Abstract
Background:
Drotaverine and Mebeverine are used for alleviating the pain of IBS, but the evidence for their efficacy is scarce. In this randomised control study, we evaluated and compared their efficacy in improving severity, frequency of pain and its associated symptoms.
Methods:
Patients fulfilling the ROME III criteria of IBS were evaluated in this randomised control trial during 4 weeks of treatment. Group A (n = 100) received 80 mg Drotaverine and Group B (n = 100) received 135 mg Mebeverine three times a day, 1 hour before meals. Primary outcome measure was, the reduction in severity of pain (>30% reduction) assessed by VAS (0 to 10 scale) & PSS (patient symptoms scores).
Results:
The pain severity score fell from 6.02 to 4.8 on day 3 in Group A as compared to decrease from 6.72 to 6.62 in Group B (p < 0.01). This significant reduction in pain severity was observed till the end of the study, reducing from 6.02 to 1.78 (74% reduction) in Group A compared to 6.72 to 3.62 (46.1% reduction) in Group B (p < 0.05). There was a significant reduction in pain frequency, straining on stool, a change in one score in Bristol stool chart (BSC), achievement of complete spontaneous smooth bowel movement in Group A, compared to Group B patients. A significant improvement in Patient's evaluation of Global Assessment of Symptoms (p < 0.05) and Patient Assessment of Constipation – Quality Of Life (PAC-QOL) (p < 0.01) was observed in Group A compared to Group B.
Conclusion:
Drotaverine was significantly superior in efficacy as compared to Mebeverine in alleviating pain severity (starting from day 3), frequency and stools-elated symptoms of IBS.
Keywords: Drotaverine, global assessment of symptoms, irritable bowel syndrome, mebeverine, patient symptom score
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder characterized by abdominal pain and altered bowel habits in the absence of specific organic pathology.[1] Studies in Asia estimate a prevalence of 3.7%-22% using Rome diagnostic criteria.[2,3,4] However, the data reported from developed countries like United States estimate the prevalence of irritable bowel syndrome at 10%-20% and the incidence of irritable bowel syndrome at 1%-2% per year. The incidence is markedly different among countries, depending upon factors like race, food habits and diagnostic criteria used. Of people with IBS, approximately 75% seek medical advice for abdominal pain or discomfort.[5]
Four bowel patterns may be seen with IBS. These patterns include IBS-D (diarrhoea predominant), IBS-C (constipation predominant), IBS-M (mixed diarrhoea and constipation), and IBS-U (unsubtyped).[1] A report by the Indian Society of Gastroenterology Task Force revealed that as high as 70% of IBS patients have abdominal pain or discomfort, significantly affecting the patient's quality of life.[6] Treatment for IBS may include medication use, stress relief, and changes in eating habits.[7]
Antispasmodics are a heterogenous group of drugs such as direct smooth muscle relaxants (eg. papaverine, drotaverine, mebeverine, peppermint oil), anti-cholinergic agents (eg. butylscopolamine, hyoscine, cimetropium bromide). They are being used in the therapy for irritable bowel syndrome. The aim of these drugs is to reduce defaecation symptoms by increasing colonic transit time, improving stool consistency and reducing stool frequency.[8] Antispasmodics viz. hyoscyamine, dicyclomine, clinidium bromide and propantheline are commonly used, but are also associated with many anticholinergic side effects, thereby restricting their usage.
Most of the clinical trials done to assess their efficacy are old. These were small studies and had methodological flaws, including diagnostic and inclusion criteria used, dosing schedules, duration of therapy and study end-points used to assess the response.[8]
Mebeverine is a commonly used drugs for treatment of IBS. It is a derivative of beta-phenylethylamine and is a smooth muscle relaxant with calcium channel blocking actions.[9,10] It is a musculotropic agent that has antispasmodic activity and regulatory effects on the bowel function.
Drotaverine hydrochloride, an isoquinoline derivative, is a potent spasmolytic drug which acts directly on the smooth muscles and is devoid of any anticholinergic side effects. Its pharmacological action is due to its surface activity with which it binds to the surface of the smooth muscle and alters its membrane potential and permeability. It exerts a two- fold antispasmodic action on the smooth muscles of the gut, one being a direct inhibition of smooth muscle contraction by inhibiting the calcium-calmodulin complex and the other being mediated via an inhibitory effect on the phosphodiesterase (PDE) enzyme system. PDE isoenzymes, especially isoenzyme IV play a regulatory role in the spasmolysis of smooth muscles of the gastrointestinal tract via regulation of intracellular cAMP. Drotaverine, by selectively inhibiting PDE isoenzyme IV in the gut, results in a powerful spasmolytic action.[11] Drotaverine is currently used for relief of spasmodic pain arising from smooth muscle diseases like IBS, and intestinal, biliary, or ureteric colic.
Recently, there have been two studies (currently in abstract form), which have shown beneficial effects of drotaverine in IBS patients. In these trials, patients of all sub-types of IBS were studied (diarrhoea, constipation and mixed types). Drotaverine significantly decreased the pain severity and frequency, along with improvement in the global assessment of symptoms of IBS compared to placebo.[12,13]
A recent randomized controlled trial, comparing drotaverine to placebo, in patients of IBS[14] revealed that drotaverine significantly improved the pain scores and decreased the pain frequency. It also showed significant improvement in global relief in abdominal pain as perceived by patient as well as the clinician. A significant improvement in stool frequency was observed in drotaverine treatment group as compared to placebo. The drug was well tolerated, without any major side effects.
In this study, we aimed to compare the efficacy of drotaverine versus mebeverine in patients of IBS, in terms of pain relief (frequency and severity), stool consistency and frequency, overall assessment of global improvement in patient's symptoms and their quality of life.
METHODS
The Primary outcome measure was percentage of patients achieving >30% improvement in severity and frequency of abdominal pain
Secondary outcome measures
Change in form of stool according to Bristol Stool Chart, straining and frequency of stools.
Assessment of Complete Smooth Bowel Movement (CSBM)
Global assessment by the patient about the illness as a whole.
Pre and post assessment of the quality of life.
Consecutive patients presenting with symptoms of IBS, aged between 18 to 60 years, fulfilling ROME III Criteria were enrolled, to assess for their inclusion in this study.
Study protocol
The Ethics committee approval was taken from each participating institution. The study was registered in Clinical Trial Registry - India (CTRI); Registration Number – CTRI/2017/11/010529. All subjects provided written informed consent to participation in the study.
In the first two weeks i.e., run-in period, all the drugs were stopped. In the second week, every patient was given a diary to fill for assessing the severity and frequency of pain, stool type (Bristol Stool Chart), frequency of stools and number of complete smooth bowel movements, on a daily basis for 7 days.
The following assessments were conducted before the entry into the trial (before day 0). and determined to be within the normal range or lacked any pathological finding
Complete medical history, signs and symptoms
Physical examination
Haematological evaluation (including complete blood count and peripheral blood film),
Routine stool examination for ova and cysts in patients with diarrhea,
Blood glucose, alanine and aspartate aminotransferase,
TSH, T3, T4
Serum tissue transglutaminase estimation (S-tTG)
Sigmoidoscopy – in patients presenting with alarm symptoms.
At the end of two weeks, baseline assessments of all these parameters were entered in a data sheet. Patients were asked to record their Global Assessment of Symptoms of IBS and the Patient Assessment of Constipation - Quality Of Life (PAC-QOL) questionnaire was filled by the investigators.
After the run-in period, patients fulfilling ROME III criteria were enrolled into a double-blinded control study of four weeks. Exclusion criteria were presence of co-morbid diseases, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF); patients taking drugs which modify or aggravate symptoms of IBS (antidepressants, calcium channel blockers etc.); patients having hypothyroidism & gluten hypersensitivity, patients with alarming symptoms, viz. history of fever, passage of blood in stool, loss of weight, any organic gastrointestinal disease in the recent past, recent change in bowel habits, patients on any other concomitant medication for abdominal pain, bowel disturbance or altering gastrointestinal motility and malignancy of any other organ.
For this investigator-initiated study, active drugs, drotaverine hydrochloride (80 mg) and Mebeverine (135 mg) were provided by Walter-Bushnell Private Limited, New Delhi. Each drug was packed in identical looking capsules.
Randomization and blinding
Patients were randomized with the use of a computer-generated randomization list using block randomization with variable block size. Adequate numbers of sequentially numbered capsule bottles were uniquely coded as per the randomization code.
Participants, investigators and outcome assessors were blinded to the treatment assigned. The randomization key was kept with the respective ethics committee of the two study centres till the analysis of results.
Procedure
Two hundred subjects fulfilling the inclusion and exclusion criteria were included in the study. After enrolment, all the subjects were advised life style changes and dietary changes.
The patients were assigned to either of the two study groups.
-
Group A -(n=100)
- Drotaverine 80 mg – Three times/day one hour before principal meals for 4 weeks.
-
Group B – (n=100)
- Mebeverine 135 mg – Three times/day one hour before principal meals for 4 weeks.
Study visits and assessments
For each patient's baseline evaluation was done (at initiation of drug treatment) i.e., at day 0 and at the end of every week for the next 4 weeks of drug treatment. Adverse events (AE) were monitored throughout the study.
Each patient was handed over a patient assessment diary for each week along with the set of drugs for each week.
PARAMETERS FOR EVALUATION OF EFFICACY
Assessment of pain seweverity
-
Pain Severity Scale (PSS)
None - Score 0
Mild - Score 1
- Self-limiting episodes
- Doesn't require medication for relief
- Doesn't interfere with normal daily routine or activities
Moderate - Score 2
- Not self-limiting
- Requires medication
- Doesn't interfere with normal daily routine or activities
Severe - Score 3
- Not self-limiting
- Requiring medication that may only provide partial relief
- Causing severe distress
- Interferes with normal routine and activities
Visual Analogue Scale (VAS)
Severity of pain was assessed by each patient on a visual analogue scale (0-10).
Assessment of pain frequency
Pain frequency was recorded as number of pain episodes per week for each patient.
Assessment of stool characteristics
-
Character of Stool
Each patient was asked to assess the type of stool according to Bristol Stool Chart[15] which would decide about the type of IBS
A change in stool character was recorded each week by the patient.
-
Frequency of Stools
Each patient was asked to record the number of stools he/she passed per day.
-
Straining
Each patient was asked to record the degree of straining while defecating each day on a scale of 1 to 5.
1 - Not at all
2 - A little bit
3 - A moderate amount
4 - A great deal
5 - An extreme amount
The number of CSBM per week was recorded.
Global assessment of symptoms
The patients were asked to give response to the following questions each week:
How would you rate your abdominal pain overall in the past 7 days?
How would you rate your constipation (for IBS -C) or diarrhoea (for IBS –D) overall for the past 7 days?
How would you rate your IBS Symptoms overall over the last 7 days.
They were asked to assess response on a LIKERT SCALE
+2 - Significantly relieved
+1 - Moderately relieved
0 - Unchanged
-1 - Moderately worse
-2 - Significantly worse
Patient assessment of constipation – Quality of life (PAC-QOL)
The PAC-QOL[16] is a validated scale to assess impact of constipation on the quality of life. The questionnaire uses 28 questions, measured on a 0 to 4 scale (Not at all to extremely) to cover seven domains (intensity of symptoms – 2 items), (effect of constipation on daily life – 4 items), (effect on eating behaviour and daily routine – 6 items), (about patient's psychological feelings – 6 items), (patient's feeling about stool character – 3 items), (impact on life with IBS symptoms – 3 items), (satisfaction level – 4 items).
Assessment of safety
The assessment of safety was done in all randomized subjects receiving study medication. Adverse events (AEs) were monitored throughout the study.
Enrolled patients were called after every 7 days to examine their symptom diary. The following AEs were inquired from the patient: Nausea, heartburn, headache, generalised weakness, chronic fatigue, poor sleep, dizziness, palpitations, flatulence, dryness of mouth and blurred vision.
Statistical analysis
Appropriate statistical analysis was performed as per protocol analysis and also intention to treat analysis at the end of the study. A P value < 0.05 was considered as significant. The efficacy parameters of the two treatment groups was calculated by the two sample “t” test and Chi-square test. Data was entered into Microsoft Excel spreadsheet and analysed by SPSS version 23.0;IBM Corp., Armonk, NY, USA.
RESULTS
Two hundred patients, who fulfilled the inclusion criteria, were included in this study. The two groups were well balanced with regard to the patient characteristics and symptoms [Table 1]. Percentage of IBS - C sub-type was relatively higher in this study (80%), unlike normal distribution of sub-type (IBS – C as 39%) in the community study from India.[6] This may probably be because patients were enrolled from tertiary care practice. Patient presenting with any alarm symptom, underwent sigmoidoscopy, to exclude any organic cause. All these patients had normal sigmoidoscopy. Thyroid function test, stool examination for Ova and Cysts and test for Gluten Hypersensitivity (StTG) were normal in patients presenting with diarrhoea. Most of the patients used Psyllium two tea spoon per day after the night meal. No other drug was used in all these patients for 4 weeks. Patients with IBS C were allowed Sodium Picosulphate 2 mg, as a rescue medication for constipation. Twenty patients in group A and 35 patients in group B used Sodium Picosulphate 2-3 times during the 4 weeks of study period. For IBS (D) and IBS (M) patients, Loperamide 2 mg was permitted as a rescue medicine. Two patients in each group needed Loperamide on 8 occasions.
Table 1.
Patient characteristics in drotaverine and mebeverine groups
| Characteristics | Drotaverine (n=100) | Mebeverine (n=100) | Significance (P) |
|---|---|---|---|
| Age - years | 42±3 | 40±2 | N.S. |
| Sex - M/F | 80:20 | 75:35 | N.S. |
| BMI | 22.6±0.9 | 21.8±1.4 | N.S. |
| IBS (Type) % | |||
| C | 80% | 76% | N.S. |
| D | 15% | 14% | N.S. |
| M | 3% | 6% | N.S. |
| U | 2% | 4% | N.S. |
*BMI – Body Mass Index, IBS – Irritable Bowel Syndrome, C – constipation, D – diarrhoea, M – mixed, U - unsubtyped
Efficacy results
Pain Severity: Drotaverine-treated patients showed a significant improvement in the pain severity (> 30%) on Day 3 compared to mebeverine. The pain severity score decreased from 6.02 to 4.8 on day 3 in the drotaverine group compared to the decrease from 6.72 to 6.62 (p < 0.01) in the mebeverine group. This reduction in pain continued till the end of the study. Both the groups showed reduction in pain score severity. At the end of study, the pain severity decreased in drotaverine (group A) from 6.02 to 1.78, a 70.4% reduction. However, in mebeverine (group B), severity of pain reduced from 6.72 to 3.62, a 46.1% reduction. The difference between groups A and B was statistically significant (p < 0.05) [Figure 1].
Figure 1.
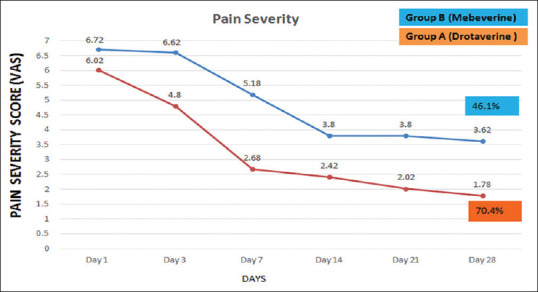
Pain Severity Score (VAS) in Group A Drotaverine, and Group B Mebeverine on basis of Days
Most of the patients had moderate to severe pain on PSS scale, which reduced to mild pain in 80% of patients in drotaverine (Group A), however, in mebeverine (Group B) the reduction was only in 40% of patients.
Pain Frequency: The Number of episodes of pain per week reduced from 3 ± 1.0 to 1.1 ± 0.2 in the drotaverine (group A) as compared to 3 ± 1.0 to 2 ± 0.1 in the mebeverine (group B). The reduction in pain frequency was significant (p < 0.01) from day 0 to day 28 [Figure 2].
Figure 2.
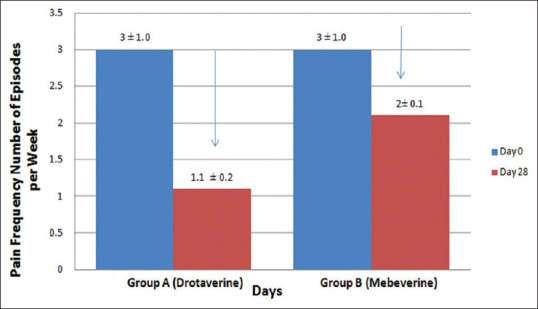
Pain frequency (number of episodes per week) in Group A and B on Day 0 and Day 28
Straining: Significant number of patients showed decrease in straining from 85% to 40%, a decrease of 45% in drotaverine group, as compared to a decrease from 80% to 65%, a decrease of 15% observed in mebeverine group (p < 0.05) [Figure 3].
Figure 3.
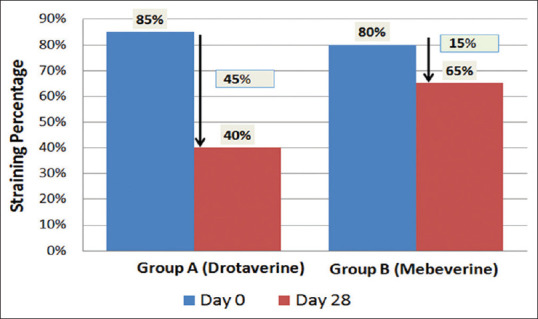
Percentage of patients presenting with straining and reduction of straining in Group A and B on Day 0 and Day 28
Change in Bristol Stool Chart (BSC): On day 28, in the drotaverine group, more number of patients (55%) achieved a change of 1 Bristol Stool Chart (BSC) form, as compared to only 30% in the mebeverine group (p < 0.01) [Figure 4].
Figure 4.
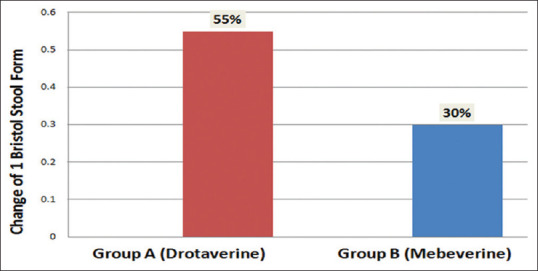
Change of 1 Bristol Stool Chart score in Group A and B on Day 28
Complete Smooth Bowel Movement (CSBM): On Day 0 only 10% of the patients had complete smooth bowel movement which increased to 55% on day 28 in drotaverine group, as compared to 8% CSBM on day 0 in the mebeverine group, which increased to 25% on day 28. The difference in achievement of CSBM was significant in the drotaverine group as compared to mebeverine (<0.01) [Figure 5].
Figure 5.
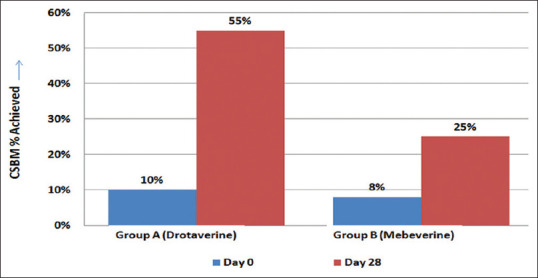
Complete smooth bowel movement (CSBM) in Group A and B on Day 0 and Day 28
Global Assessment of Symptoms: Patient's evaluation of Global Assessment of Symptoms in drotaverine group showed a greater improvement on day 28 compare to day 0. A reduction from 2.60 to 1.0 in drotaverine (Group A), which was significantly more as compared to mebeverine (Group B), and showed a reduction from 2.68 on day 0 to 1.8 on Day 28 (p < 0.05) [Figure 6].
Figure 6.
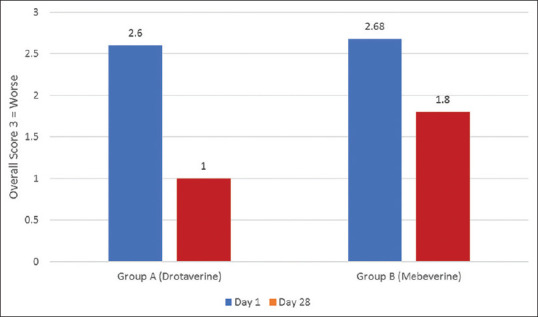
Reduction in Patients Global Assessment of Symptoms in Group A and Group B
Patient Assessment of Constipation – Quality Of Life (PAC-QOL): Improvement in pain severity score, decrease in frequency of pain, decrease in straining and attainment of complete smooth bowel movement led to improvement in quality of life of patients. The improvement in the quality of life was significantly more in drotaverine group as compared to mebeverine group.
Patient Assessment of Constipation – Quality Of Life (PAC-QOL) which was 91.54% (worsened) on day 0, remained on 33% in Group A, as compared to Group B, which was 92.08% on Day 0 and remained on 53.52% on day 28. The difference between the two groups was statistically significant (p < 0.01) [Figure 7].
Figure 7.
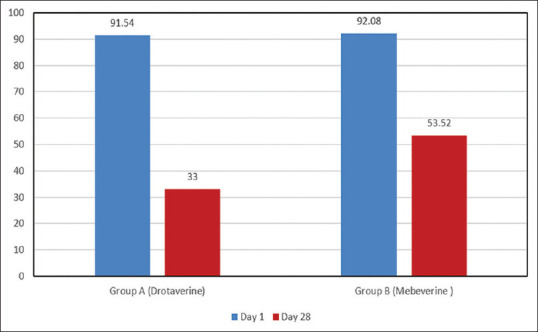
Improvement in the quality of life on Day 28 compared to Day 0 in Group A & B
Adverse events
Only 13% of patients receiving drotaverine and 12% receiving mebeverine experienced minor adverse effects. None of them required discontinuation of treatment. No anti-cholinergic side-effects were observed [Table 2].
Table 2.
Incidence of adverse events in Drotaverine and Mebeverine groups
| Adverse Effects | Drotaverine (%) (n=100) | Mebeverine (%) (n=100) |
|---|---|---|
| Nausea | 1 | 2 |
| Heartburn | 3 | 4 |
| Headache | 5 | 3 |
| Generalized weakness | 0 | 2 |
| Chronic fatigue | 0 | 0 |
| Poor sleep | 0 | 0 |
| Dizziness | 1 | 0 |
| Palpitation | 0 | 0 |
| Flatulence | 3 | 1 |
| Dry Mouth | 0 | 0 |
| Blurring of Vision | 0 | 0 |
DISCUSSION
In this randomized control trial comparing drotaverine and mebeverine, drotaverine treated patients showed significantly greater percentage of improvement in relief from abdominal pain and associated bowel symptoms, compared to mebeverine treated patients.
Abdominal pain is the key clinical feature and arguably, the most difficult symptom to treat in patients suffering from IBS. In this trial, drotaverine improved abdominal pain severity score (>30% reduction) in significant number of patients as compared to mebeverine (p < 0.05). The improvement in abdominal pain was observed from the day 3 of the treatment in drotaverine group and this improvement continued till the end of treatment; whereas as in mebeverine treated group, lesser number of patients showed improvements at Day 3 and at the end of the study. Similar result of improvement in pain relief were observed in a randomised control trail comparing drotaverine with placebo in IBS, wherein 70% of the patients showed improvement compared to placebo (30%), in a four week trial.[14]
When compared with a previous meta-analysis, mebeverine improved pain scores in 46% of our patients, comparable to the results of the meta-analysis, where mebeverine and placebo showed similar reduction (30%) in pain.[10]
In addition to reduction in abdominal pain, drotaverine resulted in significant improvement in other abdominal symptoms, including pain frequency, straining, reduction in Bristol Stool Scale and complete smooth bowel movement, compared to mebeverine treated group of patients. Most of the earlier studies with mebeverine have not reported its effects on these associated additional symptoms of IBS.[10] A similar effect has been shown in a randomised controlled trial – where drotaverine treatment resulted in significant improvement in stool frequency compared to placebo.[14]
The increased phasic contractions of colon are attributed as an important cause of pain in IBS. The improvement in IBS symptoms drotaverine-treated patients may be due to the relaxation of the intestinal smooth muscles obtained by the inhibition of PDE (Phosphodiesterase) inhibitory and Ca2 calmodulin complex which may decrease the functional obstruction.[11]
Further studies are needed to completely elucidate the mechanism that underline favourable effects of drotaverine on these additional symptoms i.e., straining, change in Bristol stool scores and CSBM.
When patients were assessed for global assessment of symptoms - drotaverine group showed greater improvement on day 28 as compared to day 0. A greater and significant improvement was observed in drotaverine group compared to mebeverine treated group (p < 0.05)
Previously published meta-analysis on efficacy and safety of mebeverine verses placebo have not shown a greater improvement in global symptom score in mebeverine group as compared to placebo group. However, this effect in global improvement in IBS was not statistically significant.[10] A significant improvement in quality of life scores were observed in the drotaverine treated patients compared to the mebeverine treated ones in the present study. This Improvement in quality of life in the drotaverine group could be because of better improvement in pain severity score, decrease in pain frequency, straining and attainment of CSBM. This could possibly be due to stronger anti-spasmodic action of drotaverine.
Both drotaverine and mebeverine are safe and well tolerated in patients of IBS. The anti-cholinergic side effects were not observed in either group. The adverse effects were comparable and none led to discontinuation of treatment or withdrawal from the trial. Similar results were observed in a recent study[14] and also in a meta-analysis of safety and efficacy of mebeverine.[10]
The recently published American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome, also suggested the use of anti-spasmodics (otilonium, pinaverium, hyoscine, cinetropium, drotaverine and dicyclomine) for overall symptom improvement in IBS patients. Mebeverine did not have a statistically significant effect on IBS symptoms, although the number of patients studied was small.[17]
The limitation of our study was that it was not conducted in multiple centers across the country, and that safety and efficacy was evaluated in all subtypes of IBS groups in a combined manner and sub-group analysis was not performed.
CONCLUSIONS
Drotaverine was significantly superior in efficacy as compared to mebeverine in alleviating pain severity (starting from day 3), frequency and stools related symptoms of IBS, in a cohort of predominantly IBS-C sub-type patients with mostly moderate to severe pain. A significant improvement in patient evaluated Global assessment of symptoms and Patient Assessment of Constipation - Quality of Life (PAC-QOL) was achieved in patients treated with drotaverine as compared to mebeverine.
Financial support and sponsorship
Walter-Bushnell Private Limited. New Delhi.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–12. doi: 10.1038/ajg.2012.254. [DOI] [PubMed] [Google Scholar]
- 2.Rey E, Talley NJ. Irritable bowel syndrome: Novel views on the epidemiology and potential risk factors. Dig Liver Dis. 2009;41:772–80. doi: 10.1016/j.dld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Gwee KA, Wee S, Wong ML, Png DJ. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an Asian urban community. Am J Gastroenterol. 1998;93:1816–22. doi: 10.1111/j.1572-0241.2004.04161.x. [DOI] [PubMed] [Google Scholar]
- 4.Drossman D, Corazziari E, Talley NJ, Thompson WG, Whitehead WE, editors. Rome II: The Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology and Treatment: A Multinational Consensus. 2nd ed. McLean, VA: Degnon Associates; 2000. [Google Scholar]
- 5.Williams RE, Black CL, Kim HY, Andrews EB, Mangel AW, Buda JJ, et al. Determinants of healthcare-seeking behaviour among subjects with irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1667–75. doi: 10.1111/j.1365-2036.2006.02928.x. [DOI] [PubMed] [Google Scholar]
- 6.Ghoshal UC, Abraham P, Bhatt C, Choudhuri G, Bhatia SJ, Shenoy KT, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: Report of the Indian society of gastroenterology task force. Indian J Gastroenterol. 2008;27:22–8. [PubMed] [Google Scholar]
- 7.Mangel AW, Northcutt AR. The safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13:77–82. doi: 10.1046/j.1365-2036.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 8.Annaházi A, Róka R, Rosztóczy A, Wittmann T. Role of antispasmodics in the treatment of irritable bowel syndrome. World J Gastroenterol. 2014;20:6031–43. doi: 10.3748/wjg.v20.i20.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell AM. Physiological and clinical assessment of the effect of the musculotropic agent mebeverine on the human colon. Br Med J. 1965;2:848–51. doi: 10.1136/bmj.2.5466.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darvish-Damavandi M, Nikfar S, Abdollahi M. A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome. World J Gastroenterol. 2010;16:547–53. doi: 10.3748/wjg.v16.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blasko G. Pharmocology: A mechanism of action and clinical significance of a convenient antispasmodic agent: Drotaverine. J Am Med Assoc India. 1998;1:63–9. [Google Scholar]
- 12.Pap A, Hamvas J, Filiczky I, Burai M, Szikszay E. Beneficial effect of drotaverine in irritable bowel syndrome (IBS) Gastroenterology. 1998;114:A818. [Google Scholar]
- 13.Misra SC, Pande RM. Efficacy of drotaverine in irritable bowel syndrome: A double blind randomized, placebo controlled clinical trial. Am J Gastroenterol. 2000;95:2544. [Google Scholar]
- 14.Rai RR, Dwivedi M, Kumar N. Efficacy and safety of drotaverine hydrochloride in irritable bowel syndrome: A randomized double-blind placebo-controlled study. Saudi J Gastroenterol. 2014;20:378–82. doi: 10.4103/1319-3767.145331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez AP, de Azevedo GR. The Bristol Stool Form Scale: Its translation to Portuguese, cultural adaptation and validation. Rev Latino-Am Enfermagem. 2012;20:583–9. doi: 10.1590/s0104-11692012000300021. [DOI] [PubMed] [Google Scholar]
- 16.Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the patient assessment of constipation quality of life questionnaire. Scand J Gastroenterol. 2005;40:540–51. doi: 10.1080/00365520510012208. [DOI] [PubMed] [Google Scholar]
- 17.Ford AC, Moayyedi P, Chey WD, Harris LA, Lacy BE, Saito YA, et al. American College of gastroenterology monograph on management of Irritable Bowel Syndrome. Am J Gastroenterol. 2018;113:1–18. doi: 10.1038/s41395-018-0084-x. [DOI] [PubMed] [Google Scholar]


