ABSTRACT
Staphylococcus aureus is both a commensal and a pathogenic bacterium for humans. Its ability to induce severe infections is based on a wide range of virulence factors. S. aureus community-acquired pneumonia (SA-CAP) is rare and severe, and the contribution of certain virulence factors in this disease has been recognized over the past 2 decades. First, the factors involved in metabolism adaptation are crucial for S. aureus survival in the lower respiratory tract, and toxins and enzymes are required for it to cross the pulmonary epithelial barrier. S. aureus subsequently faces host defense mechanisms, including the epithelial barrier, but most importantly the immune system. Here, again, S. aureus uses myriad virulence factors to successfully escape from the host’s defenses and takes advantage of them. The impact of S. aureus virulence, combined with the collateral damage caused by an overwhelming immune response, leads to severe tissue damage and adverse clinical outcomes. In this review, we summarize step by step all of the S. aureus factors implicated in CAP and described to date, and we provide an outlook for future research.
KEYWORDS: Staphylococcus aureus, virulence factors, community-acquired pneumonia, physiopathology, immune response, community-acquired infections, pathophysiology, pneumonia
INTRODUCTION
Staphylococcus aureus is detected in 30% of the population, mostly as a nasal commensal, but also in the throat, on the skin, and in the gastrointestinal tract (1). In addition to these innocuous interactions with the human host, S. aureus has the potential to develop a wide range of diseases in humans, from mild infections of the skin and soft tissues to severe and fatal infections, such as bacteremia and pneumonia (2).
S. aureus pneumonia can be divided into two categories, hospital-acquired pneumonia (very often ventilator-associated pneumonia) pertaining to nosocomial infections (3) and community-acquired pneumonia (CAP) (4). S. aureus is a major pathogen of hospital-acquired pneumonia, whereas S. aureus CAP (SA-CAP) represents only 5% of CAP cases admitted to intensive care units in the United States (5) and 1 to 5.6% in Europe (6, 7). However, the prevalence of S. aureus in CAP has increased in recent decades, mainly due to the emergence of new lineages of methicillin-resistant Staphylococcus aureus (MRSA) that have become highly prevalent in the community, notably in the United States (8–10). Moreover, despite being infrequent, SA-CAP is a severe disease; mortality ranges from 20 to 44.5%, and there are significant age-dependent disparities (6, 9, 11–14). Deciphering the molecular mechanisms involved in host-pathogen interactions is therefore central to better understand SA-CAP physiopathology.
The human body, but more specifically the airway and the lungs, have broad defense mechanisms against pathogen invasion (15–20), at the forefront of which is the pulmonary epithelium (17, 19, 21–23), followed by professional immune cells (24, 25). How S. aureus is able to challenge those mechanisms and successfully colonize tissue and replicate is thus a central question. For nearly 3 decades, the role and mechanisms of action of S. aureus toxins, adhesins, proteases, and regulatory proteins have been extensively investigated and characterized in vitro (26–33). Nonetheless, the pathological impact of each of these virulence factors on pulmonary infections in humans is not fully understood. Historically, clinical evidence of their involvement, or at least association, in the occurrence and/or outcome of pneumonia has been assessed for a very limited number of them, namely, the alpha-toxin (Hla) in 1999 (34), the superantigenic toxic shock syndrome toxin 1 (TSST-1) in 2000 (35), and Panton-Valentine leucocidin (PVL) in 2002 (12). Since then, there has been growing evidence of the implication of these three toxins, as well as other virulence factors, at different steps of pulmonary infection.
This review follows step by step the mechanisms and virulence factors deployed by S. aureus during CAP, starting with the adaptation and invasion of the pulmonary tissue, followed by immune escape and the acute inflammatory response induced. Although pathogenesis is much more complex than this sequential description, this scheme was chosen for convenience of the review. The virulence factors involved in S. aureus CAP virulence with in vitro and/or in vivo evidence are summarized in Table 1. All in vivo data reported in this review were from animal models unless otherwise specified.
TABLE 1.
Virulence factors implicated in S. aureus virulence during pneumonia with in vitro and/or in vivo evidence
| Virulence factor | Infection step | Role/mechanism(s)b | Reference(s)a |
|---|---|---|---|
| agr system | Adaptation | Virulence regulation | 140, 200 |
| Alpha-toxin (Hla) | Invasion | Pulmonary epithelial disruption | 59, 100, 103 |
| Host defense escape | Ciliary beat frequency impairment | 98, 100, 107 | |
| Macrophage digestion avoidance | 130 | ||
| Mayhem in the lung | Cytokine production induction | 101, 166, 167, 168 | |
| Beta-toxin | Host defense escape | Ciliary beat frequency impairment | 108 |
| Epithelial phagolysosome escape | 113 | ||
| Mayhem in the lung | Cytokine production induction | 163, 166 | |
| Biofilm | Adaptation | S. aureus aggregation and protection | 62, 63 |
| ClfA/ClfB | Colonization | Adhesion factor | 47, 48 |
| Host defense escape | Macrophage phagocytosis inhibition | 129 | |
| Cna | Colonization | Adhesion factor | 201, 202 |
| Complement-binding protein (Ecb) | Host defense escape | Complement inhibition | 155 |
| Delta-toxin (Hld) | Host defense escape | Epithelial phagolysosome escape | 113 |
| Enterotoxins B and C (SEB and SEC) | Mayhem in the lung | Abnormal T-lymphocyte activation | 170, 171 |
| Fibrinogen-binding protein (Efb) | Host defense escape | Complement inhibition | 155 |
| Fibrinogen-binding protein (Fnbp) | Colonization | Adhesion factor | 65 |
| Adaptation | Biofilm component | 65, 68 | |
| Fur and iron acquisition | Adaptation | Metabolism adaption | 57 |
| IgG binding protein (Sbi) | Host defense escape | Complement inhibition | 152, 153 |
| IsdB | Adaptation | Adaptation to iron deprivation | 29, 48 |
| Nuc | Host defense escape | NET DNA degradation | 146, 147, 148 |
| Panton-Valentine leucocidin (PVL) | Host defense escape | Macrophage and neutrophil lysis | 119, 122, 123, 138 |
| Mayhem in the lung | Cytokine production induction | 12, 120, 163 | |
| Phenol-soluble modulin α (PSMα) | Adaptation | Biofilm dispersion | 86 |
| Host defense escape | Epithelial and macrophage phagolysosome escape | 114 | |
| Mayhem in the lung | Necroptosis induction | 177 | |
| Host defense escape | Neutrophil lysis | 141, 142 | |
| Phevalin | Host defense escape | Epithelial phagolysosome escape | 115 |
| PhnD | Adaptation | S. aureus aggregation | 59 |
| Staphylococcal protein A (Spa) | Invasion | Pulmonary epithelial disruption | 70, 97 |
| Host defense escape | Ig binding | 157 | |
| Abnormal B lymphocyte activation and death | 157, 158, 159 | ||
| Mayhem in the lung | Cytokine production induction | 70, 160 | |
| Necroptosis induction | 177 | ||
| SElX | Host defense escape | Neutrophil phagocytosis inhibition | 143, 144 |
| Mayhem in the lung | Abnormal T-lymphocyte T activation | 143 | |
| Serine protease SplA | Invasion | Mucine degradation | 96 |
| Staphopain A (ScpA) | Host defense escape | Surfactant protein A (SP-A) degradation | 105 |
| TSST-1 | Mayhem in the lung | Cytokine production induction | 35 |
| Abnormal T-lymphocyte T activation | 170, 171 |
References for virulence factors implicated in pneumonia infection with animal models are highlighted in bold. References highlighted in italics present clinical evidence.
NET, neutrophil extracellular trap(s).
ADAPTATION AND INVASION
Very little is known about the mechanisms by which S. aureus induces lung infection in the absence of nasal carriage. However, it has been hypothesized that S. aureus reaches the lower respiratory tract from nasal colonization by air uptake during breathing by the host (1). Thus, nasal colonization by S. aureus and the associated increased risk of developing further infection has been thoroughly documented in cotton rats and mice (36, 37), as well as in clinical studies (38, 39), as reviewed by Sakr et al. (40) and by Kluytmans et al. (41); furthermore, nasal decolonization contributes to a decrease in deep S. aureus infections (42, 43). However, most studies were conducted on postoperative infections, therefore limiting our knowledge of the impact of S. aureus carriage on CAP occurrence. Nevertheless, the association between pneumonia and nasal colonization has been reported in several clinical studies (38, 41) and in two studies involving mouse models, in which previous nasal colonization increased the severity or the risk of developing pneumonia (37, 44). During nasal colonization, S. aureus upregulates several virulence factors (45), particularly adhesins such as clumping factor B (ClfB) (46, 47), wall teichoic acid (WTA) (36), and iron surface determinant A (IsdA), which is part of the iron acquisition pathway of S. aureus (29, 45). Recently, Yang et al. demonstrated that a vaccine against clumping factor A (ClfA) and IsdB, another protein from the iron acquisition pathway, reduces the severity of pneumonia in a mouse model after nasal injection (48), emphasizing the association of colonization factor expression with pneumonia. Overall, previous nasal colonization by S. aureus seems to be the main route of access to the lung. However, the mechanisms involved in the transition between the upper to the lower respiratory tract remain unclear.
Adaptation to the lung environment.
S. aureus adapts to its new environment when it reaches the lungs and therefore modifies the expression of various factors in comparison with the nasal colonization state. Indeed, the lumen of the pulmonary tissue is poor in nutriments and metal ions, particularly iron (49), and is coated with pulmonary mucus (17, 50) that is composed of mucin (51) and surfactant proteins (18, 52). The low availability in nutriments reduces the overall energy metabolism of S. aureus with, notably, an upregulation of glycolysis and a downregulation of gluconeogenesis (53). Regarding iron uptake, S. aureus uses two main mechanisms, siderophores and heme acquisition by the Isd system or the heme transport system (Hts) (29, 54). The regulation of these mechanisms occurs predominantly through the ferric uptake regulator (Fur) (29), which is a central regulator for virulence adaptation and activation (55, 56). During lung infection, in the presence of low concentrations of iron, Fur promotes the Isd system (55, 57), as well as certain virulence factors, such as Hla and HlgC of gamma-hemolysin (57), and biofilm production through the upregulation of the ica operon (55, 58).
Aggregation and biofilm formation are pertinent mechanisms of bacterial adaptation to the lung environment, and S. aureus aggregates within a very short period of time (1 h after nasal inoculation), when it first interacts with the pulmonary epithelium (59). Among the S. aureus factors contributing to aggregation, PhnD, a phosphonate ABC transporter substrate binding protein, described in S. epidermidis for its contribution to biofilm (60), stabilizes the aggregate. Its depletion makes S. aureus more vulnerable to antibiotics and partially reduced lethality in a pneumonia mouse model (59). In addition to PhnD, in the sequence type 239 (ST239) MRSA lineage from Asia, a novel gene called sasX was identified as promoting nasal colonization and large bacterial aggregates (37). Further investigation on sasX has shown that its neutralization by specific antibodies reduces lung injuries in vivo (61). In addition to providing protection, this aggregate formation is the first step in the initiation of biofilm production (62). Biofilm allows both the protection and replication of S. aureus (33, 62, 63). Its composition depends on the maturation state, the environment, and the bacterium’s genetic background (64). However, several components are conserved, such as extracellular DNA, the microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), and aggregation factors, as well as virulence factors that are mostly expressed in the last steps of the biofilm. Among the MSCRAMMs, most of them are already expressed during nasal colonization; these include fibrinogen-binding proteins (FnBPs), SdrC, and ClfB (26, 65–68). Among the other virulence factors potentially involved are protein A (Spa) (69, 70), either cell wall anchored or released into the extracellular milieu (71), as well as the CHIPS and SCIN proteins (72), both implicated in immune evasion (73, 74). Other compounds involved in biofilm formation were thoroughly described in a review by Paharik et al. (62).
Biofilm enhances resistance to phagocytosis and antimicrobial molecules (e.g., antibiotics), while also allowing S. aureus replication, leading to an increase in bacterial density and regulatory activity switching. At the beginning of lung colonization, only a few bacterial cells reach the lung epithelium, resulting in a low bacterial density and thus in an inactive quorum sensing (QS) regulatory system (agr) that leads to the low expression of its effector, RNAIII. In the absence of RNAIII, the transcriptional regulatory factor Rot represses the expression of several S. aureus virulence factors, including Hla, the PVL, and LukDE (75–77). This repression prevents a strong immune reaction in the early steps of the infection. Furthermore, Rot induces biofilm formation (78) and the expression of surface proteins (75, 79, 80) such as Spa, ClfB, and SdrC (66, 67). In the second step of colonization, biofilm formation allows S. aureus to replicate and to gradually increase its bacterial density, promoting the agr QS system. During its replication, S. aureus produces an accumulating amount of an autoinducing peptide (AIP), encoded by agrD of the agrACDB operon. Upon maturation and secretion via AgrB, AgrD AIP occurs in the form of a tailed thiolactone ring with autoinducing activity on the membrane protein, AgrC, belonging to a two-component regulatory system (TCS). The accumulation of AIP during exponential growth induces the autophosphorylation of AgrC in its cytoplasmic domain, leading to the activation of AgrA (the effector of the TCS), which in turn activates the agrACDB operon promoter in an autocatalytic circuit, as well as the divergent promoter P3 producing RNAIII (81, 82). RNAIII represses numerous cell wall-associated proteins and the global regulator Rot by an antisense RNase III-dependent mechanism (31, 83). Consequently, S. aureus expresses numerous virulence factors, such as PVL and Hla toxins (84, 85) and phenol-soluble modulins (PSM) (86, 87), which allow biofilm dispersion.
However, the agr system is not the only regulatory system involved in S. aureus virulence factor expression; SaeRS, a TCS which, unlike agr, is mainly activated by neutrophil signals (88, 89), also promotes the expression of toxins such as superantigens, hemolysins, and proteases (79, 88). In addition, a cytoplasmic regulator, SarA, activated during periods of metabolic stress or in the stationary growth phase, modulates virulence factor expression along with agr and SaeRS and upregulates the agr system (76, 90). Thus, S. aureus inaugurates lung colonization under the Rot regulation climax and later on, following bacterial replication, switches to a more aggressive state upon quorum sensing dependent activation of the agr system and SarA. This virulence state is exacerbated by the induction of the SaeRS system by the innate immune response (91). Specific mutations/variants of these regulatory systems, in particular for the agr system, have been related to more or less virulent strains in the context of pneumonia (92, 93).
This accurate adaption of S. aureus progressively enables it to reach, colonize, and then invade the pulmonary tissue, from the mucus to the extracellular matrix (ECM).
Invasion.
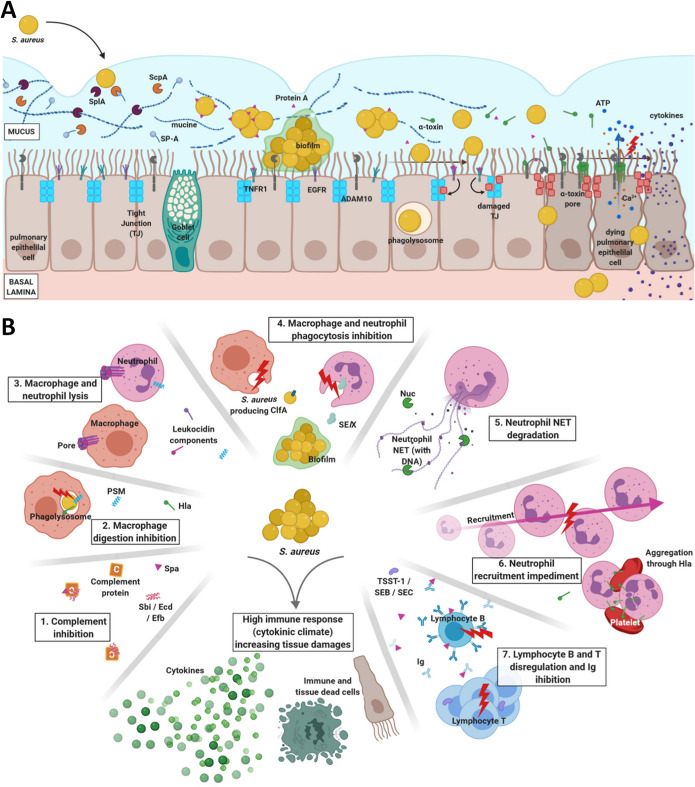
To colonize the tissue, S. aureus first passes through the mucus and then through the tight junctions (TJs) of lung epithelial cells (Fig. 1A). The mucus is produced by the goblet cells to protect the epithelium by trapping microorganisms and is therefore the first host rampart. It is composed of water, ions, lipids, surfactant proteins (18, 94), and highly glycosylated proteins such as mucins (95). To get through the mucus, S. aureus targets its major component, mucin, using the serine protease SplA, which cleaves mucin-16 from the human pulmonary cell line (Fig. 1A). A mutant with splA deleted displayed reduced lung invasion in vivo (96). Subsequently, Spa and Hla initiate the disruption of the epithelial barrier (Fig. 1A). Spa destabilizes the epithelial barrier through its interaction with the EGFR and TNFR1 receptors on the cell surface (70). Spa interaction induces the activation of the RhoA/ROCK/myosin light chain (MLC) eukaryotic cell pathway, which results in the disruption of TJ of the epithelium (97). Hla also plays an important role in tissue invasion, since it can participate in pulmonary epithelium disruption and destruction. Hla is a pore-forming toxin (PFT) (98) that targets the disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) (99), which is expressed at the epithelium surface. The interaction between ADAM10 and Hla leads to numerous molecular reactions, starting with the oligomerization of Hla proteins to form an heptameric β-barrel pore in the membrane (98). Pore formation leads to the release of ions, notably Ca2+, which allows not only TJ disruption (59, 100) but also cell lysis (Fig. 1A) (59, 99, 101, 102). Moreover, the recruitment of ADAM10 by Hla hijacks its function; therefore, ADAM10 participates in TJ degradation by cleaving it through its enzymatic activity (Fig. 1A) (103). Overall, Hla and Spa are essential S. aureus weapons when infecting the host lung, since they allow S. aureus to pass from the lung lumen to the ECM (Fig. 1A). Although the agr system is activated, repressing MSCRAMM expression, the remaining MSCRAMMs at the surface of S. aureus allow strong interaction between S. aureus and the ECM (104).
FIG 1.
(A) S. aureus epithelial barrier invasion. S. aureus first crosses the mucus, which is made of mucine and surfactant protein A (SP-A). Both host proteins are degraded by serine protease SlpA and staphopain A (ScpA), respectively. S. aureus rapidly aggregates and cleaves staphylococcal protein A (Spa) from the cell wall. The aggregation leads to biofilm formation and quorum sensing agr regulation activation. Spa simultaneously interacts with the TNFR1 and EGFR host receptors on the pulmonary epithelial cells, initiating the disruption of the epithelial barrier. Upon reaching a bacterial density threshold, the biofilm is dissipated and the bacteria produce invasins, including the alpha-toxin (Hla). Hla pursues Spa pulmonary epithelium disruption through (i) disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) hijacking that induces tight junction (TJ) degradation and (ii) pore formation, which lyses the epithelial cells. In addition, pore formation causes ATP and Ca2+ leaks that impede the ciliary beat frequency required for effective mucus clearance. Finally, epithelial cells are able to endocytose S. aureus, which can survive within the phagolysosome and escapes from it to induce the cell death. (B) S. aureus mechanisms to escape the host immune system, leading to harmful immune response. 1. Complement proteins can be trapped by Spa, Sbi, Ecd, and Efb, inhibiting S. aureus opsonization. 2. S. aureus can survive, replicate, and kill macrophages after its phagocytosis, thanks to Hla and PSMα. 3. Bicomponent leucocidin PVL, but also LukDE, LukAB, and HlgCB, can induce pore formation in neutrophil and macrophage membranes, leading to cell death. 4. S. aureus phagocytosis is prevented by biofilms and ClfA for macrophages and by biofilms and SElX for neutrophils. 5. The nuclease Nuc can degrade neutrophil NET DNA to avoid trapping. 6. SCIN, CHIPS, Spa, and Sbi are proteins capable of inhibiting neutrophil recruitment. By aggregating platelets and neutrophils, Hla can also impede neutrophil recruitment at the infection location. 7. Both lymphocytes are targeted—B cells by Spa and T cells by TSST-1, SEB, and SEC. The interactions between the toxins and the lymphocytes dysregulates lymphocyte activation and replication. Spa can also interact with Ig, preventing its interaction with the bacteria. All of these mechanisms increase the immune response, generating acute inflammation that damages the pulmonary tissue, in addition to S. aureus damage. PSM, phenol-soluble modulin(s); NET, neutrophil extracellular trap(s). Figure created with BioRender.com.
In summary, S. aureus adapts to its new environment by regulating specific metabolic and virulence pathways. Subsequently, during its passage from the mucus of the lumen to the ECM, S. aureus evades numerous host defense mechanisms provided by the epithelium and the host immune system.
HOST DEFENSE ESCAPE
The lungs are confronted by numerous microorganisms, from the commensal population to invaders. Different cells and defense mechanisms are deployed by the host to prevent invasion from pathogenic microbes. The pulmonary epithelium, along with its mucus layer, forms the first rampart, followed by the immune system, and S. aureus has developed toxins and enzymes to escape these defense mechanisms.
Pulmonary epithelium.
Within the mucus, the surfactant components are proteins with antimicrobial activities that initiate the killing of entrapped microorganisms (52) before their physical expulsion by the mucociliary escalator (19, 94). S. aureus simultaneously targets mucins with SplA (Fig. 1A) and surfactants with staphopain A (ScpA), a hydrolyzing enzyme that degrades surfactant protein A (SP-A, not to be confounded with Spa [staphylococcal protein A]) (Fig. 1A) (105), an airway immune defense effector (52). The deterioration of SP-A enables S. aureus to aggregate and adhere to the pulmonary epithelium (105).
The ciliary beat of the pulmonary epithelial cells allows the clearance of mucus-embedded microorganisms. To avoid its elimination, S. aureus inhibits the mucociliary escalator in several ways. Pore formation by the oligomerization of Hla in the membrane of epithelial cells allows the release of ions, notably Ca2+ (98, 100), and the leak of ATP in the extracellular medium (106), both of which can modify the ciliary beat frequency (Fig. 1A) (107). In addition, the synergistic action of the beta-toxin with Hla between 8 and 12 h after their contact with the epithelial cells drastically decreases the ciliary beat required for effective mucus clearance (34, 108).
Finally, although the pulmonary epithelial cells are nonspecialized phagocytes, they can capture S. aureus. It is as yet unclear whether the epithelial cells actively phagocytose S. aureus, if it is S. aureus that invades the epithelial cell, or if it is a combination of both. Indeed, epithelial cells can internalize bacteria (109–111), a mechanism mediated in part by the efflux pump Tet38 of S. aureus (Fig. 1A) (111, 112). S. aureus is able to enter pulmonary epithelial cells through the interaction between the Tet38 efflux pump and CD36 eukaryotic cell membrane protein in A549 cells (lung epithelial cell line) (112). Following internalization, S. aureus rapidly escapes from the phagolysosome (2 h) and induces cell death via several effectors, namely PSMα, PSMβ, Hld, beta-toxin (111, 113, 114), and a nonribosomal peptide (phevalin) previously identified in Streptomyces (115, 116) and which is required for full virulence in vivo (115).
Altogether, S. aureus possesses several toxins and enzymes that allow the bacteria to penetrate through the mucus and the epithelial barrier. Although the pulmonary epithelium is a major line of defense, the host also possesses other cells and mechanisms to protect itself, notably the immune response. However, even these defenses can be defeated by S. aureus.
Macrophages.
Alveolar macrophages are the resident macrophages of the pulmonary tissue and the second line of defense when pathogens succeed in reaching and crossing the epithelium (20). Their major function is to phagocytose the pathogens or their debris, a function that is impaired by S. aureus through three major mechanisms.
The first mechanism that allows S. aureus to avoid macrophage phagocytosis is to destroy the macrophages using pore-forming toxins (PFT), also known as leucocidins. Leucocidins target specific cells through specific receptors at their membrane surfaces to induce pore formation, followed by cell death (Fig. 1B) (32). Five leucocidins, namely, LukAB (LukGH) (117), LukDE (118), HlgAB, HlgCB, and PVL (119, 120), target macrophages after their interactions with their receptors, namely, CD11b for LukAB, CCR5, and CXCR1/2 receptors for LukDE (118, 121), CCR2 and CXCR1/2 for HlgAB, C5aR1/R2 for HlgCB, and C5aR1/R2 and CD45 for the PVL (119, 122, 123). Except for the PVL, whose impact on pneumonia severity has been highlighted by several studies, including clinical ones (12, 14, 124–128), the roles of LukAB, LukDE, and HlgAB/CB have not yet been elucidated.
The second way for S. aureus to a avoid phagocytosis is by protecting itself by hiding and forming agglomerates inside the biofilm (Fig. 1B) (63). In this context, ClfA, which is expressed during biofilm formation, as mentioned in the “Adaptation to the lung environment” paragraph, impairs macrophage phagocytosis (129). Although there is no direct evidence of ClfA involvement with alveolar macrophages, Yang et al. reported that immunity against ClfA reduces pneumonia severity in mice (48).
Finally, the third mechanism to counter phagocytosis is the capacity of S. aureus to survive within macrophages. The activation of the NLRP3 inflammasome by Hla leads to the recruitment of mitochondria away from the phagosome, thus preventing the digestion of the bacteria inside the phagolysosome (Fig. 1B) (130). In addition, two studies have reported the ability of S. aureus to survive, replicate, and finally kill macrophages (131), in particular due to PSMα (114).
Neutrophils.
Neutrophils are professional antimicrobial cells that use phagocytosis, degranulation, antimicrobial proteins, and neutrophil extracellular traps (NET) to clear pathogens from infected tissues (20, 24). As a result, human neutrophils are one of the main targets of S. aureus toxins (132, 133).
First, as neutrophils are nonresident immune cells, S. aureus can impair neutrophil recruitment by the expression of CHIPS and SCIN factors, which are chemotaxis inhibitors (Fig. 1B) (24, 73, 74) and which are part of an “immune evasion cluster” present in human strains but generally not in bovine strains (134). Staphopain A can also inhibit the chemotaxis of neutrophils and their activation (Fig. 1B) (135); however, the role of these three proteins in the course of pneumonia in human remains to be confirmed. Another possible mechanism preventing the proper localization of neutrophils at their target site is the aggregation of both platelets and neutrophils induced by Hla, thus disturbing their recruitment, a mechanism that may occur in the lung (Fig. 1B) (136).
Second, S. aureus is able to kill neutrophils, largely by the activity of PFT. The role of PVL is predominant in neutrophil lysis (137), notably demonstrated in rabbits and humanized mouse models of pneumonia (Fig. 1B) (124, 125, 138). The bicomponent leucocidins like HlgCB, which targets the same receptors as PVL, LukDE, and LukAB, have not been experimentally demonstrated as key players in S. aureus pneumonia so far. Conversely, the degradation products of PSM-lysed neutrophils (139) are responsible for lung injuries in mice (140). Moreover, PSMs can kill neutrophils upon S. aureus phagocytosis (141), and PSMα3 synergizes with PVL to kill neutrophils (Fig. 1B) (142).
Another way to inhibit the function of neutrophils is to block their phagocytosis. Superantigen exoprotein (SAg) is a group of toxins that includes the toxic shock syndrome toxin 1 (TSST-1) and 18 staphylococcal enterotoxins (SE). These are responsible for polyclonal T-cell receptor (TCR) activation, leading to massive T-cell expansion and cytokine secretion. In 2011, a staphylococcal enterotoxin-like protein, SElX, encoded in the core genome, was identified as being implicated in S. aureus virulence in a rabbit model of pneumonia (143). Recently, one of its mechanisms was characterized; unlike the other SAg, this mechanism does not involve interaction with T cells, but it inhibits neutrophil phagocytosis (Fig. 1B) (144), leading to increased mortality in a pneumonia rabbit model.
Finally, NET are a well-organized and structured combination of DNA and cytosolic proteins that entrap pathogens in order to kill them and prevent their dissemination (145). DNA is one of the major components of NET; therefore, the secretion of Nuc, a nuclease produced by S. aureus, is very relevant means of escaping from NET (Fig. 1B) (146). In a murine pulmonary infection model, secretion of Nuc in the lung decreased S. aureus clearance and increased mortality (147). S. aureus is also able to counteract NET by other mechanisms, such as the conversion of NET into deoxyadenosine, which induces immune cell death (148). However, this mechanism was explored in kidney abscess in a mouse model and not in pneumonia.
Opsonization and humoral response.
Phagocytosis by specialized cells such as macrophages and neutrophils can be induced by the recognition of bacterial cell walls or opsonins, such as complement proteins or antibodies (mostly IgG) (149, 150), that target both cell wall-associated proteins and exotoxins (151). In addition to its direct action on phagocytic cells, S. aureus targets the complement or antibody-mediated opsonization pathways.
S. aureus secretes proteins that inhibit complement proteins, such as Sbi, which complexes complement proteins (152, 153), and Ecb (also known as Ehp), which inhibits the convertase activity of the C3 complement protein, as does Efb through its C terminus (154). Deletion mutants of Ecb and Efb proteins are reported to provoke reduced mortality in vivo (Fig. 1B) (155). Other proteins, such as SCIN and CHIPS, which inhibit neutrophil chemotaxis through the complement, and Spa, have been reported to impede the complement; nonetheless, their involvement in pneumonia through complement inhibition has yet to be proved (156).
Spa is important in S. aureus virulence in the context of pneumonia, as mentioned previously, and, in addition to epithelium disruption, Spa impedes the humoral response. It is cleaved and released in to the extracellular environment (71) and is able to bind Ig and prevent interaction with the target epitope (157). Furthermore, it interferes in lymphocyte B activation and proliferation, leading to (i) the reduction of phagocytosis of S. aureus (157), (ii) antibody production impediment (158), and (iii) disordered activation, finally leading to the death of the B cells (Fig. 1B) (159). These mechanisms have not yet been studied in the context of pneumonia; however, due to the significant interaction between Spa and the variable heavy 3 (VH3)-type B cell receptors, these phenomena would very likely occur in the lungs.
Overall, S. aureus has developed a wide range of proteins to inhibit and escape host defenses, including the immune system. Nevertheless, the presence of S. aureus, and the lysis and dysregulation of epithelial and immune cells, lead to an acute inflammatory response. This acute inflammation can still be an advantage for S. aureus, by contributing to overall tissue damage.
MAYHEM IN THE LUNG
Cytokine production.
The acute inflammatory response first results in the accumulation of cytokines produced by the lung epithelial cells (Fig. 1B). More specifically, during S. aureus infection, the interaction between S. aureus and the Toll-like receptor 2 (TLR2) of the epithelial cell induces the NF-κB pathway, and the production of proinflammatory cytokines and tumor necrosis factor (TNF) (156). Furthermore, via its interaction with TNFR1 (70, 160), Spa primes the secretion of interleukin-8 (IL-8) and of IL-16, an immune cell chemoattractant (161), which is responsible for lung damage in vivo (160). This phenomenon is amplified by the activation of EGFR, which increases the availability of TNFR1 at the cell surface (162). Moreover, the interaction between Hla and ADAM10 induces the secretion of IL-1β by the epithelium; the knockout of ADAM10 in mice protects against lethal S. aureus pneumonia through the reduction of IL-1β production (101). The PVL toxin also leads to IL-1β production by macrophages, acting in a paracrine manner to trigger IL-8 secretion by the epithelial cells (163). Finally, TSST-1 also induces the production of TNF and IL-8 by the pulmonary epithelium and thus promotes inflammation (35). Taken together, the epithelium initiates a strong cytokinic response in order to mobilize the immune system.
The pulmonary epithelial cells produce cytokines when in contact with S. aureus toxins, as do the immune cells via the NLRP3 inflammasome pathway (Fig. 1B). The inflammasome is an intracytoplasmic multiprotein complex activated by cell stresses or infections and is responsible for the release of proinflammatory cytokines, including IL-1β (164, 165). Beta-toxin, gamma-hemolysin (25, 163, 166), PVL (25, 120, 163), and Hla (166–168) trigger the NLRP3 inflammasome in macrophages, monocytes, and neutrophils, resulting in proinflammatory IL-1β and IL-18 secretion that is responsible for necrotic injuries and severe pneumonia in vivo (167). The inhibition of the inflammasome in the pneumonia mouse model results in a decrease of cytokines in the pulmonary tissue and therefore in decreased lung injuries (169). In addition to inflammasome activation in immune cells, most of these toxins, such as PVL and gamma-hemolysin, can lyse macrophages and neutrophils (119, 122), leading to an involuntary release of cytokines and exacerbating local inflammation.
Lymphocyte response dysregulation.
In addition to this cytokine storm, S. aureus impacts several other immune mechanisms, including the activation of lymphocytes (Fig. 1B). As mentioned above, SAg TSST-1, along with the enterotoxins SEB, SEC, and SElX, induces nonspecific T-lymphocyte activation and proliferation and, as a result, increases lung damage (143, 170). This polyclonal activation of T cells participates in the cytokinic storm, as T cells promote inflammation through cytokine release. These deleterious effects are circumvented by antibodies against TSST-1, SEB, and SEC in rabbit models (171). In addition, Parker et al. have demonstrated that this T-cell activation contributes to S. aureus pathogenicity by increasing lung damage (172). Therefore, the inhibition of abnormal T-cell activation might be a therapeutic option for future development.
B lymphocytes, implicated in antibody responses, are also targeted, especially by Spa. The latter first monopolizes the immunoglobin response by promoting anti-Spa immunoglobins produced by plasma cells and B cells, instead of immunoglobins against other secreted toxins, such as Hla or PVL (158). Second, Spa, by its ability to interact with B cell receptors, reduces their proliferation, but it predominantly induces B cells apoptosis after 36 to 48 h via the caspase pathway (159) via Spa shedding from the bacterial surface (71). As immunotherapies provide good evidence of efficacy to reduce the risk of occurrence and severity of S. aureus pneumonia in animals (173), and also in humans (174), the impedance of B cells by S. aureus may contribute to the persistence of infection.
Thus, both types of lymphocyte are impaired by S. aureus, with, on the one hand, an increase of inflammation by the T lymphocytes and, on the other hand, an inefficient humoral response dependent on B lymphocytes, allowing persistent S. aureus infection.
Necroptosis induction and efferocytosis inhibition.
Necroptosis is a cellular suicide mechanism used by microorganism-infected cells to prevent the replication and spread of the intruder (175). It leads to the activation of a proinflammatory pathway (IL-6, TNF, IL-1α, and IL-1β) that recruits phagocytes to clear debris after cell death and phagocytose dying cells (efferocytosis). The latter mechanism is important for the resolution of inflammation and tissue integrity restoration (176).
S. aureus is able to impact both mechanisms. First, it increases necroptosis, leading to acute cytokine release through secretion of toxins. Indeed, Hla, LukAB, and PSM participate in necroptosis mechanism induction by activating RIP1/RIP3/MLKL signaling in macrophages. Necroptosis impairment or inhibition of these toxins in the pneumonia mouse model decreases S. aureus virulence and improves its clearance (177). Regarding efferocytosis, to date only Hla has been reported as an inhibitor; by interacting with alveolar macrophages, it reduces their ability to phagocytose dying neutrophils (178). In 2014, Greenlee et al. demonstrated that when phagocytosed by neutrophils, S. aureus survives in the phagolysosome and decreases the efferocytosis of neutrophils by macrophages. In addition, S. aureus increases the production of cytokines by macrophages, exacerbating inflammation in the tissue (179). However, the pathophysiological impact of this phenomenon has not yet been assessed in any disease models, and more studies are required to further understand the manipulation of these mechanisms by S. aureus.
Other cell death mechanisms are also impeded or diverted and were recently reviewed by Grousd et al. (180).
Taken together, S. aureus pneumonia can lead to severe outcomes due to the tissue necrosis induced by S. aureus itself, but also to immune-driven inflammation. The reduction of this inflammation is one way to prevent lung tissue damage. This has been demonstrated in pneumonia mouse models, in which cytokine production was hindered by the inhibition of NF-κB signaling (181), NLRP3 inflammasome inhibition (169), and IL-1R signaling (182). Another way is to inhibit S. aureus toxins, notably by using passive immunotherapy approaches, such as neutralization with antibodies targeting Hla, PVL, HlgACB, and LukDE. This strategy has been demonstrated to be effective to protect animal models from S. aureus pneumonia (126, 127).
DISCUSSION AND PERSPECTIVES
S. aureus CAP are rare but severe infections with a high rate of lethality (9, 13). We describe here an arsenal of virulence factors produced by S. aureus that are implicated in its adhesion and adaptation to, as well as invasion of, the lung epithelium. The adaptation includes profound metabolic changes of the bacterium, notably in response to iron and nutrient limitations (53). However, most studies have assessed the impact of a given virulence factor using isogenic mutants or specific inhibitors, and therefore by comparing the presence/absence of the protein studied. These approaches omitted the notion of protein abundance. Indeed, most virulence factors belong to the bacterial core genome, and thus in vivo their impact on the host should reasonably depend on their level of expression. Only a few studies focusing on S. aureus pneumonia have investigated this parameter. Nevertheless, they offer new perspectives in the investigation of S. aureus virulence. For instance, in 2013, a link was established between mortality in the rabbit pneumonia model and Hla and PVL concentrations in lung samples (183). In humans, a severe outcome in ventilator-associated pneumonia was associated with higher Hla production in vitro (184). Quantitative approaches assessing the full spectrum of S. aureus virulence factors remain essential to fully understand the multifactorial nature of bacterial pathogenesis.
An additional aspect that is often discussed but difficult to explore is research using human samples and associated clinical data. As described in this review, several experimental models (cell culture and animal models) have been used to study interactions between S. aureus and the lung environment. Although animal models tend to mimic the highly complex human physiopathology during pneumonia as well as possible, they remain simplistic, with obvious biases such as species-specific receptor polymorphisms, leading to great variations in susceptibility to a given toxin depending on the animal species (32). This phenomenon has been perfectly deciphered for PVL, for which, surprisingly, our closest relative (nonhuman primate) was mostly resistant to the toxin (185). Therefore, caution is necessary when drawing conclusion for human based on cellular or animal models describing S. aureus infection mechanisms.
One aspect not explored in this review is the complexity of the lung microbiota, which may impact both the transition from colonization to invasive infection and S. aureus virulence. These aspects were more explored in other settings, such as cystic fibrosis patients or hospital-acquired pneumonia, but are poorly defined in the context of CAP (186, 187). Finally, we did not develop the impact of other clinical conditions on CAP occurrence or severity, such as host comorbidity factors and the role of previous viral infection damaging the pulmonary epithelium. For example, several clinical and experimental studies have reported a consistent link between previous influenza infections and the severity of SA-CAP (44, 188–197). Influenza infections induce a switch in S. aureus to a more virulent status (44), and the virus damages the host epithelium (188, 191, 194) but also promotes S. aureus intrusion and adhesion to the pulmonary tissue (189, 192, 195). Another means for S. aureus to reach the lung is via the hematogenous route in the course of bacteremia. Clinical observations suggest that in the case of pneumonia initiated by the air route of infection only one lobe can be infected, whereas multilobar infections can be observed upon bacteremia. However, there is no experimental evidence suggesting that specific virulence factors are associated with one or the other route of infection; yet, it is of interest to note that Hla was shown to impact the severity of pneumonia by the hematogenous route in rabbit. Conversely, epidermal differentiation inhibitor B (EdinB), initially described as a potential virulence factor in skin infection by impairing the maturation of keratinocytes, was shown to increase S. aureus translocation to the blood in the course of pneumonia in mice (198, 199); however, this remains to be fully investigated.
S. aureus is able to cause severe infections, notably in the lung, through the production of an array of toxins and proteins. These virulence factors are highly efficient in counteracting the host’s defense, including the immune system, which is even used to the advantage of S. aureus. With the emergence of new strains that possess newly discovered genes or accessory genes encoding toxins, such as the MRSA USA300 clones or the ST239 lineage that are implicated in CA pneumonia infection, better understanding of S. aureus virulence mechanisms is required to develop new therapeutic strategies.
In conclusion, the present review illustrates how it is the association of several virulence factors at specific infection steps, and the host response to these factors, that leads to severe staphylococcal pneumonia.
ACKNOWLEDGMENTS
We thank Thomas Henry for critical review of the manuscript. The manuscript was proofread and corrected by a native English speaker working for Hospices Civils de Lyon (P. Robinson).
M.P. was primarily responsible for writing the original draft. K.M. and F.V. contributed to its review and editing the final version.
We report no conflict of interest.
This study was not supported by a specific grant.
Contributor Information
François Vandenesch, Email: francois.vandenesch@univ-lyon1.fr.
Paul D. Fey, University of Nebraska Medical Center
REFERENCES
- 1.Deinhardt-Emmer S, Sachse S, Geraci J, Fischer C, Kwetkat A, Dawczynski K, Tuchscherr L, Löffler B. 2018. Virulence patterns of Staphylococcus aureus strains from nasopharyngeal colonization. J Hosp Infect 100:309–315. doi: 10.1016/j.jhin.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jean S-S, Chang Y-C, Lin W-C, Lee W-S, Hsueh P-R, Hsu C-W. 2020. Epidemiology, treatment, and prevention of nosocomial bacterial pneumonia. J Clin Med 9:275. doi: 10.3390/jcm9010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L. 2015. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Miguel-Díez J, López-de-Andrés A, Hernández-Barrera V, Jiménez-Trujillo I, Méndez-Bailón M, de Miguel-Yanes JM, Jiménez-García R. 2017. Impact of COPD on outcomes in hospitalized patients with community-acquired pneumonia: analysis of the Spanish national hospital discharge database (2004–2013). Eur J Intern Med 43:69–76. doi: 10.1016/j.ejim.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Walden AP, Clarke GM, McKechnie S, Hutton P, Gordon AC, Rello J, Chiche J-D, Stueber F, Garrard CS, Hinds CJ ; on behalf of the ESICM/ECCRN GenOSept Investigators. 2014. Patients with community acquired pneumonia admitted to European intensive care units: an epidemiological survey of the GenOSept cohort. Crit Care 18:R58. doi: 10.1186/cc13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy M-E, Etienne J. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerging Infect Dis 9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardakas KZ, Matthaiou DK, Falagas ME. 2009. Incidence, characteristics and outcomes of patients with severe community acquired-MRSA pneumonia. Eur Respir J 34:1148–1158. doi: 10.1183/09031936.00041009. [DOI] [PubMed] [Google Scholar]
- 10.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 11.Masters IB, Isles AF, Grimwood K. 2017. Necrotizing pneumonia: an emerging problem in children? Pneumonia (Nathan) 9:11. doi: 10.1186/s41479-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillet Y, Issartel B, Vanhems P, Fournet J-C, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, Etienne J. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 13.Dalponte R de S, Heluany GCV, Michels M, Madeira K, Prado C de E. 2020. Surgical treatment of necrotizing pneumonia in children: a 10-year assessment. Rev Col Bras Cir 47:e20202374. [DOI] [PubMed] [Google Scholar]
- 14.Gillet Y, Tristan A, Rasigade J-P, Saadatian-Elahi M, Bouchiat C, Bes M, Dumitrescu O, Leloire M, Dupieux C, Laurent F, Lina G, Etienne J, Vanhems P, Argaud L, Vandenesch F. 2020. Risk factors of severity in community-acquired staphylococcal pneumonia. medRxiv doi: 10.1101/2020.07.31.20162875. [DOI] [Google Scholar]
- 15.White ES. 2015. Lung extracellular matrix and fibroblast function. Ann Am Thorac Soc 12:S30–S33. doi: 10.1513/AnnalsATS.201406-240MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu B-H, Madison MC, Corry D, Kheradmand F. 2018. Matrix remodeling in chronic lung diseases. Matrix Biol 73:52–63. doi: 10.1016/j.matbio.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan S, Sebo P, Osicka R. 2018. A guide to polarized airway epithelial models for studies of host-pathogen interactions. FEBS J 285:4343–4358. doi: 10.1111/febs.14582. [DOI] [PubMed] [Google Scholar]
- 18.Knudsen L, Ochs M. 2018. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol 150:661–676. doi: 10.1007/s00418-018-1747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leiva-Juárez MM, Kolls JK, Evans SE. 2018. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol 11:21–34. doi: 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinton LJ, Mizgerd JP. 2015. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Physiol 77:407–430. doi: 10.1146/annurev-physiol-021014-071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Invernizzi R, Lloyd CM, Molyneaux PL. 2020. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology 160:171–182. doi: 10.1111/imm.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bals R, Hiemstra PS. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 23.Hiemstra PS, McCray PB, Bals R. 2015. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 45:1150–1162. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riches DWH, Martin TR. 2018. Overview of innate lung immunity and inflammation. Methods Mol Biol 1809:17–30. doi: 10.1007/978-1-4939-8570-8_2. [DOI] [PubMed] [Google Scholar]
- 26.Foster TJ. 2019. Surface proteins of Staphylococcus aureus. Microbiol Spectr 7. doi: 10.1128/microbiolspec.GPP3-0046-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam K, Torres VJ. 2019. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol Spectr 7. doi: 10.1128/microbiolspec.GPP3-0039-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DYM, Schlievert PM. 2013. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammer ND, Skaar EP. 2011. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu Rev Microbiol 65:129–147. doi: 10.1146/annurev-micro-090110-102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Muir TW. 2016. Regulation of virulence in Staphylococcus aureus: molecular mechanisms and remaining puzzles. Cell Chem Biol 23:214–224. doi: 10.1016/j.chembiol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spaan AN, van Strijp JAG, Torres VJ. 2017. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo H-S, Otto M. 2015. Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim Biophys Acta 1848:3055–3061. doi: 10.1016/j.bbamem.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yun YS, Min YG, Rhee CS, Jung IH, Koh YY, Jang TY, Jung DH. 1999. Effects of alpha-toxin of Staphylococcus aureus on the ciliary activity and ultrastructure of human nasal ciliated epithelial cells. Laryngoscope 109:2021–2024. doi: 10.1097/00005537-199912000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Aubert V, Schneeberger D, Sauty A, Winter J, Sperisen P, Aubert J-D, Spertini F. 2000. Induction of tumor necrosis factor alpha and interleukin-8 gene expression in bronchial epithelial cells by toxic shock syndrome toxin 1. Infect Immun 68:120–124. doi: 10.1128/iai.68.1.120-124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burian M, Rautenberg M, Kohler T, Fritz M, Krismer B, Unger C, Hoffmann WH, Peschel A, Wolz C, Goerke C. 2010. Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis 201:1414–1421. doi: 10.1086/651619. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, Tian Y, Hu J, Yu F, Lu Y, Otto M. 2012. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med 18:816–819. doi: 10.1038/nm.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paling FP, Wolkewitz M, Bode LGM, Klouwenberg PMCK, Ong DSY, Depuydt P, de Bus L, Sifakis F, Bonten MJM, Kluytmans JAJW. 2017. Staphylococcus aureus colonization at ICU admission as a risk factor for developing S. aureus ICU pneumonia. Clin Microbiol Infect 23:49.e9-49–e14. doi: 10.1016/j.cmi.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 39.Wertheim HF, Vos MC, Ott A, Belkum A, van Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 40.Sakr A, Brégeon F, Mège J-L, Rolain J-M, Blin O. 2018. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol 9:2419. doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. doi: 10.1128/CMR.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakr A, Brégeon F, Rolain J-M, Blin O. 2019. Staphylococcus aureus nasal decolonization strategies: a review. Expert Rev Anti Infect Ther 17:327–340. doi: 10.1080/14787210.2019.1604220. [DOI] [PubMed] [Google Scholar]
- 43.Humphreys H, Becker K, Dohmen PM, Petrosillo N, Spencer M, van Rijen M, Wechsler-Fördös A, Pujol M, Dubouix A, Garau J. 2016. Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery. J Hosp Infect 94:295–304. doi: 10.1016/j.jhin.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Reddinger RM, Luke-Marshall NR, Hakansson AP, Campagnari AA. 2016. Host physiologic changes induced by influenza A virus lead to Staphylococcus aureus biofilm dispersion and transition from asymptomatic colonization to invasive disease. mBio 7:e01235-16. doi: 10.1128/mBio.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, Stover CK, Sellman BR. 2015. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 6:e02272-14. doi: 10.1128/mBio.02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wertheim HFL, Walsh E, Choudhurry R, Melles DC, Boelens HAM, Miajlovic H, Verbrugh HA, Foster T, van Belkum A. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5:e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulcahy ME, Geoghegan JA, Monk IR, O’Keeffe KM, Walsh EJ, Foster TJ, McLoughlin RM. 2012. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLoS Pathog 8:e1003092. doi: 10.1371/journal.ppat.1003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Cai C, Feng Q, Shi Y, Zuo Q, Yang H, Jing H, Wei C, Zhuang Y, Zou Q, Zeng H. 2016. Protective efficacy of the chimeric Staphylococcus aureus vaccine candidate IC in sepsis and pneumonia models. Sci Rep 6:20929. doi: 10.1038/srep20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker EH, Baines DL. 2018. Airway glucose homeostasis: a new target in the prevention and treatment of pulmonary infection. Chest 153:507–514. doi: 10.1016/j.chest.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 50.Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. 2013. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol 174:193–202. doi: 10.1111/cei.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. 2014. Muc5b is required for airway defence. Nature 505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright JR. 2005. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 53.Chaffin DO, Taylor D, Skerrett SJ, Rubens CE. 2012. Changes in the Staphylococcus aureus transcriptome during early adaptation to the lung. PLoS One 7:e41329. doi: 10.1371/journal.pone.0041329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchetti M, De Bei O, Bettati S, Campanini B, Kovachka S, Gianquinto E, Spyrakis F, Ronda L. 2020. Iron metabolism at the interface between host and pathogen: from nutritional immunity to antibacterial development. Int J Mol Sci 21:2145. doi: 10.3390/ijms21062145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson M, Sengupta M, Purves J, Tarrant E, Williams PH, Cockayne A, Muthaiyan A, Stephenson R, Ledala N, Wilkinson BJ, Jayaswal RK, Morrissey JA. 2011. Fur is required for the activation of virulence gene expression through the induction of the sae regulatory system in Staphylococcus aureus. Int J Med Microbiol 301:44–52. doi: 10.1016/j.ijmm.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porcheron G, Dozois CM. 2015. Interplay between iron homeostasis and virulence: Fur and RyhB as major regulators of bacterial pathogenicity. Vet Microbiol 179:2–14. doi: 10.1016/j.vetmic.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 57.Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, Friedman DB, Heinrichs DE, Dunman PM, Skaar EP. 2010. Staphylococcus aureus Fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun 78:1618–1628. doi: 10.1128/IAI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson M, Cockayne A, Morrissey JA. 2008. Iron-regulated biofilm formation in Staphylococcus aureus Newman requires ica and the secreted protein Emp. Infect Immun 76:1756–1765. doi: 10.1128/IAI.01635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hook JL, Islam MN, Parker D, Prince AS, Bhattacharya S, Bhattacharya J. 2018. Disruption of staphylococcal aggregation protects against lethal lung injury. J Clin Invest 128:1074–1086. doi: 10.1172/JCI95823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lam H, Kesselly A, Stegalkina S, Kleanthous H, Yethon JA. 2014. Antibodies to PhnD inhibit staphylococcal biofilms. Infect Immun 82:3764–3774. doi: 10.1128/IAI.02168-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q, Du X, Hong X, Li T, Zheng B, He L, Wang Y, Otto M, Li M. 2015. Targeting surface protein SasX by active and passive vaccination to reduce Staphylococcus aureus colonization and infection. Infect Immun 83:2168–2174. doi: 10.1128/IAI.02951-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paharik AE, Horswill AR. 2016. The staphylococcal biofilm: adhesins, regulation, and host response. Microbiol Spectr 4. doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [Google Scholar]
- 63.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moormeier DE, Bayles KW. 2017. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol 104:365–376. doi: 10.1111/mmi.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geoghegan JA, Monk IR, O’Gara JP, Foster TJ. 2013. Subdomains N2N3 of fibronectin binding protein A mediate Staphylococcus aureus biofilm formation and adherence to fibrinogen using distinct mechanisms. J Bacteriol 195:2675–2683. doi: 10.1128/JB.02128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barbu EM, Mackenzie C, Foster TJ, Höök M. 2014. SdrC induces staphylococcal biofilm formation through a homophilic interaction. Mol Microbiol 94:172–185. doi: 10.1111/mmi.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feuillie C, Formosa-Dague C, Hays LMC, Vervaeck O, Derclaye S, Brennan MP, Foster TJ, Geoghegan JA, Dufrêne YF. 2017. Molecular interactions and inhibition of the staphylococcal biofilm-forming protein SdrC. Proc Natl Acad Sci U S A 114:3738–3743. doi: 10.1073/pnas.1616805114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herman-Bausier P, El-Kirat-Chatel S, Foster TJ, Geoghegan JA, Dufrêne YF. 2015. Staphylococcus aureus fibronectin-binding protein a mediates cell-cell adhesion through low-affinity homophilic bonds. mBio 6:e00413-15. doi: 10.1128/mBio.00413-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penadés JR, Lasa I. 2009. Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol 191:832–843. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gómez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. 8. Nat Med 10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 71.Becker S, Frankel MB, Schneewind O, Missiakas D. 2014. Release of protein A from the cell wall of Staphylococcus aureus. Proc Natl Acad Sci U S A 111:1574–1579. doi: 10.1073/pnas.1317181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sultan AR, Swierstra JW, Lemmens-den Toom NA, Snijders SV, Hansenová Maňásková S, Verbon A, van Wamel WJB. 2018. Production of staphylococcal complement inhibitor (SCIN) and other immune modulators during the early stages of Staphylococcus aureus biofilm formation in a mammalian cell culture medium. Infect Immun 86:e00352-18. doi: 10.1128/IAI.00352-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Haas CJC, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJB, Heezius ECJM, Poppelier MJJG, Van Kessel KPM, van Strijp JAG. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Postma B, Poppelier MJ, van Galen JC, Prossnitz ER, van Strijp JAG, de Haas CJC, van Kessel KPM. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J Immunol 172:6994–7001. doi: 10.4049/jimmunol.172.11.6994. [DOI] [PubMed] [Google Scholar]
- 75.Killikelly A, Benson MA, Ohneck EA, Sampson JM, Jakoncic J, Spurrier B, Torres VJ, Kong X-P. 2015. Structure-based functional characterization of repressor of toxin (Rot), a central regulator of Staphylococcus aureus virulence. J Bacteriol 197:188–200. doi: 10.1128/JB.02317-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenul C, Horswill AR. 2019. Regulation of Staphylococcus aureus virulence. Microbiol Spectr 7. doi: 10.1128/microbiolspec.GPP3-0031-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balasubramanian D, Ohneck EA, Chapman J, Weiss A, Kim MK, Reyes-Robles T, Zhong J, Shaw LN, Lun DS, Ueberheide B, Shopsin B, Torres VJ. 2016. Staphylococcus aureus coordinates leukocidin expression and pathogenesis by sensing metabolic fluxes via RpiRc. mBio 7:e00818-16. doi: 10.1128/mBio.00818-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mootz JM, Benson MA, Heim CE, Crosby HA, Kavanaugh JS, Dunman PM, Kielian T, Torres VJ, Horswill AR. 2015. Rot is a key regulator of Staphylococcus aureus biofilm formation. Mol Microbiol 96:388–404. doi: 10.1111/mmi.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benson MA, Lilo S, Nygaard T, Voyich JM, Torres VJ. 2012. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J Bacteriol 194:4355–4365. doi: 10.1128/JB.00706-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saïd-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, McNamara PJ, Arvidson S, Foster TJ, Projan SJ, Kreiswirth BN. 2003. Global regulation of Staphylococcus aureus genes by Rot. J Bacteriol 185:610–619. doi: 10.1128/jb.185.2.610-619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bronesky D, Wu Z, Marzi S, Walter P, Geissmann T, Moreau K, Vandenesch F, Caldelari I, Romby P. 2016. Staphylococcus aureus RNAIII and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu Rev Microbiol 70:299–316. doi: 10.1146/annurev-micro-102215-095708. [DOI] [PubMed] [Google Scholar]
- 82.Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu Rev Genet 42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 83.Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP. 2006. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol 61:1038–1048. doi: 10.1111/j.1365-2958.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 84.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheung GYC, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Periasamy S, Joo H-S, Duong AC, Bach T-HL, Tan VY, Chatterjee SS, Cheung GYC, Otto M. 2012. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A 109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peschel A, Otto M. 2013. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11:667–673. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Q, Yeo W-S, Bae T. 2016. The SaeRS two-component system of Staphylococcus aureus. Genes (Basel) 7:81–20. doi: 10.3390/genes7100081. [DOI] [Google Scholar]
- 89.Geiger T, Goerke C, Mainiero M, Kraus D, Wolz C. 2008. The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. J Bacteriol 190:3419–3428. doi: 10.1128/JB.01927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheung AL, Nishina K, Manna AC. 2008. SarA of Staphylococcus aureus binds to the sarA promoter to regulate gene expression. J Bacteriol 190:2239–2243. doi: 10.1128/JB.01826-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reyes D, Andrey DO, Monod A, Kelley WL, Zhang G, Cheung AL. 2011. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J Bacteriol 193:6020–6031. doi: 10.1128/JB.05436-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, Daum RS. 2008. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis 198:561–570. doi: 10.1086/590157. [DOI] [PubMed] [Google Scholar]
- 93.Villaruz AE, Bubeck Wardenburg J, Khan BA, Whitney AR, Sturdevant DE, Gardner DJ, DeLeo FR, Otto M. 2009. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J Infect Dis 200:724–734. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. 2013. Primary ciliary dyskinesia: recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ridley C, Thornton DJ. 2018. Mucins: the frontline defence of the lung. Biochem Soc Trans 46:1099–1106. doi: 10.1042/BST20170402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paharik AE, Salgado-Pabon W, Meyerholz DK, White MJ, Schlievert PM, Horswill AR. 2016. The Spl serine proteases modulate Staphylococcus aureus protein production and virulence in a rabbit model of pneumonia. mSphere 1:e00208-16. doi: 10.1128/mSphere.00208-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soong G, Martin FJ, Chun J, Cohen TS, Ahn DS, Prince A. 2011. Staphylococcus aureus protein A mediates invasion across airway epithelial cells through activation of RhoA GTPase signaling and proteolytic activity. J Biol Chem 286:35891–35898. doi: 10.1074/jbc.M111.295386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von Hoven G, Qin Q, Neukirch C, Husmann M, Hellmann N. 2019. Staphylococcus aureus α-toxin: small pore, large consequences. Biol Chem 400:1261–1276. doi: 10.1515/hsz-2018-0472. [DOI] [PubMed] [Google Scholar]
- 99.Wilke GA, Bubeck Wardenburg J. 2010. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A 107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwak Y-K, Vikström E, Magnusson K-E, Vécsey-Semjén B, Colque-Navarro P, Möllby R. 2012. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect Immun 80:1670–1680. doi: 10.1128/IAI.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Becker REN, Berube BJ, Sampedro GR, DeDent AC, Bubeck Wardenburg J. 2014. Tissue-specific patterning of host innate immune responses by Staphylococcus aureus α-toxin. J Innate Immun 6:619–631. doi: 10.1159/000360006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kiedrowski MR, Paharik AE, Ackermann LW, Shelton AU, Singh SB, Starner TD, Horswill AR. 2016. Development of an in vitro colonization model to investigate Staphylococcus aureus interactions with airway epithelia. Cell Microbiol 18:720–732. doi: 10.1111/cmi.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inoshima I, Inoshima N, Wilke G, Powers M, Frank K, Wang Y, Wardenburg JB. 2011. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection. Nat Med 17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Josse J, Laurent F, Diot A. 2017. Staphylococcal adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front Microbiol 8:2433. doi: 10.3389/fmicb.2017.02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kantyka T, Pyrc K, Gruca M, Smagur J, Plaza K, Guzik K, Zeglen S, Ochman M, Potempa J. 2013. Staphylococcus aureus proteases degrade lung surfactant protein A potentially impairing innate immunity of the lung. J Innate Immun 5:251–260. doi: 10.1159/000345417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baaske R, Richter M, Möller N, Ziesemer S, Eiffler I, Müller C, Hildebrandt J-P. 2016. ATP release from human airway epithelial cells exposed to Staphylococcus aureus alpha-toxin. Toxins (Basel) 8:365–313. doi: 10.3390/toxins8120365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hayashi T, Kawakami M, Sasaki S, Katsumata T, Mori H, Yoshida H, Nakahari T. 2005. ATP regulation of ciliary beat frequency in rat tracheal and distal airway epithelium. Exp Physiol 90:535–544. doi: 10.1113/expphysiol.2004.028746. [DOI] [PubMed] [Google Scholar]
- 108.Kim CS, Jeon SY, Min YG, Rhyoo C, Kim JW, Yun JB, Park SW, Kwon TY. 2000. Effects of beta-toxin of Staphylococcus aureus on ciliary activity of nasal epithelial cells. Laryngoscope 110:2085–2088. [DOI] [PubMed] [Google Scholar]
- 109.Moldovan A, Fraunholz MJ. 2019. In or out: phagosomal escape of Staphylococcus aureus. Cell Microbiol 21:e12997. doi: 10.1111/cmi.12997. [DOI] [PubMed] [Google Scholar]
- 110.Giese B, Dittmann S, Paprotka K, Levin K, Weltrowski A, Biehler D, LâM Tn-T, Sinha B, Fraunholz MJ. 2009. Staphylococcal alpha-toxin is not sufficient to mediate escape from phagolysosomes in upper-airway epithelial cells. IAI 77:3611–3625. doi: 10.1128/IAI.01478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strobel M, Pförtner H, Tuchscherr L, Völker U, Schmidt F, Kramko N, Schnittler H-J, Fraunholz MJ, Löffler B, Peters G, Niemann S. 2016. Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain. Clin Microbiol Infect 22:799–809. doi: 10.1016/j.cmi.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 112.Truong-Bolduc QC, Khan NS, Vyas JM, Hooper DC. 2017. Tet38 efflux pump affects Staphylococcus aureus internalization by epithelial cells through interaction with CD36 and contributes to bacterial escape from acidic and nonacidic phagolysosomes. Infect Immun 85:e00862-16. doi: 10.1128/IAI.00862-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giese B, Glowinski F, Paprotka K, Dittmann S, Steiner T, Sinha B, Fraunholz MJ. 2011. Expression of δ-toxin by Staphylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of β-toxin. Cell Microbiol 13:316–329. doi: 10.1111/j.1462-5822.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- 114.Grosz M, Kolter J, Paprotka K, Winkler A-C, Schäfer D, Chatterjee SS, Geiger T, Wolz C, Ohlsen K, Otto M, Rudel T, Sinha B, Fraunholz M. 2014. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell Microbiol 16:451–465. doi: 10.1111/cmi.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blättner S, Das S, Paprotka K, Eilers U, Krischke M, Kretschmer D, Remmele CW, Dittrich M, Müller T, Schuelein-Voelk C, Hertlein T, Mueller MJ, Huettel B, Reinhardt R, Ohlsen K, Rudel T, Fraunholz MJ. 2016. Staphylococcus aureus exploits a non-ribosomal cyclic dipeptide to modulate survival within epithelial cells and phagocytes. PLoS Pathog 12:e1005857. doi: 10.1371/journal.ppat.1005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alvarez ME, White CB, Gregory J, Kydd GC, Harris A, Sun HH, Gillum AM, Cooper R. 1995. Phevalin, a new calpain inhibitor, from a Streptomyces sp. J Antibiot 48:1165–1167. doi: 10.7164/antibiotics.48.1165. [DOI] [PubMed] [Google Scholar]
- 117.Melehani JH, James DBA, DuMont AL, Torres VJ, Duncan JA. 2015. Staphylococcus aureus leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS Pathog 11:e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alonzo F, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. 2013. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493:51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spaan AN, Vrieling M, Wallet P, Badiou C, Reyes-Robles T, Ohneck EA, Benito Y, de Haas CJC, Day CJ, Jennings MP, Lina G, Vandenesch F, van Kessel KPM, Torres VJ, van Strijp JAG, Henry T. 2014. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun 5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, Duncan JA, Broglie PM, Marketon K, Austermann J, Vogl T, Foell D, Niemann S, Peters G, Roth J, Löffler B. 2012. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol 92:1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reyes-Robles T, Alonzo F, Kozhaya L, Lacy DB, Unutmaz D, Torres VJ. 2013. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14:453–459. doi: 10.1016/j.chom.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spaan AN, Henry T, van Rooijen WJM, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJC, van Kessel KPM, Vandenesch F, Lina G, van Strijp JAG. 2013. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 123.Tromp AT, Van Gent M, Abrial P, Martin A, Jansen JP, De Haas CJC, Van Kessel KPM, Bardoel BW, Kruse E, Bourdonnay E, Boettcher M, McManus MT, Day CJ, Jennings MP, Lina G, Vandenesch F, Van Strijp JAG, Jan Lebbink R, Haas P-JA, Henry T, Spaan AN. 2018. Human CD45 is an F-component-specific receptor for the staphylococcal toxin Panton-Valentine leukocidin. Nat Microbiol 3:708–717. doi: 10.1038/s41564-018-0159-x. [DOI] [PubMed] [Google Scholar]
- 124.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schlievert PM. 2009. Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J Infect Dis 200:676–678. doi: 10.1086/605333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vu TTT, Nguyen NTQ, Tran VG, Gras E, Mao Y, Jung DH, Tkaczyk C, Sellman BR, Diep BA. 2019. Protective efficacy of monoclonal antibodies neutralizing alpha-hemolysin and bicomponent leukocidins in a rabbit model of Staphylococcus aureus necrotizing pneumonia. Antimicrob Agents Chemother 64:e02220-19. doi: 10.1128/AAC.02220-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diep BA, Le VTM, Visram ZC, Rouha H, Stulik L, Dip EC, Nagy G, Nagy E. 2016. Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob Agents Chemother 60:6333–6340. doi: 10.1128/AAC.01213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diep BA, Le VTM, Badiou C, Le HN, Pinheiro MG, Duong AH, Wang X, Dip EC, Aguiar-Alves F, Basuino L, Marbach H, Mai TT, Sarda MN, Kajikawa O, Matute-Bello G, Tkaczyk C, Rasigade J-P, Sellman BR, Chambers HF, Lina G. 2016. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med 8:357ra124. doi: 10.1126/scitranslmed.aag1153. [DOI] [PubMed] [Google Scholar]
- 129.Palmqvist N, Patti JM, Tarkowski A, Josefsson E. 2004. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes Infect 6:188–195. doi: 10.1016/j.micinf.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 130.Cohen TS, Boland ML, Boland BB, Takahashi V, Tovchigrechko A, Lee Y, Wilde AD, Mazaitis MJ, Jones-Nelson O, Tkaczyk C, Raja R, Stover CK, Sellman BR. 2018. S. aureus evades macrophage killing through NLRP3-dependent effects on mitochondrial trafficking. Cell Rep 22:2431–2441. doi: 10.1016/j.celrep.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Flannagan RS, Heit B, Heinrichs DE. 2016. Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell Microbiol 18:514–535. doi: 10.1111/cmi.12527. [DOI] [PubMed] [Google Scholar]
- 132.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. 2015. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spaan AN, Surewaard BGJ, Nijland R, van Strijp JAG. 2013. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu Rev Microbiol 67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 134.van Wamel WJB, Rooijakkers SHM, Ruyken M, van Kessel KPM, van Strijp JAG. 2006. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol 188:1310–1315. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laarman AJ, Mijnheer G, Mootz JM, van Rooijen WJM, Ruyken M, Malone CL, Heezius EC, Ward R, Milligan G, van Strijp JAG, de Haas CJC, Horswill AR, van Kessel KPM, Rooijakkers SHM. 2012. Staphylococcus aureus staphopain A inhibits CXCR2-dependent neutrophil activation and chemotaxis. EMBO J 31:3607–3619. doi: 10.1038/emboj.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parimon T, Li Z, Bolz DD, McIndoo ER, Bayer CR, Stevens DL, Bryant AE. 2013. Staphylococcus aureus α-hemolysin promotes platelet-neutrophil aggregate formation. J Infect Dis 208:761–770. doi: 10.1093/infdis/jit235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prince A, Wang H, Kitur K, Parker D. 2017. Humanized mice exhibit increased susceptibility to Staphylococcus aureus pneumonia. J Infect Dis 215:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang R, Braughton KR, Kretschmer D, Bach T-HL, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. 12. Nat Med 13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 140.Zhou Y, Niu C, Ma B, Xue X, Li Z, Chen Z, Li F, Zhou S, Luo X, Hou Z. 2018. Inhibiting PSMα-induced neutrophil necroptosis protects mice with MRSA pneumonia by blocking the agr system. Cell Death Dis 9:1–14. doi: 10.1038/s41419-018-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Surewaard BGJ, de Haas CJC, Vervoort F, Rigby KM, DeLeo FR, Otto M, van Strijp JAG, Nijland R. 2013. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol 15:1427–1437. doi: 10.1111/cmi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hongo I, Baba T, Oishi K, Morimoto Y, Ito T, Hiramatsu K. 2009. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J Infect Dis 200:715–723. doi: 10.1086/605332. [DOI] [PubMed] [Google Scholar]
- 143.Wilson GJ, Seo KS, Cartwright RA, Connelley T, Chuang-Smith ON, Merriman JA, Guinane CM, Park JY, Bohach GA, Schlievert PM, Morrison WI, Fitzgerald JR. 2011. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog 7:e1002271. doi: 10.1371/journal.ppat.1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tuffs SW, James DBA, Bestebroer J, Richards AC, Goncheva MI, O’Shea M, Wee BA, Seo KS, Schlievert PM, Lengeling A, van Strijp JA, Torres VJ, Fitzgerald JR. 2017. The Staphylococcus aureus superantigen SElX is a bifunctional toxin that inhibits neutrophil function. PLoS Pathog 13:e1006461. doi: 10.1371/journal.ppat.1006461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Papayannopoulos V. 2018. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 146.Schilcher K, Andreoni F, Uchiyama S, Ogawa T, Schuepbach RA, Zinkernagel AS. 2014. Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin. J Infect Dis 210:473–482. doi: 10.1093/infdis/jiu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Berends ETM, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. 2010. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun 2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gordon S. 2016. Phagocytosis: an immunobiologic process. Immunity 44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 150.Flannagan RS, Heit B, Heinrichs DE. 2015. Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 4:826–868. doi: 10.3390/pathogens4040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van den Berg S, Bowden MG, Bosma T, Buist G, van Dijl JM, van Wamel WJ, de Vogel CP, van Belkum A, Bakker-Woudenberg IAJM. 2011. A multiplex assay for the quantification of antibody responses in Staphylococcus aureus infections in mice. J Immunol Methods 365:142–148. doi: 10.1016/j.jim.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 152.Burman JD, Leung E, Atkins KL, O’Seaghdha MN, Lango L, Bernadó P, Bagby S, Svergun DI, Foster TJ, Isenman DE, van den Elsen JMH. 2008. Interaction of human complement with Sbi, a staphylococcal immunoglobulin-binding protein: indications of a novel mechanism of complement evasion by Staphylococcus aureus. J Biol Chem 283:17579–17593. doi: 10.1074/jbc.M800265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Haupt K, Reuter M, van den Elsen J, Burman J, Hälbich S, Richter J, Skerka C, Zipfel PF. 2008. The Staphylococcus aureus protein Sbi acts as a complement inhibitor and forms a tripartite complex with host complement Factor H and C3b. PLoS Pathog 4:e1000250. doi: 10.1371/journal.ppat.1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jongerius I, Köhl J, Pandey MK, Ruyken M, van Kessel KPM, van Strijp JAG, Rooijakkers SHM. 2007. Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med 204:2461–2471. doi: 10.1084/jem.20070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jongerius I, von Köckritz-Blickwede M, Horsburgh MJ, Ruyken M, Nizet V, Rooijakkers SHM. 2012. Staphylococcus aureus virulence is enhanced by secreted factors that block innate immune defenses. J Innate Immun 4:301–311. doi: 10.1159/000334604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parker D, Ahn D, Cohen T, Prince A. 2016. Innate immune signaling activated by MDR bacteria in the airway. Physiol Rev 96:19–53. doi: 10.1152/physrev.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Falugi F, Kim HK, Missiakas DM, Schneewind O. 2013. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 4:e00575-13. doi: 10.1128/mBio.00575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pauli NT, Kim HK, Falugi F, Huang M, Dulac J, Henry Dunand C, Zheng N-Y, Kaur K, Andrews SF, Huang Y, DeDent A, Frank KM, Charnot-Katsikas A, Schneewind O, Wilson PC. 2014. Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J Exp Med 211:2331–2339. doi: 10.1084/jem.20141404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goodyear CS, Silverman GJ. 2003. Death by a B cell superantigen: in vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J Exp Med 197:1125–1139. doi: 10.1084/jem.20020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ahn DS, Parker D, Planet PJ, Nieto PA, Bueno SM, Prince A. 2014. Secretion of IL-16 through TNFR1 and calpain-caspase signaling contributes to MRSA pneumonia. Mucosal Immunol 7:1366–1374. doi: 10.1038/mi.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cruikshank WW, Kornfeld H, Center DM. 2000. Interleukin-16. J Leukoc Biol 67:757–766. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- 162.Gómez MI, Seaghdha MO, Prince AS. 2007. Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. EMBO J 26:701–709. doi: 10.1038/sj.emboj.7601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perret M, Badiou C, Lina G, Burbaud S, Benito Y, Bes M, Cottin V, Couzon F, Juruj C, Dauwalder O, Goutagny N, Diep BA, Vandenesch F, Henry T. 2012. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell Microbiol 14:1019–1036. doi: 10.1111/j.1462-5822.2012.01772.x. [DOI] [PubMed] [Google Scholar]
- 164.Sanford BA, Thomas VL, Ramsay MA. 1989. Binding of staphylococci to mucus in vivo and in vitro. Infect Immun 57:3735–3742. doi: 10.1128/IAI.57.12.3735-3742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Franchi L, Muñoz-Planillo R, Núñez G. 2012. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Muñoz-Planillo R, Franchi L, Miller LS, Núñez G. 2009. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol 183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Craven RR, Gao X, Allen IC, Gris D, Wardenburg JB, McElvania-TeKippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus α-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu S, Huang J. 2017. Resveratrol alleviates Staphylococcus aureus pneumonia by inhibition of the NLRP3 inflammasome. Experimental and Therapeutic Medicine 14:6099–6104. doi: 10.3892/etm.2017.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strandberg KL, Rotschafer JH, Vetter SM, Buonpane RA, Kranz DM, Schlievert PM. 2010. Staphylococcal superantigens cause lethal pulmonary disease in rabbits. J Infect Dis 202:1690–1697. doi: 10.1086/657156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Spaulding AR, Lin Y-C, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. 2012. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine 30:5099–5109. doi: 10.1016/j.vaccine.2012.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Parker D, Ryan CL, Alonzo F, Torres VJ, Planet PJ, Prince AS. 2015. CD4+ T cells promote the pathogenesis of Staphylococcus aureus pneumonia. J Infect Dis 211:835–845. doi: 10.1093/infdis/jiu525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stulik L, Rouha H, Labrousse D, Visram ZC, Badarau A, Maierhofer B, Groß K, Weber S, Kramarić MD, Glojnarić I, Nagy G, Croisier D, Nagy E. 2019. Preventing lung pathology and mortality in rabbit Staphylococcus aureus pneumonia models with cytotoxin-neutralizing monoclonal IgGs penetrating the epithelial lining fluid. Sci Rep 9:5339. doi: 10.1038/s41598-019-41826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.François B, Mercier E, Gonzalez C, Asehnoune K, Nseir S, Fiancette M, Desachy A, Plantefève G, Meziani F, de Lame P-A, Laterre P-F, MASTER 1 study group . 2018. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med 44:1787–1796. doi: 10.1007/s00134-018-5229-2. [DOI] [PubMed] [Google Scholar]
- 175.Sridharan H, Upton JW. 2014. Programmed necrosis in microbial pathogenesis. Trends in Microbiology 22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 176.Kourtzelis I, Hajishengallis G, Chavakis T. 2020. Phagocytosis of apoptotic cells in resolution of inflammation. Front Immunol 11:553. doi: 10.3389/fimmu.2020.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. 2015. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathogens 11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cohen TS, Jones-Nelson O, Hotz M, Cheng L, Miller LS, Suzich J, Stover CK, Sellman BR. 2016. S. aureus blocks efferocytosis of neutrophils by macrophages through the activity of its virulence factor alpha toxin. Sci Rep 6:1–8. doi: 10.1038/srep35466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. 2014. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol 192:4709–4717. doi: 10.4049/jimmunol.1302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grousd JA, Rich HE, Alcorn JF. 2019. Host-pathogen interactions in gram-positive bacterial pneumonia. Clin Microbiol Rev 32:e00107-18. doi: 10.1128/CMR.00107-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shaukat A, Guo Y-F, Jiang K, Zhao G, Wu H, Zhang T, Yang Y, Guo S, Yang C, Zahoor A, Akhtar M, Umar T, Shaukat I, Rajput SA, Hassan M, Deng G. 2019. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced acute lung injury through attenuating NF-κB and MAPK activation. Microb Pathog 132:302–312. doi: 10.1016/j.micpath.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 182.Labrousse D, Perret M, Hayez D, Da Silva S, Badiou C, Couzon F, Bes M, Chavanet P, Lina G, Vandenesch F, Croisier-Bertin D, Henry T. 2014. Kineret®/IL-1ra blocks the IL-1/IL-8 inflammatory cascade during recombinant Panton Valentine leukocidin-triggered pneumonia but not during S. aureus infection. PLoS One 9:e97546. doi: 10.1371/journal.pone.0097546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stulik L, Malafa S, Hudcova J, Rouha H, Henics BZ, Craven DE, Sonnevend AM, Nagy E. 2014. α-Hemolysin activity of methicillin-susceptible Staphylococcus aureus predicts ventilator-associated pneumonia. Am J Respir Crit Care Med 190:1139–1148. doi: 10.1164/rccm.201406-1012OC. [DOI] [PubMed] [Google Scholar]
- 185.Spaan AN, Schiepers A, de Haas CJC, van Hooijdonk DDJJ, Badiou C, Contamin H, Vandenesch F, Lina G, Gerard NP, Gerard C, van Kessel KPM, Henry T, van Strijp JAG. 2015. Differential interaction of the staphylococcal toxins Panton-Valentine leukocidin and γ-hemolysin CB with human C5a receptors. J Immunol 195:1034–1043. doi: 10.4049/jimmunol.1500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yin Y, Hountras P, Wunderink RG. 2017. The microbiome in mechanically ventilated patients. Curr Opin Infect Dis 30:208–213. doi: 10.1097/QCO.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 187.Granchelli AM, Adler FR, Keogh RH, Kartsonaki C, Cox DR, Liou TG. 2018. Microbial interactions in the cystic fibrosis airway. J Clin Microbiol 56:e00354-18. doi: 10.1128/JCM.00354-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deinhardt-Emmer S, Haupt KF, Garcia-Moreno M, Geraci J, Forstner C, Pletz M, Ehrhardt C, Löffler B. 2019. Staphylococcus aureus pneumonia: preceding influenza infection paves the way for low-virulent strains. Toxins (Basel) 11:734. doi: 10.3390/toxins11120734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.de Bentzmann S, Tristan A, Etienne J, Brousse N, Vandenesch F, Lina G. 2004. Staphylococcus aureus isolates associated with necrotizing pneumonia bind to basement membrane type I and IV collagens and laminin. J Infect Dis 190:1506–1515. doi: 10.1086/424521. [DOI] [PubMed] [Google Scholar]
- 190.Siemens N, Oehmcke-Hecht S, Mettenleiter TC, Kreikemeyer B, Valentin-Weigand P, Hammerschmidt S. 2017. Port d’entrée for respiratory infections—does the influenza a virus pave the way for bacteria? Front Microbiol 8:2602. doi: 10.3389/fmicb.2017.02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bloes DA, Haasbach E, Hartmayer C, Hertlein T, Klingel K, Kretschmer D, Planz O, Peschel A. 2017. Phenol-soluble modulin peptides contribute to influenza A virus-associated Staphylococcus aureus pneumonia. Infect Immun 85:e00620-17. doi: 10.1128/IAI.00620-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Collins LF, Anderson BD, Gray GC. 2017. A case of influenza A (H3N2) complicated by community-acquired pneumonia and death in a young healthy adult during the 2013–2014 season. Front Public Health 5:1. doi: 10.3389/fpubh.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kobayashi SD, Olsen RJ, LaCasse RA, Safronetz D, Ashraf M, Porter AR, Braughton KR, Feldmann F, Clifton DR, Kash JC, Bailey JR, Gardner DJ, Otto M, Brining DL, Kreiswirth BN, Taubenberger JK, Parnell MJ, Feldmann H, Musser JM, DeLeo FR. 2013. Seasonal H3N2 influenza A virus fails to enhance Staphylococcus aureus co-infection in a non-human primate respiratory tract infection model. Virulence 4:707–715. doi: 10.4161/viru.26572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abood RN, McHugh KJ, Rich HE, Ortiz MA, Tobin JM, Ramanan K, Robinson KM, Bomberger JM, Kolls JK, Manni ML, Pociask DA, Alcorn JF. 2019. IL-22-binding protein exacerbates influenza, bacterial super-infection. Mucosal Immunol 12:1231–1243. doi: 10.1038/s41385-019-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rowe HM, Meliopoulos VA, Iverson A, Bomme P, Schultz-Cherry S, Rosch JW. 2019. Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat Microbiol 4:1328–1336. doi: 10.1038/s41564-019-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jia L, Zhao J, Yang C, Liang Y, Long P, Liu X, Qiu S, Wang L, Xie J, Li H, Liu H, Guo W, Wang S, Li P, Zhu B, Hao R, Ma H, Jiang Y, Song H. 2018. Severe pneumonia caused by coinfection with influenza virus followed by methicillin-resistant Staphylococcus aureus induces higher mortality in mice. Front Immunol 9:3189. doi: 10.3389/fimmu.2018.03189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Beumer MC, Koch RM, van Beuningen D, OudeLashof AM, van de Veerdonk FL, Kolwijck E, van der Hoeven JG, Bergmans DC, Hoedemaekers CWE. 2019. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J Crit Care 50:59–65. doi: 10.1016/j.jcrc.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Crémieux A-C, Saleh-Mghir A, Danel C, Couzon F, Dumitrescu O, Lilin T, Perronne C, Etienne J, Lina G, Vandenesch F. 2014. α-Hemolysin, not Panton-Valentine leukocidin, impacts rabbit mortality from severe sepsis with methicillin-resistant Staphylococcus aureus osteomyelitis. J Infect Dis 209:1773–1780. doi: 10.1093/infdis/jit840. [DOI] [PubMed] [Google Scholar]
- 199.Courjon J, Munro P, Benito Y, Visvikis O, Bouchiat C, Boyer L, Doye A, Lepidi H, Ghigo E, Lavigne J-P, Vandenesch F, Lemichez E. 2015. EDIN-B promotes the translocation of Staphylococcus aureus to the bloodstream in the course of pneumonia. Toxins (Basel) 7:4131–4142. doi: 10.3390/toxins7104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Heyer G, Saba S, Adamo R, Rush W, Soong G, Cheung A, Prince A. 2002. Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect Immun 70:127–133. doi: 10.1128/IAI.70.1.127-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Valotteau C, Prystopiuk V, Pietrocola G, Rindi S, Peterle D, De Filippis V, Foster TJ, Speziale P, Dufrêne YF. 2017. Single-cell and single-molecule analysis unravels the multifunctionality of the Staphylococcus aureus collagen-binding protein Cna. ACS Nano 11:2160–2170. doi: 10.1021/acsnano.6b08404. [DOI] [PubMed] [Google Scholar]
- 202.Kang M, Ko Y-P, Liang X, Ross CL, Liu Q, Murray BE, Höök M. 2013. Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of Gram-positive bacteria inhibit complement activation via the classical pathway. J Biol Chem 288:20520–20531. doi: 10.1074/jbc.M113.454462. [DOI] [PMC free article] [PubMed] [Google Scholar]