Abstract
Objective:
The majority of smokers who make a quit attempt experience their first lapse within the first week of quitting, yet limited research to-date has examined how the strength and direction of the relationship between smoking risk factors and lapse may change over longer periods of time. Time-varying effect modeling (TVEM) was used to address this gap.
Methods:
A diverse sample (N=325) of adult smokers completed ecological momentary assessments of risk factors for lapse for 28 days after quitting. TVEM was used to examine the relationship between risk factors (abstinence self-efficacy, positive affect, positive coping expectancies, smoking expectancies, motivation, negative affect, stress, and urge) and lapse for 28 days post-quit.
Results:
Some associations were stable (e.g., negative affect, motivation), whereas others varied over time. Abstinence self-efficacy, positive affect, and positive coping expectancies were most strongly associated with lapse between days 3–8 post-quit. The association of urge with lapse was strongest between days 4 and 10, as well as near the end of the quit attempt. Stress was also most strongly associated with lapse near the beginning and end of the post-quit period and was the only predictor associated with lapse on quit date. The strength of the association between smoking expectancies and lapse increased over time.
Conclusions:
There may be periods during a quit attempt when certain risk factors are more strongly related to lapse. This work has relevance for tailoring interventions designed to deliver intervention components in particular contexts or times of need.
Keywords: time-varying effects model, lapse, smoking cessation, ecological momentary assessment
Introduction
Nearly 40 million (15.5%) adults in the United States smoke cigarettes (Drope et al., 2018). Smoking causes about a half a million deaths per year, which is more than can be attributed to other modifiable risk factors, including but not limited to overweight/obesity, poor diet, cardiovascular disease, alcohol use, accidents, and violence (Danaei et al., 2009; Samet, 2013). Many current smokers have a desire to quit, with nearly half reporting quitting for at least one day in the last 12 months (Ahluwalia et al., 2018; Dube, Asman, Malarcher, & Carabollo, 2009), and an increasing proportion of ever smokers who successfully quit (Agaku, King, & Dube, 2014). Most smokers who attempt to quit experience a sequence of failed attempts before achieving long-term abstinence, with the first lapse most often occurring within the first week of a quit attempt (Hughes, Keely, & Naud, 2004; Zhou et al., 2009). This has resulted in a large body of smoking cessation research examining quitting as a process that unfolds over time, which can help clarify dynamic factors that influence the process at various points in time (Baker et al., 2011; Shiffman, 2005).
Theoretical models such as Marlatt and Gordon’s Relapse Prevention Model and its updated conceptualizations have outlined key factors that influence behavior change (Marlatt, 1985; Marlatt & Donovan, 2005; Witkiewitz & Marlatt, 2004). The model posits that behavior change is influenced by the interplay between a person, their environment, and cognitive factors. For example, cues to smoke or interoceptive cues such as negative affect can increase urge and motivation to smoke. Whether high risk situations result in lapsing or refraining from smoking is influenced by cognitive factors such as self-efficacy, expectancies about the consequences of smoking, and coping outcome expectancies. A key tenant of the model is that these processes are very dynamic and can change over short periods of time (Witkiewitz & Marlatt, 2004). Seminal work by Shiffman and colleagues introduced ecological momentary assessment (EMA) as a method for studying smoking behavior using real time, real world measures of hypothesized risk factors (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). EMA is advantageous for understanding dynamic processes because data are less influenced by recall bias, are more ecologically valid, and better elucidate proximal antecedents and sequelae of events or behaviors compared to retrospective recall data (Shiffman, Stone, & Hufford, 2008). Early work demonstrated the utility of using EMA and showed that factors like negative mood, urge to smoke, and situational cues were related to smoking lapse in-the-moment (Shiffman et al., 1996). Since, then, the burgeoning EMA research on smoking has demonstrated the acute effects of urge, negative affect and stress, positive affect, motivation, abstinence self-efficacy, smoking expectancies, and coping outcome expectancies on smoking lapse (Businelle et al., 2016; Cambron et al., 2019; Gwaltney, Bartolomei, Colby, & Kahler, 2008; Lam et al., 2014; Shiffman et al., 2007; Vinci et al., 2017).
Traditionally, multilevel models (MLMs) have been used to examine within-person relationships in EMA data. However, a limitation of MLMs is that they are “fixed with respect to time,” thereby assuming that the strength and direction of relationships are constant over time. Traditional MLMs are also parametric and estimate simple linear or quadratic associations (Lanza, Vasilenko, & Russell, 2016; Shiyko, Lanza, Tan, Li, & Shiffman, 2012; Tan, Shiyko, Li, Li, & Dierker, 2012). As such, these models are less able to fully explicate the rich, dynamic nature of intensive longitudinal EMA data, which may be vital for understanding the process of smoking cessation (Shiffman et al., 2002). Importantly, withdrawal symptoms typically “peak” within a few days of quitting (Hughes, 2007), but may continue for 2–4 weeks or longer (Herd & Borland, 2009; Hughes et al., 2010). In addition, withdrawal symptoms (e.g., negative affect and urge) have been shown to be variable following the quit date, such that they show intermittent and prominent spikes over time (Piper et al., 2011). Although an implicit assumption in theoretical models and most smoking cessation research is that the association between risk factors and lapse is constant over time, there may be periods when specific risk factors (e.g., urge, self-efficacy, negative affect) are more or less influential in their impact on risk for lapse.
Time-varying effect modeling (TVEM) is a non-parametric extension of the MLM that is designed to use all available data to flexibly estimate natural trajectories, such as whether the strength and direction of a relationship changes as a function of continuous time (Li, Root, & Shiffman, 2006; Shiyko et al., 2012). TVEM research to-date has enhanced the understanding of smoking cessation as a dynamic process. Li and colleagues were among the first to demonstrate with TVEM that the effect of negative affect on smoking may differ before and after a quit attempt (Li et al., 2006). Others have shown that the effect of smoking cessation treatment on the association between negative affect and urge may dissipate shortly after the quit attempt (Lanza, Vasilenko, Liu, Li, & Piper, 2014), that level of urge across a quit attempt may differ between those who relapse and those who successfully quit (Shiyko et al., 2012), and that the strength of the association of craving and negative affect with lapse may increase following a quit attempt (Koslovsky et al., 2018; Vasilenko et al., 2014).
The purpose of this study is to replicate and extend prior research by investigating the time varying association of abstinence self-efficacy, positive affect, positive coping expectancies, smoking expectancies, motivation, negative affect, stress, and urge with lapse. This study adds to the literature by exploring associations for a longer duration than prior research (i.e., 28 days following a quit attempt). Although previous research suggests that risk factors may have differing effects on various aspects of smoking behavior during different phases of a smoking “career” (e.g., negative affect predicts lapse during an active quit attempt but may not be a primary driver during normal maintenance of smoking; Shiffman et al., 2002; Shiffman et al., 1996), there is limited work examining time varying effects within the context of a quit attempt. Moreover, models of drug dependence typically do not detail specific hypotheses about differing strengths of association between risk factors and drug outcomes over time. However, previous TVEM research (Koslovsky et al., 2018; Vasilenko et al., 2014) suggests that the strength of association of negative affect and urge with lapse may increase over time. Assuming that is the case, it could also be hypothesized that other risk factors might show similar patterns.
Method
Participants
Data for the current study were collected in a National Institute on Drug Abuse-funded longitudinal study examining the effects of race/ethnicity and social/environmental influences on smoking cessation. Previous studies from these data have included models linking socioeconomic status to relapse, as well as longitudinal associations of socioeconomic status, pro-smoking social context factors, and affective precipitants with smoking lapse (Businelle et al., 2010; Cambron et al., 2019; Cambron, Lam, Cinciripini, Li, & Wetter, 2019; Kendzor et al., 2010; Vinci et al., 2017). Participants were recruited between 2005 and 2007 from the Houston, TX area using media (e.g., public service announcements and print media) and community outreach (e.g., flyers in medical offices or at public health fairs). Participants were at least 21 years of age, smoked at least five cigarettes per day for the last year, were motivated to quit within the next month, had a home address and functioning telephone number, and could read, write, and speak English at least at a sixth-grade level. Exclusion criteria included regular use of other tobacco products (e.g., cigars, pipes, smokeless tobacco), contraindication for use of nicotine patch or use of smoking cessation products other than the patch (e.g., nicotine gum), and having a household member enrolled in the study or being enrolled in another smoking cessation study in the past 90 days. Interested participants were screened for eligibility over the phone (n=944). Eligible participants (n=837) were invited to attend an in-person orientation session to give written informed consent, complete a series of assessments, and be trained on EMA procedures. Of those eligible, 424 attended the orientation visit and were enrolled in the study. Additional details about the study have been published elsewhere (Kendzor et al., 2008)
Enrolled participants attended six additional in-person laboratory visits at 1 week prior to quit date (baseline; week −1), on their quit date (week 0), and at 1, 2, 4, and 26 weeks post-quit. Participants received six smoking cessation counseling sessions (10–20 minutes each) during in-person visits at weeks −1, 0, 1, 2, and 4, and an additional session via telephone at week 3 post-quit. Counseling was based on the Treating Tobacco Use and Dependence: Clinical Practice Guideline (Fiore, 2008), in addition to self-help materials. Counseling sessions and self-help materials provided psychoeducation about nicotine dependence and quitting smoking, encouraged participants to identify and plan for high-risk situations, and taught cognitive and behavioral techniques (e.g., distraction, modifying routines) for avoiding and coping with high-risk situations. All participants received six weeks of nicotine patch therapy that were distributed at weeks −1, 0, 1, 2, and 4. Patch therapy for participants who smoked more than 10 cigarettes per day consisted of four weeks of 21 mg patches, one week of 14 mg patches, and one week of 7 mg patches. Patch therapy for those who smoked 5–10 cigarettes per day consisted of four weeks of 14 mg patches and two weeks of 7 mg patches. At every in-person visit, participants filled out a series of questionnaires assessing theory-based risk factors for smoking and other psychosocial and contextual variables. Participants received financial compensation at each in-person visit in the form of one $20 gift card per in-person visit through week 2, and one $40 gift card per in-person visit for weeks 4 and 26 (i.e., up to $180 in gift cards for attending every in-person visit). Participants were also eligible to receive additional compensation for completing EMAs. This additional compensation was prorated for each participant based on the percentage of random EMAs completed; participants could receive up to $250 in additional gift cards for compliance with EMA procedures. All study procedures were approved by the University of Texas MD Anderson Cancer Center Institutional Review Board.
Measures
EMAs were collected using a pre-programed Palmtop Personal Computer (PPC) that participants carried with them starting one week prior to the quit date until their in-person visit at week four (i.e., five contiguous weeks or 35 days). The PPC was a pen-based, touchscreen system that allowed participants to self-initiate or answer random survey questions, was extremely user friendly, and did not require any previous computer or typing skills. The PPC was small (roughly the size of a pack of cigarettes) and participants typically reported no difficulty carrying it with them at all times. Four random EMAs were schedule to be delivered each day during typical waking hours. Participants were also instructed to self-initiate non-random EMAs when they were about to smoke (smoking assessment), experienced an urge to smoke (urge assessment), or had already slipped (slip assessment). Among the 424 participants who enrolled in the study, 43 did not complete any EMAs and were excluded. The remaining participants who completed any EMA (n=391) demonstrated an overall compliance rate of 75.8% for random EMAs (i.e., completed 40,198 out of 53,047 random EMAs) and additionally completed 30,093 self-initiated EMAs in response to urges or smoking. Data for the current study included participants who completed any random and non-random assessments and did not meet the Society for Research on Nicotine and Tobacco (SRNT) criterion for relapse (i.e., smoke for seven consecutive days; Piper et al., 2019) during the 28 days of post-quit monitoring (N=325). Participants provided a total of 38,097 observations from random (68.3%), smoking (0.7%), urge (28.2%), and slip (2.83%) assessments. Across the 325 participants 2,356 lapse events were reported during the 28 day post-quit period. Predictor variables were assessed during random EMAs.
Smoking
Smoking was assessed using random and non-random (i.e., smoking, urge, and slip assessments) EMAs. A single “lapse” variable was created to indicate whether a participant smoked 1 or more times in the interval between two EMAs, with 1 indicating “lapse” and 0 indicating “no lapse.”
Abstinence self-efficacy
Abstinence self-efficacy was assessed with the item, “I am confident in my ability not to smoke.” Participants indicated their level of agreement with the item on a scale of 1 (strongly disagree) to 5 (strongly agree).
Affect
Affect was assessed with the items “I feel enthusiastic,” “I feel happy,” “I feel relaxed,” “I feel bored,” “I feel sad,” “I feel angry,” “I feel anxious, ”and “I feel restless” that were rated on a scale of 1 (strongly disagree) to 5 (strongly agree). A composite of “enthusiastic, happy, and relaxed” were used to create the positive affect variable (Reliability = .67), and “bored, sad, angry, anxious, and restless” to create the negative affect variable (Reliability = .67).
Expectancies
Expectancies were assessed with the items, “I am confident that I could do something other than smoke to improve my mood,” (hereinafter “positive coping expectancies”) and “I am confident that smoking would improve my mood” (hereinafter “smoking expectancies”). Participants responded on a scale of 1 (strongly disagree) to 5 (strongly agree).
Motivation to quit
Motivation to quit was assessed with the items “My desire to be a non-smoker is very strong,” and “I am extremely motivated to be smoke free” that were answered on a scale of 1 (strongly disagree) to 5 (strongly agree). Items were summed to create a total motivation score (Correlation = .67)
Stress
Stress was assessed with the item “I feel stressed” that was rated on a scale of 1 (strongly disagree) to 5 (strongly agree).
Urge
Urge was assessed with the items “I have an urge to smoke,” “I really want to smoke,” and “I need a cigarette.” Participants responded on a scale of 1 (strongly disagree) to 5 (strongly agree). The mean of the items was used as the total urge score (Reliability = .87).
Analytic Plan
TVEMs were used to estimate the time-varying association of smoking risk factors with smoking lapse (SL) likelihood. For example, consider abstinence self-efficacy:
| 1 |
In equation 1, SLij (smoking lapse) and ASEij (abstinence self-efficacy) were intensively measured for subject i at several times tij. β0 is the intercept parameter and represents the odds of smoking lapse over time when ASE is zero. β1 is the slope parameter and represents the time-varying association between ASE and SL likelihood. Models were estimated as a function of minutes since the start of the quit attempt, resulting in the formation of curves of coefficients of the relationship between ASE and SL changing along a time continuum of minutes since quitting. TVEM models examined the relationship between ASE at time t and lapse in the interval between time t and time t+1, while also controlling for lapse during the t-1 to t interval.
A set of intercept-only models were estimated to show the mean level of risk factors over the post-quit monitoring period. Then, separate TVEMs were used to estimate the time-varying association between each risk factor (i.e., abstinence self-efficacy, positive affect, positive coping expectancies, smoking expectancies, motivation, negative affect, stress, and urge) and lapse likelihood. To understand whether the relationship between the predictor at time t and lapse in the interval between t and t+1 differed depending on having lapsed in the prior EMA interval (i.e., time t-1 to t), models with an interaction term between the predictor and lapse in the t-1 to t interval were estimated. TVEM parameter estimates and confidence intervals are plotted to graphically summarize results. To aide interpretation, results are plotted in terms of days since quitting. All models included age, education, partner status, race/ethnicity, gender, time to first cigarette, and number of cigarettes smoked per day as time-invariant covariates. TVEMs were fit using the penalized truncated power spline (p-spline) approach with 10 knots, which is a recommended approach because it automatically identifies the best-fitting model and utilizes sandwich standard errors to account for within-subject correlation. Technical details about data preparation, the p-spline approach, and how to download the %TVEM SAS macro version 3.0.4 for free can be found at methodology.psu.edu (Tan et al., 2012).
Results
The final sample (n=325) was 54.8% female, about a third White (33.8%), a third African American (33.8%) and a third Latino (32.5%) with a mean age of 41.5 years. Over a third (36.0%) reported a total income of less than $20,000 per year and the average number of years of education completed was thirteen. At baseline, participants reported smoking 21 cigarettes per day on average and nearly half the sample (47.4%) reported smoking their first cigarette within less than five minutes after waking. Across all participants, the average number of lapse events during the post-quit monitoring was 5.94 (range 0–47).
Mean level of risk factors for lapse across quit attempt
Figures 1a–h display the intercept function (i.e., average level) of risk factors across the post-quit monitoring period. Average levels of abstinence self-efficacy, positive affect, positive coping expectancies, motivation, negative affect, and stress did not change over the post-quit period (Figures 1a–1c, 1e–1g). However, average levels of smoking expectancies and urge slightly declined over the post-quit period (Figures 1d and 1h).
Figure 1.
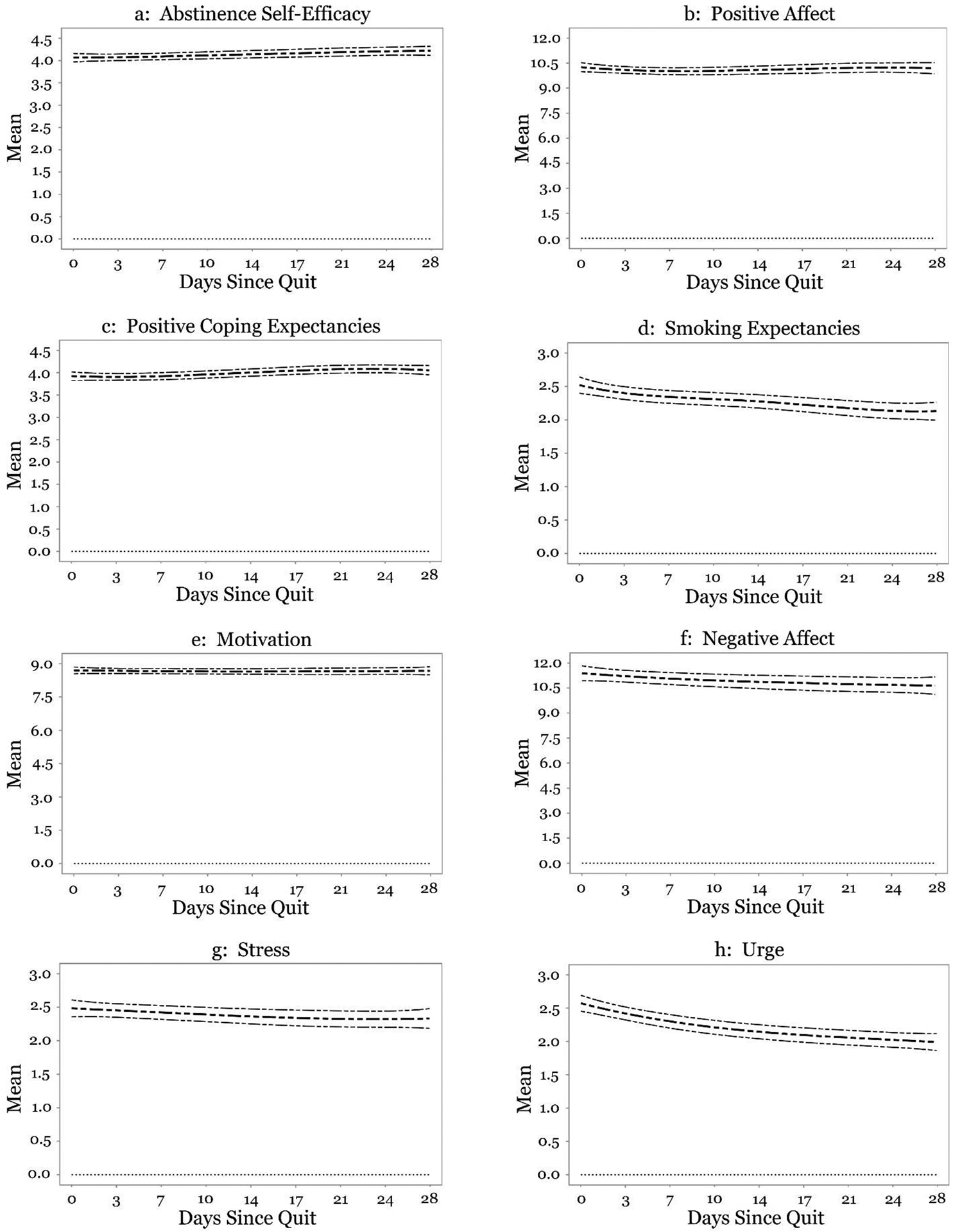
(Panels a-h) Time-varying mean level of risk factors.
Time varying relationship between risk factors and lapse across quit attempt
The relationships between risk factors and lapse are displayed in Figures 2a–h. The curved lines represent the odds ratios and corresponding 95% CIs. A point on the line represents the time-specific association between the risk factor and lapse (i.e., the odds ratio of lapse for each unit change in the risk factor at a specific time). An odds ratio greater than one indicates that a higher level of the risk factor is associated with a greater odds of lapse, whereas an odds ratio less than one indicates that a higher level of the risk factor is associated with reduced odds of lapse. If at any time the CI does not include one (horizontal dashed line), there is a statistically significant association between the risk factor and lapse at that specific time.
Figure 2.
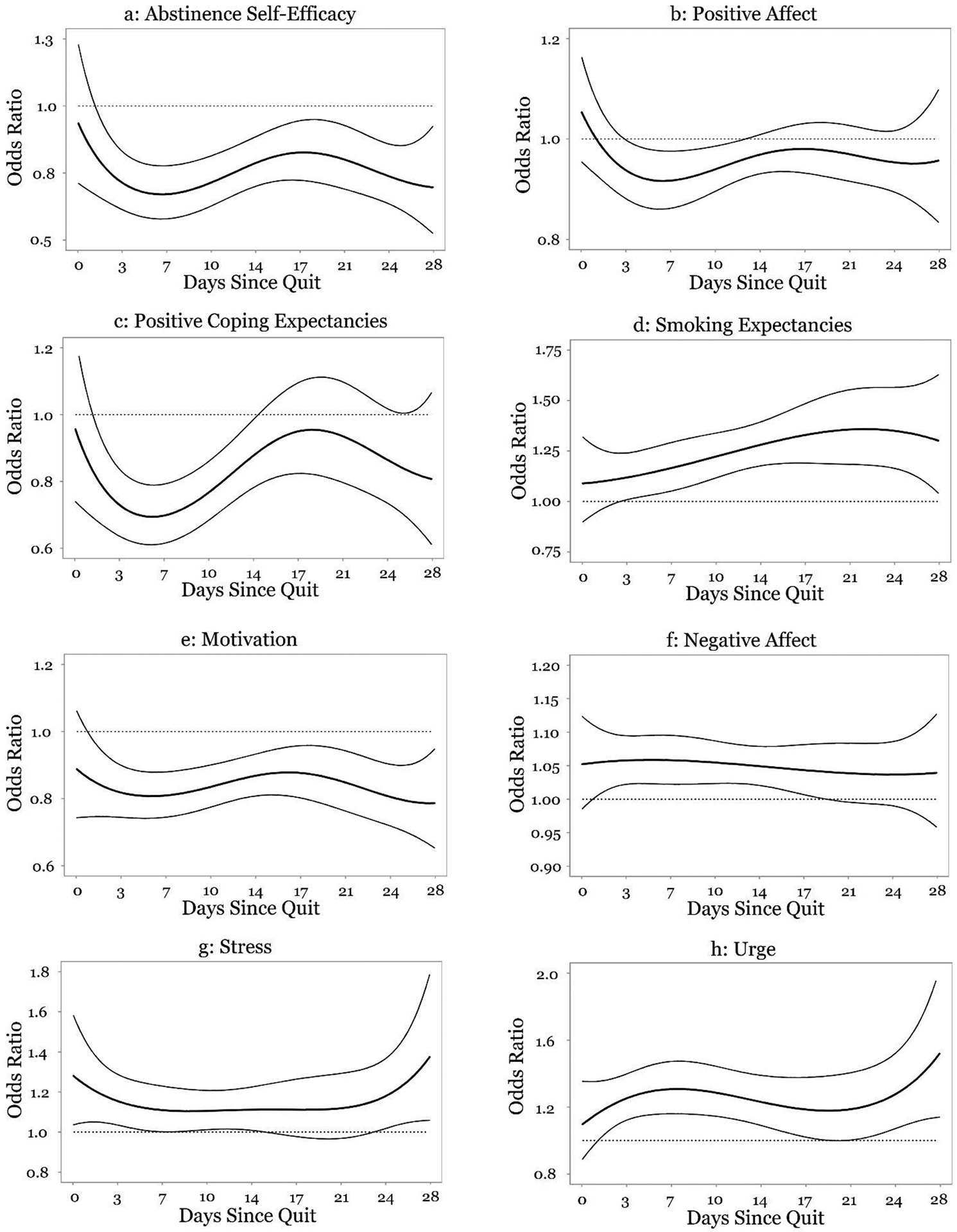
(Panels a-h) Time-varying effect of risk factors on lapse.
Plots of the interaction between risk factors and prior lapse (i.e., lapse in the t-1 to t interval) suggested that there was inadequate power to reliably estimate time varying associations when there was a prior lapse. In particular, when there was a prior lapse there was generally no association between risk factors and odds of lapse during the entire post-quit period. However, the confidence intervals were very wide suggesting that standard errors were very large. Several trajectories also showed extreme deviations in the strength and direction of associations in unexpected directions. As such, meaningful conclusions could not be made about the association between risk factors and lapse when there was a lapse in the prior interval using the present sample. Therefore, results are presented only for the time varying association between risk factors and lapse when there was no lapse in the prior interval.
Figure 2a shows that higher levels of abstinence self-efficacy were related to lower odds of lapse for nearly the entire post-quit period. On day one post-quit, a one unit increase in abstinence self-efficacy was associated with a 19% decrease in odds of lapse (OR = 0.81, 95% CI [0.67, 0.99]), which strengthened to a 33% decrease in odds of lapse by day six (OR = 0.67, 95% CI [0.58, 0.78]). The association then weakened and by day 18, a one unit increase in abstinence self-efficacy was associated with a 17% decrease in odds of lapse (OR = 0.83, 95% CI [0.72, 0.95]). The association again strengthened and by day 28, a one unit increase in abstinence self-efficacy was associated with a 30% decrease in odds of lapse (OR = 0.70, 95% CI [0.51, 0.94]).
There was a significant relationship between positive affect and odds of lapse between days three and 11 post-quit, such that higher levels of positive affect were associated with decreased odds of lapse (Figure 2b). This relationship was strongest on day six post-quit, when a one unit increase in positive affect was associated with a 8% decrease in odds of lapse (OR = 0.92, 95% CI [0.86, 0.98]). By day 12, the association became non-significant.
Figure 2c shows a significant relationship between positive coping expectancies and odds of lapse between days one and 13 post-quit. The association strengthened between day one (OR = 0.83, 95% CI [0.69, 0.99]) and day five, when a one unit increase in positive coping expectancies was associated with a 31% decrease in odds of lapse (OR = 0.69, 95% CI [0.61, 0.79]). This association then weakened and reached non-significance on day 13 post-quit.
Figure 2d shows a significant association between smoking expectancies and odds of lapse. On day three, a unit increase in smoking expectancies was associated with a 12% increase in odds of lapse (OR = 1.12, 95% CI [1.01, 1.24]). The strength of this association then increased and was strongest on day 22 post-quit, when a one unit increase in smoking expectancies was associated with a 36% increase in odds of lapse (OR = 1.36, 95% CI [1.18, 1.56]).
Figure 2e shows a significant negative association between motivation to quit and odds of lapse for nearly the entire post-quit period that was fairly stable over time. On day one, a unit increase in motivation was associated with a 14% decrease in odds of lapse (OR = 0.86, 95% CI [0.75, 0.98]). This association weakened slightly between days five (OR = 0.81, 95% CI [0.74, 0.88]) and 16 (OR = 0.88, 95% CI [0.81, 0.95]), but then strengthened slightly such that by day 28, a one unit increase in motivation to quit was associated with a 21% decrease in odds of lapse (OR = 0.79, 95% CI [0.65, 0.96]).
There was a significant positive association between negative affect and lapse between day one and day 17 post-quit. This association was relatively stable over time but was strongest on day five post-quit, when a one unit increase in negative affect was associated with a 6% increase in odds of lapse (OR = 1.06, 95% CI [1.02, 1.10]; Figure 2f).
There was a significant association between stress and odds of lapse between quit day and day five post-quit, days nine to 14 post-quit, as well as between days 23 and 28 post-quit (Figure 2g). On quit day, a one unit increase in stress was associated with a 28% increase in odds of lapse (OR = 1.28, 95% CI [1.04, 1.58]). The strength of the association decreased and became non-significant on day 5 post-quit. The association was again significant between day nine (OR = 1.10, 95% CI [1.01, 1.21]) and day 14 post-quit (OR = 1.11, 95% CI [1.01, 1,23]) and remained fairly consistent during this period. On day 23 post-quit, a one unit increase in stress was associated with a 16% increase in odds of lapse (OR = 1.16, 95% CI [1.01, 1.34]). The association then strengthened and by day 28 post-quit, a one unit increase in stress was associated with a 40% increase in odds of lapse (OR = 1.40, 95% CI [1.06, 1.85]).
Figure 2h shows a significant association between urge and odds of lapse between day one and day 18 and between days 21 and 28 post-quit. The strength of the association increased from day one (OR = 1.17, 95% CI [1.02, 1.36]) until day seven post-quit, when a one unit increase in urge was associated with a 31% increase in odds of lapse (OR = 1.31, 95% CI [1.16, 1.47]). The association weakened and became non-significant between days 18 and 20 post-quit. The association was again significant on day 21 post-quit (OR = 1.19, 95% CI [1.01, 1.41]) and increased until the end of the post-quit period, when a one unit increase in urge was associated with a 55% increase in odds of lapse (OR = 1.55, 95% CI [1.14, 2.10]).
Discussion
This study examined the time varying relationship between momentary risk factors and lapse risk during a smoking quit attempt. There were several key findings. First, all of the associations of predictors with lapse were in the hypothesized direction. Second, there were several different patterns of associations over time. The magnitude of the association between some predictors and lapse remained fairly stable (i.e., negative affect, motivation), whereas others (i.e., abstinence self-efficacy, positive coping expectancies, positive affect) displayed their strongest association between days three and eight post-quit. Urge displayed a similar pattern, such that the association was strong between days four and 10 post-quit. However, the association of urge with lapse increased near the end of the quit attempt. Higher levels of smoking expectancies were associated with increased odds of lapse for nearly the entire post-quit period and the strength of this association increased over time. Stress was most strongly associated with lapse near the beginning and end of the post-quit period and was the only predictor that was significant on quit date (i.e., most became significant between days one and three post-quit).
A key tenant of social cognitive models of drug use is that risk factors for smoking are dynamic (Marlatt, 1985; Marlatt & Witkiewitz, 2005; Witkiewitz & Marlatt, 2004). Although a growing body of EMA work has supported the role of proximal factors predicting smoking lapse (Businelle et al., 2016; Cambron et al., 2019; Gwaltney et al., 2008; Gwaltney, Shiffman, Balabanis, & Paty, 2005; Minami, Yeh, Bold, Chapman, & McCarthy, 2014; Shiffman et al., 1996; Vinci et al., 2017), there is very limited work that has tested the implicit assumption in theories and prior EMA studies that the association between risk factors and lapse is constant over time. As such, results of the current study may inform theoretical models by clarifying more precisely which risk factors exert a stronger influence on smoking cessation during certain periods of a quit attempt.
Several predictors displayed associations with lapse that were stable over time. For example, negative affect displayed a stable positive association with odds of lapse from day one to day 17 post-quit (Figure 2f), in line with a recent TVEM study showing an association between negative affect and lapse from quit date to day six post-quit (Koslovsky et al., 2018). However, the current results are in contrast to another TVEM study suggesting that the strength of the association of negative affect with lapse increases over time (Vasilenko et al., 2014). In order to clarify the time periods when negative affect may exert a stronger influence on lapse, more research of this kind is warranted. Similarly, motivation displayed a significant negative association with lapse that was relatively stable between day one post-quit and the end of the post-quit period (Figure 2e). These results are in contrast to a recent study showing that motivation was not a significant predictor of lapse between quit day and day six post-quit (Koslovsky et al., 2018). Prior EMA studies suggest that motivation may predict quit-day abstinence (Businelle et al., 2014) and reduces the risk of smoking lapse over 12 hours (Minami et al., 2014). These studies, as well as the results of the current study, support theoretical models suggesting that motivation is an important proximal determinant of behavior (Ajzen, 1991; Witkiewitz & Marlatt, 2004). However, the current study extends existing work by showing that motivation may continue to drive behavior change at nearly all stages of a quit attempt. Given that most smokers have a desire to quit and take action to do so (i.e., about half of smokers report having made a quit attempt in the past year; Ahluwalia et al., 2018; Dube et al., 2009), the current results may inform research exploring how cultivating motivation during periods when other risk factors exert a stronger influence on lapse likelihood (e.g., smoking expectancies or urges later in the quit attempt, discussed herein) may aid cessation success.
Several predictors displayed associations with lapse that varied over time. For example, abstinence self-efficacy was associated with lower odds of lapse for nearly all of the post-quit period, but the association varied over time and was strongest on day six, when a unit increase in abstinence self-efficacy was associated with a 33% decrease in odds of lapse (Figure 2a). Similarly, positive coping expectancies were related to lower odds of lapse between days one and 13 post-quit, but the association was strongest on day five, when a unit increase in positive coping expectancies was associated with a 31% decrease in odds of lapse (Figure 2c). These results reproduce a recent study showing that higher levels of abstinence self-efficacy and positive coping expectancies were associated with decreased odds of lapse for five to six days post-quit (Koslovsky et al., 2018). The current results extend prior research suggesting that abstinence self-efficacy varies across situations, as well as EMA studies suggesting that self-efficacy and positive coping expectancies are proximal predictors of lapse (Cambron et al., 2019; Gwaltney et al., 2005; Gwaltney et al., 2001), by showing that they may play the strongest role in protecting against lapse during the first week post-quit yet continue to exert an effect for several weeks. By specifying particular periods of the quit attempt when these factors influence lapse, this work may help extend social learning and motivational models of drug use positing that drug use may be less likely with increasing beliefs that other forms of coping (i.e., non-drug related behaviors) are available (Cooper, Frone, Russell, & Mudar, 1995), as well as when individuals are confident in their ability to resist temptations to smoke (Marlatt, 1985).
Positive affect was associated with lower odds of lapse between days three and 11 post-quit but was strongest on day six (Figure 2b). These results are in contrast to a recent TVEM study showing that positive affect was related to smoking during the pre-quit but not the post-quit period (Koslovsky et al., 2018). Although positive affect has received less attention in the cessation literature than negative affect, theories of positive emotion (e.g., Broaden and Build, Upward Spiral Theory) posit that positive emotion may enhance resiliency, regulate responses to threat, and reinforce positive health behaviors to help facilitate long-term behavior change (Ferrer & Mendes, 2018; Fredrickson, 2004; Fredrickson & Joiner, 2002). This has been has been demonstrated across a variety of behaviors (e.g., exercise, diet; Van Cappellen, Rice, Catalino, & Fredrickson, 2018) including smoking, such as in studies linking positive affect to making a quit attempt, first lapse, and smoking cessation success (Branstrom, Penilla, Perez-Stable, & Munoz, 2010; Vinci et al., 2017). In the current study, positive affect was associated with decreased odds of lapse earlier in the quit attempt, which is a period of high vulnerablility to lapse (Hughes et al., 2004). Intervention strategies that enhance positive emotion (e.g., mindfulness) may improve resiliency to stress or other threats to abstinence early in the quitting process (Brown & Ryan, 2003; McCarthy, Piasecki, Fiore, & Baker, 2006).
Higher levels of urge were associated with increased odds of lapse starting on day one post-quit but were most strongly associated with lapse between days four (26% increase) and 10 (29% increase), as well as near the end of the quit attempt. By day 27, a unit increase in urge was associated with a 55% increase in odds of lapse (Figure 2h). Urge has been noted as a predictor of smoking relapse in studies of long-term smoking cessation outcomes (Blevins, Farris, Brown, Strong, & Abrantes, 2016) as well as in studies conducted in daily life (Businelle et al., 2016; Cambron et al., 2019; Shiffman et al., 1997). In the present study, mean level of urge declined over time (Figure 1h), consistent with prior work showing a natural decline in urge and other withdrawal symptoms (Piper et al., 2011; Shiffman et al., 1997; Shiffman, West, Gilbert, & With, 2004). However, despite its decline over time, urge does not lose its predictive power for lapse (Shiffman et al., 1997). Other research suggests that lapses occurring later in the quit attempt are strongly linked to interoceptive and contextual cues, and it may be that urges in response to these cues are driving the strong association of urge with lapse (Ferguson, Shiffman, & Blizzard, 2017). As such, interventions might benefit by training individuals to take urges very seriously and enact coping strategies even after urge frequency and severity have diminished later in the quit attempt (Hagger et al., 2013).
There was a significant positive association between smoking expectancies and odds of lapse that steadily increased over time (Figure 2d). These results support theoretical models suggesting that affect regulation is an important motive for substance use (Cooper et al., 1995; Marlatt, 1985), as well as studies showing that affect regulation expectancies predict long term cessation success (Wetter et al., 2004), next-day lapse (Gwaltney et al., 2005), and lapse in the moment (Cambron et al., 2019). In line with the findings by Gwaltney and colleagues, level of smoking expectancies in the current study declined over time (Figure 1d). However, similar to urge, smoking expectancies were more strongly related to lapse later in the quit attempt. Although relapse models emphasize that smoking expectancies may be dynamic, few studies have examined the time varying association of smoking expectancies with lapse. As such, the current results underscore the need for more research of this kind, as results could inform interventions targeting factors that may activate smoking expectancies (e.g., craving) later in the quit attempt (Gwaltney et al., 2005).
As expected, stress was associated with increased odds of lapse, but this association was strongest near the beginning and end of the quit attempt (Figure 2g). These results are in line with a compelling body of work showing that stress is an influential proximal predictor of cessation success, as well as with models of relapse prevention suggesting that stress may activate other precipitants of smoking lapse (Businelle et al., 2016; Businelle et al., 2014; Cambron et al., 2019; Kassel, Stroud, & Paronis, 2003; Witkiewitz & Marlatt, 2004). However, this study extends prior work by showing that lapses occurring earlier and later in the quit attempt may be more strongly tied to stress. Unlike other predictors in the current study, stress was associated with lapse on the quit date. Quitting smoking is generally perceived to be “emotionally wrenching” and it could be that the quit day is simply so stressful that the stress-lapse relationship is more prominent than that of other predictors with lapse (Cohen & Lichtenstein, 1990). The current results suggest that interventions focusing on cultivating stress management skills immediately upon quitting and maintain those skills later in the quitting process may be necessary for achieving smoking cessation success.
This study has several limitations. The non-parametric trajectories modeled in this study were driven by the available data, thus caution should be taken when generalizing to other studies that may have variation in populations, study durations, and data quality (Shiyko et al., 2012). Participants in the current study were broadly representative of the community from which the sample was recruited and of cigarette users in the U.S. (e.g., lower socioeconomic status, racially and ethnically diverse). Although slight variation in trajectories would be expected due to sample differences, several of the trajectories in this study were in line with prior research (Koslovsky et al., 2018; Vasilenko et al., 2014). Further replication of these findings will improve the understanding of how specific proximal risk factors may exert more or less influence on lapse during particular periods to time across populations. When there was a lapse in the prior interval, the relationship between predictors and lapse were generally not significant, displayed very wide confidence intervals, and inconsistent trajectories. It is likely there was not enough power in this sample to reliably determine time varying associations when there was a lapse in the prior interval, thus further exploration of these associations is warranted. TVEM is a powerful approach because it uses all available data, however this does not account for the fact that the study population may change due to drop out or that there may be differences in EMA compliance across participants. Unfortunately, the procedure is not yet capable of incorporating weights to account for the fact that participants may be providing different amounts of data to the model (Lanza et al., 2016; Tan et al., 2012). Additionally, the time varying associations modeled in this work are still correlational in nature, and do not imply causation. Because only 52 participants met the SRNT criterion for relapse, there was a lack of adequate power to estimate reliable TVEMs among this group. Since the time of data collection, public attitudes towards smoking have changed and rates of electronic cigarette use/vaping (e-cig) have increased substantially. Caution should be used in generalizing these results to users of multiple products or e-cigs. Nevertheless, the vast majority of tobacco users (81%) continue to use only a single product (Wang et al., 2018). Thus, data on smokers alone remain very relevant to today’s tobacco landscape. Future studies should investigate how changes in public attitudes towards smoking (e.g., more stringent workplace smoking policies) and tobacco/nicotine product use might influence the association of risk factors with lapse in various contexts. Finally, all participants received nicotine patch therapy, however the current study did not collect data on patch compliance. This limits the ability to test whether patch compliance influenced outcomes. Given that patch therapy is associated with reductions in withdrawal symptoms (Hooper, Dietz, & Wilson, 2017), it is possible that the results are influenced by patch compliance. Similarly, participants received tobacco cessation counseling, which could have influenced the variables under study. However, it is unclear whether patch compliance or counseling would reduce “mean” symptom severity or whether it would influence the strength of the associations as well.
This study is one of only several that uses TVEM to investigate whether there are particular periods of time in a quit attempt when certain risk factors may be more strongly related to lapse likelihood. Whereas many prior studies with intensive longitudinal data focus on average momentary correlations between predictors and lapse likelihood (Businelle et al., 2016; Lam et al., 2014; Shiffman et al., 2007), this approach cannot reveal how the strength of associations may vary across time. Finally, the current results may inform interventions tailored to emphasize specific intervention components at particular time intervals during a quit attempt.
Acknowledgments
Research reported in this publication was supported by awards from the National Institute on Drug Abuse (R01DA014818), National Cancer Institute (P30CA042014; the Cancer Biostatistics Shared Resource at Huntsman Cancer Institute; F32CA232796), the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002538; 5TL1TR002540), and the Huntsman Cancer Foundation.
Footnotes
All authors have directly participated in the planning, execution, or analysis of this study and we have no conflicts of interest to disclose.
References
- Agaku IT, King BA, & Dube SR (2014). Current Cigarette Smoking Among Adults - United States, 2005–2012. Morbidity and Mortality Weekly Report, 63(2), 29–34. [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia IB, Smith T, Arrazola RA, Palipudi KM, de Quevedo IG, Prasad VM, … Armour BS (2018). Current Tobacco Smoking, Quit Attempts, and Knowledge About Smoking Risks Among Persons Aged >= 15 Years - Global Adult Tobacco Survey, 28 Countries, 2008–2016. Morbidity and Mortality Weekly Report, 67(38), 1072–1076. doi: 10.15585/mmwr.mm6738a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajzen I (1991). The theory of planned behavior. Organizational Behavior and Human Decision Processes, 50(2), 179–211. doi: 10.1016/0749-5978(91)90020-t [DOI] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, … Fiore MC (2011). New Methods for Tobacco Dependence Treatment Research. Annals of Behavioral Medicine, 41(2), 192–207. doi: 10.1007/s12160-010-9252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CE, Farris SG, Brown RA, Strong DR, & Abrantes AM (2016). The Role of Self-Efficacy, Adaptive Coping, and Smoking Urges in Long-term Cessation Outcomes. Addictive Disorders & Their Treatment, 15(4), 183–189. doi: 10.1097/adt.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branstrom R, Penilla C, Perez-Stable EJ, & Munoz RF (2010). Positive Affect and Mood Management in Successful Smoking Cessation. American Journal of Health Behavior, 34(5), 553–562. [PubMed] [Google Scholar]
- Brown KW, & Ryan RM (2003). The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology, 84(4), 822–848. doi: 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li YS, … Wetter DW (2010). Mechanisms Linking Socioeconomic Status to Smoking Cessation: A Structural Equation Modeling Approach. Health Psychology, 29(3), 262–273. doi: 10.1037/a0019285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Frank SG, Wetter DW, & Vidrine DJ (2016). Using Intensive Longitudinal Data Collected via Mobile Phone to Detect Imminent Lapse in Smokers Undergoing a Scheduled Quit Attempt. Journal of Medical Internet Research, 18(10), 10. doi: 10.2196/jmir.6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Reitzel LR, Chen MX, Lam CY, … Wetter (2014). Predicting Quit Attempts Among Homeless Smokers Seeking Cessation Treatment: An Ecological Momentary Assessment Study. Nicotine & Tobacco Research, 16(10), 1371–1378. doi: 10.1093/ntr/ntu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambron C, Haslam AK, Baucom BRW, Lam C, Vinci C, Cinciripini P, … Wetter DW (2019). Momentary Precipitants Connecting Stress and Smoking Lapse During a Quit Attempt. Health Psychology, 38(12), 1049–1058. doi: 10.1037/hea0000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambron C, Lam CY, Cinciripini P, Li L, & Wetter DW (2019). Socioeconomic status, social context, and smoking lapse during a quit attempt: An ecological momentary assessment study. Annals of Behavioral Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Lichtenstein E (1990). Perceived stress, quitting smoking, and smoking relapse. Health Psychology, 9(4), 466–478. doi: 10.1037/0278-6133.9.4.466 [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, & Mudar P (1995). Drinking to regulate positive and negative emotions. A motivational model of alcohol use. Journal of Personality and Social Psychology, 69(5), 990–1005. doi: 10.1037/0022-3514.69.5.990 [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, & Ezzati M (2009). The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. Plos Medicine, 6(4). doi: 10.1371/journal.pmed.1000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drope J, Liber AC, Cahn Z, Stoklosa M, Kennedy R, Douglas CE, & Henson R (2018). Who’s Still Smoking? Disparities in Adult Cigarette Smoking Prevalence in the United States. Ca-a Cancer Journal for Clinicians, 68(2), 106–115. doi: 10.3322/caac.21444 [DOI] [PubMed] [Google Scholar]
- Dube SR, Asman K, Malarcher A, & Carabollo R (2009). Cigarette Smoking Among Adults and Trends in Smoking Cessation-United States, 2008 (Reprinted from MMWR, vol 58, pg 1227–1232, 2009). Jama-Journal of the American Medical Association, 302(24), 2651–2654. [Google Scholar]
- Ferguson SG, Shiffman S, & Blizzard L (2017). Triggers of Smoking Lapses Over the Course of a Quit Attempt. Journal of Smoking Cessation, 12(4), 205–212. doi: 10.1017/jsc.2016.21 [DOI] [Google Scholar]
- Ferrer RA, & Mendes WB (2018). Emotion, health decision making, and health behaviour. Psychology & Health, 33(1), 1–16. doi: 10.1080/08870446.2017.1385787 [DOI] [PubMed] [Google Scholar]
- Fiore MC (2008). Treating tobacco use and dependence: 2008 update. In T. U. a. D. G. Panel; (Ed.). Rockville, MD. [Google Scholar]
- Fredrickson BL (2004). The broaden-and-build theory of positive emotions. Philosophical Transactions of the Royal Society B-Biological Sciences, 359(1449), 1367–1377. doi: 10.1098/rstb.2004.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, & Joiner T (2002). Positive emotions trigger upward spirals toward emotional well-being. Psychological Science, 13(2), 172–175. doi: 10.1111/1467-9280.00431 [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Bartolomei R, Colby SM, & Kahler CW (2008). Ecological momentary assessment of adolescent smoking cessation: A feasibility study. Nicotine & Tobacco Research, 10(7), 1185–1190. doi: 10.1080/14622200802163118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, & Paty JA (2005). Dynamic self-efficacy and outcome expectancies: Prediction of smoking lapse and relapse. Journal of Abnormal Psychology, 114(4), 661–675. doi: 10.1037/0021-843x.114.4.661 [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Norman GJ, Paty JA, Kassel JD, Gnys M, … Balabanis M (2001). Does smoking abstinence self-efficacy vary across situations? Identifying context-specificity within the relapse situation efficacy questionnaire. Journal of Consulting and Clinical Psychology, 69(3), 516–527. doi: 10.1037//0022-006x.69.3.516 [DOI] [PubMed] [Google Scholar]
- Hagger MS, Leaver E, Esser K, Leung CM, Pas NT, Keatley DA, … Chatzisarantis NLD (2013). Cue-Induced Smoking Urges Deplete Cigarette Smokers’ Self-Control Resources. Annals of Behavioral Medicine, 46(3), 394–400. doi: 10.1007/s12160-013-9520-8 [DOI] [PubMed] [Google Scholar]
- Hooper MW, Dietz NA, & Wilson JC (2017). Smoking urges during treatment and long-term cessation among low-income African Americans. Ethnicity & Disease, 27(4), 395–402. doi: 10.18865/ed.27.4.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, & Naud S (2004). Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction, 99(1), 29–38. doi: 10.1111/j.1360-0443.2004.00540.x [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, & Paronis CA (2003). Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin, 129(2), 270–304. doi: 10.1037/0033-2909.129.2.270 [DOI] [PubMed] [Google Scholar]
- Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Cofta-Woerpel LM, … Wetter DW (2010). Financial Strain and Smoking Cessation Among Racially/Ethnically Diverse Smokers. American Journal of Public Health, 100(4), 702–706. doi: 10.2105/ajph.2009.172676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzor DE, Costello TJ, Li YS, Vidrine JI, Mazas CA, Reitzel LR, … Wetter DW (2008). Race/Ethnicity and Multiple Cancer Risk Factors among Individuals Seeking Smoking Cessation Treatment. Cancer Epidemiology Biomarkers & Prevention, 17(11), 2937–2945. doi: 10.1158/1055-9965.epi-07-2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslovsky MD, Hebert ET, Swartz MD, Chan W, Leon-Novelo L, Wilkinson AV, … Businelle MS (2018). The Time-Varying Relations Between Risk Factors and Smoking Before and After a Quit Attempt. Nicotine & Tobacco Research, 20(10), 1231–1236. doi: 10.1093/ntr/ntx225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CY, Businelle MS, Aigner CJ, McClure JB, Cofta-Woerpel L, Cinciripini PM, & Wetter DW (2014). Individual and Combined Effects of Multiple High-Risk Triggers on Postcessation Smoking Urge and Lapse. Nicotine & Tobacco Research, 16(5), 569–575. doi: 10.1093/ntr/ntt190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Vasilenko SA, Liu XY, Li RZ, & Piper ME (2014). Advancing the Understanding of Craving During Smoking Cessation Attempts: A Demonstration of the Time-Varying Effect Model. Nicotine & Tobacco Research, 16, S127–S134. doi: 10.1093/ntr/ntt128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Vasilenko SA, & Russell MA (2016). Time-Varying Effect Modeling to Address New Questions in Behavioral Research: Examples in Marijuana Use. Psychology of Addictive Behaviors, 30(8), 939–954. doi: 10.1037/adb0000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Root TL, & Shiffman S (2006). A local linear estimation procedure for functional multilevel modeling. In Walls TA & Schafer JL (Eds.), Models for Intensive Longitudinal Data (pp. 63–83). New York: Oxford: University Press. [Google Scholar]
- Marlatt GA (1985). Relapse prevention: Theoretical rationale and overview of the model. In Marlatt GA & Gordon JR (Eds.), Relapse Prevention (pp. 250–280). New York: Guilford Press. [Google Scholar]
- Marlatt GA, & Donovan DM (2005). Relapse prevention: Maintenance strategies in the treatment of addictive behaviors: The Guilford Press. [Google Scholar]
- Marlatt GA, & Witkiewitz K (2005). Relapse prevention for alcohol and drug problems. In Marlatt GA & Donovan DM (Eds.), Relapse prevention: Maintenance strategies in the treatment of addictive behaviors (pp. 1–44). New York: The Guilford Press. [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, & Baker TB (2006). Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology, 115(3), 454–466. doi: 10.1037/0021-843x.115.3.454 [DOI] [PubMed] [Google Scholar]
- Minami H, Yeh VM, Bold KW, Chapman GB, & McCarthy DE (2014). Relations Among Affect, Abstinence Motivation and Confidence, and Daily Smoking Lapse Risk. Psychology of Addictive Behaviors, 28(2), 376–388. doi: 10.1037/a0034445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Bullen C, Krishnan-Sarin S, Rigotti NA, Steinberg ML, Streck JM, & Joseph AM (2019). Defining and measuring abstinence in clinical trials of smoking cessation interventions: An updated review. Nicotine & Tobacco Research, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh W-Y, … Baker TB (2011). Tobacco withdrawal components and their relations with cessation success. Psychopharmacology, 216(4), 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM (2013). Tobacco Smoking The Leading Cause of Preventable Disease Worldwide. Thoracic Surgery Clinics, 23(2), 103–+. doi: 10.1016/j.thorsurg.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Shiffman S (2005). Dynamic influences on smoking relapse process. Journal of Personality, 73(6), 1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, … Paton SM (2007). Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence, 91(2–3), 159–168. doi: 10.1016/j.drugalcdep.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, & Hickcox M (1997). A Day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology, 106(1), 104–116. doi: 10.1037/0021-843x.106.1.104 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, … Gnys M (2002). Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology, 111(4), 531–545. doi: 10.1037/0021-843x.111.4.531 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, & Hickcox M (1996). First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology, 64(2), 366–379. doi: 10.1037/0022-006x.64.2.366 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. In Annual Review of Clinical Psychology (Vol. 4, pp. 1–32). Palo Alto: Annual Reviews. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG, & With S. W. G. A. C. (2004). Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research, 6(4), 599–614. doi: 10.1080/14622200410001734067 [DOI] [PubMed] [Google Scholar]
- Shiyko MP, Lanza ST, Tan XM, Li RZ, & Shiffman S (2012). Using the Time-Varying Effect Model (TVEM) to Examine Dynamic Associations between Negative Affect and Self Confidence on Smoking Urges: Differences between Successful Quitters and Relapsers. Prevention Science, 13(3), 288–299. doi: 10.1007/s11121-011-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan XM, Shiyko MP, Li RZ, Li YL, & Dierker L (2012). A Time-Varying Effect Model for Intensive Longitudinal Data. Psychological Methods, 17(1), 61–77. doi: 10.1037/a0025814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cappellen P, Rice EL, Catalino LI, & Fredrickson BL (2018). Positive affective processes underlie positive health behaviour change. Psychology & Health, 33(1), 77–97. doi: 10.1080/08870446.2017.1320798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilenko SA, Piper ME, Lanza ST, Liu XY, Yang JY, & Li RZ (2014). Time-Varying Processes Involved in Smoking Lapse in a Randomized Trial of Smoking Cessation Therapies. Nicotine & Tobacco Research, 16, S135–S143. doi: 10.1093/ntr/ntt185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci C, Li L, Wu C, Lam CY, Guo L, Correa-Fernandez V, … Wetter DW (2017). The Association of Positive Emotion and First Smoking Lapse: An Ecological Momentary Assessment Study. Health Psychology, 36(11), 1038–1046. doi: 10.1037/hea0000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, … King BA (2018). Tobacco Product Use Among Adults - United States, 2017. Morbidity and Mortality Weekly Report, 67(44), 1225–1232. doi: 10.15585/mmwr.mm6744a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Welsch SK, Smith SS, Fouladi RT, Fiore MC, & Baker TB (2004). Prevalence and predictors of transitions in smoking behavior among college students. Health Psychology, 23(2), 168–177. doi: 10.1037/0278-6133.23.2.168 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, & Marlatt GA (2004). Relapse prevention for alcohol and drug problems - That was Zen, this is Tao. American Psychologist, 59(4), 224–235. doi: 10.1037/0003-066x.59.4.224 [DOI] [PubMed] [Google Scholar]
- Zhou XL, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, & West R (2009). Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addictive Behaviors, 34(4), 365–373. doi: 10.1016/j.addbeh.2008.11.013 [DOI] [PubMed] [Google Scholar]


