Abstract
Familial hypercholesterolemia (FH) is an inherited disorder with retarded clearance of plasma LDL caused by mutations of the genes involved in the LDL receptor-mediated pathway and most of them exhibit autosomal dominant inheritance. Homozygotes of FH (HoFH) may have plasma LDL-C levels, which are at least twice as high as those of heterozygous FH (HeFH) and therefore four times higher than normal levels. Prevalence of HoFH had been estimated as 1 in 1,000,000 before but more recent genetic analysis surveys predict 1 in 170,000 to 300,000. Since LDL receptor activity is severely impaired, HoFH patients do not or very poorly respond to medications to enhance activity, such as statins, and have a poorer prognosis compared to HeFH. HoFH should therefore be clinically distinguished from HeFH. Thorough family studies and genetic analysis are recommended for their accurate diagnosis.
Fatal cardiovascular complications could develop even in the first decade of life for HoFH, so aggressive lipid-lowering therapy should be initiated as early as possible. Direct removal of plasma LDL by lipoprotein apheresis has been the principal measure for these patients. However, this treatment alone may not achieve stable LDL-C target levels and combination with drugs should be considered. The lipid-lowering effects of statins and PCSK9 inhibitors substantially vary depending on the remaining LDL receptor activity of individual patients. On the other hand, the action an MTP inhibitor is independent of LDL receptor activity, and it is effective in most HoFH cases.
This review summarizes the key clinical issues of HoFH as well as insurance coverage available under the Japanese public healthcare system.
Keywords: Homozygous familial hypercholesterolemia, Family study, Genetic diagnosis, Lipoprotein apheresis, MTP inhibitor, PCSK9 inhibitor, Cutaneous and Tendon Xanthoma, Aortic Supra-valvular stenosis
Introduction
Familial hypercholesterolemia (FH) is an inherited disorder of lipoprotein metabolism caused by mutations of the genes involved in the LDL receptor-mediated pathway for cellular uptake of LDL. Most FH patients show an autosomal dominant trait. Hyper-LDL-cholesterolemia remains throughout their lives and causes premature coronary heart disease unless properly treated 1 - 3) .
Early diagnosis and initiation of lipid-lowering therapy is essential for preventing development of cardiovascular complications, even in Japan where cardiovascular disease is not the leading cause of death and its prevalence among FH patients seems to be somewhat lower than in Western countries. Heterozygous FH (HeFH) patients who carry the mutated gene in a single allele have plasma LDL cholesterol (LDL-C) levels double normal or higher and may experience the first cardiovascular event as early as their thirties. With mutations in both alleles, homozygous FH (HoFH) exhibits LDL-C levels twice those of HeFH, or even higher, and patients develop cardiovascular complications even in the first decade of their lives. Most HoFH is refractory to statins and other standard lipid-lowering drugs as most of them depend on remaining LDL receptor activity. Thus, HoFH has a poor prognosis compared to HeFH and therefore, earlier diagnosis and more aggressive treatments are required to prevent premature death.
1. Clinical Manifestations of HoFH
Familial hypercholesterolemia (FH) is a disease with a triad of clinical characteristics: elevated LDL-C, cutaneous and/or tendon xanthomas, and premature atherosclerotic cardiovascular disease (ASCVD). A genetic defect in LDL receptor function is the cause of FH, and activity of the LDL receptor is completely or almost completely absent in HoFH patients. HoFH includes homozygotes or compound/double heterozygotes of autosomal-dominant disease-causing mutations in the related genes, and their parents are HeFH, but there are rare exceptions that exhibit autosomal recessive inheritance 4 , 5) .
1) Elevated LDL-C Levels
HoFH patients have very high LDL-C levels from birth, which puts them at very high-risk of coronary heart disease. Plasma LDL-C levels are more than 500 mg/dL in many cases of genetically confirmed HoFH, but there is considerable variation in lipid levels among patients. Those with LDL-C levels over 370 mg/dL (or total cholesterol levels over 450 mg/dL) in the fasting steady state should consult with specialists as they are probably cases of HoFH.
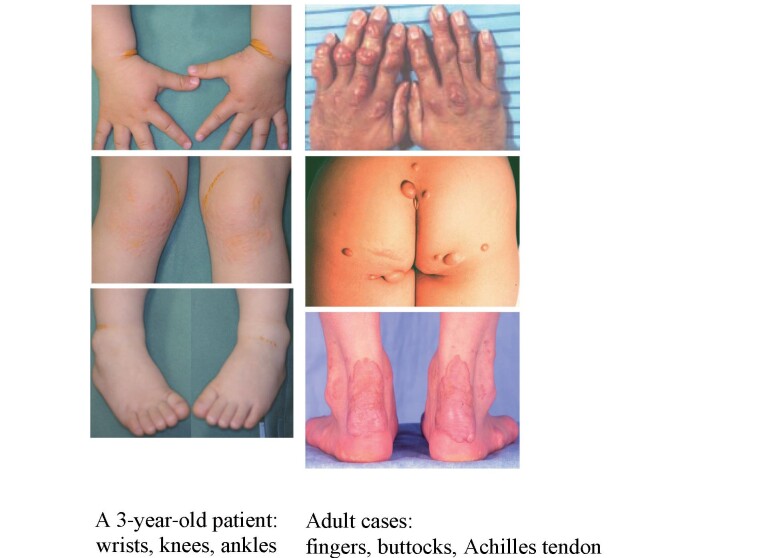
2) Cutaneous and Tendinous Xanthomas ( Fig.1 )
Fig. 1. Cutaneous and Tendon Xanthomas in HoFH .
Presence of cutaneous xanthomas from childhood is strongly suggestive of HoFH, and tendon xanthomas are generally prominent in adults with HoFH.
Characteristic cutaneous xanthomas that have developed since infancy are physical findings suggestive of HoFH. They are commonly found on the extensor surfaces of the elbows/knees and wrist/gluteal regions and parents often take a child to a doctor for the first visit because of them 6) . Tendon xanthomas are pathognomonic for both HeFH and HoFH, but not apparent during childhood and gradually appear around puberty. Xanthomas can be repressed or made to regress with continuous aggressive lipid-lowering treatment.
3) ASCVD
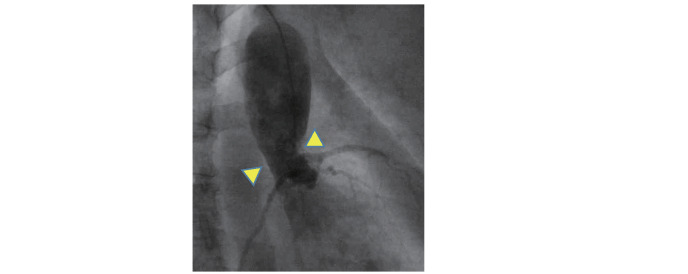
The prognosis for untreated HoFH is extremely poor. It is difficult for patients to live beyond 30 years without treatment. The LDL-C accumulation threshold hypothesis, which uses a calculation of [LDL-C x years of life], has been proposed as a rational explanation for the coronary risk of FH, and according to it, the coronary threshold of HoFH would be around 11 years old even for individuals with lower LDL-C levels 7) . Angina or myocardial infarction from infancy, as well as aortic supravalvular and/or valvular stenosis are often noted in HoFH ( Fig.2 ) , and may become the main cause of death in patients. Even with substantially effective treatment, systemic atherosclerosis, with such manifestations as aortic aneurysms, peripheral artery disease, and cerebrovascular disease, develops along with aging. Aggressive LDL-lowering treatments should be started as young as possible to prevent these atherosclerotic complications.
Fig. 2. Aortic Supra-valvular stenosis in HoFH .
Supra-valvular stenosis is a pathognomonic finding of HoFH, and one of the causes of premature cardiac death in this disease.
2. Prevalence
In the 1970s, prevalence of HeFH was estimated as 1 in 500 in the general population, and accordingly, that of HoFH as 1 in 1,000,000 8) . However, more recent molecular genetic studies have revealed that FH is more common than previously expected. HeFH is now estimated as 1 in 200 to 300 and HoFH as 1 in 170,000 to 300,000 in many countries including Japan 9 - 13) .
3. Genetics
LDL receptor-related disease-causing mutations are identified only in 60 to 80% of clinically diagnosed HeFH 14 - 16) . Most of them are in the LDL receptor gene ( LDLR ), and those in apolipoprotein B-100 (coded as APOB gene), the main ligand for the LDL receptor, have also been reported mainly in Caucasian populations 17) . In 2003, gain-of-function mutations in PCSK9 , the gene coding proprotein convertase subtilistin/kexin-type 9 and enhancer of LDL receptor degradation, were found. This was considered to be the second major FH-related gene, explaining 5% of HeFH in Japan 18) .
Families with FH caused by mutations in LDLR , APOB , and PCSK9 all show autosomal dominant inheritance. Since LDLR accounts for the majority of the FH mutations, most individuals with HoFH are “true homozygotes” or “compound heterozygotes” for two different mutations in LDLR . Some patients are “double heterozygotes”, having combined mutations in LDLR and PCSK9 4) , for example. The parents of HoFH children who are “true or compound heterozygotes” mostly show the HeFH phenotype. On the other hand, the family traits of HoFH “double heterozygotes” may not apparently exhibit simple Mendelian inheritance since the heterozygotes for PCSK9 show a variety of phenotypes.
It should be noted that HeFH is underdiagnosed and undertreated in the general population 3) . In the background of one suspected case of HoFH, there are many family members with HeFH , and they must be properly cared for in time to prevent their premature death.
Besides the mutations discussed above, those causing, autosomal recessive hypercholesterolemia (ARH), a rare unique type of FH have been reported in Japan 4 , 5) . ARH is caused by mutations in a gene coding low-density lipoprotein receptor adaptor protein 1 ( LDLRAP1 ). LDLRAP1 is an adaptor protein making a complex with clathrin and LDL receptors for efficient endocytosis of LDL receptors. Heterozygous carriers of this mutation do not exhibit the HeFH phenotype so it apparently shows autosomal recessive inheritance. If the parents in a probable case of HoFH have normolipidemia, ARH may be considered.
Disease-causing mutations cannot be identified in 20% to 40% of clinically diagnosed HeFH 19) . This may be due to still-unknown FH-related genes or limitations of analytic technologies. A clinical diagnosis of typical HoFH cannot be excluded even if two pathogenic FH gene variants are not identified. A substantial portion of HeFH cases without “known” FH-gene mutations could be “polygenic hypercholesterolemia” 20) or “oligogenic FH” 21 - 23) , though these concepts of FH have not become established. Although genetic analysis provides a definitive diagnosis of HoFH, the possibility of such cases cannot be excluded. Therefore, clinical assessment including thorough physical examination and familial studies is essential. For information, Japanese public healthcare insurance does not cover the expenses for HoFH genetic testing as of February 2021.
4. Pathophysiology
LDL receptor activity is completely or nearly all lost in HoFH. Severely elevated LDL-C levels from birth (more exactly, from fetal period 24) ) often cause fatal cardiovascular disease even in infancy. As an example of cholesterol deposition in tissues, pathognomonic skin xanthomas develop from infancy in HoFH and tendon xanthomas become apparent later, and they are more prominent than those in HeFH.
5. Diagnostic Criteria in Japan
1) Clinical Diagnosis
A clinical diagnosis of HoFH can be made on the basis of skin or tendon xanthomas since infancy, and untreated LDL-C levels of twice those of HeFH or higher. Diagnostic criteria for FH in Japanese guidelines apply not only HeFH but also HoFH 1 , 4) ( Table 1 , Table 2 ) . In cases with very high LDL-C and/or prominent xanthomas, HoFH should be suspected 25 , 26) .
Table 1. Diagnostic criteria for FH in children.
|
1.Elevated serum LDL cholesterol levels: untreated LDL-C level of ≥ 140 mg/dL (If total cholesterol level is ≥ 220 mg/dL, measure LDL-C level) 2. Family history of FH or premature CAD (within second-degree relatives) |
|---|
|
・Secondary hyperlipidemia should be ruled out. ・If a patient meets both of the above-mentioned criteria, FH is diagnosed. ・As LDL-C levels fluctuate during growth due to dietary and hormonal influences, careful examination is required. ・Clinical symptoms and findings including angina, xanthomas, and corneal arcus are rare in heterozygous FH children. Therefore, family history of FH is important in making diagnosis ・Premature CAD is defined as occurrence of CAD in men <55 years old or in women <65 years old. ・Homozygous FH should be suspected if patient has xanthomas. |
Table 2. Diagnostic criteria for heterozygous FH in adults (15 years of age or older).
|
1. Hyper-LDL-cholesterolemia (an untreated LDL-C level of >= 180 mg/dL) 2. Tendon xanthoma (tendon xanthoma on the backs of the hands, elbows, knees, etc. or Achilles tendon hypertrophy) or xanthoma tuberosum 3. Family history of FH or premature CAD (within the patient's second-degree relatives) |
|---|
|
・The diagnosis should be made after excluding secondary hyperlipidemia. ・If a patient meets two or more of the above-mentioned criteria, the condition should be diagnosed as FH. In cases of suspected FH, obtaining a diagnosis using genetic testing is desirable. ・Xanthoma palpebrarum is not included in xanthoma tuberosum. ・Achilles tendon hypertrophy is diagnosed if the Achilles tendon thickness is >= 9 mm on X- ray imaging. ・An LDL-C level of >= 250 mg/dL strongly suggests FH. ・If a patient is already receiving drug therapy, the lipid level that led to treatment should be used as the reference for diagnosis. ・Premature CAD is defined as the occurrence of CAD in men <55 years of age or women<65 years of age, respectively. ・If FH is diagnosed, it is preferable to also examine the patient’s family members. |
Typical HoFH exhibits total cholesterol levels of more than 600 mg/dL in total 1) , but there is considerable overlapping of levels between HoFH and severe HeFH 28) . LDL-C levels over 370 mg/dL (or total cholesterol levels over 450 mg/dL) in the fasting steady state would be sufficient for a diagnosis of probable HoFH and the patient should be referred to specialists. In pediatric patients, plasma LDL-C levels may fluctuate and should be measured multiple times.
Measurement of LDL receptor activity in the fibroblasts of patients provides useful information in diagnosing HoFH, but is currently not routinely available at the laboratories of commercial or research facilities in Japan. Assaying LDL receptor activity in lymphocytes is feasible 29) but less reliable than fibroblast measurements.
Skin xanthomas since infancy are pathognomonic for HoFH, and sometimes the chief complaint for the first consultation with doctors 4) ( Fig.1 ) . Skin xanthomas in pediatric HoFH are frequently found in flexures of the wrist and ankles, and also in other regions having mechanical stress. Tendon xanthomas are more prominent than in HeFH 27) , but become apparent later than skin xanthomas.
2) Genetic Diagnosis
Genetic testing is recommended for suspected HoFH cases, but is not covered by Japanese public healthcare insurance as of February 2021. Genetic diagnosis indicates the potential efficacy of drug treatment and thereby therapeutic strategies 30 , 31) . Conversely, drug ineffectiveness (for example, refractory to PCSK9 inhibitors) may suggest the HoFH genotype so genetic tests should be considered in such cases 32) .
The possibility of detecting an FH-causative mutation in HeFH is 60 to 80%. Therefore, diagnosis should be carefully made together with clinical examinations and detailed familial studies even in cases of suspected HoFH where the mutations detected are apparently in 0 alleles or only 1 allele, because a genetic test does not exclude the possibility of HoFH due to unknown gene mutations. Consultation with experienced specialists is required in such cases.
6. Screening/Follow-Up for ASCVD
Most HoFH patients may die of ASCVD before 30 years old if untreated 33 , 34) . Once HoFH is suspected, extensive examination for ASCVD should be carried out. In HoFH, the potentially fatal disorders of angina pectoris, myocardial infarction, and aortic supra-valvular and valvular stenosis could occur even in childhood ( Fig.2 ) . Aortic supra-valvular and valvular stenosis in HoFH is sometimes difficult to treat and lethal 35) . If patients are left untreated until around 20 years old, ASCVD risk is extremely high. Heart and aortic disease proceeds and systemic atherosclerosis develops later in life.
Non-invasive tests such as cardiac ultrasonography, carotid ultrasonography, and electrocardiograms should be conducted first, and enhanced CT for the aorta and coronary artery and coronary angiography should be considered when necessary 36) . Exercise stress tests should be carefully performed with consideration for patient safety.
7. Differential Diagnosis
A differential diagnosis should be made using high LDL-C levels and prominent xanthomas. LDL-C may be elevated to HoFH levels by secondary hypercholesterolemia such as hypothyroidism or nephrotic syndrome. Xanthomas may develop due to hypercholesterolemia in primary biliary cholangitis. Sitosterolemia, caused by the ATP-binding cassette transporter ABCG5/ABCG8 gene ( ABCG5/ABCG8 ) mutations 37) , sometimes exhibits elevation of LDL-C and skin xanthomas comparable to HoFH during the suckling stage 38) . Although LDL-C elevation and skin xanthomas subside after weaning from breastfeeding, elevated levels of plasma plant-sterols (including sitosterol) persist in these patients. There is also cerebrotendinous xanthomatosis (CTX), an autosomal recessive disease caused by sterol 27-hydroxylase gene ( CYP27A ) mutations, which is characterized by prominent xanthomatosis in the tendon and brain and sometimes accompanied by central nervous symptoms (mental retardation, cognitive impairment, or motor ataxia) 39) . CTX is clinically diagnosed by elevated plasma cholestanol levels. A differential diagnosis should be carefully made because these diseases require specific therapeutic approaches.
8. Treatment of HoFH
HoFH patients may suffer fatal ASCVD from infancy so initiation of aggressive LDL-C lowering as early as possible is essential for preventing their premature deaths 1 , 4) ( Fig.3 , Fig.4 ) . Specific therapeutic strategies for preventing ASCVD development in individual cases should be planned in addition to LDL lowering.
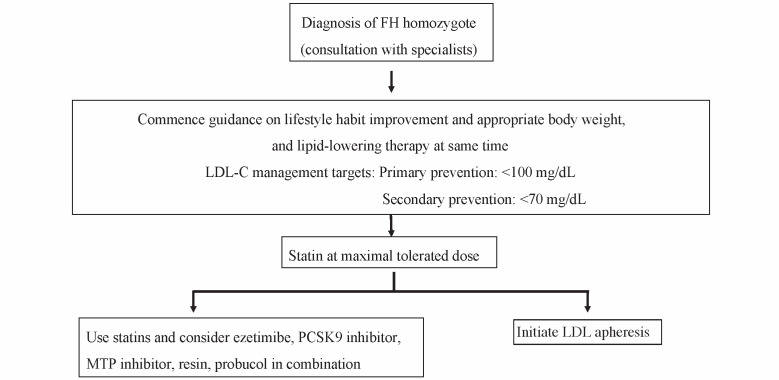
Fig. 3. Algorithm for treatment of adult (15 years of age or older) FH homozygotes .
In HoFH, powerful LDL-C lowering therapy is required from a young age to prevent the onset and progression of CAD. Combination of lipid-lowering drugs, including MTP inhibitor, and lipoprotein apheresis is required in many patients with HoFH.
(Adapted from Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 20171) “LDL apheresis” means “lipoprotein apheresis” in this review)
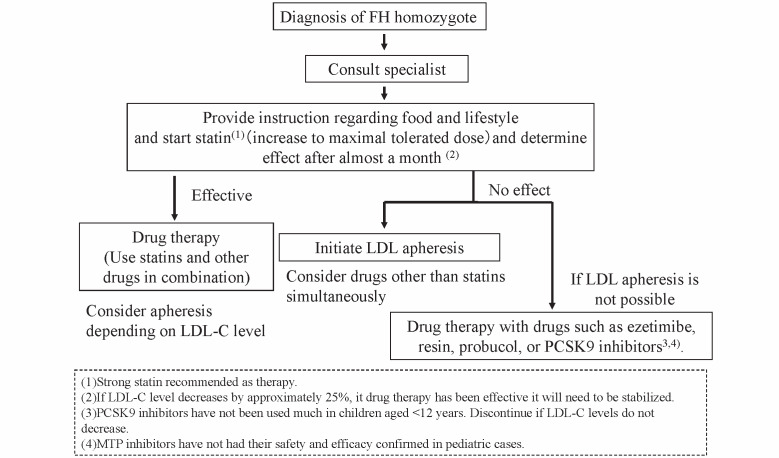
Fig. 4. Algorithm for treatment of pediatric FH homozygotes .
Lifestyle interventions and maximally tolerated statin therapy should be started at the initial diagnosis. Since LDL-C targets are rarely achieved, lipoprotein apheresis therapy is recommended, and should be commenced by age 5 ideally.
Patients as well as their families will be burdened by various sources of stress such as anxiety about prognosis, concern about heredity, and costs of treatments. Supportive information should be provided, including that on possible financial aid, the therapeutic options available, and genetic counseling. Financial aid is available under The Program for Designated Intractable Diseases of the Japanese public healthcare system. Pediatric FH patients can receive support separately under The Program of Medical Aid for Chronic Pediatric Diseases of Specified Categories, which covers both HeFH and HoFH. In practice, consultation with specialists should strongly be advised.
1) Adult HoFH (15 years of age or older)
The LDL-C management goal is less than 100 mg/dL in primary prevention, and less than 70 mg/dL in secondary prevention 1) in the Japanese guideline ( Fig.3 ) . Medication should start with statins at appropriate doses 40) , followed by increasing them to maximal tolerated doses and combination with other drugs 41 - 43) . Achievement of these goals with drug treatment only is, however, not easy in HoFH.
Statins, ezetimibe and PCSK9 inhibitors all act by enhancing LDL receptor activity 44 , 45) , so their effectiveness depends on residual LDL receptor activity in the individual HoFH patient. Many HoFH patients are refractory to these drugs. However, if any of them are effective in lowering LDL-C, they should be continued together with the additional treatments described below.
MTP inhibitors (lomitapide) are a class of oral drug that is independent of LDL receptor activity. Clinically, they are indicated only for HoFH, and a decrease in LDL-C to approximately half of the pretreatment level can be achieved in many HoFH cases, if tolerated 46 - 49) . The main adverse effects are gastrointestinal symptoms and liver enzyme elevation accompanied by fat accumulation, similar to the symptoms observed in patients with MTP deficiency 50) . The MTP inhibitor should be started with the lowest dose, taking adequate measures in accordance with nutritional guidance (restricted fat and alcohol intake), and then the dose should be carefully increased gradually.
Lipoprotein apheresis directly removes LDL from plasma through selective absorption or filtration of LDL using an extra-corporal circulation system, and this has been the core therapy for HoFH to date 51 - 53) . LDL-C levels can be precisely decreased by this procedure, depending upon the treated plasma volume, when properly performed. Lipoprotein apheresis not only removes LDL but also has potential pleiotropic effects in preventing atherosclerosis through removal of cell adhesion molecules 54) , coagulation factors 55) , and inflammatory cytokines 56) . Lipoprotein apheresis for HoFH once every 1 to 2 weeks is covered by Japanese public healthcare insurance. The use of ACE inhibitors is contra-indicated for patients treated by lipoprotein apheresis with the Liposorber system and selective absorption of LDL by dextran sulfate-cellulose because shock may occur due to an increase in bradykinin activation.
Another option is liver transplantation. While this is highly invasive, it has been shown be a feasible therapeutic option for reversing atherosclerotic changes in HoFH patients that are uncontrollable with conservative therapy 57 , 58) .
2) Pediatric HoFH
Even in the suckling stage, children with HoFH must be referred to specialists for initiation of lipid-lowering therapy, as this is the key to a better prognosis 4) . The target LDL-C level should be the same as for adult HoFH in secondary prevention (though not clearly stated in the current pediatric guideline 4)) . In pediatric HeFH, it is set at less than 140 mg/dL for primary prevention.
Statins and life-style interventions must be started at the time of diagnosis. Statins must be up-titrated and efficacy should be evaluated within a 1-month interval in order to titrate up to the maximal tolerated doses. Combination therapy with ezetimibe, bile sequestering resins, and PCSK9 inhibitors should be considered. These strategies may be effective in cases where there is a response in residual LDL receptor activity. Probucol should also be considered as it could reduce LDL in HoFH due to an unknown mechanism. Administration of an MTP inhibitor could be considered but only with extreme caution because no results of clinical trials in children have been reported. Drug therapy should be conducted until it becomes possible to commence lipoprotein apheresis.
In any case, lipoprotein apheresis must be considered since LDL-C targets are seldom achieved in HoFH only with drug treatment. This extracorporeal circulation therapy is generally commenced at the age of 5 or older, though it has reportedly been started in a patient who was 3.5 years old. In Japan, the main method is selective LDL absorption by dextran sulfate cellulose. However, with the aim of reducing extracorporeal volume, simple plasma exchange can be selected for children with a bodyweight under 30 kg.
3) Treatment during Pregnancy in HoFH
Female HoFH patients could be treated in the same way as for secondary prevention on reaching childbearing age. During pregnancy, LDL and VLDL are generally increased, and continuation of LDL-lowering therapy would be important. Statins and many other drugs are contraindicated during pregnancy and breastfeeding, and only bile sequestering resins can be used, taking proper care, but their LDL-lowering effects are limited 59) . It has been reported that lipoprotein apheresis is effective and feasible during pregnancy, and a case of cardiovascular death caused by suspension of apheresis during pregnancy 60) has been reported. Consultation with experienced specialists is recommended for pregnancy in HoFH.
Future Perspectives
Therapeutic options are still limited for HoFH, and all-out efforts should be made to achieve the best combination of feasible therapies in many cases. However, each therapeutic strategy has its advantages and disadvantages so new therapies are awaited.
Inclisiran, a new siRNA drug that inhibits translation of PCSK9, is being developed 61) . As the effect of one injection lasts 6 months to 1 year, this drug may be a good option for HoFH patients who respond to PCSK9 inhibitors. Inclisiran was approved in the European Union in December 2020.
Evinacumab, an anti-ANGPTL3 antibody, is a new class of drug that is reportedly effective even in HoFH 62) , and clinical trials are ongoing in Japan and other countries 63) . The LDL-C lowering effect of evinacumab is independent of LDL receptor activity.
Mipomersen (Kynamro), an antisense drug for the APOB gene, has moderate LDL-lowering effects but has the adverse effects of liver damage and injection site reactions. It was approved by the FDA for HoFH in 2013 64) but rejected by the European Medicines Agency, and FDA approval was eventually withdrawn in 2019.
Gene therapy for HoFH has been investigated, but is still in the experimental stage. Since HoFH is a relatively rare disease, even if approved, demand for therapies would not be very high anywhere in the world. Lomitapide had been approved only for the treatment of HoFH in just 38 countries as of March 2021, including those in North and South America, the European Economic Area, and only Japan in East Asia. Lipoprotein-apheresis, which requires specific equipment, is available in a limited number of countries. Wider availability of therapeutic measures for HoFH should encourage its more active diagnosis worldwide.
Early diagnosis of FH is essential for preventing premature death in patients, whether they are hetero- or homozygotes 65 - 67) . Universal screening of plasma lipid levels during childhood has been trialed in some parts of Japan and we expect that such trials on local universal screening will lead to a nationwide system. In addition, reverse cascade screening, in which family members are screened on the basis of child probands, and continued worldwide registry research should improve the effectiveness of finding FH 68 - 76) .
Acknowledgments and Notice of Grant Support
This work has been supported by Health, Labour and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases (H30-nanji-ippan-003).
Conflicts of Interest
Atsushi Nohara has nothing to disclose. Hayato Tada has nothing to disclose. Masatsune Ogura has received honoraria from Amgen Inc., Astellas Pharma Inc. Sachiko Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Koh Ono has nothing to disclose. Hitoshi Shimano has nothing to disclose. Hiroyuki Daida has received honoraria from Amgen Inc., Daiichi-Sankyo Co., Ltd., Kowa Co., Ltd., and MSD K.K., Novartis Pharma K.K., Bayer Yakuhin, Ltd. and received clinical research funding from Canon Medical Systems Corporation, Philips Japan, Ltd., Toho Holdings Co., Ltd., Asahi Kasei Corporation, and Inter Reha Co., Ltd. HD has also received scholarship grants from Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., MSD K.K., Daiichi-Sankyo Co., Ltd., Pfizer Co., Ltd., Mitsubishi Tanabe Pharma Corp., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma, Ltd., Shionogi & Co., Ltd., Actelion Pharmaceuticals, Ltd., Actelion Ltd., Kowa Co., Ltd., Bayer Yakuhin, Ltd. HD has also courses endowed by companies, including Philips Japan, Ltd., ResMed, Fukuda Denshi Co., Ltd., and Paramount Bed Co., Ltd. Kazushige Dobashi has nothing to disclose. Toshio Hayashi has nothing to disclose. Mika Hori has nothing to disclose. Kota Matsuki has nothing to disclose. Tetsuo Minamino has nothing to disclose. Shinji Yokoyama has nothing to disclose. Mariko Harada-Shiba has received stock holdings or options from Liid Pharma, honoraria from Amgen Inc., Astellas Pharma Inc., Sanofi, and scholarship grants from Aegerion Pharmaceuticals, Inc., Recordati Rare Diseases Japan, and Kaneka Corporation. Katsunori Ikewaki has nothing to disclose. Yasushi Ishigaki has nothing to disclose. Shun Ishibashi has received honoraria from Kowa Co., Ltd., and a scholarship grant from Ono Pharmaceutical Co., Ltd. Kyoko Inagaki has nothing to disclose. Hirotoshi Ohmura has nothing to disclose. Hiroaki Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Masa-aki Kawashiri has nothing to disclose. Masayuki Kuroda has nothing to disclose. Masahiro Koseki has received clinical research funding from Kowa Company, Ltd., Rohto Pharmaceutical Co., Ltd. Takanari Gotoda has nothing to disclose. Shingo Koyama has nothing to disclose. Yoshiki Sekijima has nothing to disclose. Manabu Takahashi has nothing to disclose. Yasuo Takeuchi has nothing to disclose. Misa Takegami has nothing to disclose. Kazuhisa Tsukamoto has received honoraria from Bayer Yakuhin, Ltd., MSD Ltd., Takeda Pharmaceutical Company Ltd., and scholarship grants from Mitsubishi Tanabe Pharma Corporation., Bayer Yakuhin, Ltd., Sanofi K.K. Atsuko Nakatsuka has nothing to disclose. Kimitoshi Nakamura has nothing to disclose. Satoshi Hirayama has nothing to disclose. Hideaki Bujo has nothing to disclose. Daisaku Masuda has received clinical research funding from MSD K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kowa Co., Ltd. Takashi Miida has nothing to disclose. Yoshihiro Miyamoto has nothing to disclose. Takeyoshi Murano has nothing to disclose. Takashi Yamaguchi has nothing to disclose. Shizuya Yamashita has received honoraria from Kowa Company, Ltd., MSD K.K. Masashi Yamamoto has nothing to disclose. Koutaro Yokote has received honoraria from Kowa Company, Ltd., MSD K.K., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corp., Amgen K.K., Takeda Pharmaceutical Company Limited, Sanofi K.K., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Daiichi-Sankyo Co., Ltd., Novartis Pharma K.K., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., and received clinical research funding from Taisho Pharmaceutical Co., Ltd. KY has also received scholarship grants from Mitsubishi Tanabe Pharma Corp., Takeda Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd., Kao Corporation, Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi-Sankyo Co., Ltd., Teijin Pharma, Ltd., Shionogi Co., Ltd., Bayer Yakuhin, Ltd. Jun Wada has nothing to disclose.
References
- 1).Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K and Working Group by Japan Atherosclerosis Society for Making Guidance of Familial H. Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. The Metabolic & Molecular Bases of Inherited Disease. eighth edition. Vol. 2, Mac Graw Hill. 2863-2913 [Google Scholar]
- 3).Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS. Familial hypercholesterolemia. Nat Rev Dis Primers, 2017, 3: 17093 [DOI] [PubMed] [Google Scholar]
- 4).Harada-Shiba M, Takagi A, Miyamoto Y, Tsushima M, Ikeda Y, Yokoyama S and Yamamoto A. Clinical features and genetic analysis of autosomal recessive hypercholesterolemia. J Clin Endocrinol Metab, 2003; 88: 2541-2547 [DOI] [PubMed] [Google Scholar]
- 5).Tada H, Kawashiri MA, Ikewaki K, Terao Y, Noguchi T, Nakanishi C, Tsuchida M, Takata M, Miwa K, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H and Yamagishi M. Altered metabolism of low-density lipoprotein and very-low-density lipoprotein remnant in autosomal recessive hypercholesterolemia: results from stable isotope kinetic study in vivo. Circ Cardiovasc Genet, 2012; 5: 35-41 [DOI] [PubMed] [Google Scholar]
- 6).Harada-Shiba M, Ohta T, Ohtake A, Ogura M, Dobashi K, Nohara A, Yamashita S, Yokote K, Joint Working Group by Japan Pediatric S and Japan Atherosclerosis Society for Making Guidance of Pediatric Familial H. Guidance for Pediatric Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 539-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A and European Atherosclerosis Society Consensus P. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34: 3478-3490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Goldstein JL, Schrott HG, Hazzard WR, Bierman EL and Motulsky AG. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest, 1973; 52: 1544-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J and Hokuriku FHSG. Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]
- 10).Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J, 2016; 37: 1384-1394 [DOI] [PubMed] [Google Scholar]
- 11).de Ferranti SD, Rodday AM, Mendelson MM, et al. Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation, 2016; 133: 1067-1072 [DOI] [PubMed] [Google Scholar]
- 12).Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J Am Coll Cardiol, 2020; 75: 2553-2566 [DOI] [PubMed] [Google Scholar]
- 13).Hu P, Dharmayat KI, Stevens CAT, Sharabiani MTA, Jones RS, Watts GF, Genest J, Ray KK, Vallejo-Vaz AJ. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation, 2020; 141: 1742-1759 [DOI] [PubMed] [Google Scholar]
- 14).Mabuchi H. Half a Century Tales of Familial Hypercholesterolemia (FH) in Japan. J Atheroscler Thromb, 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H and Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J, 2017; 38: 1573-1579 [DOI] [PubMed] [Google Scholar]
- 16).Hori M, Ohta N, Takahashi A, Masuda H, Isoda R, Yamamoto S, Son C, Ogura M, Hosoda K, Miyamoto Y and Harada-Shiba M. Impact of LDLR and PCSK9 pathogenic variants in Japanese heterozygous familial hypercholesterolemia patients. Atherosclerosis, 2019; 289: 101-108 [DOI] [PubMed] [Google Scholar]
- 17).Hori M, Takahashi A, Son C, Ogura M, Harada-Shiba M. The first Japanese cases of familial hypercholesterolemia due to a known pathogenic APOB gene variant, c.10580 G>A: p.(Arg3527Gln). J Clin Lipidol, 2020; 14: 482-486 [DOI] [PubMed] [Google Scholar]
- 18).Noguchi T, Katsuda S, Kawashiri MA, Tada H, Nohara A, Inazu A, Yamagishi M, Kobayashi J and Mabuchi H. The E32K variant of PCSK9 exacerbates the phenotype of familial hypercholesterolaemia by increasing PCSK9 function and concentration in the circulation. Atherosclerosis, 2010; 210: 166-172 [DOI] [PubMed] [Google Scholar]
- 19).Tada H, Hori M, Nomura A, Hosomichi K, Nohara A, Kawashiri MA and Harada-Shiba M. A catalog of the pathogenic mutations of LDL receptor gene in Japanese familial hypercholesterolemia. J Clin Lipidol, 2020; 14: 346-351 e9 [DOI] [PubMed] [Google Scholar]
- 20).Talmud PJ, Shah S, Whittall R, Futema M, Howard P, Cooper JA, Harrison SC, Li K, Drenos F, Karpe F, Neil HA, Descamps OS, Langenberg C, Lench N, Kivimaki M, Whittaker J, Hingorani AD, Kumari M and Humphries SE. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet, 2013; 381: 1293-1301 [DOI] [PubMed] [Google Scholar]
- 21).Tada H, Kawashiri MA, Nomura A, Teramoto R, Hosomichi K, Nohara A, Inazu A, Mabuchi H, Tajima A, Yamagishi M. Oligogenic familial hypercholesterolemia, LDL cholesterol, and coronary artery disease. J Clin Lipidol, 2018; 12: 1436-1444 [DOI] [PubMed] [Google Scholar]
- 22).Tada H, Nohara A, Kawashiri MA. Monogenic, polygenic, and oligogenic familial hypercholesterolemia. Curr Opin Lipidol, 2019; 30: 300-306 [DOI] [PubMed] [Google Scholar]
- 23).Tada H, Okada H, Nomura A, Yashiro S, Nohara A, Ishigaki Y, Takamura M, Kawashiri MA. Rare and Deleterious Mutations in ABCG5/ABCG8 Genes Contribute to Mimicking and Worsening of Familial Hypercholesterolemia Phenotype. Circ J, 2019; 83: 1917-1924 [DOI] [PubMed] [Google Scholar]
- 24).de Gennes JL, Daffos F, Dairou F, Forestier F, Capella-Pavlosky M, Truffert J, Gaschard JC, Darbois Y. Direct fetal blood examination for prenatal diagnosis of homozygous familial hypercholesterolemia. Arteriosclerosis, 1985; 5: 440-442 [DOI] [PubMed] [Google Scholar]
- 25).Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E, Tybjærg-Hansen A, Watts GF, Averna M, Boileau C, Borén J, Catapano AL, Defesche JC, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Ray KK, Stalenhoef AF, Stroes E, Taskinen MR, Wiegman A, Wiklund O, Chapman MJ; European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J, 2014; 35: 2146-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, Ose L, Averna M, Boileau C, Borén J, Bruckert E, Catapano AL, Defesche JC, Descamps OS, Hegele RA, Hovingh GK, Humphries SE, Kovanen PT, Kuivenhoven JA, Masana L, Nordestgaard BG, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AF, Steinhagen-Thiessen E, Stroes ES, Taskinen MR, Tybjærg-Hansen A, Wiklund O; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J, 2015; 36: 2425-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Kawashiri MA, Okada H, Tada H. Severe calcification of the Achilles’ tendon. Eur Heart J, 2021 in press [DOI] [PubMed] [Google Scholar]
- 28).Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, Baum SJ, Catapano AL, Chapman MJ, Defesche JC, Folco E, Freiberger T, Genest J, Hovingh GK, Harada-Shiba M, Humphries SE, Jackson AS, Mata P, Moriarty PM, Raal FJ, Al-Rasadi K, Ray KK, Reiner Z, Sijbrands EJ, Yamashita S and International Atherosclerosis Society Severe Familial Hypercholesterolemia P. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol, 2016; 4: 850-861 [DOI] [PubMed] [Google Scholar]
- 29).Tada H, Kawashiri MA, Noguchi T, Mori M, Tsuchida M, Takata M, Nohara A, Inazu A, Kobayashi J, Yachie A, Mabuchi H, Yamagishi M. A novel method for determining functional LDL receptor activity in familial hypercholesterolemia: application of the CD3/CD28 assay in lymphocytes. Clin Chim Acta, 2009; 400: 42-47 [DOI] [PubMed] [Google Scholar]
- 30).Raal FJ, Hovingh GK, Blom D, Santos RD, Harada-Shiba M, Bruckert E, Couture P, Soran H, Watts GF, Kurtz C, Honarpour N, Tang L, Kasichayanula S, Wasserman SM, Stein EA. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol, 2017; 5: 280-290 [DOI] [PubMed] [Google Scholar]
- 31).Thedrez A, Blom DJ, Ramin-Mangata S, Blanchard V, Croyal M, Chemello K, Nativel B, Pichelin M, Cariou B, Bourane S, Tang L, Farnier M, Raal FJ, Lambert G. Homozygous Familial Hypercholesterolemia Patients With Identical Mutations Variably Express the LDLR (Low-Density Lipoprotein Receptor): Implications for the Efficacy of Evolocumab. Arterioscler Thromb Vasc Biol, 2018; 38: 592-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Sturm AC, Knowles JW, Gidding SS, Ahmad ZS, Ahmed CD, Ballantyne CM, Baum SJ, Bourbon M, Carrié A, Cuchel M, de Ferranti SD, Defesche JC, Freiberger T, Hershberger RE, Hovingh GK, Karayan L, Kastelein JJP, Kindt I, Lane SR, Leigh SE, Linton MF, Mata P, Neal WA, Nordestgaard BG, Santos RD, Harada-Shiba M, Sijbrands EJ, Stitziel NO, Yamashita S, Wilemon KA, Ledbetter DH, Rader DJ; Convened by the Familial Hypercholesterolemia Foundation. Clinical Genetic Testing for Familial Hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol, 2018; 72: 662-680 [DOI] [PubMed] [Google Scholar]
- 33).Gautschi M, Pavlovic M, Nuoffer JM. Fatal myocardial infarction at 4.5 years in a case of homozygous familial hypercholesterolaemia. JIMD Rep, 2012; 2: 45-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Widhalm K, Binder CB, Kreissl A, Aldover-Macasaet E, Fritsch M, Kroisboeck S, Geiger H. Sudden death in a 4-year-old boy: a near-complete occlusion of the coronary artery caused by an aggressive low-density lipoprotein receptor mutation (W556R) in homozygous familial hypercholesterolemia. J Pediatr, 2011; 158: 167 [DOI] [PubMed] [Google Scholar]
- 35).Zhang R, Xie J, Zhou J, Xu L, Pan Y, Qu Y, Li R, Chong M, Song L, Wen W, Wu Y, Li J, Wang L, Yang Y. Supravalvular Aortic Stenosis and the Risk of Premature Death Among Patients With Homozygous Familial Hypercholesterolemia. Am J Cardiol, 2021 in press [DOI] [PubMed] [Google Scholar]
- 36).Luirink IK, Kuipers IM, Hutten BA, Planken RN, Backx APCM, Groothoff JW, Wiegman A. Coronary computed tomography angiography and echocardiography in children with homozygous familial hypercholesterolemia. Atherosclerosis, 2019; 285: 87-92 [DOI] [PubMed] [Google Scholar]
- 37).Tada H, Nohara A, Inazu A, Sakuma N, Mabuchi H, Kawashiri MA. Sitosterolemia, Hypercholesterolemia, and Coronary Artery Disease. J Atheroscler Thromb, 2018; 25: 783-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Tada H, Kawashiri MA, Takata M, Matsunami K, Imamura A, Matsuyama M, Sawada H, Nunoi H, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H and Yamagishi M. Infantile Cases of Sitosterolaemia with Novel Mutations in the ABCG5 Gene: Extreme Hypercholesterolaemia is Exacerbated by Breastfeeding. JIMD Rep, 2015; 21: 115-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Sekijima Y, Koyama S, Yoshinaga T, Koinuma M, Inaba Y. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet, 2018; 63: 271-280 [DOI] [PubMed] [Google Scholar]
- 40).Stein EA, Dann EJ, Wiegman A, Skovby F, Gaudet D, Sokal E, Charng MJ, Mohamed M, Luirink I, Raichlen JS, Sundén M, Carlsson SC, Raal FJ, Kastelein JJP. Efficacy of Rosuvastatin in Children With Homozygous Familial Hypercholesterolemia and Association With Underlying Genetic Mutations. J Am Coll Cardiol, 2017; 70: 1162-1170 [DOI] [PubMed] [Google Scholar]
- 41).Gagné C, Gaudet D, Bruckert E; Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation, 2002; 105: 2469-2475 [DOI] [PubMed] [Google Scholar]
- 42).Yamamoto A, Harada-Shiba M, Endo M, Kusakabe N, Tanioka T, Kato H, Shoji T. The effect of ezetimibe on serum lipids and lipoproteins in patients with homozygous familial hypercholesterolemia undergoing LDL-apheresis therapy. Atherosclerosis, 2006; 186: 126-131 [DOI] [PubMed] [Google Scholar]
- 43).Gotto AM Jr, Moon JE. Pharmacotherapies for lipid modification: beyond the statins. Nat Rev Cardiol, 2013; 10: 560-570 [DOI] [PubMed] [Google Scholar]
- 44).Santos RD, Stein EA, Hovingh GK, Blom DJ, Soran H, Watts GF, López JAG, Bray S, Kurtz CE, Hamer AW, Raal FJ. Long-Term Evolocumab in Patients With Familial Hypercholesterolemia. J Am Coll Cardiol, 2020; 75: 565-574 [DOI] [PubMed] [Google Scholar]
- 45).Blom DJ, Harada-Shiba M, Rubba P, Gaudet D, Kastelein JJP, Charng MJ, Pordy R, Donahue S, Ali S, Dong Y, Khilla N, Banerjee P, Baccara-Dinet M, Rosenson RS. Efficacy and Safety of Alirocumab in Adults With Homozygous Familial Hypercholesterolemia: The ODYSSEY HoFH Trial. J Am Coll Cardiol, 2020; 76: 131-142 [DOI] [PubMed] [Google Scholar]
- 46).Harada-Shiba M, Ikewaki K, Nohara A, Otsubo Y, Yanagi K, Yoshida M, Chang Q and Foulds P. Efficacy and Safety of Lomitapide in Japanese Patients with Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb, 2017; 24: 402-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Nohara A, Otsubo Y, Yanagi K, Yoshida M, Ikewaki K, Harada-Shiba M and Jurecka A. Safety and Efficacy of Lomitapide in Japanese Patients with Homozygous Familial Hypercholesterolemia (HoFH): Results from the AEGR-733-301 Long-Term Extension Study. J Atheroscler Thromb, 2019; 26: 368-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Aljenedil S, Alothman L, Bélanger AM, Brown L, Lahijanian Z, Bergeron J, Couture P, Baass A, Ruel I, Brisson D, Khoury E, Gaudet D, Genest J. Lomitapide for treatment of homozygous familial hypercholesterolemia: The Quebec experience. Atherosclerosis, 2020; 310: 54-63 [DOI] [PubMed] [Google Scholar]
- 49).Underberg JA, Cannon CP, Larrey D, Makris L, Blom D, Phillips H. Long-term safety and efficacy of lomitapide in patients with homozygous familial hypercholesterolemia: Five-year data from the Lomitapide Observational Worldwide Evaluation Registry (LOWER). J Clin Lipidol, 2020; 14: 807-817 [DOI] [PubMed] [Google Scholar]
- 50).Yang XP, Inazu A, Yagi K, Kajinami K, Koizumi J, Mabuchi H. Abetalipoproteinemia caused by maternal isodisomy of chromosome 4q containing an intron 9 splice acceptor mutation in the microsomal triglyceride transfer protein gene. Arterioscler Thromb Vasc Biol, 1999; 19: 1950-1955 [DOI] [PubMed] [Google Scholar]
- 51).Makino H, Koezuka R, Tamanaha T, Ogura M, Matsuki K, Hosoda K and Harada-Shiba M. Familial Hypercholesterolemia and Lipoprotein Apheresis. J Atheroscler Thromb, 2019; 26: 679-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Luirink IK, Determeijer J, Hutten BA, Wiegman A, Bruckert E, Schmitt CP, Groothoff JW. Efficacy and safety of lipoprotein apheresis in children with homozygous familial hypercholesterolemia: A systematic review. J Clin Lipidol, 2019; 13: 31-39 [DOI] [PubMed] [Google Scholar]
- 53).Luirink IK, Hutten BA, Greber-Platzer S, Kolovou GD, Dann EJ, de Ferranti SD, Taylan C, Bruckert E, Saheb S, Oh J, Driemeyer J, Farnier M, Pape L, Schmitt CP, Novoa FJ, Maeser M, Masana L, Shahrani A, Wiegman A, Groothoff JW. Practice of lipoprotein apheresis and short-term efficacy in children with homozygous familial hypercholesterolemia: Data from an international registry. Atherosclerosis, 2020; 299: 24-31 [DOI] [PubMed] [Google Scholar]
- 54).von Bauer R, Oikonomou D, Sulaj A, Kopf S, Fleming T, Rudofsky G, Nawroth P. Pleiotropic Effect of Lipoprotein-Apheresis on the Soluble Form of Activated Leukocyte Cell Adhesion Molecule (sALCAM) in Familial Hypercholesterolaemia. Exp Clin Endocrinol Diabetes, 2019; 127: 276-280 [DOI] [PubMed] [Google Scholar]
- 55).Ramunni A, Burzo M, Vernò L, Brescia P. Pleiotropic effects of LDL apheresis. Atheroscler Suppl, 2009; 10: 53-55 [DOI] [PubMed] [Google Scholar]
- 56).Lappegård KT, Enebakk T, Thunhaug H, Ludviksen JK, Mollnes TE, Hovland A. LDL apheresis activates the complement system and the cytokine network, whereas PCSK9 inhibition with evolocumab induces no inflammatory response. J Clin Lipidol, 2016; 10: 1481-1487 [DOI] [PubMed] [Google Scholar]
- 57).Cephus CE, Qureshi AM, Sexson Tejtel SK, Alam M, Moodie DS. Coronary artery disease in a child with homozygous familial hypercholesterolemia: Regression after liver transplantation. J Clin Lipidol, 2019; 13: 880-886 [DOI] [PubMed] [Google Scholar]
- 58).Ishigaki Y, Kawagishi N, Hasegawa Y, Sawada S, Katagiri H, Satomi S, Oikawa S. Liver Transplantation for Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb, 2019; 26: 121-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Botha TC, Pilcher GJ, Wolmarans K, Blom DJ, Raal FJ. Statins and other lipid-lowering therapy and pregnancy outcomes in homozygous familial hypercholesterolaemia: A retrospective review of 39 pregnancies. Atherosclerosis, 2018; 277: 502-507 [DOI] [PubMed] [Google Scholar]
- 60).Ogura M, Makino H, Kamiya C, Yoshimatsu J, Soran H, Eatough R, Perrone G, Harada-Shiba M and Stefanutti C. Lipoprotein apheresis is essential for managing pregnancies in patients with homozygous familial hypercholesterolemia: Seven case series and discussion. Atherosclerosis, 2016; 254: 179-183 [DOI] [PubMed] [Google Scholar]
- 61).Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, Wijngaard PLJ, Curcio D, Jaros MJ, Leiter LA, Kastelein JJP; ORION-9 Investigators. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N Engl J Med, 2020; 382: 1520-1530 [DOI] [PubMed] [Google Scholar]
- 62).Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, Ali S, Banerjee P, Chan KC, Gipe DA, Khilla N, Pordy R, Weinreich DM, Yancopoulos GD, Zhang Y, Gaudet D; ELIPSE HoFH Investigators. Evinacumab for Homozygous Familial Hypercholesterolemia. N Engl J Med, 2020; 383: 711-720 [DOI] [PubMed] [Google Scholar]
- 63).Harada-Shiba M, Ali S, Gipe DA, Gasparino E, Son V, Zhang Y, Pordy R, Catapano AL. A randomized study investigating the safety, tolerability, and pharmacokinetics of evinacumab, an ANGPTL3 inhibitor, in healthy Japanese and Caucasian subjects. Atherosclerosis, 2020; 314: 33-40 [DOI] [PubMed] [Google Scholar]
- 64).Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet, 2010; 375: 998-1006 [DOI] [PubMed] [Google Scholar]
- 65).Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N Engl J Med, 2019; 381: 1547-1556 [DOI] [PubMed] [Google Scholar]
- 66).Tada H, Okada H, Nomura A, Nohara A, Yamagishi M, Takamura M, Kawashiri MA. Prognostic Impact of Cascade Screening for Familial Hypercholesterolemia on Cardiovascular Events. J Clin Lipidol, 2021 in press [DOI] [PubMed] [Google Scholar]
- 67).Representatives of the Global Familial Hypercholesterolemia Community, Wilemon KA, Patel J, Aguilar-Salinas C, Ahmed CD, Alkhnifsawi M, Almahmeed W, Alonso R, Al-Rasadi K, Badimon L, Bernal LM, Bogsrud MP, Braun LT, Brunham L, Catapano AL, Cillikova K, Corral P, Cuevas R, Defesche JC, Descamps OS, de Ferranti S, Eisele JL, Elikir G, Folco E, Freiberger T, Fuggetta F, Gaspar IM, Gesztes AG, Gro.elj U, Hamilton-Craig I, Hanauer-Mader G, Harada-Shiba M, Hastings G, Hovingh GK, Izar MC, Jamison A, Karlsson GN, Kayikcioglu M, Koob S, Koseki M, Lane S, Lima- Martinez MM, Lopez G, Martinez TL, Marais D, Marion L, Mata P, Maurina I, Maxwell D, Mehta R, Mensah GA, Miserez AR, Neely D, Nicholls SJ, Nohara A, Nordestgaard BG, Ose L, Pallidis A, Pang J, Payne J, Peterson AL, Popescu MP, Puri R, Ray KK, Reda A, Sampietro T, Santos RD, Schalkers I, Schreier L, Shapiro MD, Sijbrands E, Soffer D, Stefanutti C, Stoll M, Sy RG, Tamayo ML, Tilney MK, Tokgozoglu L, Tomlinson B, Vallejo-Vaz AJ, Vazquez-Cardenas A, de Luca PV, Wald DS, Watts GF, Wenger NK, Wolf M, Wood D, Zegerius A, Gaziano TA, Gidding SS. Reducing the Clinical and Public Health Burden of Familial Hypercholesterolemia: A Global Call to Action. JAMA Cardiol, 2020; 5: 217-229 [DOI] [PubMed] [Google Scholar]
- 68).Wu X, Pang J, Wang X, Peng J, Chen Y, Wang S, Watts GF, Lin J. Reverse cascade screening for familial hypercholesterolemia in high-risk Chinese families. Clin Cardiol, 2017; 40: 1169-1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Vinson A, Guerra L, Hamilton L, Wilson DP. Reverse Cascade Screening for Familial Hypercholesterolemia. J Pediatr Nurs, 2019; 44: 50-55 [DOI] [PubMed] [Google Scholar]
- 70).Pérez de Isla L, Alonso R, Mata N, Fernández-Pérez C, Muñiz O, Díaz-Díaz JL, Saltijeral A, Fuentes-Jiménez F, de Andrés R, Zambón D, Piedecausa M, Cepeda JM, Mauri M, Galiana J, Brea Á, Sanchez Muñoz-Torrero JF, Padró T, Argueso R, Miramontes-González JP, Badimón L, Santos RD, Watts GF, Mata P. Predicting Cardiovascular Events in Familial Hypercholesterolemia: The SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation, 2017; 135: 2133-2144 [DOI] [PubMed] [Google Scholar]
- 71).Ceska R, Latkovskis G, Ezhov MV, Freiberger T, Lalic K, Mitchenko O, Paragh G, Petrulioniene Z, Pojskic B, Raslova K, Shek AB, Vohnout B, Altschmiedova T, Todorovova V. The Impact of the International Cooperation On Familial Hypercholesterolemia Screening and Treatment: Results from the ScreenPro FH Project. Curr Atheroscler Rep, 2019; 21: 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Tada H, Okada H, Yoshida S, Shimojima M, Nomura A, Tsuda T, Mori M, Takashima SI, Kato T, Usui S, Sakata K, Hayashi K, Fujino N, Inazu A, Takahara S, Imai Y, Matsubara T, Nohara A, Miwa K, Namura M, Terai H, Yoshida T, Araki T, Minamoto M, Aburao T, Ito Y, Nakanishi C, Kawasaki S, Todo Y, Koizumi J, Kita Y, Matsumoto H, Shintaku H, Hodatsu A, Ino H, Higashikata T, Takata M, Misawa K, Yamaguchi M, Noji Y, Osato K, Mabuchi T, Ichise T, Kaku B, Katsuda S, Fujimoto M, Uchiyama K, Fujioka K, Nakahashi T, Nozue T, Michishita I, Usuda K, Otowa K, Okeie K, Hirota S, Aburadani I, Kurokawa K, Takatori O, Hondo S, Oda H, Takata S, Murai H, Kinoshita M, Nagai H, Sekiguchi Y, Sakagami S, Omi W, Fujita C, Katsuki T, Ootsuji H, Igarashi A, Nakano M, Okura S, Maeno K, Mitamura Y, Sugimoto N, Yamamoto M, Akao H, Kajinami K, Takamura M, Kawashiri MA. Hokuriku-plus familial hypercholesterolaemia registry study: rationale and study design. BMJ Open, 2020; 10: e038623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Duell PB, Gidding SS, Andersen RL, Knickelbine T, Anderson L, Gianos E, Shrader P, Kindt I, O’Brien EC, McCann D, Hemphill LC, Ahmed CD, Martin SS, Larry JA, Ahmad ZS, Kullo IJ, Underberg JA, Guyton J, Thompson P, Wilemon K, Roe MT, Rader DJ, Cuchel M, Linton MF, Shapiro MD, Moriarty PM, Knowles JW. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: The CASCADE FH registry. Atherosclerosis, 2019; 289: 85-93 [DOI] [PubMed] [Google Scholar]
- 74).Alves AC, Alonso R, Diaz-Diaz JL, Medeiros AM, Jannes CE, Merchan A, Vasques-Cardenas NA, Cuevas A, Chacra AP, Krieger JE, Arroyo R, Arrieta F, Schreier L, Corral P, Bañares VG, Araujo MB, Bustos P, Asenjo S, Stoll M, Dell’Oca N, Reyes M, Ressia A, Campo R, Magaña-Torres MT, Metha R, Aguilar-Salinas CA, Ceballos-Macias JJ, Morales ÁJR, Mata P, Bourbon M, Santos RD. Phenotypical, Clinical, and Molecular Aspects of Adults and Children With Homozygous Familial Hypercholesterolemia in Iberoamerica. Arterioscler Thromb Vasc Biol, 2020; 40: 2508-2515 [DOI] [PubMed] [Google Scholar]
- 75).Mehta R, Martagon AJ, Galan Ramirez GA, Antonio- Villa NE, Vargas-Vazquez A, Elias-Lopez D, Gonzalez- Retana G, Rodriguez-Encinas B, Ceballos-Macias JJ, Romero-Zazueta A, Martinez-Alvarado R, Morales- Portano JD, Alvarez-Lopez H, Sauque-Reyna L, Gomez- Herrera LG, Simental-Mendia LE, Garcia-Aguilar H, Ramirez-Cooremans E, Pena-Aparicio B, Mendoza- Zubieta V, Carrillo-Gonzalez PA, Ferreira-Hermosillo A, Caracas-Portilla N, Jimenez-Dominguez G, Ruiz-Garcia AY, Arriaga-Cazares HE, Gonzalez-Gonzalez JR, Mendez- Valencia CV, Padilla FG, Madriz-Prado R, De Los Rios- Ibarra MO, Vazquez-Cardenas A, Arjona-Villicana RD, Acevedo-Rivera KJ, Allende-Carrera R, Alvarez JA, Amezcua-Martinez JC, de Los Reyes Barrera-Bustillo M, Carazo-Vargas G, Contreras-Chacon R, Figueroa-Andrade MH, Flores-Ortega A, Garcia-Alcala H, Garcia de Leon LE, Garcia-Guzman B, Garduno-Garcia JJ, Garnica- Cuellar JC, Gomez-Cruz JR, Hernandez-Garcia A, Holguin-Almada JR, Juarez-Herrera U, Lugo-Sobrevilla F, Marquez-Rodriguez E, Martinez-Sibaja C, Medrano- Rodriguez AB, Morales-Oyervides JC, Perez-Vazquez DI, Reyes-Rodriguez EA, Robles-Osorio ML, Rosas-Saucedo J, Torres-Tamayo M, Valdez-Talavera LA, Vera-Arroyo LE, Zepeda-Carrillo EA, Aguilar-Salinas CA; Mexican Familial Hypercholesterolemia Group. Familial hypercholesterolemia in Mexico: Initial insights from the national registry. J Clin Lipidol, 2021 in press [Google Scholar]
- 76).de Ferranti SD, Shrader P, Linton MF, Knowles JW, Hudgins LC, Benuck I, Kindt I, O’Brien EC, Peterson AL, Ahmad ZS, Clauss S, Duell PB, Shapiro MD, Wilemon K, Gidding SS, Neal W. Children with Heterozygous Familial Hypercholesterolemia in the United States: Data from the Cascade Screening for Awareness and Detection-FH Registry. J Pediatr, 2021; 229: 70-77 [DOI] [PubMed] [Google Scholar]