ABSTRACT
Studies have found that cell pyroptosis is involved in the occurrence and development of rheumatoid arthritis (RA). Hsa_circ_0044235 has been found to be significantly low-expressed in RA patients. The purpose of this research was to reveal the regulatory mechanism of hsa_circ_0044235 in the pyroptosis pathway of RA. Serum expressions of hsa_circ_0044235 and SIRT were detected by RT-qPCR, and the relationship of the two genes was analyzed by Pearson. Next, a collagen-induced arthritis (CIA) mouse model was constructed to examine the effect of hsa_circ_0044235 on knee joint injury. The number of apoptotic cells and the level of inflammatory cytokines in synovial tissue were detected by TUNEL and ELISA. Fibroblast-like synoviocytes (FLSs) were extracted as in vitro study subject. Functional assays including flow cytometry and immunofluorescence staining, molecular experiments including RT-qPCR, Western blot and dual luciferase assay, and bioinformatics analysis were performed to analyze the mechanism of hsa_circ_0044235 in pyroptosis in FLSs. Hsa_circ_0044235 and SIRT1 expressions were suppressed in RA patients and the two were positively correlated. Overexpressed hsa_circ_0044235 attenuated joint inflammation, cell apoptosis, and joint damage, reduced foot pad thickness, clinical case scores, inhibited the NLRP3-mediated pyroptosis pathway but promoted SIRT1 expression in CIA mice. Overexpressed hsa_circ_0044235 inhibited caspase-1 content and the NLRP3-mediated pyroptosis pathway. Moreover, hsa_circ_0044235 promoted SIRT1 expression by sponging miR-135b-5p in FLSs. Additionally, the effect of overexpressed hsa_circ_0044235 on FLSs was reversed by miR-135b-5p mimic and siSIRT1, while the effect of siSIRT1 was reversed by miR-135b-5p inhibitor. Hsa_circ_0044235 regulated NLRP3-mediated pyroptosis through miR-135b-5p-SIRT1 axis to regulate the development of RA.
KEYWORDS: Hsa_circ_0044235, rheumatoid arthritis, pyroptosis
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by joint swelling and joint tenderness caused by many factors [1]. The main pathological features of RA are hyperplasia of synovial tissues, thickening of the joint lining layer, and continuous erosion and destruction of articular cartilage [2]. Studies have shown that RA is mainly resulted from the decrease of pH value of synovial fluid, which is caused by the accumulation of inflammatory metabolites in the articular cavity of RA patients’ affected joints [3]. In addition, the pro-inflammatory cytokines, including IL-1β, IL-6, and IL-18, in the synovial fluid play an important role in the pathological process such as chondrocyte apoptosis and articular cartilage destruction in RA patients [4].
Pyroptosis, which is a programmed cell death accompanied by inflammation [5], is highly dependent on the activation of Caspase-1, accompanied by the synthesis and release of a large number of pro-inflammatory cytokines [6]. It has been found that cell pyroptosis is involved in the occurrence and development of RA, and there may be articular cartilage cell pyroptosis during the development of RA [7,8]. Therefore, studying the mechanism of pyroptosis in RA patients could help identify new therapeutic targets.
SIRT1 is closely related to the pyroptosis pathway [9]. For example, studies showed that down-regulated miR-29a inhibits oxidative stress and NLRP3-mediated pyroptosis and improves myocardial ischemia-reperfusion damage through SIRT1 [10]. In addition, some scholars have detected abnormal expression of SIRT1 in fibroblast-like synovial cells (FLS) and synovial tissues in RA patients [11]. Silencing SIRT1 reduced the proliferation and potential adhesion of FLS, suggesting that SIRT1 is also an important molecular marker in RA [12].
Circular RNAs (circRNAs) are newly discovered endogenous non-coding RNAs with a covalently closed circular structure [13]. CircRNAs act as miRNA molecular sponges to regulate gene expressions and interact with proteins [14]. The application value of circRNA as a biomarker for early diagnosis and prognostic evaluation of various diseases and also its involvement in the occurrence and development of diseases has been previously reported [15]. However, the current research on the relationship between circRNA and RA is still in its infancy [16]. Therefore, the discovery and identification of circRNA with high specificity in early diagnosis is very necessary for the clinical diagnosis of RA, as it can also provide a new direction for the research on the pathogenesis of RA. Hsa_circ_0044235 has been shown to be significantly low-expressed in RA patients [17], but the underlying mechanism involved in the pathogenesis of RA is still unknown. Therefore, the main purpose of this article was to reveal the regulation mechanism of hsa_circ_0044235 in the pyroptosis pathway in RA.
Materials and methods
Ethics statement
The collection of blood samples from clinical RA patients (n = 48) or healthy control people (n = 36) was approved by the Ethics Committee of Ganzhou People’s Hospital (approval number: HR20191006002). Written informed consents were signed by the recruited subjects. All of the animal protocols were conducted following the guidelines of the China Council on Animal Care and Use under the approval of Committee of Experimental Animals of our hospital (DA20191102011).
RA mouse model establishment
A total of 36 male DBA/1 J mice (30–35 g, 8–10 weeks old, 218, Charles River, China) were placed in a SPF-level animal house under controlled temperature and light and were provided with free access to food and water. A collagen-induced arthritis (CIA) mouse model was established by secondary immunization as previously described [18]. As characteristics of CIA animal comparing with control animal have been frequently reported before [19,20], the experiments on control mice were not shown in this report. The mice were randomly divided into 3 groups (n = 12): CIA, CIA+adenovirus-empty vector (Ad-Vec) and CIA+Ad-hsa_circ_0044235 groups. Adenovirus-empty vector and Ad-hsa_circ_0044235 were purchased from Shanghai GenePharma Company (China). To be brief, DBA/1 J mice were immunized with emulsified 100 μg collagen II (CII) (234,184-M, Sigma-Aldrich, USA) and an equal volume of complete Freund’s adjuvant (CFA) (344,289, Sigma-Aldrich). After 21 days, CII emulsified with incomplete Freund’s adjuvant (IFA) (344,291, Sigma-Aldrich) was injected as a booster. At 15, 20 and 25 days after the first immunization, 108 pfu of Ad-Vec or Ad-hsa_circ_0044235 were injected intra-articularly into the knee joint. The mice were sacrificed with intraperitoneal sodium pentobarbital (100 mg/kg, P3761, Sigma-Aldrich, USA) at the 36th day to harvest the synovial tissues for further experimental analysis.
According to the method described in a previous study [18], the symptoms of arthritis were daily monitored, and the severity of the disease of each paw was scored on the 18th, 21st, 24th, 28th, 32nd, and 36th days. The score ranges from 0 to 3, and the highest score for each mouse is 12.
Isolation and treatment of fibroblast-like synoviocytes (FLSs)
Fibroblast-like synoviocytes (FLSs) were extracted as previously described [21]. Briefly, the synovial tissues were washed twice to three times with phosphate buffered saline (PBS) (P3813, Sigma-Aldrich, USA), cut into small pieces, and digested with 0.1% CII for 30 minutes (min). After separation, FLSs were centrifuged at 1500 × g for 4 min and cultured in DMEM (30–2002, American Type Culture Collection, USA) supplemented with 10% FBS (30–2020, ATCC, USA) in a 37°C incubator (SCO6WE-2, SHELLAB, USA) with 5% CO2.
To induce cell pyroptosis, the extracted FLSs were first treated with 1 μg/ml lipopolysaccharide (LPS) (L2630, Sigma-Aldrich) for 6 hours (h), and then incubated with 3 mM ATP (A6559, Sigma-Aldrich) for 1 h. To assess the effect of target genes, after transfection of hsa_circ_0044235 overexpression or siSIRT1 or the corresponding blank vector (NC for hsa_circ_0044235, and siNC for siSIRT1), or co-transfection of miR-135b-5p mimic (M) (or miR-135b-5p mimic control (MC)) and has_circ_0044235 overexpression plasmid, or co-transfection of miR-135b-5p inhibitor (I) (or miR-135b-5p inhibitor control (IC)) and siSIRT1 using Lipofectamine 3000 (L3000015, Invitrogen, USA), the transfected FLSs were incubated with LPS/ATP. Cells without any treatment were considered as Control group. All plasmids and RNAs were purchased from Shanghai GenePharma Company (China).
Real-time quantitative PCR (RT-qPCR)
Total RNAs were extracted from serums or synovial tissues or FLSs with Trizol reagent (15,596,018, Invitrogen, USA). Linear RNAs were firstly removed by Rnase R (Epicenter; Illumina, Inc., USA). After determining RNA concentration by a NanoDrop One machine (Thermo, USA), cDNA was synthesized by FastKing one-step RT-PCR kit (KR123 TIANGEN, China) or miRNA cDNA Kit (KR211, TIANGEN, China). QPCR was performed in ABI PRISM 7300 (Applied Biosystems, USA) using TIANGEN FastFire qPCR PreMix (FP207, China). Relative mRNA levels were determined by the 2−ΔΔCt method [21]. The primer sequences were as follows (5'-3ʹ): hsa_circ_0044235: TGAGTTTGGTGATTCAGCTTGC, AACAAGGCTTCTTCTGAGTGT; β-actin: GTGACGTTGACATCCGTAAAGA, GCCGGACTCATCGTACTCC; U6: CTCGCTTCGGCAGCACA, AACGCTTCACGAATTTGCGT; SIRT1: TGATTGGCACCGATCCTCG, CCACAGCGTCATATCATCCAG; NLRP3: ATCAACAGGCGAGACCTCTG, GTCCTCCTGGCATACCATAGA; ASC: GACAGTACCAGGCAGTTCGT, AGTAGGGCTGTGTTTGCCTC; IL-1β: GAAATGCCACCTTTTGACAGTG, TGGATGCTCTCATCAGGACAG; miR-135b-5p: TATGGCTTTTCATTCCTATG, GCGAGCACAGAATTAATACGAC.
Histopathological assessment
The synovial tissues were isolated from the mice (n = 6), fixed in 4% paraformaldehyde (P0099, Beyotime, China) for 48 h, followed by decalcification and paraffin-embedding. Sections (5 μm) were prepared and stained with hematoxylin-eosin (H&E) staining or TUNEL staining for joint damage examination. The protocols were performed by H&E Staining Kit (BL700A, Biosharp, China) and In Situ Cell Death Detection Kit (11,684,795,910, Roche Applied Science, USA), according to the manufacturer’s recommendations. Finally, the pathological changes of synovial tissues were photographed with a DMD108 microscope (Leica Microsystems GmbH, Germany) (Magnification ×200).
Enzyme-linked immunosorbent assay (ELISA)
The synovial tissues of the knee joint of mice (n = 6) were collected. Next, ELISA method was performed to detect the levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α. The ELISA kits of IL-1β (PI301), IL-6 (PI326) and TNF-α (PT512) were purchased from Beyotime Company (China). The specific steps were performed in accordance with manufacturer’s instructions.
Western blot
RIPA lysis buffer (P0013B, Beyotime, China) containing protease inhibitors (P1005, Beyotime, China) and PMSF (ST505, Beyotime, China) was used for the collection of total proteins from synovial tissues or FLSs. The total proteins were quantified by BCA kit (P0011, Beyotime, China) and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) method. Next, the proteins were transferred onto PVDF membrane (160–0184, BIO-RAD, USA). Afterward, the membrane was incubated with primary antibodies such as SIRT (ab110304, 1 µg/ml, 110 kDa, Abcam, UK), ASC (ab188865, 1/1000, 50 kDa, Abcam, UK), NLRP3 (ab232401, 1/5000, 118 kDa, Abcam, UK), cleaved caspase-1 (PA5-105,049, 1/1000, 10 kDa, Invitrogen, USA), IL-1β (P420B, 1 µg/ml, 31 kDa, Invitrogen, USA) and β-actin (ab8226, 1 µg/ml, 42 kDa, Abcam, UK), and was then incubated with secondary antibodies such as Mouse IgG (ab205719) or Rabbit IgG (ab205718). Subsequently, ECL chemiluminescent solution (PE0010, Solarbio, China) and ChemiDoc MP Imager (BIO-RAD, USA) were applied to visualization.
Caspase-1 assay
Caspase-1 assay could indicate the degree of cell pyroptosis. Caspase-1 contents were assessed by flow cytometry and immunofluorescence staining. FLICA 660 Caspase-1 Assay Kit (9122, ImmunoChemistry Technologies, USA) was used to perform flow cytometry. Caspase-1 fluorochrome inhibitor and propidium iodide (PI) were added into FLSs. After performing FLICA/PI staining, pyroptosis was detected by flow cytometry (DxFLEX, Beckman, USA) and CytExpert software (Beckman, USA). PI (+) and caspase-1 FLICA (+) were defined as pyroptosis. For immunofluorescence staining protocol, FLSs were fixed in 4% paraformaldehyde for 10 min, and then incubated with FITC-conjugated caspase-1 antibody (sc-392,736 FITC, Santa Cruz Biotechnology, China), followed by further incubation with 4',6-diamidino-2-phenylendole (DAPI) (10,236,276,001, Roche, Switzerland). After that, the images were assessed by a fluorescence microscope (TCS SP8, Leica-Microsystem, Germany).
Bioinformatics assay
The starBase (http://starbase.sysu.edu.cn/index.php) was used to screen the miRNA that can be adsorbed by hsa_circ_0044235. TargetScan v7.2 (http://www.targetscan.org/vert_72/), miRDB (http://mirdb.org/) and miRWalk (http://mirwalk.umm.uni-heidelberg.de/) websites were used to predict the miRNA targeting SIRT1.
Dual-luciferase assay
The wild-type and mutant sequences of has_circ_0044235 were created, cloned into the pmirGLO vector (E1330, Promega, USA), and co-transfected with miR-135b-5p mimic (M) or mimic control (MC) into FLSs to examine the relationship between has_circ_0044235 and miR-135b-5p. To detect the relationship between SIRT1 and miR-135b-5p, FLSs were transfected with wild-type and mutant SIRT1 together with M or MC. Finally, luciferase activities were determined by luciferase detection kit (E1910, Promega, USA) and a microplate reader (Fluoroskan Ascent FL, Thermo, USA).
Data analysis
Graphpad prism 8.0 (Graphpad software, USA) was used for statistical analysis, and Pearson was used for correlation analysis. The measurement data were expressed as mean ± standard deviation; independent sample t test was used for comparison between two groups; one-way analysis of variance was used for comparison among multiple groups. P < 0.05 was defined as statistically significant.
Results
The expressions of hsa_circ_0044235 and SIRT1 in RA patients were suppressed and the two were positively correlated
Studies have found that hsa_circ_0044235 may be a new biomarker for the diagnosis of RA [17], but its specific role and mechanism still need to be further explored. This study found that compared with healthy samples, the expression of has_circ_0044235 in the serum of RA patients was lower (P < 0.001, Figure 1(a)). SIRT1 has a close relationship with the pyroptosis pathway, and pyroptosis is an integral part of the development of RA. We found that the expression of SIRT1 was suppressed in the RA group (P < 0.001, Figure 1(b)). Further detection showed a significant positive correlation between the expressions of has_circ_0044235 and SIRT1 (r = 0.485, P < 0.001, Figure 1(c)), suggesting that has_circ_0044235 could affect the pyroptosis pathway mediated by NLRP3 inflammasomes through indirect regulation of SIRT1.
Figure 1.
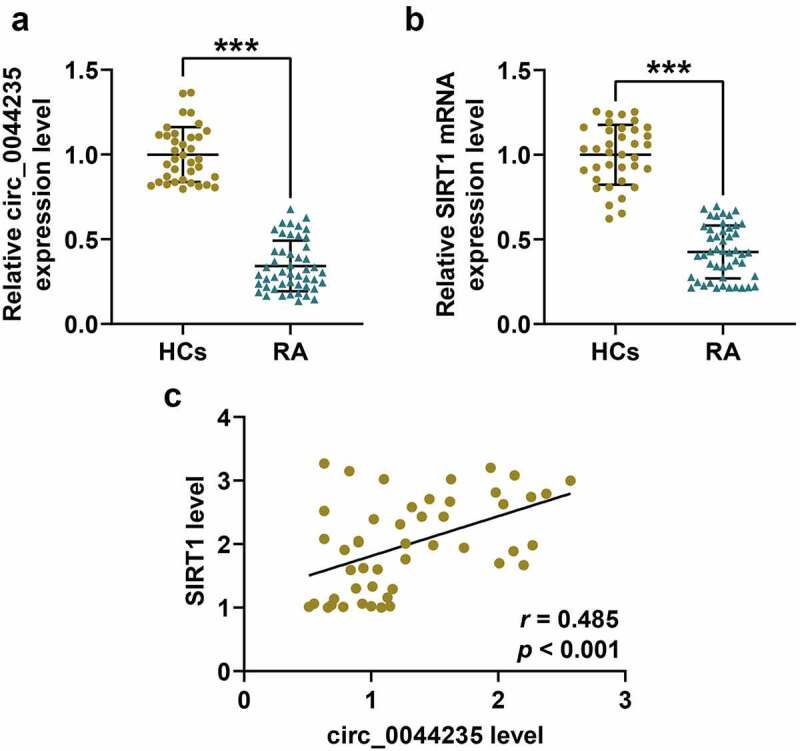
The expressions and correlation detection of hsa_circ_0044235 and SIRT1 in the serum of RA patients. (a-b) RT-qPCR results showed that has_circ_0044235 and SIRT1 were low-expressed in the serum of RA patients. (c) Pearson analysis found that there was a significant positive correlation between the expressions of has_circ_0044235 and SIRT1 in the serum of RA patients. RA: Rheumatoid arthritis. RT-qPCR: real-time quantitative PCR. experiments were repeated in triplicates. ***P < 0.001 vs HCs
Effects of hsa_circ_0044235 on CIA mouse model
The possible role of hsa_circ_0044235 in the CIA mouse model was analyzed (Figure 2(a)). As expected, overexpression of has_circ_0044235 strongly reduced the clinical case score of CIA mice (P < 0.001, Figure 2(b)). As shown in Figure 2(c), the joint inflammation and the thickness of the foot pads of the CIA mice were both significantly reduced in the CIA+Ad-circ_0044235 group. Subsequently, we observed the histopathological changes of the mice in each group, and the results showed that overexpression of hsa_circ_0044235 greatly reduced synovial hyperplasia, cartilage damage, and inflammatory cell infiltration, at the same time, it also significantly reduced cell apoptosis in the synovial tissues of CIA mice (Figure 2(d-e)). Consistently, by detecting the levels of inflammatory factors in each group of CIA mice, overexpression of has_circ_0044235 was found to be able to reduce the levels of IL-1β, IL-6 and TNF-α in synovial tissue (P < 0.001, Figure 2(f-h)). In addition, we verified that overexpression of has_circ_0044235 locally increased the expression of has_circ_0044235 in the synovial tissues of CIA mice (P < 0.001, Figure 2(i)). Overexpression of has_circ_0044235 noticeably promoted SIRT1 expression but significantly inhibited NLRP3, ASC, cleaved caspase-1 and IL-1β expressions in CIA mouse synovial tissues (P < 0.01, Figure 3(a-j)).
Figure 2.
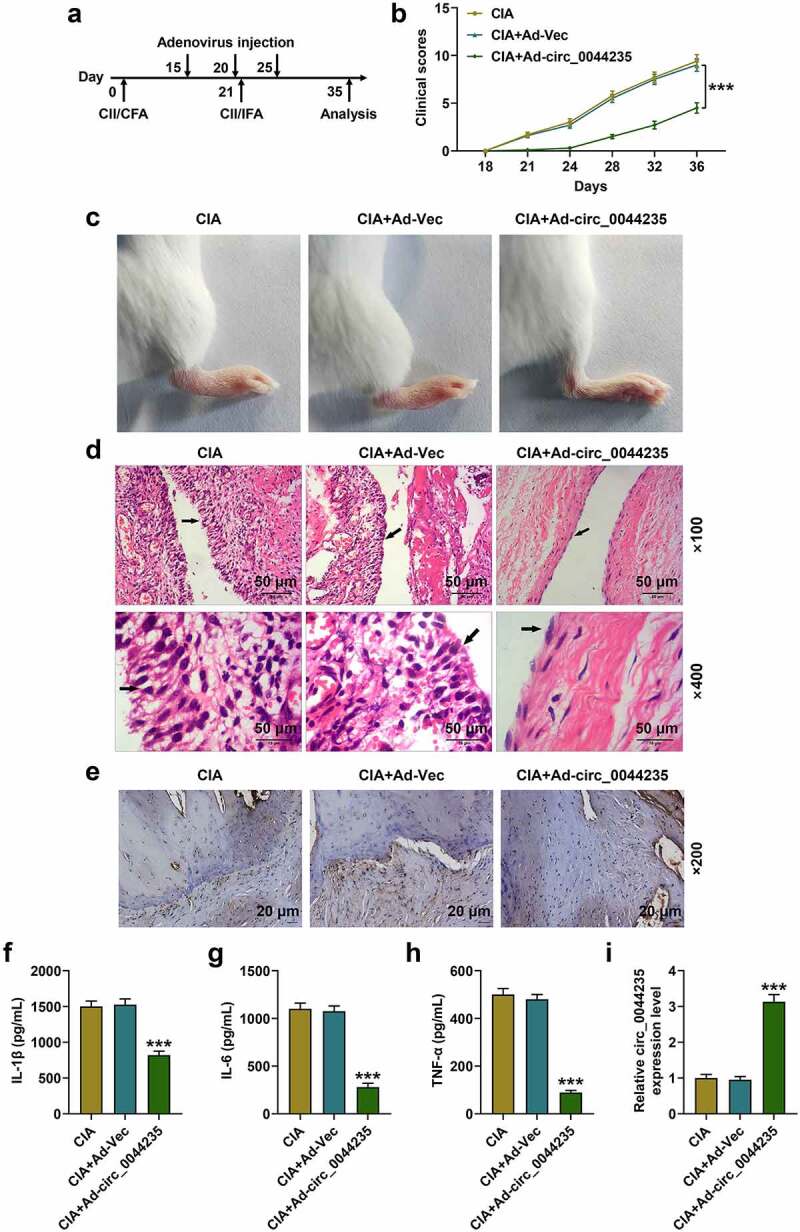
Effects of hsa_circ_0044235 on CIA mouse model. (a) Induction of CIA and hsa_circ_0044235 injection in DBA/1 J mice immunized with CII. (b) Clinical case scores of mice in CIA, CIA+Ad-Vec (adenovirus-vector), CIA+Ad-hsa_circ_0044235 groups. (c) Representative left knee joints of mice in each group on day 32 after the first CII immunization. (d) Hematoxylin and eosin (H&E) staining was performed to detect knee joint injuries in each group of mice. (e) The TUNEL method was constructed to measure the number of apoptotic cells in synovial tissues. (f-h) The levels of IL-1β, IL-6 and TNF-α in the synovial tissues of the knee joint were detected by ELISA. (i) The expression of hsa_circ_0044235 in the knee joint synovial tissues of each group of mice was detected by RT-qPCR. CIA: collagen-induced arthritis; CII: bovine type II collagen. ELISA: enzyme-linked immunosorbent assay; IL: interleukin; TNF: Tumor necrosis factor. ***P < 0.001 vs. CIA+Ad-Vector
Figure 3.
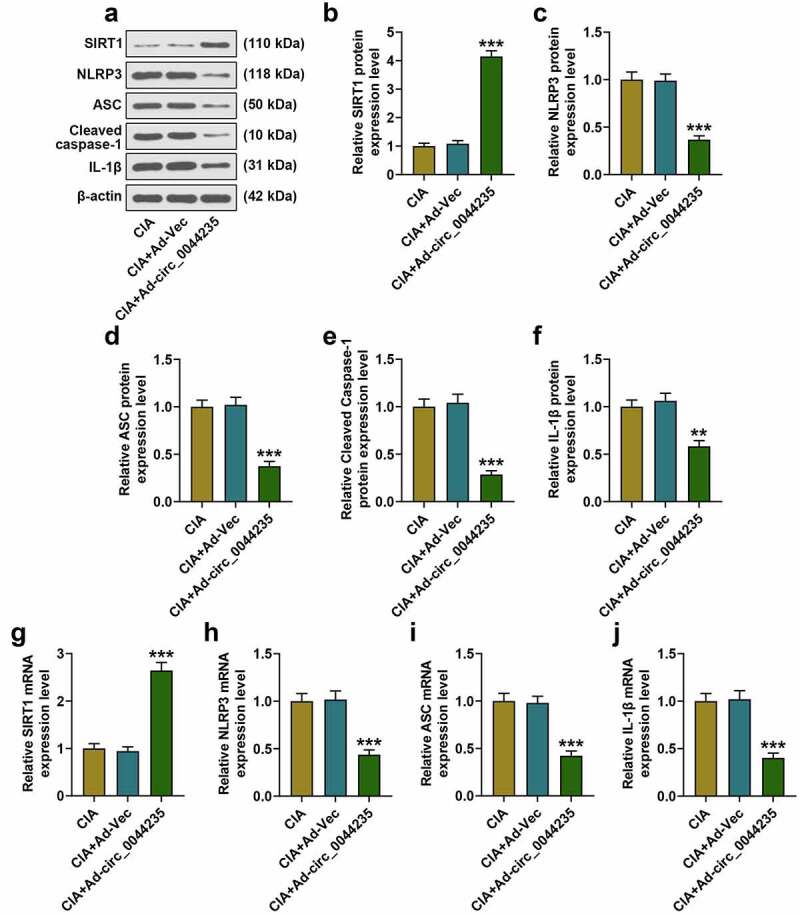
The expressions of SIRT1, NLRP3, ASC, cleaved caspase-1 and IL-1β in the synovial tissues of mice in the CIA, CIA+Ad-Vec, CIA+Ad-hsa_circ_0044235 groups were detected by RT-qPCR and Western blot as required. **P < 0.01, ***P < 0.001 vs. CIA+Ad-Vector
The effects of overexpressed has_circ_0044235 and silent SIRT1 on RA-FLSs
To further study the effects of has_circ_0044235 and SIRT1 on RA, we used LPS/ATP to induce a pyroptosis model. As we expected, LPS/ATP reduced has_circ_0044235 expression, and overexpression of has_circ_0044235 could partially relieve the inhibition of LPS/ATP on has_circ_0044235 expression, but siSIRT1 had no effect on has_circ_0044235 expression (P < 0.001, Figure 4(a)). Subsequently, we analyzed the effects of each group on SIRT1, and found that similarly, LPS/ATP inhibited SIRT1 expression, and overexpressed has_circ_0044235 could partially reverse the regulation of LPS/ATP, and siSIRT1 promoted the inhibitory effect of LPS/ATP on SIRT1 expression, but siSIRT1 reduced the effect of overexpression has_circ_0044235 on promoting SIRT1 expression (P < 0.001, Figure 4(b-d)). Flow cytometry and immunofluorescence were carried out to determine the caspase-1 content in each group of cells. It was found that the caspase-1 content was increased in the LPS/ATP+NC group, and that overexpressed hsa_circ_0044235 reversed the effect of LPS/ATP on promoting the caspase-1 content, also, siSIRT1 effectively increased caspase-1 content induced by has_circ_0044235 overexpression (P < 0.001, Figure 4(e-g)). In addition, LPS/ATP promoted the pyroptosis pathway mediated by NLRP3, and the expressions of NLRP3, ASC, cleaved caspase-1 and IL-1β in the LPS/ATP+circ_0044235 group were greatly reduced compared with the LPS/ATP+NC group. Moreover, siSIRT1 partially abolished the inhibitory effect of has_circ_0044235 overexpression on the NLRP3-mediated pyroptosis pathway (P < 0.01, Figure 5(a-h)).
Figure 4.
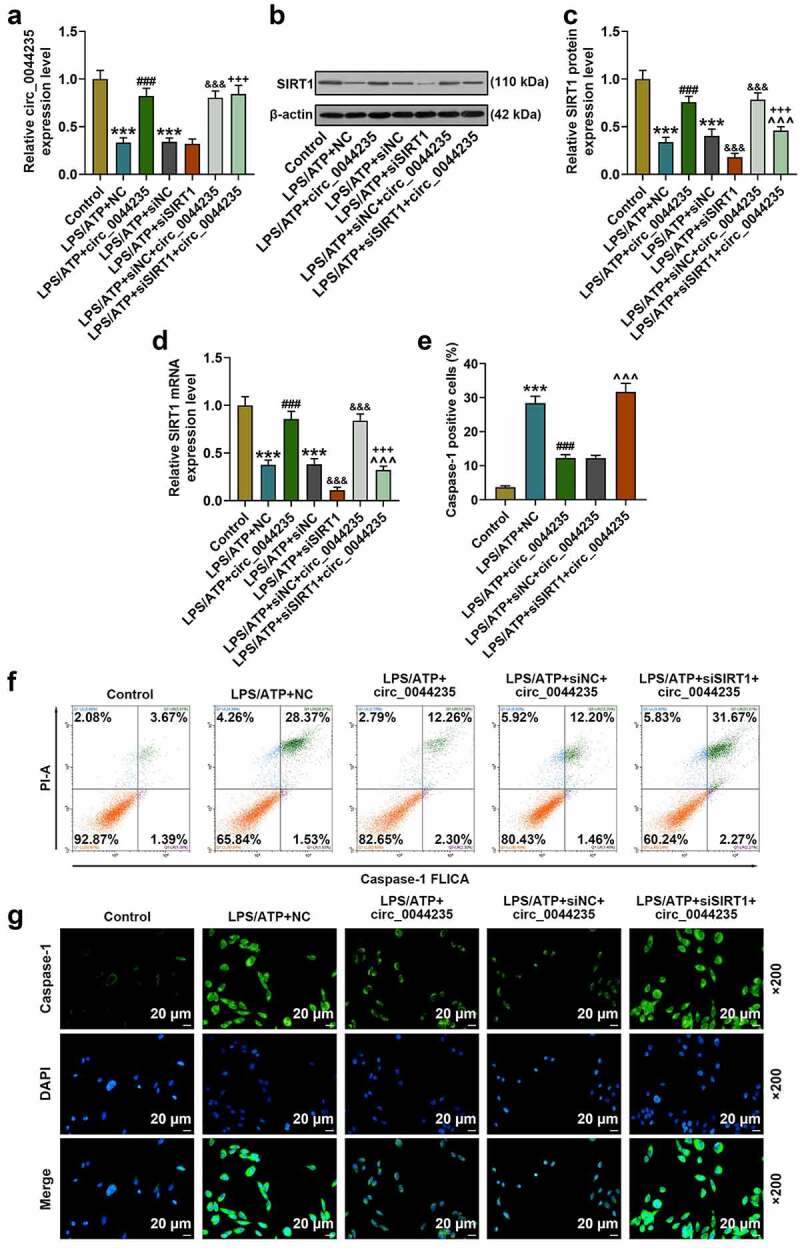
The effect of hsa_circ_0044235 overexpression and SIRT1 silencing on RA-FLSs cells. After transfection with hsa_circ_0044235 overexpression or siSIRT1 or the corresponding blank vector, the cells were incubated with LPS/ATP as needed (synovial cells were first treated with 1 μg/ml LPS for 6 h, and then incubated with 3 mM ATP for 1 h). (a) RT-qPCR was used to detect the expression of hsa_circ_0044235 in each group of cells. (b-d) the expression of SIRT1 in each group of cells was determined by RT-qPCR and Western blot. (e-f) The number of caspase-1 positive cells in each group was evaluated by flow cytometry. (g) The content of caspase-1 in cells of each group was detected by immunofluorescence. experiments were repeated in triplicates. ***P < 0.001 vs. control; ###P < 0.001 vs. LPS/ATP+NC; &&&P < 0.001 vs. LPS/ATP+siNC; +++P < 0.001 vs. LPS/ATP+circ_0044235; ^^^P < 0.001 vs. LPS/ATP+siNC+circ_0044235
Figure 5.
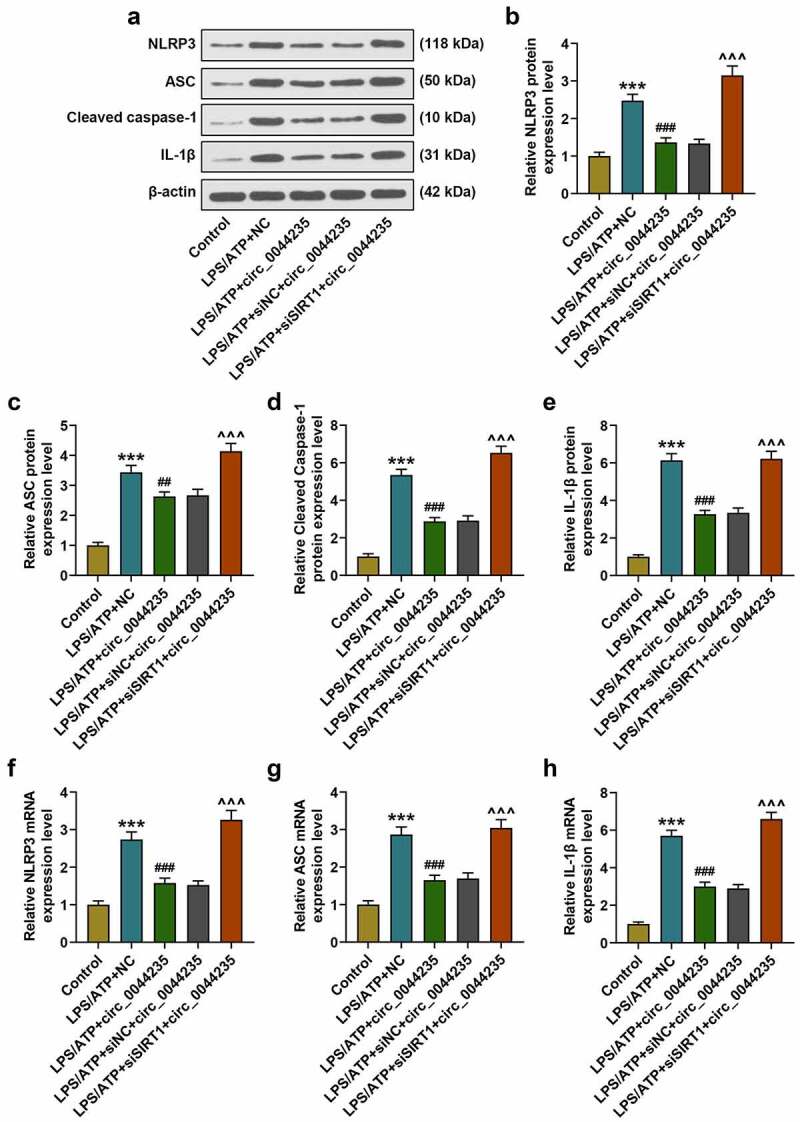
The effects of hsa_circ_0044235 overexpression and SIRT1 silencing on the expressions of NLRP3, ASC, cleaved caspase-1 and IL-1β in RA-FLSs cells were detected by RT-qPCR and Western blot as required. experiments were repeated in triplicates. ***P < 0.001 vs. control; ##P < 0.01, ###P < 0.001 vs. LPS/ATP+NC; ^^^P < 0.001 vs. LPS/ATP+siNC+circ_0044235
Hsa_circ_0044235 regulated SIRT1 expression via sponging miR-135b
We screened 9 miRNAs that can be adsorbed by hsa_circ_0044235 through starBase, and obtained 45, 202 and 1917 miRNAs that targeted to SIRT1 through TargetScan, miRDB and miRWalk, respectively (Figure 6(a)). Subsequently, miR-135b-5p was determined by taking the intersection of the above-mentioned databases through the Venn diagram (Figure 6(a)). As shown in Figure 6(b-c), hsa_circ_0044235 could bind to miR-135b-5p (P < 0.001). Figure 6(d-e) showed that miR-135b-5p targeted SIRT1 (P < 0.01). Next, we found that miR-135b-5p expression was increased in RA patients and LPS/ATP-induced FLSs, and that overexpression of has_circ_0044235 could partially reduce miR-135b-5p expression in LPS/ATP-induced FLSs (P < 0.001, Figure 6(f-g)).
Figure 6.
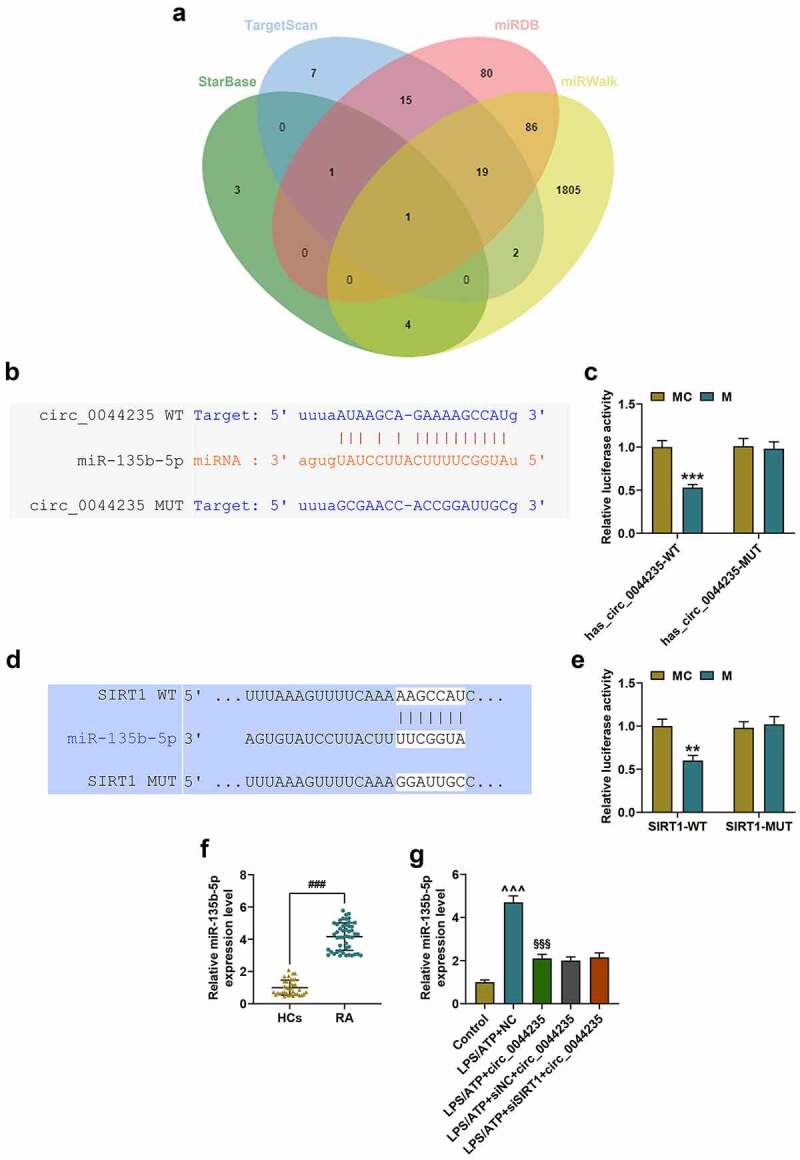
Hsa_circ_0044235 adsorbed miR-135b to regulate SIRT1 expression. (a) The venn diagram showed the intersection of the adsorbable miRNAs of hsa_circ_0044235 screened by starbase and the possible miRNAs predicted to target SIRT1 by TargetScan, miRDB and miRWalk. (b-c) the binding site of has_circ_0044235 and miR-135b-5p was predicted by starBase and verified by dual-luciferase. (d-e) TargetScan prediction and dual-luciferase verification results showed that miR-135b-5p targeted SIRT1. (f-g) the expression of miR-135b-5p in tissues and cells in each group was determined by RT-qPCR. **P < 0.01, ***P < 0.001 vs.MC; ###P < 0.001 vs. HCs; ^^^P < 0.001 vs. control; §§§P < 0.001 vs. LPS/ATP+NC
The effects of has_circ_0044235-miR-135b-5p-SIRT1 axis on cell pyroptosis
To further verify the role of has_circ_0044235 in RA via the miR-135b-5p-SIRT1 axis, exogenous up-regulation or down-regulation of miR-135b-5p was transfected into cells with circ_0044235 or siSIRT1, respectively, and then cells were treated with LPS/ATP. Our results showed that miR-135b-5p mimic partially reversed the inhibitory effect of has_circ_0044235 overexpression on miR-135b-5p expression (P < 0.001, Figure 7(a)). At the same time, we found that miR-135b-5p mimic partially relieved the inhibition of has_circ_0044235 overexpression on caspase-1 content, while miR-135b-5p inhibitor significantly reduced the promotion of siSIRT1 on caspase-1 content (P < 0.001, Figure 7(b-d)). In addition, miR-135b-5p mimic partially reversed the inhibitory effect of overexpressed has_circ_0044235 on the expressions of NLRP3, ASC, cleaved caspase-1 and IL-1β, and miR-135b-5p inhibitor noticeably reduced the effect of siSIRT1 on promoting the activation of NLRP3 cell pyroptosis pathway (P < 0.05, Figure 8(a-h)).
Figure 7.
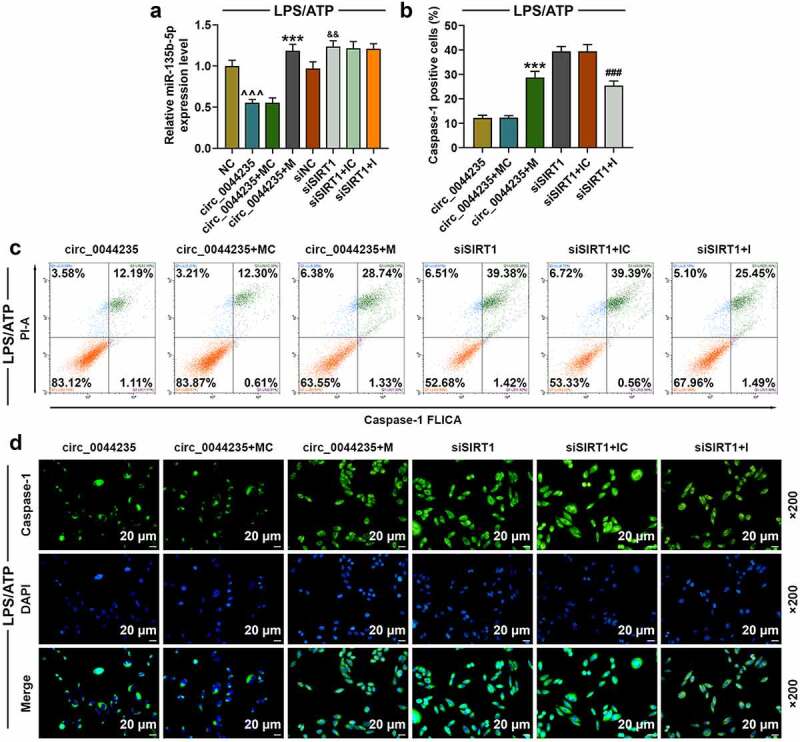
The effects of has_circ_0044235-miR-135b-5p-SIRT1 axis on cell pyroptosis in cells treated with LPS/ATP. (a) miR-135b-5p mimic partially reversed the inhibitory effect of has_circ_0044235 overexpression on miR-135b-5p expression. (b-c) the number of caspase-1 positive cells in circ_0044235, circ_0044235+ MC, circ_0044235 + M, siSIRT1, siSIRT1+ IC, siSIRT1 + I group was determined by flow cytometry. (d) the content of caspase-1 in cells of each group was detected by immunofluorescence.^^^P< 0.001 vs. NC; &&P < 0.01 vs.siNC; ***P < 0.001 vs. circ_0044235+ MC; ###P < 0.001 vs. siSIRT1+ IC. IC, miR-135b-5p inhibitor control; I, miR-135b-5p inhibitor; MC, miR-135b-5p inhibitor control; M, miR-135b-5p mimic control
Figure 8.
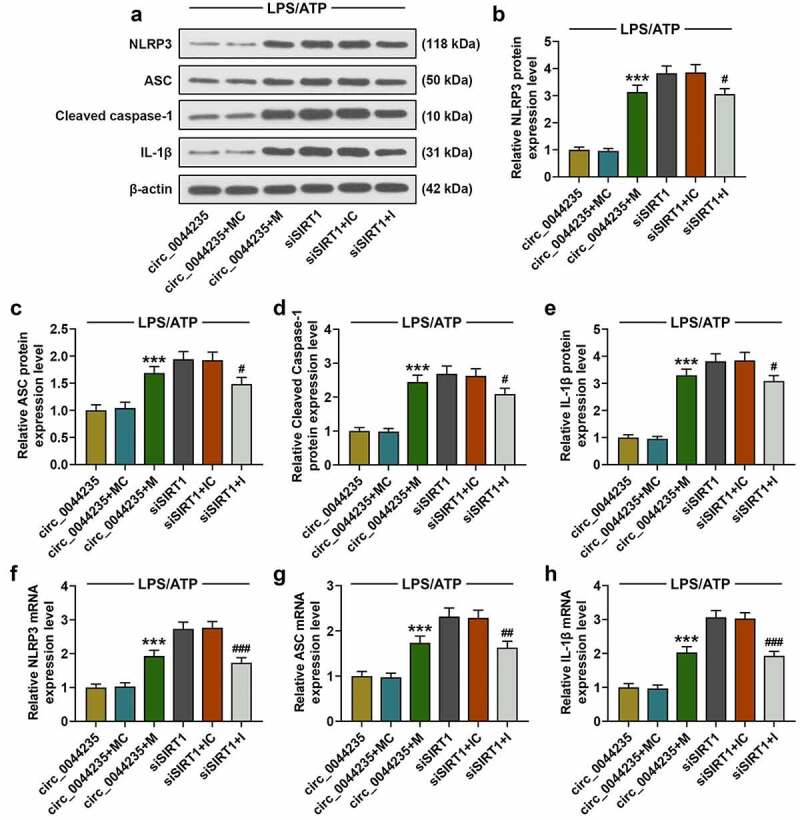
The effects of has_circ_0044235-miR-135b-5p-SIRT1 axis on the expressions of NLRP3, ASC, cleaved caspase-1 and IL-1β in cells treated with LPS/ATP were detected by RT-qPCR and Western blot as required. experiments were repeated in triplicates. ***P < 0.001 vs. circ_0044235+ MC; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. siSIRT1+ IC
Discussion
RA is an autoimmune disease characterized by chronic synovitis, and its pathogenesis is affected by many factors [1]. In recent years, studies have shown that RA is closely related to cell pyroptosis and apoptosis, but its underlying mechanism is still unclear [4,8]. Nowadays, increasing reports have revealed that circRNAs are related to a variety of human diseases, such as cancer and autoimmune diseases, indicating that circRNAs may become a new generation of biomarkers or therapeutic targets [15,22]. But so far, few studies have explored the relationship between circRNA and RA. In addition, there is no research on the effects of hsa_circ_0044235 on NLRP3-mediated articular chondrocyte pyroptosis. In this study, we confirmed for the first time that hsa_circ_0044235 acts on NLRP3-mediated pyroptosis through miR-135b-5p-SIRT1 axis to regulate the development of RA.
Some scholars have discussed the relationship between circRNAs and RA [23]. Zheng et al. used microarray chips to detect the expression of cricRNA in peripheral blood mononuclear cells in 10 RA patients and 10 healthy controls, and found that 255 circRNA expressions were significantly up-regulated and 329 cricRNAs were significantly down-regulated [16]. Also, Tang et al. reported that ciRS-7 was greatly high-expressed in the peripheral blood of RA patients, and indicated that ciRS-7 may act as a “sponge” of miR-7 and “absorb” a certain amount of miR-7 to promote the expression of mTOR [24]. In this study, we found that hsa_circ_0044235 was low-expressed in RA patients, which is consistent with previous reports. In addition to that, our research also showed that over-expression of hsa_circ_004423 can significantly attenuate knee joint injury and reduce the release of inflammatory factors in CIA mice.
So far, little research has been carried out on hsa_circ_0044235. In addition to hsa_circ_0044235 as a new type of biomarker in RA, there is another study on systemic lupus erythematosus (SLE) [17,25], which proved that the level of hsa_circ_0044235 is sharply reduced in SLE patients, and may therefore play a role through negative regulation of miRNA-892a [25]. However, our study found that hsa_circ_0044235 was positively correlated with the expression of SIRT1, and may be regulated by adsorption of miR-135b-5p. Research on miR-135b-5p mainly focuses on cancer [26]. For example, Zhang et al. showed that miR-135b-5p promotes pancreatic cancer cell metastasis and EMT through NR3C2 [25]. Only one study demonstrated that miR-135b-5p is related to RA, and suggest that miR-135b-5p level is up-regulated in RA patients and is positively correlated with disease activity and inflammation level of RA [27]. We observed that miR-135b-5p expression was promoted in RA patients and FLSs induced by LPS/ATP.
Caspase-1-mediated pyroptosis pathway is a typical inflammasome pathway [28,29]. Inflammasome is a multi-protein complex assembled by receptor protein, ASC and pro-caspase-1 [28]. NLRP3 is one of the most widely studied receptor proteins of inflammasomes [30]. Inflammasomes mainly rely on the binding between domains to activate caspase-1 [31]. Caspase-1 can also promote the maturation, activity, and secretion of pro-IL-1β and pro-IL-18 from cells to further induce the synthesis of other inflammatory cytokines and chemokines, and stimulate the inflammatory response [32]. Studies have found that PTX3 and C1q act synergistically by coordinating and enhancing the overactivation of NLRP3 inflammasomes and inducing gasdermin D lysis and pyroptosis to participate in RA [33]. In this study, we confirmed that overexpressed hsa_circ_0044235 positively promoted the expression of SIRT1 by adsorbing miR-135b-5p, thereby inhibiting the activation of the NLRP3-mediated pyroptosis pathway. In addition, the effect of overexpressed hsa_circ_0044235 on the pyroptosis pathway was reversed by miR-135b-5p mimic, while the promotion of caspase-1, NLRP3, ASC and IL-1β expressions by siSIRT1 was reversed by miR-135b-5p inhibitor.
In conclusion, our findings showed that hsa_circ_0044235 participated in the development of RA, and revealed that hsa_circ_0044235 acted on the NLRP3-mediated pyroptosis pathway through the miR-135b-5p-SIRT1 axis. In the future study, we will focus on verifying the current results at an animal level and further explore other molecular mechanisms regulated by hsa_circ_0044235 in RA.
Acknowledgments
Not applicable.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet. 2016. Oct 22;388(10055):2023–2038. PubMed PMID: 27156434. [DOI] [PubMed] [Google Scholar]
- [2].Kumar LD, Karthik R, Gayathri N, et al. Advancement in contemporary diagnostic and therapeutic approaches for rheumatoid arthritis. Biomed Pharmacother. 2016. Apr;79:52–61. PubMed PMID: 27044812. [DOI] [PubMed] [Google Scholar]
- [3].Favalli EG, Biggioggero M, Crotti C, et al. Sex and management of rheumatoid arthritis. Clin Rev Allergy Immunol. 2019. Jun;56(3):333–345. PubMed PMID: 29372537. [DOI] [PubMed] [Google Scholar]
- [4].Chen Z, Bozec A, Ramming A, et al. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019. Jan;15(1):9–17. PubMed PMID: 30341437. [DOI] [PubMed] [Google Scholar]
- [5].Vande WL, Lamkanfi M. Pyroptosis. Curr Biol. 2016. Jul 11;26(13):R568–R572. PubMed PMID: 27404251. [DOI] [PubMed] [Google Scholar]
- [6].Lacey CA, Mitchell WJ, Dadelahi AS, et al. Caspase-1 and caspase-11 mediate pyroptosis, inflammation, and control of brucella joint infection. Infect Immun. 2018. Sep;86(9).DOI: 10.1128/IAI.00361-18. PubMed PMID: 29941463; PubMed Central PMCID: PMCPMC6105886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu X, Ren G, Zhou R, et al. The role of Ca(2+) in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest. 2019. Apr;99(4):499–513. PubMed PMID: 30487596. [DOI] [PubMed] [Google Scholar]
- [8].Spel L, Martinon F. Inflammasomes contributing to inflammation in arthritis. Immunol Rev. 2020. Mar;294(1):48–62. PubMed PMID: 31944344. [DOI] [PubMed] [Google Scholar]
- [9].Zhao MW, Yang P, Zhao LL. Chlorpyrifos activates cell pyroptosis and increases susceptibility on oxidative stress-induced toxicity by miR-181/SIRT1/PGC-1alpha/Nrf2 signaling pathway in human neuroblastoma SH-SY5Y cells: implication for association between chlorpyrifos and parkinson’s disease. Environ Toxicol. 2019. Jun;34(6):699–707. PubMed PMID: 30835941. [DOI] [PubMed] [Google Scholar]
- [10].Ding S, Liu D, Wang L, et al. Inhibiting microRNA-29a protects myocardial ischemia-reperfusion injury by targeting SIRT1 and suppressing oxidative stress and NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther. 2020. Jan;372(1):128–135. PubMed PMID: 31481517. [DOI] [PubMed] [Google Scholar]
- [11].Engler A, Tange C, Frank-Bertoncelj M, et al. Regulation and function of SIRT1 in rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl). 2016. Feb;94(2):173–182. PubMed PMID: 26298564. [DOI] [PubMed] [Google Scholar]
- [12].Li G, Xia Z, Liu Y, et al. SIRT1 inhibits rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and inflammatory response via suppressing NF-kappaB pathway. Biosci Rep. 2018. Jun 29;38(3). DOI: 10.1042/BSR20180541. PubMed PMID: 29784872; PubMed Central PMCID: PMCPMC6013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsiao KY, Sun HS, Tsai SJ. Circular RNA - new member of noncoding RNA with novel functions. Exp Biol Med (Maywood). 2017. Jun;242(11):1136–1141. PubMed PMID: 28485684; PubMed Central PMCID: PMCPMC5478007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Panda AC. Circular RNAs act as miRNA sponges. Adv Exp Med Biol. 2018;1087:67–79. PubMed PMID: 30259358. [DOI] [PubMed] [Google Scholar]
- [15].Han B, Chao J, Circular YH. RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018. Jul;187:31–44. PubMed PMID: 29406246. [DOI] [PubMed] [Google Scholar]
- [16].Zheng F, Yu X, Huang J, et al. Circular RNA expression profiles of peripheral blood mononuclear cells in rheumatoid arthritis patients, based on microarray chip technology. Mol Med Rep. 2017. Dec;16(6):8029–8036. PubMed PMID: 28983619; PubMed Central PMCID: PMCPMC5779885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Luo Q, Zhang L, Li X, et al. Identification of circular RNAs hsa_circ_0044235 in peripheral blood as novel biomarkers for rheumatoid arthritis. Clin Exp Immunol. 2018;Oct;194(1):118–124. PubMed PMID: 30216431; PubMed Central PMCID: PMCPMC6156811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun J, Li L, Li L, et al. Metallothionein-1 suppresses rheumatoid arthritis pathogenesis by shifting the Th17/Treg balance. Eur J Immunol. 2018. Sep;48(9):1550–1562. PubMed PMID: 30055006. [DOI] [PubMed] [Google Scholar]
- [19].Dai H, Wang X, Yin S, et al. Atrial fibrillation promotion in a rat model of rheumatoid arthritis. J Am Heart Assoc. 2017. Dec 21;6(12). DOI: 10.1161/jaha.117.007320. PubMed PMID: 29269354; PubMed Central PMCID: PMCPMC5779041. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen J, Wu W, Zhang M, et al. Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int Immunopharmacol. 2019. May;70:274–283. PubMed PMID: 30851708; eng. [DOI] [PubMed] [Google Scholar]
- [21].Zhang L, Zhang L, Huang Z, et al. Increased HIF-1alpha in knee osteoarthritis aggravate synovial fibrosis via fibroblast-like synoviocyte pyroptosis. Oxid Med Cell Longev. 2019; 6326517. 2019. 10.1155/2019/6326517. PubMed PMID: 30755787; PubMed Central PMCID: PMCPMC6348923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luo Q, Zhang L, Xiong L, et al. Peripheral blood circular RNA hsa_circ_0082688-hsa_circ_0008675 can be used as a candidate biomarker of systemic lupus erythematosus with renal involvement. Clin Exp Rheumatol. 2020. Sep 16;38(5):822–833. PubMed PMID: 32940208. [PubMed] [Google Scholar]
- [23].Wang J, Yan S, Yang J, et al. Non-coding Rnas in Rheumatoid Arthritis: from bench to bedside. Front Immunol. 2019;10:3129. PubMed PMID: 32047497; PubMed Central PMCID: PMCPMC6997467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tang X, Wang J, Xia X, et al. Elevated expression of ciRS-7 in peripheral blood mononuclear cells from rheumatoid arthritis patients. Diagn Pathol. 2019. Feb 2;14(1):11. PubMed PMID: 30711014; PubMed Central PMCID: PMCPMC6359828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luo Q, Zhang L, Li X, et al. Identification of circular RNAs hsa_circ_0044235 and hsa_circ_0068367 as novel biomarkers for systemic lupus erythematosus. Int J Mol Med. 2019. Oct;44(4):1462–1472. PubMed PMID: 31432107; PubMed Central PMCID: PMCPMC6713423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shao L, Chen Z, Soutto M, et al. Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. Faseb J. 2019. Jan;33(1):264–274. PubMed PMID: 29985646; PubMed Central PMCID: PMCPMC6355059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu C, Pan A, Chen X, et al. MiR-5571-3p and miR-135b-5p, derived from analyses of microRNA profile sequencing, correlate with increased disease risk and activity of rheumatoid arthritis. Clin Rheumatol. 2019. Jun;38(6):1753–1765. PubMed PMID: 30707326. [DOI] [PubMed] [Google Scholar]
- [28].Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015. Oct 29;526(7575):660–665. PubMed PMID: 26375003. [DOI] [PubMed] [Google Scholar]
- [29].Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017. Apr;42(4):245–254. PubMed PMID: 27932073. [DOI] [PubMed] [Google Scholar]
- [30].Fu Q, Wu J, Zhou XY, et al. NLRP3/caspase-1 pathway-induced pyroptosis mediated cognitive deficits in a mouse model of sepsis-associated encephalopathy. Inflammation. 2019. Feb;42(1):306–318. PubMed PMID: 30276509; PubMed Central PMCID: PMCPMC6394578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015. May;265(1):6–21. PubMed PMID: 25879280; PubMed Central PMCID: PMCPMC4400844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015. May;265(1):35–52. PubMed PMID: 25879282; PubMed Central PMCID: PMCPMC4400874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu XY, Li KT, Yang HX, et al. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J Autoimmun. 2020. Jan;106:102336. PubMed PMID: 31601476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.


