Abstract
Purpose
To determine whether computed tomography (CT)-based bone density and strength measurements from the thoracic spine are associated with incident vertebral fracture. Further, compare the performance of thoracic and lumbar bone measurements to predict incident vertebral fracture.
Methods
This case-control study of community-based men and women (age 74.6 ± 6.6), included 135 cases with incident vertebral fracture at any level and 266 age- and sex-matched controls. We used baseline CT scans to measure integral and trabecular volumetric bone mineral density (BMD) and vertebral strength (via finite element analysis, FEA) at the T8 and L2 levels. Association between these measurements and vertebral fracture was determined by conditional logistic regression. Sensitivity and specificity for predicting incident vertebral fracture were determined for lumbar spine and thoracic bone measurements.
Results
Bone measurements from T8 and L2 predicted incident vertebral fracture equally well, regardless of fracture location. Specifically, for predicting vertebral fracture at any level, the odds ratio (per 1SD decrease) for the BMD and strength measurements at L2 and T8 ranged from 2.0 to 2.7 (p<0.0001) and 1.8 to 2.8 (p<0.0001), respectively. Results were similar when predicting fracture only in the thoracic versus the thoracolumbar spine. Lumbar and thoracic spine bone measurements had similar sensitivity and specificity for predicting incident vertebral fracture.
Conclusion
These findings indicated that like those from the lumbar spine, CT-based bone density and strength measurements from the thoracic spine may be useful for identifying individuals at high risk for vertebral fracture.
Keywords: Vertebral fracture, Osteoporosis, QCT, Fracture prediction, Finite element analysis
Mini-abstract
In a population-based study, we found that CT-based bone density and strength measures from the thoracic spine predicted new vertebral fracture as well as measures from the lumbar spine, suggesting that CT scans at either the thorax or abdominal regions are useful to assess vertebral fracture risk.
Introduction
Vertebral fractures (VF) are the most common type of osteoporotic fractures, with a prevalence of 3050% among those over the age of 50 years [1]. Furthermore, they are robust predictors of future fractures; individuals with a VF have a 2- to 5-fold higher risk of any fracture compared to those without vertebral fracture, independently of bone mineral density (BMD) [2].
Despite the fact that osteoporotic fractures are a major and growing health concern [1] and that studies have shown it is cost-effective to diagnose and treat osteoporosis [3], the disease remains underdiagnosed and undertreated [4,5]. Thus, there is an urgent need to identify individuals at high risk of fractures and ultimately offer treatments to reduce fractures.
BMD assessment by dual-energy X-ray absorptiometry (DXA) combined with evaluation of clinical risk factors is the current standard for assessing fracture risk. However, BMD by DXA has low to modest sensitivity (26–44%) for fracture risk assessment [6,7]. Further, DXA of the spine may provide artificially elevated BMD values in those who have scoliosis, degenerative conditions in the spine (e.g., osteophytes) and/or aortic calcification. Moreover, annual DXA screening rates of osteoporosis are low, less than 10% for women and 2% for men in the U.S. Medicare population [8]. Computed tomography (CT) is an alternative method to assess BMD and bone strength. Diagnostic CT scans of chest, abdomen and pelvis can be utilized to perform ancillary bone health assessments which provides an opportunity to expand osteoporosis screening without any additional radiation dose, imaging or patient time [9]. Previous studies have used CT scans of the spine to demonstrate an association between incident vertebral fracture and volumetric BMD (vBMD) and vertebral strength estimated by finite element analysis (CT-FEA) [10–14]. However, as these previous studies mostly measured BMD and strength at the lumbar spine, there are limited data testing whether incident vertebral fractures are also well predicted using measurements taken at the thoracic spine. This information is important to acquire to give insight into whether CT scans only covering the thorax or chest are useful for bone health assessments. Furthermore, although osteoporosis diagnostic thresholds for both CT-based trabecular vBMD [15] and CT-FEA strength [11], defined to be analogous to the BMD-based WHO-criteria [16], have been established in the lumbar spine, such thresholds remain to be defined for the thoracic spine which is essential so that bone measurements from this region can be used clinically to diagnose osteoporosis and assess fracture risk.
Thus, we aimed to test the hypothesis that bone measurements, including integral and trabecular vBMD and vertebral strength (estimated by CT-FEA), from the thoracic spine would predict incident vertebral fracture as well as bone measurements from the lumbar spine. Furthermore, we posited that the association would be independent of the location of the incident fracture. Accordingly, we compared the ability of CT-based bone measurements at the thoracic (T8) and lumbar spine (L2) to predict incident vertebral fracture in a community-based cohort of women and men. To be able to utilize diagnostic thresholds previously established for the lumbar spine, we generated “L2-estimated measures” for trabecular vBMD and vertebral strength from the T8 measures using linear regression. We then applied the osteoporosis diagnostic thresholds for trabecular vBMD [15] and vertebral strength [11] to both the lumbar measurements and the “estimated lumbar” measurements derived from the thoracic measurements.
Material and Methods
Study Participants
We performed a case-control study utilizing data from a prospective population-based cohort, the Age, Gene/Environment Susceptibility Reykjavik study (AGES)[17]. Participants in the AGES study underwent CT scans of the chest and abdomen in 2002–2006 (baseline) and 2007–2011 (follow-up), with an average of 5 yrs between scans. Written informed consent was obtained from all participants at the time of data collection, and the study was approved by the Icelandic National Bioethics Committee and by the Institutional Review Boards of Boston University Medical Center, Beth Israel Deaconess Medical Center and Hebrew SeniorLife.
CT protocol
Participants underwent CT on a multidetector CT (MDCT) scanner (Somatom Sensation 4, Siemens Medical Systems, Erlangen, Germany) [18,19]. Thoracic CT imaging was obtained to assess coronary calcification (140 kVp, a reference exposure of 50 mAs, in-plane pixel size 0. 68 × 0.68 mm and slice thickness 2.5 mm, field of view (FOV): 35 cm) and included approximately vertebral levels T6–T10. The lumbar spine CT included vertebral levels L1–L2 (120 kVp, a reference exposure of 150 mAs, in-plane pixel size of 0.98 by 0.98 and slice thickness of 1.0 mm, FOV: 50 cm). In addition, lateral scout images were acquired, covering levels T6–L5. The scans were performed at the same visit and each subject was scanned with two hydroxyapatite phantoms (Image Analysis, Inc., Lexington, KY, USA), one placed in the lumbar region and the second one in the thorax region, to enable the scan data to be calibrated into equivalent mineral density (mg/cm3 of hydroxyapatite).
Vertebral fracture identification
Incident vertebral fracture cases included both clinical (identified from patient medical records and confirmed radiographically via CT scans) and morphologic (identified from the CT scans) fractures, as described elsewhere [11]. The incident vertebral fracture cases overlap with those reported by Kopperdahl et al [11], except that worsening fracture cases were excluded in the present study. Briefly, incident fractures were identified for each vertebral level from T6–L4 from the lateral scout views of the baseline and followup CT scans (5 yrs apart). First the follow-up lateral scout images for all participants (N=897 in AGES) were reviewed and each vertebra was graded according the semiquantitative technique of Genant et al. [20] as no fracture (SQ0), mild (SQ1), moderate (SQ2), or severe (SQ3) fracture. If a fracture was identified on the follow-up scout, then the baseline lateral CT scout was examined to classify the fracture at follow-up as prevalent or incident. Prevalent vertebral fracture was defined as any vertebra graded as SQ1 or higher at baseline. Incident fracture was defined as a vertebra that was graded SQ0 at baseline and SQ1 or higher at follow-up. Similar to prior reports [21,22], fractures in vertebral levels T6–T10 were classified as “thoracic” fractures, whereas fractures in levels T11–L4 were classified as “thoracolumbar” fractures. The reasoning for the definition of the spinal regions is based on findings from epidemiology studies and biomechanical factors. Firstly, the occurrence of vertebral fracture has bimodal distribution along the spine [21–24] and this division provides a separation between the two peaks in fracture incidence. Secondly, T11 and T12 vertebral levels have floating ribs which cause these levels to be more mobile and the rib cage does not help supporting loads at these levels compared with the thoracic levels above. Therefore from a mechanical point of view T11 and T12 biomechanically behave more similarly to the lumbar levels than the thoracic levels above. The final fracture sample consisted of 135 (92 women and 43 men) incident fracture cases with total of 167 incident fractures as some had more than one fracture. Two controls selected from participants who had no incident vertebral fracture on follow-up CT scan were matched by age (± 4 yrs) and sex with each fracture case. Four controls were excluded because the baseline CT scan was unsuitable for analysis, resulting in 266 controls (180 women and 86 men).
Bone density and strength assessment
Volumetric bone density measurements and nonlinear finite element analysis (FEA) were performed on baseline CT scans by an operator who was blinded to fracture status using VirtuOst software (O.N. Diagnostics, Berkeley, CA, USA), a process that is described elsewhere [11]. Integral volumetric bone mineral density (vBMD) (mg/cm3) was defined as the average density over the whole vertebral body, including endplates but excluding posterior elements. Trabecular vBMD (mg/cm3) was defined as the average density of an anterior ellipsoidal volume placed inside the trabecular compartment in the middle 10mm of the vertebral body. The nonlinear FEA was performed to virtually load the vertebral bodies to failure in a uniform axial compressive overload to estimate vertebral compressive strength (N). Vertebral measurements were performed on T8 (96% of analyses) and L1/L2 (98% of analyses) vertebrae, except in individuals who had a prevalent fracture at either T8 or L2 or those levels were not suitable for analysis. The closest vertebral level that could be analyzed was then used and scaled to an equivalent T8 or L2 value. The scale factor was determined from a small independent cohort of 22 women and 3 men (age 50 to 81 years) for whom bone density and strength were measured at all vertebral levels.
Statistical analyses
We calculated standard descriptive statistics and compared baseline characteristics between incident vertebral fracture cases and controls using a two-sample t-test for continuous variables and a chi-square test of independence for categorical variables. We determined the association between bone measurements (vBMD and strength) in the thoracic and lumbar spine using Pearson’s correlations. The associations of bone measurements with incident fracture were determined using conditional logistic regression and the odds ratios (OR) were computed, along with 95% confidence intervals, for a 1 standard deviation (SD) decrease in vertebral bone density or strength. Models were adjusted for age (years), height (cm) and weight (kg). The models were further adjusted for prevalent vertebral fracture and results reported in supplementary materials. Incident vertebral fracture cases at all levels (T6–L4) were included in the main analysis, followed by sub-analyses of vertebral fracture location. One sub-analysis included incident fracture cases occurring only in the thoracic spine (T6–T10) while another sub-analysis included fracture cases occurring only in the thoracolumbar spine (T11–L4).
Finally, we calculated the sensitivity and specificity as well as positive and negative predictive values of CT-based FEA and CT-based vBMD using two diagnostic thresholds proposed for the lumbar spine: 1) osteoporosis by trabecular vBMD (80 mg/cm3) [15] and 2) fragile bone strength by CT-FEA (Women, 4500 N; Men, 6500 N) [11]. To be able to apply these diagnostic thresholds to the thoracic measurements we converted each thoracic measurement to an estimated lumbar measurement by a linear regression between L2 and T8 measurements. Finally, we performed stratified analyses including only moderate/severe (SQ2+) fracture cases and their controls, results reported in supplementary materials. All statistical analyses were performed using R software (R-3.2.5, www.r-project.org) and p-values less than 0.05 were considered statistically significant.
Results
Among the 135 vertebral fracture cases, 39 (29%) had fractures only in the thoracic spine (T6–T10), 82 (61%) only in the thoracolumbar spine (T11–L4), and 14 (10%) in both thoracic and thoracolumbar regions. Thirty-nine percent of the vertebral fractures were classified as moderate (SQ2) or severe (SQ3). Individuals with an incident vertebral facture did not differ from controls in terms of height, weight and BMI, although the vertebral fracture cases were more likely to have a prevalent vertebral fracture (Table 1). Specifically, 18% of controls versus 40% of cases had at least one prevalent vertebral fracture (p<0.0001). There were 167 incident vertebral fractures among the 135 cases (Sup.Figure 1).
Table 1:
Baseline characteristics of cases and controls (mean ± SD, or n, %).
| Controls |
All Cases |
SQ2, SQ3 Cases |
|||||
|---|---|---|---|---|---|---|---|
| % Difference | p-value | % Difference | p-valuea | ||||
| Sample size | 266 | 135 | - | - | 52 | - | - |
| Women (n, %) | 180 (68%) | 92 (68%) | - | - | 40 (77%) | - | - |
| Age (Years) | 75.7 (5.2) | 76.3 (5.4) | 0.7 | 0.33 | 77.1 (4.9) | 0.8 | 0.49 |
| Height (cm) | 165.6 (8.1) | 165.4 (9.9) | −0.1 | 0.86 | 162.2 (7.5) | −1.5 | 0.57 |
| Weight (kg) | 74.6 (13.4) | 72.7 (14.0) | −2.5 | 0.20 | 68.2 (10.2) | −7.8 | 0.004 |
| BMI (kg/m2) | 27.1 (4.2) | 26.5 (4.3) | −2.3 | 0.17 | 25.9 (3.7) | −4.8 | 0.057 |
| Prev Fx, any (yes/no) (n, %) | 48 (18%) | 54 (40%) | 22 | <0.0001 | 18 (35%) | 15 | 0.064 |
| L Int vBMD (mg/cm3) | 175 (44) | 152 (34) | −13.3 | <0.0001 | 146 (36) | −16.6 | <0.0001 |
| L Trab vBMD (mg/cm3) | 82 (34) | 64 (28) | −22.3 | <0.0001 | 59 (26) | −25.7 | <0.0001 |
| L Strength (N) | 5,990 (2,560) | 4,710 (1,900) | −21.3 | <0.0001 | 4,240 (1670) | −26.7 | <0.0001 |
| T Int vBMD (mg/cm3) | 183 (43) | 161 (36) | −11.8 | <0.0001 | 156 (30) | −14.6 | <0.0001 |
| T Trab vBMD (mg/cm3) | 97 (36) | 80 (31) | −17.6 | <0.0001 | 78 (27) | −20.0 | 0.0002 |
| T Strength (N) | 5,130 (2,280) | 4,120 (1,760) | −19.5 | <0.0001 | 3,640 (1,200) | −27.7 | <0.0001 |
| SQ1 (n, %) | - | 83 (61%) | - | - | - | - | - |
| SQ2 (n, %) | - | 52 (39%) | - | - | 52 (100%) | - | - |
BMI = body mass index, Prev Fx = prevalent vertebral fracture, Int vBMD = integral volumetric bone mineral density, Trab vBMD = trabecular volumetric bone mineral density
The p-values comparing SQ2+ cases versus matched controls (n=102).
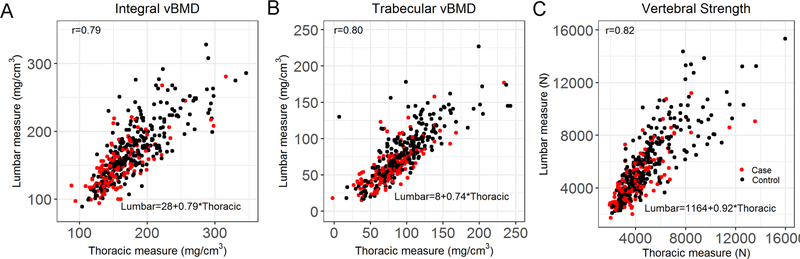
Integral vBMD, trabecular vBMD and vertebral strength, in both lumbar and thoracic spine, were significantly lower in the fracture cases than controls (p<0.0001, Table 1). Bone measures in the lumbar and thoracic spine were −13.3% to −21.3% and −11.8% to −19.5% lower, respectively, in the cases than controls. Bone density and strength measurements at T8 and L2 were strongly correlated with each other (r = 0.79 to 0.82, p<0.0001, Figure 1).
Figure 1:
Association between lumbar measurement and thoracic measurement (A) Integral vBMD, r = 0.79 (p<0.0001), L2=28+0.79xT8; (B) Trabecular vBMD, r = 0.80 (p<0.0001), L2=8+0.74xT8; (C) Vertebral Strength by CT-based finite element analysis, r = 0.82 (p<0.0001) L2=1164+0.92xT8. r= Pearson’s correlation coefficient
When considering incident fracture at any location of the spine, all thoracic and lumbar bone measures were significantly associated with incident vertebral fracture (Table 2). On average, odd ratios were consistently numerically higher for strength than vBMD but did not reach statistical significance. Specifically, the odds ratios (OR per 1 SD decrease) for L2 integral and trabecular vBMD were both 2.0, while the OR for L2 vertebral strength was 2.7. The findings were similar for thoracic bone measures, with ORs for integral vBMD, trabecular vBMD and vertebral strength equal to 2.0, 1.8 and 2.8, respectively (Table 2). The results were similar after a further adjustment for prevalent vertebral fracture, but the ORs were slightly attenuated (Sup Table 1). When only considering moderate and severe incident fractures (SQ2+), the ORs were slightly increased and highest for vertebral strength in the thoracic spine (Sup. Table 2).
Table 2:
Odds ratios per 1 SD decrease (OR, 95% CI) for associations of incident vertebral fracture (VF) with bone measurements at T8 and L2 vertebral level, adjusted for age, height and weight. Thoracic VF defined as T6-T10 and thoracolumbar VF as T11-L4.
| Any Level (n=135) | *Thoracic (n=53) | *Thoracolumbar (n=96) | |
|---|---|---|---|
| Lumbar (L2) measurements | |||
| Int vBMD (mg/cm3) | 2.0 (1.5–2.8) | 2.9 (1.5–5.7) | 2.0 (1.4–2.8) |
| Trab vBMD (mg/cm3) | 2.0 (1.5–2.7) | 2.8 (1.5–5.3) | 1.9 (1.3–2.7) |
| Strength (N) | 2.7 (1.8–4.0) | 3.5 (1.6–7.5) | 2.7 (1.7–4.3) |
| Thoracic (T8) measurements | |||
| Int vBMD (mg/cm3) | 2.0 (1.4–2.8) | 2.4 (1.3–4.3) | 2.1 (1.4–3.0) |
| Trab vBMD (mg/cm3) | 1.8 (1.4–2.4) | 2.3 (1.3–4.0) | 1.8 (1.3–2.5) |
| Strength (N) | 2.8 (1.8–4.3) | 2.9 (1.4–6.1) | 3.4 (1.9–6.0) |
Prev Fx = prevalent vertebral fracture, Int vBMD = integral volumetric bone mineral density, Trab vBMD = trabecular volumetric bone mineral density
Among the 135 cases, 53 had fracture in the thoracic spine and of those 14 had also fracture in the thoracolumbar region. 96 cases had fracture in the thoracolumbar spine and of those 14 had also fracture in the thoracic region.
When examining incident fracture only occurring in the thoracic spine (T6–T10), all bone measures for both lumbar and thoracic regions were again significantly associated with vertebral fracture, with ORs for L2 and T8 bone outcomes ranging from 2.9 to 3.5 and 2.3 to 2.9, respectively (Table 2). Similar patterns were observed when examining incident fracture occurring only in the thoracolumbar spine (T11–L4), with slight decrements in the ORs (Table 2). ORs for associations for incident vertebral fracture in both the thoracic and thoracolumbar spine remained significant after adjustment for prevalent vertebral fracture (Sup Table 1).
The performance of lumbar measurements and estimated lumbar measurements (thoracic measurements mapped with the linear regressions reported in Figure. 1) for predicting incident vertebral fracture was similar (Table 3). In particular, osteoporosis based on trabecular vBMD (≤ 80 mg/cm3) for L2 measurements identified incident fracture with a sensitivity of 79 %, whereas the trabecular vBMD with estimated L2 trabecular vBMD (from T8 measurements) had a sensitivity of 76%. Sensitivity of fragile bone strength thresholds (women ≤ 4500N, men ≤ 6500N) was also similar for the L2 measurement and estimated L2 measurement, 64 % and 63% respectively. Furthermore, there were no differences in specificity for lumbar and estimated lumbar measurements, 47–48% for trabecular vBMD and 60–62% for fragile bone strength. For both L2 and estimated L2 measurements, trabecular vBMD exhibited greater sensitivity than strength (p<0.03) whereas strength had higher specificity (p<0.01). The same pattern was found when only considering moderate and severe incident fractures (SQ2+), however the sensitivity was higher for both trabecular vBMD and fragile bone strength (Sup. Table 3).
Table 3:
Sensitivity and specificity for predicting incident vertebral fracture at any level according to trabecular vBMD and vertebral strength measured at L2 and estimated L2 from T8 measurements.
| Lumbar Trab vBMD (≤80 mg/cm2) | Lumbarestimated Trab vBMD (≤80 mg/cm2) | |
| Sensitivity | 0.79 (0.71, 0.85) | 0.76 (0.68, 0.83) |
| Specificity | 0.47 (0.41, 0.54) | 0.44 (0.38, 0.51) |
| Pos. Predictive Value | 0.43 (0.37, 0.50) | 0.41 (0.35, 0.47) |
| Neg. Predictive Value | 0.81 (0.74, 0.87) | 0.79 (0.71, 0.85) |
| Lumbar Vertebral strength | Lumbarestimated Vertebral Strength | |
| Women ≤ 4500 N | Women ≤ 4500 N | |
| Men ≤ 6500 N | Men ≤ 6500 N | |
| Sensitivity | 0.64 (0.56, 0.72) | 0.63 (0.54, 0.71) |
| Specificity | 0.60 (0.54, 0.66) | 0.62 (0.56, 0.68) |
| Pos. Predictive Value | 0.45 (0.38, 0.52) | 0.45 (0.38, 0.53) |
| Neg. Predictive Value | 0.77 (0.70, 0.82) | 0.77 (0.70, 0.82) |
Discussion
We compared the ability of CT-based vBMD and FEA-estimated vertebral strength from the thoracic and lumbar spine to predict incident vertebral fracture in older men and women. We found that vertebral vBMD and compressive strength from both the thoracic and lumbar spine regions predicted incident vertebral fracture risk equally well, irrespective of fracture location. As prior work has demonstrated that both CT-based vBMD and FEA-estimated vertebral strength can be reliably measured from CT scans using patient-specific phantomless calibration [25], the current findings suggest that ancillary bone health assessment from diagnostic CT scans at either the thorax or abdominal regions can be used to identify patients at high risk for vertebral fracture.
A novel aspect of this study is the availability of bone measures in both the thoracic and lumbar spine, which allowed us to assess whether the location of bone measurement was preferentially associated with fractures at different locations along the spine. Although the majority of vertebral fractures occur in the thoracic and thoracolumbar regions, it is not possible to perform DXA scans in these regions because of the overlying ribcage. Our finding that bone measures from both the thoracic and lumbar spine predict incident fracture equally well, irrespective of fracture location, is not surprising given the strong correlation between the bone measures from T8 and L2 in this and prior studies [26–28].
Several studies have measured thoracic bone density from CT images [26–28], however, few have tested whether thoracic bone measurements can predict incident vertebral fractures. One small study (30 fractures in 199 subjects) used CT scans from women aged 50 to 64 years to show that CT attenuation values measured at any vertebral level from T10 to L5 could discriminate incident fracture cases from controls [29]. This finding is consistent with our results showing that both thoracic and lumbar measurements predict incident vertebral fracture.
Although no prior studies have specifically compared the association of thoracic versus lumbar bone measures with location of incident vertebral fracture, one large study in postmenopausal women (223 incident vertebral fractures) found that low lumbar spine aBMD by DXA was more strongly associated with incident vertebral fracture in the thoracic (OR: 2.1, 95% CI: 1.7–2.6) than in the thoracolumbar (OR: 1.5, 95% CI: 1.3–1.8) region [21]. We found a similar trend in that lumbar vBMD and vertebral strength had numerically higher ORs when predicting incident fractures in the thoracic versus thoracolumbar region, but the ORs were not statistically significantly different.
Our findings have implications for use of routine CT scans for osteoporosis screening as our scan parameters were similar to those used for diagnostic imaging. Notably, though only applicable to scans without contrast agents, our results indicate that diagnostic CT scans from either the chest or abdominal region would be sufficient to identify those at a risk of any incident vertebral fracture. There are many clinical scenarios in which patients requiring a CT scan may also be at a high risk of osteoporosis and fragility fractures, including inflammatory bowel disease (IBD) and prostate cancer, yet BMD screening still remains underutilized in these patients [30,31]. In the present study, a hydroxyapatite phantom was scanned simultaneously with the subject, allowing conversion of the CT values to BMD and also correcting for scanner instabilities. In diagnostic clinical CT scans, such phantoms are not routinely scanned with the patient. While use of uncalibrated CT attenuation values is not recommended due to wide variation between scanners and scanning protocols [32], both asynchronous calibration [33,34] and phantomless calibration [25] provide accurate BMD and bone strength assessments [34,25].
Another unique aspect of this study is that we examined the performance of the proposed diagnostic thresholds for trabecular vBMD and fragile bone strength [15,11] in the thoracic spine by converting the thoracic measurements to estimated lumbar measurements. This step is necessary because, although lumbar and thoracic bone measures are highly correlated, the absolute values used for the diagnostic thresholds would differ without employing conversion equations. Accordingly, it is necessary to establish an approach so that currently proposed diagnostic thresholds can be applied to routine CT scans at different spinal locations. Notably, our estimated lumbar measurements and actual lumbar measurements had similar capability to predict incident vertebral fractures. The CT-based bone measurements correctly identified 63–79% of fracture cases, suggesting that they might provide better sensitivity than DXA-BMD t-score (≤−2.5), as only 32–39% of individuals with a prevalent or an incident vertebral fracture had aBMD in the osteoporotic range [6,35,36]. One small study using CT from men and women aged ≥50 years reported that the lumbar threshold for CT-FEA vertebral strength had better sensitivity and similar specificity than trabecular vBMD for identifying those with a new vertebral fracture [13]. This finding differs from our observation that, compared to trabecular vBMD, vertebral strength had lower sensitivity (64% vs 79 %, p<0.01) but higher specificity (60% vs 47%, p<0.01) for predicting incident fractures. The lack of consistency between the two studies may be due to differences in the age of the participants, as the performance of diagnostic thresholds might depend on age, and the prior study included people ≥50 years, whereas our study included individuals ≥65 years of age. Further studies are needed to verify the proposed conversion equations needed to generate estimated L2 measurements from T8 measurements, as well as investigate whether the performance of different bone measurements and associated diagnostic thresholds are associated with age.
One challenge using thoracic or abdominal CT scans is the frequent use of intravenous contrast, which has been shown to induce notable measurement bias in the lumbar spine vBMD [37]. Whereas some have suggested that this systematic bias could be accounted for [38], others have shown that the vBMD measurements are sensitive to the time since IV contrast agent injection [39]. Therefore, further investigations are needed to establish guidance for reliable use of spine vBMD and strength measurements in cases where CT scans include contrast agents.
This study has a few limitations. First, we did not have aBMD measurements by DXA, which the clinical standard for the diagnosis of osteoporosis [16]. In addition, the definition of spinal regions is somewhat arbitrary, but provides a separation between the two peaks in fracture incidence; groups T11 and T12 with the lumbar levels because those levels are not supported by the rib cage like the other thoracic levels; overlaps with the regions included in chest and abdominal scans; and is consistent with the classification done by others [21,22]. Also, this study only measured one level at each spinal region but combining two or more measurements probably would provide better reliability. Additionally our conclusions are limited to non-contrast scans and because the cohorts were primarily white, we cannot make any conclusion regarding possible associations in other racial and ethnic groups. Further, this study focused on prediction of vertebral fractures, which while important, represent only a portion of the fractures associated with skeletal fragility. Future studies should examine the ability of lumbar and thoracic spine CT to predict all clinical fractures and test the generality of the reported linear regressions between L2 and T8 measurements. Despite these limitations, this study has several important strengths. In particular, the study sample is drawn from a community-based cohort of both men and women, and included a robust number of incident vertebral fractures. In addition, this study is among the few to test whether CT-based BMD and bone strength measurements from the thoracic spine can predict incident vertebral fractures. These findings are important given the growing interest in ancillary evaluation of diagnostic CT scans for assessment of skeletal health.
In conclusion, our findings indicate that CT-based bone density and strength from either the thoracic or lumbar spine predict incident vertebral fracture risk in a community-based population of men and women, irrespective of fracture location. These findings suggest that non-contrast diagnostic CT scans of the thorax or abdomen may be useful for identifying people at high risk of vertebral fracture.
Supplementary Material
Acknowledgements
The researchers are indebted to the participants for their willingness to participate in the study.
Funding information
This study has been funded by National Institutes of Health (NIH) contract N01-AG-1-2100, NIH grant R01 AR053986, R44 AR052234, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association) and the Althingi (the Icelandic Parliament). The contents are solely the responsibility of the authors, and do not necessarily represent the views of the NIH.
Declarations
Conflict of Interest
F.J., B.A., S.S., M.A.B., D.E.A., E.J.S., D.P.K., T.B.H., V.G.G. and M.L.B. declare that they have no conflict of interest. D.L.K. is an employee of O.N. Diagnostics and has a financial interest in O.N. Diagnostics. T.M.K. has received consulting fees from Amgen and O.N. Diagnostics and has a financial interest in O.N. Diagnostics. DPK has received grant funding from Radius Health and the National Dairy Council, and serves on a scientific advisory board for Solarea Bio. He also received royalties for publication by Wolters Kluwer for contributions to UpToDate.
Abbreviations
- CT
computed tomography
- DXA
dual-energy X-ray absorptiometry
- FEA
finite element analysis
- vBMD
volumetric bone mineral density
- VF
vertebral fracture
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Fjola Johannesdotti, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA; Department of Orthopedic Surgery, Harvard Medical School, Boston, MA, USA.
Brett Allaire, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA.
David L. Kopperdahl, O.N. Diagnostics LLC, Berkeley, CA, USA.
Tony M. Keaveny, Department of Mechanical Engineering, University of California, Berkeley, CA, USA; Department of Bioengineering, University of California, Berkeley, CA, USA.
Sigurdur Sigurdsson, Icelandic Heart Association, Kopavogur, Iceland.
Miriam A. Bredella, Department of Radiology, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Dennis E. Anderson, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA; Department of Orthopedic Surgery, Harvard Medical School, Boston, MA, USA.
Elizabeth J. Samelson, Institute for Aging Research, Hebrew SeniorLife, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA; Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Douglas P. Kiel, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA; Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Vilmundur G. Gudnason, Department of Medicine, University of Iceland, Reykjavik, Iceland; Icelandic Heart Association, Kopavogur, Iceland.
Mary L. Bouxsein, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA; Department of Orthopedic Surgery, Harvard Medical School, Boston, MA, USA.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22 (3):465–475. doi: 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Arden NK, Palermo L, Pearson J, Cummings SR (1999) Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 14 (5):821–828. doi: 10.1359/jbmr.1999.14.5.821 [DOI] [PubMed] [Google Scholar]
- 3.Agten CA, Ramme AJ, Kang S, Honig S, Chang G (2017) Cost-effectiveness of Virtual Bone Strength Testing in Osteoporosis Screening Programs for Postmenopausal Women in the United States. Radiology 285 (2):506–517. doi: 10.1148/radiol.2017161259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla S, Shane E (2016) A Crisis in the Treatment of Osteoporosis. J Bone Miner Res 31 (8):1485–1487. doi: 10.1002/jbmr.2888 [DOI] [PubMed] [Google Scholar]
- 5.Miller PD (2016) Underdiagnosis and Undertreatment of Osteoporosis: The Battle to Be Won. J Clin Endocrinol Metab 101 (3):852–859. doi: 10.1210/jc.2015-3156 [DOI] [PubMed] [Google Scholar]
- 6.Cosman F, Krege JH, Looker AC, Schousboe JT, Fan B, Sarafrazi Isfahani N, Shepherd JA, Krohn KD, Steiger P, Wilson KE, Genant HK (2017) Spine fracture prevalence in a nationally representative sample of US women and men aged >/=40 years: results from the National Health and Nutrition Examination Survey (NHANES) 2013–2014. Osteoporos Int 28 (6):1857–1866. doi: 10.1007/s00198-017-3948-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34 (1):195–202. doi: 10.1016/j.bone.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Delzell E, Zhao H, Laster AJ, Saag KG, Kilgore ML, Morrisey MA, Wright NC, Yun H, Curtis JR (2012) Central DXA utilization shifts from office-based to hospital-based settings among medicare beneficiaries in the wake of reimbursement changes. J Bone Miner Res 27 (4):858–864. doi: 10.1002/jbmr.1534 [DOI] [PubMed] [Google Scholar]
- 9.Gausden EB, Nwachukwu BU, Schreiber JJ, Lorich DG, Lane JM (2017) Opportunistic Use of CT Imaging for Osteoporosis Screening and Bone Density Assessment: A Qualitative Systematic Review. J Bone Joint Surg Am 99 (18):1580–1590. doi: 10.2106/jbjs.16.00749 [DOI] [PubMed] [Google Scholar]
- 10.Chalhoub D, Orwoll ES, Cawthon PM, Ensrud KE, Boudreau R, Greenspan S, Newman AB, Zmuda J, Bauer D, Cummings S, Cauley JA (2016) Areal and volumetric bone mineral density and risk of multiple types of fracture in older men. Bone 92:100–106. doi: 10.1016/j.bone.2016.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopperdahl DL, Aspelund T, Hoffmann PF, Sigurdsson S, Siggeirsdottir K, Harris TB, Gudnason V, Keaveny TM (2014) Assessment of Incident Spine and Hip Fractures in Women and Men using Finite Element Analysis of CT Scans. J Bone Miner Res 29 (3):570–580. doi: 10.1002/jbmr.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Sanyal A, Cawthon PM, Palermo L, Jekir M, Christensen J, Ensrud KE, Cummings SR, Orwoll E, Black DM, Keaveny TM (2012) Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res 27 (4):808–816. doi: 10.1002/jbmr.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allaire BT, Lu D, Johannesdottir F, Kopperdahl D, Keaveny TM, Jarraya M, Guermazi A, Bredella MA, Samelson EJ, Kiel DP, Anderson DE, Demissie S, Bouxsein ML (2018) Prediction of incident vertebral fracture using CT-based finite element analysis. Osteoporos Int. doi: 10.1007/s00198-018-4716-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Löffler MT, Jacob A, Valentinitsch A, Rienmüller A, Zimmer C, Ryang Y-M, Baum T, Kirschke JS (2019) Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. European Radiology 29 (9):4980–4989. doi: 10.1007/s00330-019-06018-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACR-SPR-SSR Practice parameters for the performance of musculoskeletal quantitative computed tomography (QCT) (2018). American College of Radiology. doi:https://www.acr.org//media/ACR/Files/Practice-Parameters/QCT.pdf [Google Scholar]
- 16.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42 (3):467–475. doi: 10.1016/j.bone.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 17.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V (2007) Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 165 (9):1076–1087. doi: 10.1093/aje/kwk115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF (2006) Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK). Bone 39 (3):644–651. doi: 10.1016/j.bone.2006.03.020 [DOI] [PubMed] [Google Scholar]
- 19.Gudmundsson EF, Gudnason V, Sigurdsson S, Launer LJ, Harris TB, Aspelund T (2012) Coronary artery calcium distributions in older persons in the AGES-Reykjavik study. Eur J Epidemiol 27 (9):673–687. doi: 10.1007/s10654-012-9730-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8 (9):1137–1148. doi: 10.1002/jbmr.5650080915 [DOI] [PubMed] [Google Scholar]
- 21.Nevitt MC, Ross PD, Palermo L, Musliner T, Genant HK, Thompson DE (1999) Association of prevalent vertebral fractures, bone density, and alendronate treatment with incident vertebral fractures: effect of number and spinal location of fractures. The Fracture Intervention Trial Research Group. Bone 25 (5):613–619 [DOI] [PubMed] [Google Scholar]
- 22.Anderson DE, Demissie S, Allaire BT, Bruno AG, Kopperdahl DL, Keaveny TM, Kiel DP, Bouxsein ML (2014) The associations between QCT-based vertebral bone measurements and prevalent vertebral fractures depend on the spinal locations of both bone measurement and fracture. Osteoporos Int 25 (2):559–566. doi: 10.1007/s00198-013-2452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schousboe JT (2016) Epidemiology of Vertebral Fractures. J Clin Densitom 19 (1):8–22. doi: 10.1016/j.jocd.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Van der Klift M, De Laet CE, McCloskey EV, Hofman A, Pols HA (2002) The incidence of vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res 17 (6):1051–1056. doi: 10.1359/jbmr.2002.17.6.1051 [DOI] [PubMed] [Google Scholar]
- 25.Lee DC, Hoffmann PF, Kopperdahl DL, Keaveny TM (2017) Phantomless calibration of CT scans for measurement of BMD and bone strength-Inter-operator reanalysis precision. Bone 103:325–333. doi: 10.1016/j.bone.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budoff MJ, Hamirani YS, Gao YL, Ismaeel H, Flores FR, Child J, Carson S, Nee JN, Mao S (2010) Measurement of thoracic bone mineral density with quantitative CT. Radiology 257 (2):434–440. doi: 10.1148/radiol.10100132 [DOI] [PubMed] [Google Scholar]
- 27.Weishaupt D, Schweitzer ME, DiCuccio MN, Whitley PE (2001) Relationships of Cervical, Thoracic, and Lumbar Bone Mineral Density by Quantitative CT. J Comput Assist Tomogr 25 (1):146–150. doi: 10.1097/00004728-200101000-00027 [DOI] [PubMed] [Google Scholar]
- 28.Mao SS, Li D, Syed YS, Gao Y, Luo Y, Flores F, Child J, Cervantes M, Kalantar-Zadeh K, Budoff MJ (2017) Thoracic Quantitative Computed Tomography (QCT) Can Sensitively Monitor Bone Mineral Metabolism: Comparison of Thoracic QCT vs Lumbar QCT and Dual-energy X-ray Absorptiometry in Detection of Agerelative Change in Bone Mineral Density. Acad Radiol 24 (12):1582–1587. doi: 10.1016/j.acra.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Franconeri A, Boos J, Nimhuircheartaigh J, Zhang Z, Brook A, Brook OR (2018) Opportunistic Bone Density Measurement on Abdomen and Pelvis Computed Tomography to Predict Fracture Risk in Women Aged 50 to 64 Years Without Osteoporosis Risk Factors. J Comput Assist Tomogr 42 (5):798–806. doi: 10.1097/RCT.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 30.Lassemillante A-CM, Doi SAR, Hooper JD, Prins JB, Wright ORL (2014) Prevalence of osteoporosis in prostate cancer survivors: a meta-analysis. Endocrine 45 (3):370–381. doi: 10.1007/s12020-013-0083-z [DOI] [PubMed] [Google Scholar]
- 31.Targownik LE, Bernstein CN, Leslie WD (2014) Risk factors and management of osteoporosis in inflammatory bowel disease. Curr Opin Gastroenterol 30 (2):168–174. doi: 10.1097/mog.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 32.Engelke K (2017) Quantitative Computed Tomography-Current Status and New Developments. J Clin Densitom 20 (3):309–321. doi: 10.1016/j.jocd.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 33.Pickhardt PJ, Bodeen G, Brett A, Brown JK, Binkley N (2015) Comparison of Femoral Neck BMD Evaluation Obtained Using Lunar DXA and QCT With Asynchronous Calibration From CT Colonography. Journal of Clinical Densitometry 18 (1):5–12. doi: 10.1016/j.jocd.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 34.Bodeen G, Brown J, Brett A (2014) PROSPECTIVE COMPARISON OF QCT BMD MEASUREMENT USING EITHER ASYNCHRONOUS OR SIMULTANEOUS CALIBRATION AT THE FEMORAL NECK AND LUMBAR SPINE. Osteoporosis International 25:S294–S294 [Google Scholar]
- 35.Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007) Low bone mineral density and fracture burden in postmenopausal women. Canadian Medical Association Journal 177 (6):575–580. doi: 10.1503/cmaj.070234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18 (11):1947–1954. doi: 10.1359/jbmr.2003.18.11.1947 [DOI] [PubMed] [Google Scholar]
- 37.Bauer JS, Henning TD, Müeller D, Lu Y, Majumdar S, Link TM (2007) Volumetric Quantitative CT of the Spine and Hip Derived from Contrast-Enhanced MDCT: Conversion Factors. AJR Am J Roentgenol 188 (5):1294–1301. doi: 10.2214/AJR.06.1006 [DOI] [PubMed] [Google Scholar]
- 38.Kaesmacher J, Liebl H, Baum T, Kirschke JS (2017) Bone Mineral Density Estimations From Routine Multidetector Computed Tomography: A Comparative Study of Contrast and Calibration Effects. J Comput Assist Tomogr 41 (2):217–223. doi: 10.1097/rct.0000000000000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acu K, Scheel M, Issever AS (2014) Time dependency of bone density estimation from computed tomography with intravenous contrast agent administration. Osteoporos Int 25 (2):535–542. doi: 10.1007/s00198-013-2440-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.