of 669.2 pg/mL vs. 891.7 pg/mL). In the UDCA + Mesalazine group, IL-23 levels at post-treatment week 4 were significantly lower than those at baseline (medians of 437.7 pg/mL vs. 990.4 pg/mL). IL-17 levels of both treatment groups were significantly decreased. From baseline to post-treatment week 1, in the Mesalazine group, medians decreased from 6.26 pg/mL to 4.53 pg/mL; and in the UDCA + Mesalazine group, from 6.60 pg/mL to 3.66 pg/mL. IL-17 levels were further decreased from post-treatment week 1 to post-treatment week 4, In the Mesalazine group medians decreased from 4.53 pg/mL to 2.94 pg/mL, and in the UDCA + Mesalazine group, from 3.66 pg/mL to 2.67 pg/mL (all P < 0.05). At post-treatment week 4, IL-23 and IL-17 levels were significantly lower in patients of the UDCA + Mesalazine group, compared to those in patients of the Mesalazine group, with P < 0.001 for IL-23 and P = 0.038 for IL-17 [Table 3].
Table 3.
Change trends of IL-23 and IL-17 levels from baseline to post-treatment 4 weeks in patients with UC of the two treatment groups
| Patients with UC |
P | |||
|---|---|---|---|---|
| Mesalazine (n=20) | UDCA+Mesalazine (n=20) | |||
| IL-23 (pg/mL) | Baseline | 962.2 (631.2, 1,477.6) | 990.4 (427.3, 1,450.1) | 0.914 |
| Post-treatment 1 week | 891.7 (860.6, 935.4) | 651.4 (241.6, 1,107.8) | 0.130 | |
| Post-treatment 4 weeks | 669.2 (635.7, 708.9)b | 437.7 (393.8, 471.0)a | <0.001* | |
| IL-17 (pg/mL) | Baseline | 6.26 (4.65, 8.98) | 6.60 (4.78, 7.88) | 0.808 |
| Post-treatment 1 week | 4.53 (3.78, 5.63)a | 3.66 (2.80, 4.84)a | 0.079 | |
| Post-treatment 4 weeks | 2.94 (2.50, 3.61)ab | 2.67 (1.86, 2.98)ab | 0.038* | |
| Data are presented by medians and IQR. | ||||
*Indicates a significant difference between the two treatment groups. sIndicates a significant change compared to baseline level within group. bIndicates a significant change compared to post-treatment 1 week within group
Intestinal microflora before treatment
The Healthy individual group included 11 males and 9 females, with median age of 42.5 years [Table 1]. In the Healthy individual group, the most frequent intestinal microflora was Firmicutes, followed by Bacteroidetes and Bacteroides, with medians of 53.99%, 24.17%, and 5.03%, respectively. In patients with UC before treatment, the most frequent intestinal microflora was Bacteroidetes, followed by Bacteroides and Proteobacteria, with medians of 57.31%, 33.82%, and 25.78%, respectively. Percentages of Bacteroidetes, Bacteroides, Proteobacteria, and Fusobacteria were significantly higher in patients with UC, compared to those in the healthy individuals, and the percentages of Firmicutes and F. prausnitzii were significantly lower in patients with UC, compared to those in the healthy individuals. The differences in the percentages of Escherichia-Shigella, Actinobacteria, prevotella_9, Fusobacterium, and Roseburia between the healthy individuals and patients with UC did not obtain any statistical significance [Figure 1].
Figure 1.
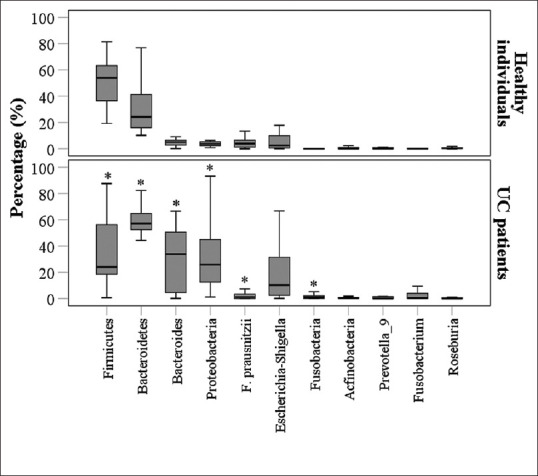
Comparisons of intestinal flora between patients with UC (n= 40, at baseline before treatment) and healthy individuals (n= 20). *P< 0.0045 (0.05/11) indicates a statistically significant difference between patients with UC and the healthy individuals by Bonferroni correction
Treatment effects on intestinal microflora in patients with UC
Bacteroidetes
Both treatment groups showed significant decreases at post-treatment week 4 compared to those at baseline and post-treatment week 1. In the Mesalazine group, medians of percentages were decreased from 57.9% at baseline to 44.9% and 22.0% at post-treatment 1 and 4 weeks, respectively (both P < 0.05). In the UDCA + Mesalazine group, medians of percentages were decreased from 56.7% at baseline to 55.2% and 40.8% at post-treatment 1 and 4 weeks, respectively (both P < 0.05). The differences of Bacteroidetes percentages between the two treatment groups did not obtain any statistical significance at each time point [Table 4].
Table 4.
The treatment effects of Mesalazine and UDCA+Mesalazine on intestinal microflora in patients with UC
| Patients with UC |
P | |||
|---|---|---|---|---|
| Mesalazine (n=20) | UDCA+Mesalazine (n=20) | |||
| Bacteroidetes | Baseline | 57.9 (52.0, 63.3) | 56.7 (52.7, 68.4) | 0.779 |
| Post treatment 1 week | 44.9 (25.4, 54.9) | 55.2 (47.7, 60.3) | 0.040 | |
| Post treatment 4 weeks | 22.0 (12.6, 39.0) ab | 40.8 (31.2, 49.0) ab | 0.013 | |
| Firmicutes | Baseline | 21.3 (17.2, 33.7) | 40.3 (22.2, 62.0) | 0.026 |
| Post treatment 1 week | 26.2 (18.7, 35.7) | 39.7 (25.8, 46.7) | 0.055 | |
| Post treatment 4 weeks | 28.7 (22.7, 47.8) | 61.0 (43.6, 72.3) ab | <0.001* | |
| Proteobacteria | Baseline | 45.0 (21.9, 60.9) | 18.4 (11.6, 30.5) | 0.005 |
| Post treatment 1 week | 31.6 (21.8, 44.7) a | 3.9 (2.6, 9.3) a | <0.001* | |
| Post treatment 4 weeks | 17.3 (11.6, 22.7) ab | 3.7 (2.5, 5.1) a | <0.001* | |
| Fusobacteria | Baseline | 0.57 (0.00, 2.77) | 1.01 (0.08, 1.98) | 0.472 |
| Post treatment 1 week | 0.03 (0.01, 0.12) a | 0.04 (0.01, 0.11) a | 0.752 | |
| Post treatment 4 weeks | 0.01 (0.00, 0.05) a | 0.01 (0.00, 0.02) a | 0.638 | |
| Actinobacteria | Baseline | 0.45 (0.21, 1.43) | 0.38 (0.22, 0.83) | 0.351 |
| Post treatment 1 week | 0.53 (0.17, 1.05) | 1.63 (0.68, 2.87) a | 0.004* | |
| Post treatment 4 weeks | 0.53 (0.24, 1.13) | 0.82 (0.17, 1.43) | 0.516 | |
| Bacteroides | Baseline | 27.42 (4.04, 50.05) | 34.69 (5.25, 52.99) | 0.465 |
| Post treatment 1 week | 4.16 (1.35, 15.22) a | 5.27 (2.01, 7.01) a | 0.931 | |
| Post treatment 4 weeks | 28.73 (4.31, 41.94) b | 6.22 (4.05, 22.88) a | 0.250 | |
| Escherichia-Shigella | Baseline | 10.17 (4.43, 31.36) | 10.87 (0.72, 32.28) | 0.952 |
| Post treatment 1 week | 6.94 (1.79, 30.49) | 2.62 (0.56, 9.66) a | 0.104 | |
| Post treatment 4 weeks | 4.09 (2.09, 8.96) ab | 1.06 (0.15, 4.85) | 0.032 | |
| F. prausnitzii | Baseline | 0.82 (0.01, 3.15) | 1.10 (0.01, 3.35) | 0.856 |
| Post treatment 1 week | 2.12 (0.88, 3.75) | 3.49 (2.15, 5.63) a | 0.052 | |
| Post treatment 4 weeks | 3.96 (1.41, 4.84) ab | 3.25 (2.13, 4.02) a | 0.654 | |
| Prevotella_9 | Baseline | 0.29 (0.00, 4.37) | 0.03 (0.00, 0.85) | 0.406 |
| Post treatment 1 week | 0.09 (0.00, 0.75) | 0.02 (0.00, 0.14) | 0.231 | |
| Post treatment 4 weeks | 2.19 (0.76, 4.69) b | 0.01 (0.00, 0.36) | <0.001* | |
| Roseburia | Baseline | 0.01 (0.00, 0.24) | 0.17 (0.00, 1.53) | 0.046 |
| Post treatment 1 week | 0.19 (0.05, 0.73) | 0.00 (0.00, 0.11) a | <0.001* | |
| Post treatment 4 weeks | 0.11 (0.02, 0.27) | 0.00 (0.00, 0.02) a | <0.001* | |
| Data are presented by medians and IQR. | ||||
* Because of Bonferroni correction, P<0.0045 (0.05/11) indicates a significant difference between the two treatment groups. aIndicates a significant change compared to baseline level within group. b Indicates a significant change compared to post-treatment 1 week within group
Firmicutes
Percentage of Firmicutes in patients of the UDCA + Mesalazine group remained stable from baseline to post-treatment week 1 with medians of 40.3% and 39.7%, respectively, but were significantly increased to 61.0% at post-treatment 4 weeks, compared to baseline. In the UDCA + Mesalazine group, medians of percentages were significantly higher than those of the Mesalazine group at post-treatment week 4 (61.0% vs. 28.7%, p < 0.001).
Proteobacteria
Both treatment groups showed significant decreases at post-treatment 1 and 4 weeks, compared to baseline. In the Mesalazine group, medians of percentages were decreased from 45.0% at baseline to 31.6% at post-treatment 1 week and 17.3% at post-treatment 4 weeks (both P < 0.05). In the UDCA + Mesalazine group, medians of percentages were decreased from 18.4% at baseline to 3.9% at post-treatment 1 week and 3.7% at post-treatment 4 weeks (both P < 0.05). The UDCA + Mesalazine group showed significantly lower percentage of Proteobacteria compared to the Mesalazine group at post-treatment 1 and 4 weeks (both P < 0.001) [Table 4].
Fusobacteria
Percentages of Fusobacteria in both treatment groups showed significant decreases at post-treatment 1 and 4 weeks, compared to baseline. Medians of percentages were decreased from 0.57% at baseline to 0.03% at post-treatment 1 week and 0.01% at post-treatment 4 weeks in the Mesalazine group, and from 1.01% at baseline to 0.04% at post-treatment 1 week and 0.01% at post-treatment 4 weeks in the UDCA + Mesalazine group, no other significant difference was found between the two treatment groups at each time point [Table 4].
Actinobacteria
In the UDCA + Mesalazine group, percentages of Actinobacteria were significantly increased at post-treatment 1 week, compared to baseline, with medians of 0.38% vs. 1.63%. Percentages of Actinobacteria in the UDCA + Mesalazine group were also significantly higher than those in the Mesalazine group, with medians of 1.63% vs. 0.53% at post-treatment 1 week (P = 0.004). At post-treatment 4 weeks, no significant difference between the two treatment groups was found [Table 4].
Bacteroides
Percentages of Bacteroides in the Mesalazine group were significantly decreased from medians of 27.42% at baseline to 4.16% at post-treatment 1 week, then significantly increased to a median of 28.73% at post-treatment 4 weeks. Percentages of Bacteroides in the UDCA + Mesalazine group also showed significant decreases from baseline to post-treatment 1 week, with the medians from 34.69% to 5.27%, then remained stable to post-treatment 4 weeks. No significant difference was found between the two treatment groups at each time point [Table 4].
Escherichia-Shigella
The differences between the two treatment groups did not obtain any statistical significance at each time point. Percentages of Escherichia-Shigella in the UDCA + Mesalazine group showed significant decreases from baseline to post-treatment 1 week, with medians from 10.87% to 2.62%. Percentage of Escherichia-Shigella in patients of the Mesalazine group at post-treatment 4 weeks showed significant decreases, compared to those at baseline and post-treatment 1 week, with medians of 4.09% vs. 10.17% at baseline and 6.94% at post-treatment 1 week, (both P < 0.05) [Table 4].
F. prausnitzii
The differences between the two treatment groups did not obtain any statistical significance at each time point. Compared to baseline, percentages of F. prausnitzii in the UDCA + Mesalazine group showed significant increases at post-treatment 1 and 4 weeks, with medians of 1.10% vs. 3.49% and 3.25%, respectively. In the Mesalazine group, percentages of F. prausnitzii at post-treatment 4 weeks showed significant increases compared to those at baseline and post-treatment 1 week, with medians of 3.96% vs. 0.82% at baseline and 2.12% at post-treatment 1 week [Table 4].
Prevotella_9
The differences between the two treatment groups did not obtain any statistical significance at baseline and post-treatment 1 week. At post-treatment 4 weeks, percentages of Prevotella_9 in the UDCA + Mesalazine group were significantly lower than those in the Mesalazine group, with medians of 0.01% vs. 2.19% (P < 0.001). Only those in the Mesalazine group showed significant increases at post-treatment 4 weeks compared to those at post-treatment 1 week [Table 4].
Roseburia
The differences between the two treatment groups did not obtain any statistical significance at baseline. At post-treatment 1 and 4 weeks, percentages of Roseburia in the UDCA + Mesalazine group were significantly decreased compared to those at baseline. Significant difference between the two treatments were found at post-treatment 1 and 4 week, with medians of 0.0% vs. 0.19% and 0.0% vs. 0.11%, respectively [Table 4].
DISCUSSION
Based on the changes of Mayo and IBDQ scores, additional UDCA could provide significantly better therapeutic effects than mesalazine alone, possibly due to the changes of IL-23 and IL-17 related immune responses, because at post-treatment 4 weeks, IL-23 and IL-17 levels were significantly lower in the UDCA + Mesalazine group compared to those in the Mesalazine group. The proportional distribution of the intestinal microflora was different between patients with UC and the healthy individuals. Percentages of Bacteroidetes, Bacteroides, Proteobacteria, and Fusobacteria were significantly higher, and percentage of Firmicutes and F. prausnitzii were significantly lower in patients with UC, compared to those in the healthy individuals. In patients with UC, after treatment, the percentages of Firmicutes in the UDCA + Mesalazine group was significantly higher than that in the Mesalazine group, and the UDCA + Mesalazine group showed a significantly lower percentage of Proteobacteria compared to that in the Mesalazine group.
IL-23/IL-17 axis is one of the key pro-inflammatory signaling pathways and has been increasingly considered as the leading cause of chronic intestinal inflammation and other chronic autoimmune inflammatory diseases.[27,28,29,30,31,32] Furthermore, the level of IL-23 and IL-17 has been reported to correlate with the disease activity of UC.[6,7,33] In UC, IL-23 may induce more Th17 responses after receiving specific stimuli and increases the level of IL-17 that is secreted mainly by CD 4 effectors, further activating neutrophils and causing damag to endothelial cells and tissue, thus inducing inflammation.[7,33] One of the stimuli is the antigen from the intestinal microflora. Bacterial translocation can cause epithelial barrier dysfunction and increases intestinal permeability, thereby aggravat UC symptoms.[34,35,36,37,38]
In the present study, before treatment, the differences in the percentages of Escherichia-Shigella, Actinobacteria, prevotella_9, Fusobacterium, and Roseburia between the healthy individuals and UC patients did not obtain statistical significance. In those with significant difference at baseline, after treatment, Bacteroidetes, Proteobacteria, and Bacteroides were significantly decreased, and Firmicutes was increased, which is consistent with previous studies.[39,40,41] Furthermore, comparing the results of the two treatment groups, the percentages of Firmicutes was higher and Proteobacteria was lower significantly in the UDCA + Mesalazine group.
Proteobacteria is one of the most abundant bacteria in the intestine, and is considered as a potential marker of intestinal microbial instability.[42] Studies focusing on the changes in the proportions of the intestinal microflora during IBD have shown that Proteobacteria usually proliferate more under these conditions of IBD, compared to others.[43,44] During intestinal inflammation, epithelial cells reduce β-oxidation due to an increase in oxygen supply, which is thought to promote stunting and is associated with the proliferation of Proteobacteria.[36,41,43,44,45,46,47] It has been known that in patients with UC and UC mouse models, the abundance of Firmicutes was decreased, compared to the healthy individuals,[40] and significantly increased after Akkermansia muciniphia treatment, accompanied with the amelioration of mucosal inflammation and reduction of weight loss, colon length shortening and histopathology scores in a UC mouse model.[48]
The possible mechanism of UDCA on the changes of the proportions of Proteobacteria and Firmicutes needs more molecular examinations to clarify in the future. Speculating from previous studies of UDCA, phosphatidylcholine contents in the patients with UC can be restored by UDCA through inhibiting activity of phospholipase.[21] The distribution of intestinal commensal microbiota is changed probably due to the different phospholipase activity borne by each bacterial strain.[36,49,50,51] The study has some limitations. The fecal microbiota cannot fully reflect the intestinal microbiota. While there were no significant differences in age and gender among the study groups, our results may have been affected by external environmental and other factors. Since the albumin and fecal calprotectin levels are important indicators in patients with UC, this information may provide valuable reference in future studies. Patients enrolled in this study were newly diagnosed with UC, therefore, it was not possible to observe differences among disease stages and the possible effects of other medications for UC such as anti-TNF agents, before UDCA treatment. Investigation of the main microflora in the feces of patients with UC identified a positive effect of short-term use of UDCA; however, these findings need to be further verified in a large independent cohort study.
In conclusion, UDCA combined with mesalazine had better therapeutic effects and quality of life on patients with UC compared to those with mesalazine alone. Regulation of the IL-23/IL-17 axis may be one of the mechanisms of these effects. UDCA may play a positive role in the balance of intestinal microflora in patients with UC. A larger, well-designed randomized controlled trial with extensive follow-up is likely to verify and update the results of this analysis.
Financial support and sponsorship
This work was supported by the Natural Science Foundation of Fujian Province of China (grant number 2015Y5005).
Acknowledgments
The authors thank Jianqiang Liu for his help in the inclusion of participants and valuable discussion.
REFERENCES
- 1.Zheng K, Zhang S, Wang C, Zhao W, Shen H. Health-related quality of life in Chinese patients with mild and moderately active ulcerative colitis. PLoS One. 2015;10:e0124211. doi: 10.1371/journal.pone.0124211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danese S. Immune and nonimmune components orchestrate the pathogenesis of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G716–22. doi: 10.1152/ajpgi.00472.2010. [DOI] [PubMed] [Google Scholar]
- 3.Chen ML, Sundrud MS. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:1157–67. doi: 10.1097/MIB.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SM, Choi HS, Kim DH. The mixture of anemarrhena asphodeloides and coptis chinensis attenuates high-fat diet-induced colitis in mice. Am J Chin Med. 2017;45:1033–46. doi: 10.1142/S0192415X17500550. [DOI] [PubMed] [Google Scholar]
- 5.Fragoulis GE, Siebert S, McInnes IB. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med. 2016;67:337–53. doi: 10.1146/annurev-med-051914-021944. [DOI] [PubMed] [Google Scholar]
- 6.Mańkowska-Wierzbicka D, Swora-Cwynar E, Poniedziałek B, Adamski Z, Dobrowolska A, et al. Usefulness of selected laboratory markers in ulcerative colitis. Eur Cytokine Netw. 2015;26:26–37. doi: 10.1684/ecn.2015.0363. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–9. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 8.Hlavaty T, Batovsky M, Balakova D, Pav I, Celec P, Gregus M, et al. The impact of thiopurine-S-methyltransferase genotype on the adverse drug reactions to azathioprine in patients with inflammatory bowel diseases. Bratisl Lek Listy. 2013;114:199–205. doi: 10.4149/bll_2013_042. [DOI] [PubMed] [Google Scholar]
- 9.Kreijne JE, de Veer RC, de Boer NK, Dijkstra G, West R, Moorsel SAW, et al. Real-life study of safety of thiopurine-allopurinol combination therapy in inflammatory bowel disease: Myelotoxicity and hepatotoxicity rarely affect maintenance treatment. Aliment Pharmacol Ther. 2019;50:407–15. doi: 10.1111/apt.15402. [DOI] [PubMed] [Google Scholar]
- 10.Laukens D, Devisscher L, Van den Bossche L, Hindryckx P, Vandenbroucke RE, Vandewynckel YP, et al. Tauroursodeoxycholic acid inhibits experimental colitis by preventing early intestinal epithelial cell death. Lab Invest. 2014;94:1419–30. doi: 10.1038/labinvest.2014.117. [DOI] [PubMed] [Google Scholar]
- 11.Kim DJ, Yoon S, Ji SC, Yang J, Kim Y-K, Lee S, et al. Ursodeoxycholic acid improves liver function via phenylalanine/tyrosine pathway and microbiome remodelling in patients with liver dysfunction. Scientific Reports. 2018;8:11874. doi: 10.1038/s41598-018-30349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang R, Wei Y, Li Y, Chen W, Chen H, Wang Q, et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67:534–41. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 13.Webb BJ, Brunner A, Lewis J, Ford CD, Lopansri BK. Repurposing an old drug for a new epidemic: Ursodeoxycholic acid to prevent recurrent clostridioides difficile infection. Clin Infect Dis. 2019;68:498–500. doi: 10.1093/cid/ciy568. [DOI] [PubMed] [Google Scholar]
- 14.Benz, Angermüller, Otto, Sauer, Stremmel, Stiehl Effect of tauroursodeoxycholic acid on bile acid-induced apoptosis in primary human hepatocytes. Eur J Clin Invest. 2000;30:203–9. doi: 10.1046/j.1365-2362.2000.00615.x. [DOI] [PubMed] [Google Scholar]
- 15.Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–9. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Li C. Analysis of changes in intestinal flora and intravascular inflammation and coronary heart disease in obese patients. Exp Ther Med. 2018;15:4538–42. doi: 10.3892/etm.2018.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio CA, Schmidt PT. Severe defects in the macrophage barrier to gut microflora in inflammatory bowel disease and colon cancer. Anticancer Res. 2018;38:3811–5. doi: 10.21873/anticanres.12664. [DOI] [PubMed] [Google Scholar]
- 18.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada T, Nowak JA, Milner DA, Jr, Song M, Ogino S. Integration of microbiology, molecular pathology, and epidemiology: A new paradigm to explore the pathogenesis of microbiome-driven neoplasms. J Pathol. 2019;247:615–28. doi: 10.1002/path.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stremmel W, Staffer S, Schneider MJ, Gan-Schreier H, Wannhoff A, Stuhrmann N, et al. Genetic mouse models with intestinal-specific tight junction deletion resemble an ulcerative colitis phenotype. J Crohns Colitis. 2017;11:1247–57. doi: 10.1093/ecco-jcc/jjx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stremmel W, Staffer S, Stuhrmann N, Gan-Schreier H, Gauss A, Burger N, et al. Phospholipase A(2) of microbiota as pathogenetic determinant to induce inflammatory states in ulcerative colitis: Therapeutic implications of phospholipase A(2) inhibitors. Inflamm Intest Dis. 2018;2:180–7. doi: 10.1159/000486858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapoor S. Ursodeoxycholic acid and its emerging role in attenuation of tumor growth in gastrointestinal malignancies. J Cachexia Sarcopenia Muscle. 2012;3:277–8. doi: 10.1007/s13539-012-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13:567–79. doi: 10.1038/nrgastro.2016.128. [DOI] [PubMed] [Google Scholar]
- 26.Paschos P, Katsoula A, Salanti G, Giouleme O, Athanasiadou E, Tsapas A. Systematic review with network meta-analysis: The impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther. 2018;48:1174–85. doi: 10.1111/apt.15005. [DOI] [PubMed] [Google Scholar]
- 27.Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20:3394. doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison PJ, Ballantyne SJ, Kullberg MC. Interleukin-23 and T helper 17-type responses in intestinal inflammation: From cytokines to T-cell plasticity. Immunology. 2011;133:397–408. doi: 10.1111/j.1365-2567.2011.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng JS, Yang Z, Zhu YZ, Liu Z, Guo CC, Zheng XB. Serum IL-17 and IL-6 increased accompany with TGF-β and IL-13 respectively in ulcerative colitis patients. Int J Clin Exp Med. 2014;7:5498–504. [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 31.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Q, Zheng P, Chen X, Zhou F, He Q, Yang Y. Andrographolide presents therapeutic effect on ulcerative colitis through the inhibition of IL-23/IL-17 axis. Am J Transl Res. 2018;10:465–73. [PMC free article] [PubMed] [Google Scholar]
- 33.Dragasevic S, Stankovic B, Sokic-Milutinovic A, Milosavljevic T, Milovanovic T, Lukic S, et al. Importance of TLR9-IL23-IL17 axis in inflammatory bowel disease development: Gene expression profiling study. Clin Immunol. 2018;197:86–95. doi: 10.1016/j.clim.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 35.Dillon SM, Rogers LM, Howe R, Hostetler LA, Buhrman J, McCarter MD, et al. Human intestinal lamina propria CD1c+dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J Immunol. 2010;184:6612–21. doi: 10.4049/jimmunol.1000041. [DOI] [PubMed] [Google Scholar]
- 36.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 37.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. Isme J. 2013;7:1256–61. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera-Chávez F, Lopez CA, Bäumler AJ. Oxygen as a driver of gut dysbiosis. Free Radic Biol Med. 2017;105:93–101. doi: 10.1016/j.freeradbiomed.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 39.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, et al. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–11. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Xu H, Chen S, He J, Zhou Y, Nie Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15222. doi: 10.1111/jgh. 15222. [DOI] [PubMed] [Google Scholar]
- 42.Shin NR, Whon TW, Bae JW. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJO. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–41. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehman A, Lepage P, Nolte A, Hellmig S, Schreiber S, Ott SJ. Transcriptional activity of the dominant gut mucosal microbiota in chronic inflammatory bowel disease patients. J Med Microbiol. 2010;59:1114–22. doi: 10.1099/jmm.0.021170-0. [DOI] [PubMed] [Google Scholar]
- 45.Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, et al. Microbial Respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe. 2017;21:208–19. doi: 10.1016/j.chom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litvak Y, Byndloss MX, Tsolis RM, Bäumler AJ. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr Opin Microbiol. 2017;39:1–6. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: A common factor in human diseases. BioMed Res Int. 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, et al. Administration of akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02259. doi: 10.3389/fmicb.2019.02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibiino G, Lopetuso LR, Scaldaferri F, Rizzatti G, Binda C, Gasbarrini A. Exploring bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig Liver Dis. 2018 Jul;50:635–9. doi: 10.1016/j.dld.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Wexler AG, Goodman AL. An insider's perspective: Bacteroides as a window into the microbiome. Nat Microbiol. 2017;2:17026. doi: 10.1038/nmicrobiol.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Zhi F. Lower level of bacteroides in the gut microbiota is associated with inflammatory bowel disease: A meta-analysis. Biomed Res Int. 2016;2016:5828959. doi: 10.1155/2016/5828959. [DOI] [PMC free article] [PubMed] [Google Scholar]


