ABSTRACT
Background
hsa_circ_0006168 is an oncogenic circular RNA in esophageal cancer. However, its role remains unclarified in tumor progression of gliomas, especially in glioblastoma (GBM).
Methods
Cell counting kit-8 assay, transwell assays, western blotting, and xenograft experiment, as well as colony formation assay and flow cytometry were performed to measure cell proliferation and motility. Expression of hsa_circ_0006168, microRNA (miR)-628-3p, insulin‑like growth factor 1 receptor (IGF1R), and Ras/extracellular signal regulated kinases (Erk)-related proteins were determined by quantitative real-time polymerase chain reaction and western blotting. The physical interaction was confirmed by dual-luciferase reporter assay and RNA pull-down assay.
Results
hsa_circ_0006168 and IGF1R were upregulated, and miR-628-5p was downregulated in human GBM tissues and cells. Functionally, blocking hsa_circ_0006168 and overexpressing miR-628-5p suppressed cell proliferation, migration, invasion, and expression of Vimentin and Snail (mesenchymal markers) in A172 and LN229 cells, accompanied with increased E-cadherin (epithelial marker), decreased colony formation, and promoted apoptosis rate. Silencing miR-628-5p counteracted the suppression of hsa_circ_0006168 deficiency on these behaviors, and restoring IGF1R blocked miR-628-5p-mediated inhibition as well. More importantly, hsa_circ_0006168 knockdown could delay xenograft tumor growth in vivo and lower Ras and phosphorylated Erk1/2 expression in vitro and in vivo. Mechanically, hsa_circ_0006168 targeted and sponged miR-628-5p, and IFG1R was a novel target for miR-628-5p. Inhibiting miR-628-5p could abrogate in vitro role of hsa_circ_0006168 knockdown, and similarly IGF1R upregulation counteracted miR-628-5p role.
Conclusion
Silencing hsa_circ_0006168 might suppress GBM proliferation and motility via serving as competitive endogenous RNA for miR-628-5p and regulating IGF1R/Ras/Erk pathway.
KEYWORDS: Hsa_circ_0006168, miR-628-5p, glioblastoma, IGF1R
Introduction
MicroRNAs (miRNAs) are short, endogenous noncoding RNAs that take a remarkable part in gene expression in physiological and pathological processes, including gliomagenesis [1]. Glioblastomas (GBM), constituting 60–70% of malignant gliomas, are a highly aggressive (WHO grade IV) form of diffuse gliomas [2]. Less than 8% GBM patients survive for 5 years after diagnosis [3]. Since GBM are endowed with tremendous proliferative and invasive capacity, drug resistance and tumor recurrence are inevitable [4]. miRNAs are emerging multifaceted players in GBM progression [5], as well as biomarkers for the diagnosis and prognosis [6]. Moreover, miRNA-based therapeutics have been intricately linked with management of GBM [7].
miRNA (miR)-628-5p is an extensive tumor suppressor in human cancers, such as gastric cancer, pancreatic cancer [8,9], and glioma [10]. Functionally, miR-628-5p exhibits dominant regulatory activities in malignant proliferation and cell cycle in GBM through miRNA/mRNA regulatory network [11]. Insulin-like growth factor 1 receptor (IGF1R) is one of receptor tyrosine kinases (RTKs) [12], and alterations of RTKs link to the initiation, maintenance and progression of many different tumor including GBM [13,14]. IGF1R is frequently activated in GBM and has been a new prognostic biomarker for poor survival and temozolomide resistance in GBM patients [15]. Furthermore, RTKs and RTKs-activated Ras/Raf/Mek/extracellular signal regulated kinases (Erk) signaling pathway are central to oncogenesis, and show potent clinical implications [16].
Circular RNAs (circRNAs) are generally endogenous noncoding RNAs which were discovered in the early 1970s as unconsidered transcriptional by-product. However, circRNAs have been emerging as key regulators in human diseases including gliomas due to their stability, conservation and specificity [17,18]. Plenty of circRNAs, miRNAs and their downstream target genes form competitive endogenous RNA (ceRNA) network to mediate gliomas biological functions [19]. Recently, abnormally expressed circRNAs are screened in GBM patients according to Gene expression omnibus dataset (GEO; accession GSE146463), and hsa_circ_0006168 is one of the top 10 abnormally upregulated circRNAs. Besides, circbank software predicts that hsa_circ_0006168 contains miR-628-5p response elements. Thus, we intended to investigate the role of hsa_circ_0006168 in cell progression and Ras/Erk pathway in human GBM, and the involvement among hsa_circ_0006168, miR-628-5p and IGF1R.
Materials and methods
Gene expression omnibus (GEO) dataset
In the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/), “GEO DataSets” was searched using key words “glioblastoma and circRNA”, and the first result “Development of circRNA expression signatures for glioblastoma cells.” was displayed, namely GSE146463 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE146463). The 11 samples including 8 biological replicates of GBM cells and 3 biological replicates of normal neural progenitor cells were analyzed by clicking “Analyze with GEO2R”. The top 10 upregulated and downregulated circRNAs were captured. Then, the circBase ID of these circRNAs was identified using Basic Local Alignment Search Tool (blast) in circBase (http://www.circbase.org/cgi-bin/webBlat).
GBM patients
All patients’ tissue samples, including 33 primary GBM tissues and 16 normal brain tissues, were obtained from consented patients undergoing surgical treatment at The First Affiliated Hospital of Xi’an Jiaotong University. GBM patients were diagnosed blindly, independently by two senior pathologists, and none of them had undergone chemotherapy or radiotherapy prior to this surgery. Control patients were diagnosed with open craniocerebral trauma caused by car accidents, and none of them had any congenital diseases. The study was approved by the Research Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University. All the tissues were subsequently subjected to total RNA or protein isolation. The clinical pathologic characteristics of GBM patients were showed, as well as the correlation between hsa_circ_0006168 and these characteristics, as shown in Table 1.
Table 1.
Correlation between hsa_circ_0006168 expression and clinical pathologic characteristics of GBM patients
| Clinicopathological features |
No. of cases |
hsa_circ_0006168 |
P Value |
|
| High† |
Low |
|||
| Gender | ||||
| Male | 17 | 9 | 8 | 0.866 |
| Female | 16 | 8 | 8 | |
| Age | ||||
| ≤60 | 14 | 8 | 6 | 0.579 |
| >60 | 19 | 9 | 10 | |
| WHO Grade | ||||
| I–II | 20 | 15 | 5 | 0.001* |
| III–IV | 13 | 2 | 11 | |
| T stage | ||||
| T1-T2 | 19 | 15 | 4 | 0.000* |
| T3-T4 | 14 | 2 | 12 | |
| N stage | ||||
| N0 | 20 | 14 | 6 | 0.008* |
| N1 | 13 | 3 | 10 | |
| M stage | ||||
| M0 | 24 | 15 | 9 | 0.039* |
| M1 | 9 | 2 | 7 | |
The median expression level of hsa_circ_0006168 was used as the cutoff.
*Pearson’s chi-square tests were used to analyze the correlation between hsa_circ_0006168 expression and clinical features, and *, P < 0.05.
Note: WHO, world health organization.
Cells and cell culture
Human GBM cell lines A172 (CRL-1620) and LN229 (CRL-2611) were originally from American Type Culture Collection (Manassas, VA, USA); human normal astrocyte cell line HEB (BNCC338123) and embryonic kidney cell line 293 T (BNCC353535) were from BeNa Culture Collection (Beijing, China). All the cells were cultured in 10% fetal bovine serum (R&D SYSTEMS, Minneapolis, MN, USA) diluted in DMEM (M22650; R&D SYSTEMS), which contained 4500 mg/L D-glucose, 25 mM HEPES buffer, L-glutamine, and sodium pyruvate. All cell lines were maintained in a humidified chamber containing 5% CO2 at 37°C.
Total RNA isolation and quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues and cells using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA), and was reverse-transcribed into cDNA with Revent Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific; for circRNA/mRNA) and miScript reverse transcription kit (GenePharma, Shanghai, China; for miRNA). The amplification of cDNA was carried out using SYBR® Premix Ex Taq™ II Kit (Takara, Otsu, Japan; for circRNA/mRNA) and miRNA Real-Time PCR Assay Kit (GenePharma; for miRNA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 snoRNA (U6) were the internal controls for circRNA/mRNA and miRNA, respectively. The sequences of primers were shown as follows: hsa_circ_0006168, forward primer (F) 5ʹ-TGAACTTGGTCGGCTCTTCC-3ʹ and reverse primer (R) 5ʹ-ATTGGCTACCTCCTCTGCTG-3ʹ; miR-628-5p, F 5ʹ-GCGGGCATGCTGACATATTT-3ʹ and R 5ʹ-GGGTCCGAGGTATTCGCACT-3ʹ; IGF1R, F 5ʹ-CCTACAGGTTTGAGGGCTGG-3ʹ and R 5ʹ-GTCGTGGATCACAAACCCCT-3ʹ; GAPDH, F 5ʹ-AGAAGGCTGGGGCTCATTTG-3ʹ and R 5ʹ-AGGGGCCATCCACAGTCTTC-3ʹ; U6, F 5ʹ-CTCGCTTCGGCAGCACATATACT-3ʹ and R 5ʹ-ACGCTTCACGAATTTGCGTGTC-3ʹ. Four independent PCR amplifications were performed for each experiment, and the relative expression of target genes was determined using the 2−∆∆Ct method.
Ribonuclease R (RNase R) treatment
Total RNA (1.5 μg) extracted from A172 and LN229 cells was incubated with 6 units of RNase R (Duma, Shanghai, China) for 15 min at 37°C. Then, the expression of hsa_circ_0006168 and GAPDH was detected by RT-qPCR. GAPDH was served as the endogenous control.
Cell transfection
Specific small interfering RNAs (siRNAs) objecting hsa_circ_0006168 junction sites (si-hsa_circ_0006168#1, #2 and #2) and siRNA scrambled control (si-NC), miR-628-5p mimic and its negative control (mimic NC), and miR-628-5p inhibitor and its negative control (inhibitor NC) were generated and synthesized by Hanbio (Shanghai, China). Short hairpin RNA (shRNA) objecting hsa_circ_0006168 junction sites (sh-hsa_circ_0006168) and shRNA scrambled control (sh-NC) were cloned into pGreenPuro vector (YouBio, Changsha, China); The IGF1R overexpression vector (pcDNA-IGF1R) was constructed by inserting coding domain sequence of IGF1R (NM_000875.5) into pcDNA3.1 (+) vector (YouBio). The sequences of above oligonucleotides were listed: si-NC, 5ʹ-GCUGUUACUAUAAUUCGCCUU-3ʹ, si-hsa_circ_0006168, 5ʹ-UUAGUCUUUCAAACCUAGAGU-3ʹ (#1), 5ʹ-UAGUCUUUCAAACCUAGAGUU-3ʹ (#2) and 5ʹ-GCAUCCCUAUUAGUCUUUCAA-3ʹ (#3); sh-NC, 5ʹ-AAGGCGAAUUAUAGUAACAGC-3ʹ and 5ʹ-GCUGUUACUAUAAUUCGCCUU-3ʹ, sh-hsa_circ_0006168, 5ʹ-CUCUAGGUUUGAAAGACUAAU-3ʹ and 5ʹ-AGUCUUUCAAACCUAGAGUU-3; mimic NC, 5ʹ-GGUUCGUACGUACACUGUUCA-3ʹ, miR-628-5p mimic, 5ʹ-AUGCUGACAUAUUUACUAGAGG-3ʹ; inhibitor NC, 5ʹ-GGUUCGUACGUACACUGUUCA-3ʹ, miR-628-5p inhibitor, 5ʹ-CCUCUAGUAAAUAUGUCAGCAU-3ʹ. To transfect these nucleotides into A172 and LN229 cells, Lipofectamine 2000 (Invitrogen) was used according to the manufacturer’s instructions for 48 h. Transfected A172 and LN229 cells at 4 h were added with 20 μM salirasib (Santa Cruz Biotechnology, Dallas, TX, USA) or 0.1% dimethylsulfoxide (DMSO) for another 48 h, followed with total protein isolation.
Cell counting kit (CCK)-8 assay and colony formation assay
Transfected cells were collected and dispersed into single-cell suspensions to a density of 2.5 × 104 cells/mL. For CCK-8 assay, 100 μL (2,500 cells) cells were added into wells of 96-well plates, and four paralleled wells were set in each transfection group. The inoculated cells were cultured for another 0, 24, 48, and 72 h in optimal condition (37°C and 5% CO2). After that, 10 μL CCK-8 reagent (Beyotime, Shanghai, China) were then added into each well for 1.5 h incubation, and colorimetric absorbance at 450 nm was measured by a microplate reader (Molecular devices, Shanghai, China). For colony formation assay, 10 μL cells (250 cells) were added into wells of 6-well plates with three repeats. The inoculated cells were cultured for another 14 days with medium renewal every three days. Subsequently, formed colonies were stained with 0.5% crystal violet (Beyotime). The colonies were counted via a microscope and colony formation rate was calculated (100%×colony number/250).
Flow cytometry (FCM)
eBioscience™ Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Invitrogen, Carlsbad, CA, USA) was used to measure apoptotic cells on flow cytometer. Transfected A172 and LN229 cells (5 × 104) were severally collected and re-suspended in 200 µL Binding Buffer (1×); 5 µL Annexin V-FITC and 10 µL Propidium Iodide (PI) were added in cells in sequence for staining in the dark. Percentage of apoptosis rate was determined by FACS analysis.
Transwell migration and invasion assays
After transfection, A172 and LN229 cells were harvested in serum-free medium at a density of 5 × 105 cells/mL. For migration assay, 100 µL cells were inoculated in the top chamber (Corning, Corning, NY, USA) located in 24-well plate. The bottom compartment was filled with medium containing 10% serum, and no touching with then the bottom surface of chamber was allowed. These cells were cultivated for another 48 h, and migrated cells into bottom surface of chamber were stained with 0.5% crystal violet (Beyotime). Stained cell numbers were counted via a microscope from five independent fields of each chamber. It should be noted that transwell chambers (Corning) were pre-incubated with Matrigel (BD Biosciences, San Jose, CA, USA) for invasion assay.
Total protein isolation and western blotting
Total protein was isolated from tissues and cells using RIPA reagent (Beyotime), and separated by SDS-polyacrylamide gel electrophoresis. Proteins were then transferred onto polyvinylidene fluoride membrane, and blocked with 3% bull serum albumin. The primary antibodies were used to probe corresponding proteins, and then probed with HRP-conjugated secondary antibodies. Eventually, the immune-reactivity was measured on chemiluminescence detection system (Pierce, Dallas, TX, USA) following visualization of Immobilon ECL HRP substrate (Millipore, Billerica, MA, USA). The antibodies were purchased from CST (Shanghai, China) and Sangon (Shanghai, China) and listed as follows: IGF1R (#3027), E-cadherin (#14,472), Vimentin (#3932), Snail (#4719), Ras (#3339), Erk1/2 (#9107), phosphorylated Erk1/2 (p-Erk1/2; #4376), GAPDH (D110016), HRP-conjugated anti-rabbit (D110065), and HRP-conjugated anti-mouse (D110098).
Dual-luciferase reporter assay and RNA pull-down assay
The wild-type (wt) and mutant (mut) of miR-628-5p-binding sites in 3ʹUTR fragment (position 1–3001) of IGF1R (IGF1R 3ʹUTR) or hsa_circ_0006168 cDNA were severally cloned into pmirGLO luciferase reporter vectors (Promega, Madison, WI, USA), respectively. For dual-luciferase reporter assay, reporter vectors (IGF1R 3ʹUTR wt, IGF1R 3ʹUTR mut, hsa_circ_0006168 wt, and hsa_circ_0006168 mut) were co-transfected with mimics (miR-628-5p mimic and mimic NC) and Renilla luciferase reporter vector (Promega) in 293 T cells. After transfection for 48 h, Firefly luciferase activity was determined using a Dual-Luciferase Reporter Assay System (Promega) with normalization to Renilla luciferase activity.
The ceRNAs for miR-628-5p were captured in RNA pull-down assay using Pierce Magnetic RNA-Protein Pull-Down Kit (Pierce). Biotinylated miR-628-5p (Bio-miR-628-5p; biotin-AUGCUGACAUAUUUACUAGAGG) and biotinylated miR-NC (Bio-NC; biotin-GGUUCGUACGUACACUGUUCA) were synthesized and transfected into A172 and LN229 cells. Post-transfection for 48 h, cell lysate was collected in RIP lysis buffer, and then incubated with streptavidin-coated magnetic beads for 24 h. The expression of hsa_circ_0006168 and IGF1R in biotin-captured RNA complex was analyzed by RT-qPCR.
Xenograft tumor models
LN229 cells transfected with sh-NC or sh-hsa_circ_0006168 were harvested at a concentration of 1 × 107 cells/mL. BALB/c athymic nude mice (4–5 weeks old) were acquired from Vital River (Beijing, China) and randomly divided into two groups (N = 6) for cell inoculation. Transfected LN229 cells were subcutaneously injected into posterior flanks of mice prior to another 35 days feeding. The length measure (L) and width measure (W) were examined and monitored every 7 days, and weight of xenograft tumors was examined on the last day. The animal experiment was approved by the Use and Care of Animals Committee of The First Affiliated Hospital of Xi’an Jiaotong University, and was performed following the Guide for the Care and Use of Laboratory Animals (GB/T35892-2018; Standardization Administration of the People's Republic of China). The cell proliferation curve was drawn according to tumor volume calculated using 0.5 × L× W2 formula.
Statistical analysis
The results were expressed as mean ± standard deviation from at least three independent experiments, and statistical significance of these results was analyzed by Student’s t-test or one-way analysis of variance (ANOVA) on GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). Tukey’s post-hoc test was performed following ANOVA, and correlation between RNA expression levels was determined by Pearson correlation coefficient analysis. P < 0.05 denoted a statistical significance (*).
Results
Hsa_circ_0006168 was highly expressed in human GBM tissues and cells
According to GSE146463 dataset, top 10 upregulated and downregulated circRNAs were obtained in GBM patients-derived cells (Figure S1A); among them, hsa_circRNA_103670 (another name for hsa_circ_0006168) was a novel-identified upregulated circRNA in GBM patients. hsa_circ_0006168 (395 bp spliced sequence length) was derived from exon 2–4 of CNOT6L in chromosome 4 (chr4): 78,694,234–78,697,546 (Figure S1B). Expression of hsa_circ_0006168 in GBM patients and cells was testified using RT-qPCR, and hsa_circ_0006168 level was higher in human GBM tissues and cell lines (A172 and LN229) than normal brain tissues and human astrocyte cell line (HEB) (Figure 1a and Figure 1b). What’s more, hsa_circ_0006168 expression was unaffected to RNase R treatment (Figure S1C). Clinically, hsa_circ_0006168 expression was significantly associated with WHO grade, T stage, N stage and M stage in these GBM cases (Table 1). These data manifested that hsa_circ_0006168 was an abnormally upregulated circRNA in GBM.
Figure 1.
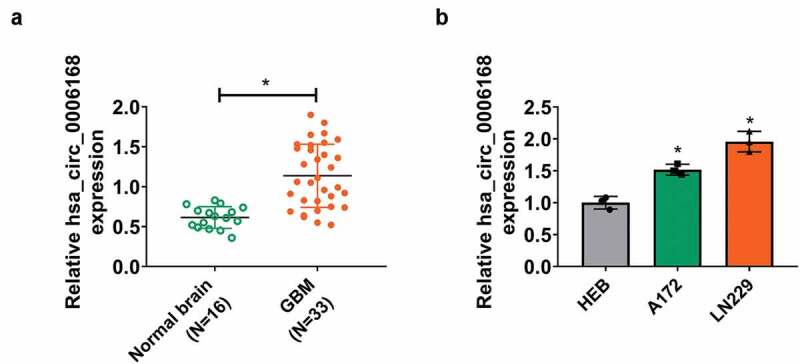
Expression of hsa_circ_0006168 in human GBM tissues and cells. (a) RT-qPCR detected expression of hsa_circ_0006168 in brain tissues from GBM patients (GBM; N = 33) and control patients (Normal brain; N = 16) with normalization to the control in normal brain group. (b) RT-qPCR detected expression of hsa_circ_0006168 in A172 and LN229 with normalization to HEB cells. *P < 0.05
Silencing hsa_circ_0006168 antagonized cell proliferation and motility of human GBM cells in vitro
Transfection of siRNAs targeting hsa_circ_0006168 caused inhibition of hsa_circ_0006168 in both A172 and LN229 cells (Figure 2a). Silencing hsa_circ_0006168 via si-hsa_circ_0006168#1 or si-hsa_circ_0006168#2 slowed down the proliferation of A172 and LN229 cells, as indicated by lower colorimetric absorption in consecutive 3 days after inoculation of transfected cells (Figure 2b). Numbers of transwell migrated cells and invaded cells were declined by depleting hsa_circ_0006168 via si-hsa_circ_0006168 (#1 and #2) transfection (Figure 2c and Figure 2d), as accompanied with increased expression of E-cadherin (epithelial marker) and decreased expression of Vimentin and Snail (mesenchymal markers) (Figure 2e). These results showed an antagonistic effect of hsa_circ_0006168 knockdown on cell proliferation and motility of human GBM cells in vitro. Consistent with that, colony formation of A172 and LN229 cells was reduced, and apoptosis was elevated due to hsa_circ_0006168 siRNAs (Figure S2A and S2B).
Figure 2.
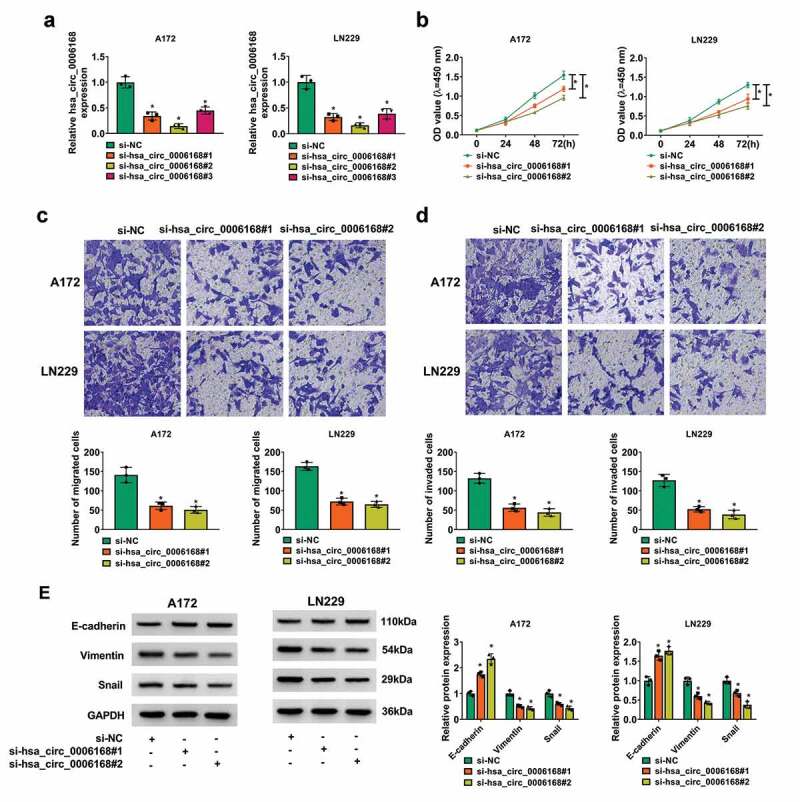
Role of hsa_circ_0006168 in cell proliferation and motility of human GBM cells in vitro. A172 and LN229 cells were transfected with siRNAs for 48 h. (a) RT-qPCR detected hsa_circ_0006168 expression with normalization to si-NC group. (b) CCK-8 assay monitored optical density (OD) value at 450 nm after inoculation of transfected cells for 0–72 h. (c, d) Transwell assays determined migrated cells and invaded cells (Upper) after inoculation of transfected cells for 48 h, and the corresponding cell counting quantification (Lower) in per field at 100× was performed. (e) Western blotting measured expression of GAPDH, E-cadherin, Vimentin and Snail, and the corresponding gray density quantification was corrected with GAPDH; then relative protein expression was determined with normalization to si-NC group. *P < 0.05
Hsa_circ_0006168 functioned as miR-628-5p sponge in human GBM cells
Circbank (http://www.circbank.cn/searchMiRNA.html) predicted a novel binding site of miR-628-5p in hsa_circ_0006168 wt, and accordingly hsa_circ_0006168 mut was synthesized (Figure 3a). Luciferase activity of reporter vector carrying hsa_circ_0006168 wt was diminished, whereas hsa_circ_0006168 mut was little affected by miR-628-5p overexpression via its mimic transfection (Figure 3b). Moreover, hsa_circ_0006168 expression was highly enriched in Bio-miR-628-5p-mediated pull-down complex from A172 and LN229 cells (Figure 3c). Additionally, expression of miR-628-5p was significantly downregulated in GBM patients’ tumors and human GBM cells (Figure 3d and Figure 3e). Notably, miR-628-5p expression was negatively, linearly correlated to hsa_circ_0006168 expression in these GBM patients (figure 3f). These outcomes suggested that miR-628-5p was abnormally downregulated in GBM and was targeted by hsa_circ_0006168.
Figure 3.
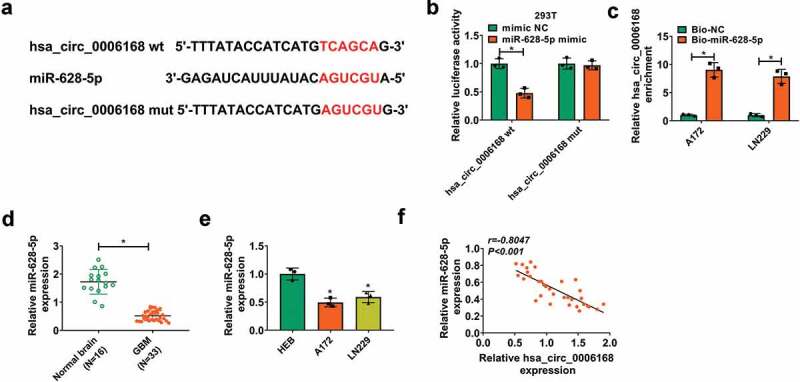
Function of hsa_circ_0006168 as miR-628-5p sponge in human GBM cells. (a) The predicted binding sites of miR-628-5p in hsa_circ_0006168 wt were mutated. (b) Dual-luciferase reporter assay measured luciferase activity of hsa_circ_0006168 wt and mut in 293 T cells transfected with miR-628-5p mimic or mimic NC. (c) RT-qPCR detected the enrichment of hsa_circ_0006168 in cell lysate incubated with Bio-miR-628-5p or Bio-NC in A172 and LN229 cells. (d, e) RT-qPCR detected miR-628-5p expression in Normal brain and GBM tissues, as well as cells including A172, LN229 and HEB. (f) Pearson correlation coefficient analysis validated the linear correlation between hsa_circ_0006168 and miR-628-5p expression in GBM tissues. *P < 0.05
miR-628-5p downregulation canceled the suppressive role of hsa_circ_0006168 deficiency in human GBM cell proliferation and motility in vitro
Rescue experiments were performed to identify the effects of hsa_circ_0006168 and miR-628-5p downregulation in GBM cell progression, and miR-628-5p inhibitor was used to silence itself expression in A172 and LN229 cells (Figure 4a). Blocking hsa_circ_0006168 via si-hsa_circ_0006168#2 transfection resulted in the upregulation of miR-628-5p, and this upregulation was abated with miR-628-5p inhibitor co-transfection (Figure 4b). The inhibitory effect of hsa_circ_0006168 knockdown on OD values in A172 and LN229 cells were counteracted by concurrently silencing miR-628-5p (Figure 4c). Blocking hsa_circ_0006168 exerted suppressive effects on migration, invasion and epithelial-to-mesenchymal transition (EMT) in A172 and LN229 cells, and these suppressions were overall abrogated when hsa_circ_0006168 and miR-628-5p were synchronously depleted, as evidenced by the decrease of transwell migrated cells and invaded cells and E-cadherin level, and the elevation of Vimentin and Snail levels (Figure 4d-Figure 4f). These results demonstrated a counteractive role between hsa_circ_0006168 knockdown and miR-628-5p downregulation in GBM cell proliferation and motility in vitro. By the way, hsa_circ_0006168 silencing-mediated inhibiting effect on colony formation and promoting effect on apoptosis were mitigated by inhibiting miR-628-5p (Figure S2C-S2D).
Figure 4.
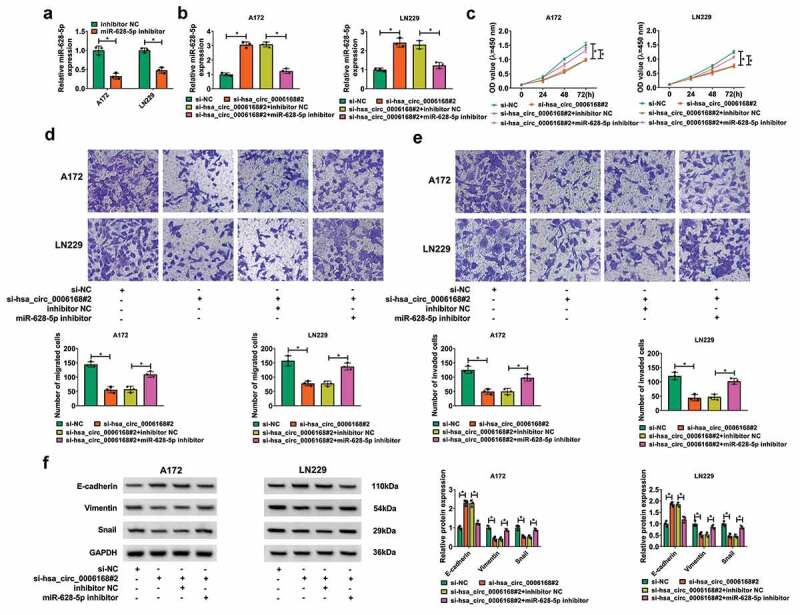
Effect of miR-628-5p downregulation on role of hsa_circ_0006168 deficiency in cell proliferation and motility of human GBM cells in vitro. A172 and LN229 cells without and with hsa_circ_0006168 knockdown were transfected with miR-628-5p inhibitor or inhibitor NC for 48 h. (a, b) RT-qPCR detected miR-628-5p expression. (c) CCK-8 assay monitored OD value at 450 nm after inoculation of transfected cells for 0–72 h. (d, e) Transwell assays determined numbers of migrated cells and invaded cells after inoculation of transfected cells for 48 h. (f) Western blotting measured relative protein expression of E-cadherin, Vimentin and Snail. *P < 0.05
miR-628-5p could target IGF1R in human GBM cells
The target genes of miR-628-5p were searched on starBase (http://starbase/miRNA&mRNA/=hsa-miR-628-5p), and IGF1R was selected as a potent candidate due to its crucial role in GBM. The complementary binding sites of miR-628-5p in IGF1R 3ʹUTR wt were presented and further mutated to construct IGF1R 3ʹUTR mut (Figure 5a). Dual-luciferase reporter assay revealed a reduction of luciferase activity in 293 T cells co-transfected with IGF1R 3ʹUTR wt and miR-628-5p mimic (Figure 5b). RNA pull-down assay depicted an enrichment of hsa_circ_0006168 by Bio-miR-628-5p in A172 and LN229 cells (Figure 5c). In addition, expression levels of IGF1R were significantly upregulated in GBM patients’ tumors and human GBM cells (Figure 5d-Figure 5f). There was also an inversely correlation between miR-628-5p and IGF1R expression in these GBM patients (Figure 5g). Notably, expression of IGF1R was lower in hsa_circ_0006168-silenced A172 and LN229 cells, and this downregulation was attenuated with co-transfection of si-hsa_circ_0006168#2 and miR-628-5p inhibitor (Figure 5h and Figure 5i). These outcomes suggested that IGF1R was targeted by miR-628-5p and regulated by hsa_circ_0006168 via miR-628-5p.
Figure 5.
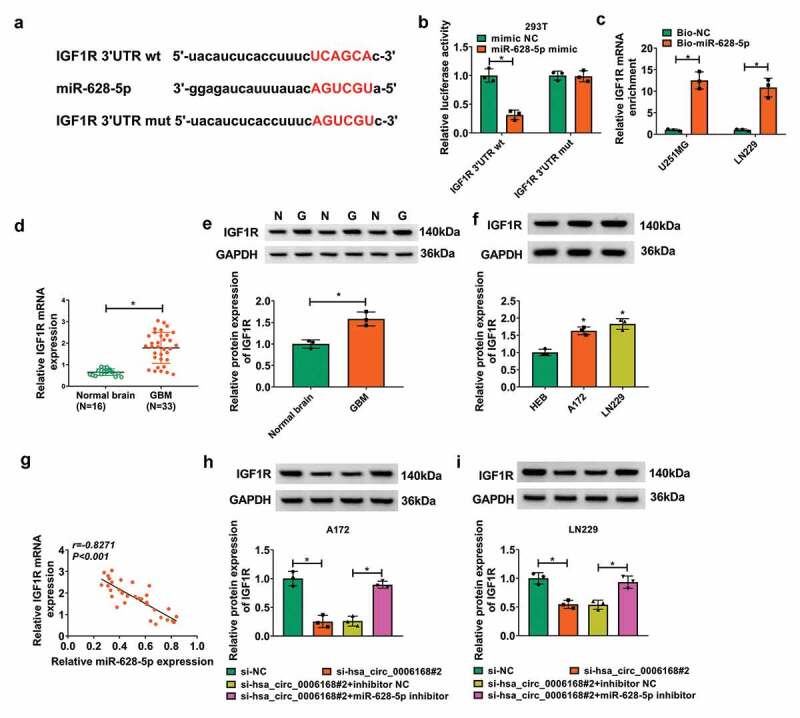
Target relationship of miR-628-5p and IGF1R in human GBM cells. (a) The predicted binding sites of miR-628-5p in IGF1R 3ʹUTR wt were mutated. (b) Dual-luciferase reporter assay measured luciferase activity of IGF1R 3ʹUTR wt and mut in 293 T cells transfected with miR-628-5p mimic or mimic NC. (c) RT-qPCR detected the enrichment of hsa_circ_0006168 in cell lysate incubated with Bio-miR-628-5p or Bio-NC in A172 and LN229 cells. (d-f) RT-qPCR and western blotting measured IGF1R mRNA expression and protein expression in Normal brain and GBM tissues, as well as cells including A172, LN229 and HEB. (g) Pearson correlation coefficient analysis validated the linear correlation between miR-628-5p and IGF1R expression in GBM tissues. (h, i) Western blotting measured IGF1R protein expression in A172 and LN229 cells transfected with si-NC, si-hsa_circ_0006168#2, si-hsa_circ_0006168#2 and inhibitor NC, si-hsa_circ_0006168#2 and miR-628-5p inhibitor. *P < 0.05
There was a counteractive effect between miR-628-5p overexpression and IGF1R upregulation in cell proliferation and motility of human GBM cells in vitro
The role of miR-628-5p and IGF1R in GBM cell progression was subsequently figured out. Transfection of miR-628-5p mimic and pcDNA-IGF1R led to miR-628-5p overexpression and IGF1R upregulation in A172 and LN229 cells, respectively (Figure 6a-Figure 6b). Furthermore, miR-628-5p overexpression mediated the inhibition of IGF1R, which could be improved by pcDNA-IGF1R transfection (Figure 6c). Functionally, restoring miR-628-5p suppressed colorimetric absorptions after inoculation of transfected A172 and LN229 cells (Figure 6d and Figure S2E). Transwell migrated cells and invaded cells were lessened when miR-628-5p was forcedly overexpressed in A172 and LN229 cells (Figure 6d and Figure 6f), accompanied with promoted E-cadherin expression, and depressed Vimentin and Snail expression (Figure 6g). These data demonstrated a suppressive role of miR-628-5p in cell proliferation and motility of GBM cells in vitro, allied with this was inhibited colony formation and enhanced apoptosis rate (Figure S2E and S2F) More importantly, the suppression of miR-628-5p overexpression was overall abrogated with pcDNA-IGF1R synchronously administration (Figure 6d-Figure 6g and Figure S2E-S2F). These results demonstrated a counteractive role between miR-628-5p overexpression and IGF1R upregulation in GBM cell proliferation and motility in vitro., and
Figure 6.
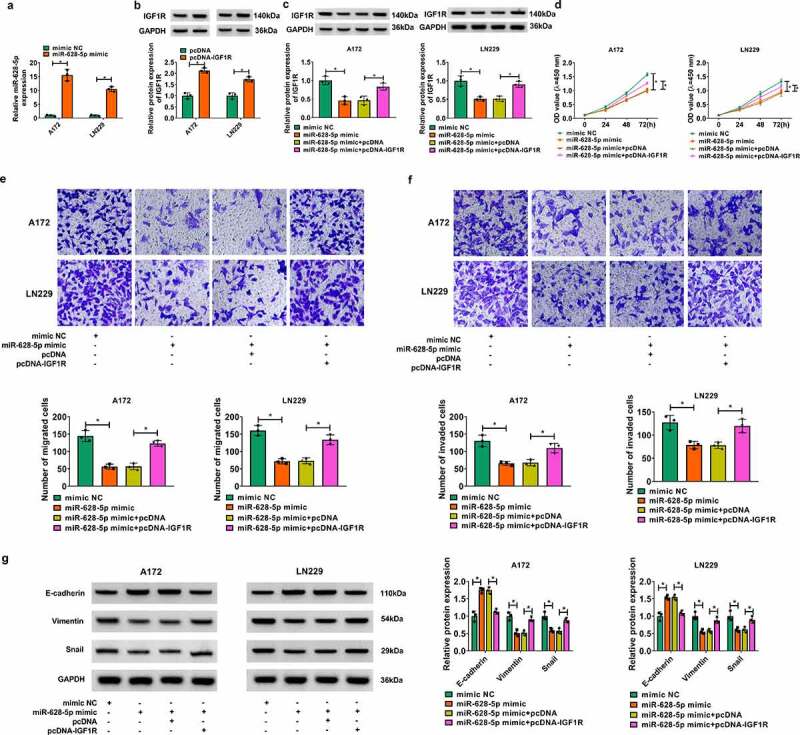
Effects of miR-628-5p and IGF1R overexpression in cell proliferation and motility of human GBM cells in vitro. (a) RT-qPCR detected miR-628-5p expression in A172 and LN229 cells transfected with miR-628-5p mimic or mimic NC. (b-i) A172 and LN229 cells without and with miR-628-5p overexpression were co-transfected with pcDNA-IGF1R vector or pcDNA empty vector for 48 h. (b, c) Western blotting detected relative IGF1R protein expression. (d) CCK-8 assay monitored OD value at 450 nm after inoculation of transfected cells for 0–72 h. (e, f) Transwell assays determined numbers of migrated cells and invaded cells after inoculation of transfected cells for 48 h. (g) Western blotting measured relative protein expression of E-cadherin, Vimentin and Snail. *P < 0.05
Blocking hsa_circ_0006168 suppressed cell proliferation of human GBM cells in vivo
Xenograft tumor models were established in nude mice by subcutaneously injecting LN229 cells with stable shRNA transfection, and A172 cells were not tumorigenic in immunosuppressed mice (https://www.atcc.org/Products/All/CRL-1620.aspx#characteristics). During cell inoculation for 35 days, tumor growth was retarded in sh-hsa_circ_0006168 group (N = 6), as evidenced by the decline of tumor volume and weight (Figure 7a and Figure 7b). Expression of hsa_circ_0006168 in xenograft tumor tissues was downregulated in sh-hsa_circ_0006168 group than sh-NC group, accompanied with higher miR-628-5p and lower IGF1R (Figure 7c).
Figure 7.
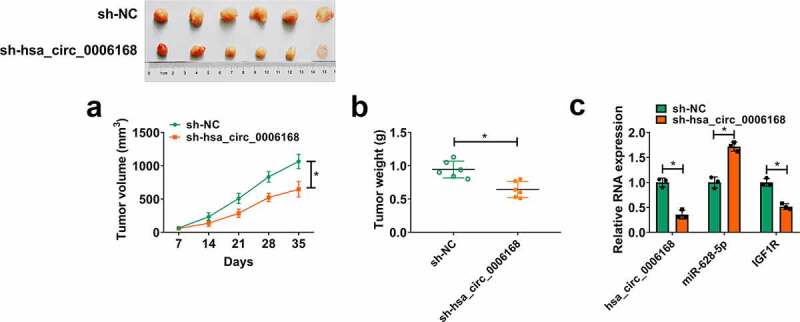
Role of hsa_circ_0006168 in cell proliferation and motility in human GBM cells in vivo. LN229 cells stably transfected with sh-NC or sh-hsa_circ_0006168 were inoculated in nude mice (N = 6). (a, b) Tumor volume and weight were measured with calipers and electronic scales, respectively. (c, d) RT-qPCR detected RNA expression of hsa_circ_0006168, miR-628-5p and IGF1R in tumor tissues from nude mice. *P < 0.05
Interfering hsa_circ_0006168 might inactivate Ras/extracellular signal regulated kinases (Erk) signaling pathway via regulation of miR-628-5p and IGF1R
Western blotting revealed that blocking hsa_circ_0006168 suppressed protein expression of Ras and p-Erk1/2 in in vitro GBM cells (Figure S1A and S1B). Besides, the inhibition of hsa_circ_0006168 deficiency on Ras/Erk pathway in GBM cells in vitro was probably diminished by additionally introducing miR-628-5p inhibitor or pcDNA-IGF1R (Figure S1C and S1D). Notably, depressed protein expression levels of IGF1R, Vimentin, Snail, Ras, and p-Erk1/2 were discovered in hsa_circ_0006168 silencing group in vivo (Figure S1E). Collectively, the results might indicate that hsa_circ_0006168 could modulate cell proliferation and motility of human GBM cells both in vitro and in vivo by regulating miR-628-5p/IGF1R axis via Ras/Erk signaling pathway.
Discussion
The protean world of circRNAs in GBM had been verified to play a role in gliomagenesis and chemoresistance [20,21], as well as early diagnosis of GBM [22]. Deregulated circRNAs were discovered in human GBM tissues [23,24], and quite a few circRNAs exerted oncogenic role in GBM cells by acting as ceRNAs, such as hsa_circ_0001801 [25]. hsa_circ_0006168 was firstly discovered to be remarkably increased in esophageal squamous cell cancer (ESCC) patients and cells [26]. Moreover, hsa_circ_0006168 sponged miRNAs and regulated proliferation, invasion and migration of ESCC cells [26,27]. However, the expression and role of hsa_circ_0006168 in gliomas remained to be disclosed. Here, we retrieved GEO datasets to predict its expression in GBM patients’ tissues and launched RT-qPCR analysis to validate that. Loss-of-functional experiments were performed to investigate the role of hsa_circ_0006168 knockdown in cell proliferation and motility both in vitro and in vivo.
hsa_circ_0006168 level was distinctively upregulated in this cohort of GBM specimens, as well as cell lines derived from GBM patients. Expression of hsa_circ_0006168 in GBM was resistant with RNase R digestion, which had been previously observed in ESCC [26,27]. Functionally, our data showed that forcedly silencing hsa_circ_0006168 in GBM cells suppress cell proliferation, migration, invasion and EMT both in vitro and in vivo. Additionally, hsa_circ_0006168 blockage was responsible to apoptosis promotion and colony formation inhibition in this study. Except for these roles, glycolysis was also found to be inhibited with hsa_circ_0006168 repression in ESCC cells [27]; instead of studying aerobic glycolysis, we confirmed hsa_circ_0006168 role in EMT that was a hallmark of GBM and a driver of changing carcinoma, microenvironment, survive, invasion, metastasis, and resistance to chemo- and/or radio-therapy [28,29]. Notably, in vivo anti-tumor role of hsa_circ_0006168 might be firstly demonstrated in this study.
We considered miR-628-5p as a novel-identified target of hsa_circ_0006168, and its expression was downregulated in GBM specimens and cells. This downregulation had been consistent with other results [10,11,30,31]. Our findings depicted that GBM cell proliferation, colony formation, EMT, migration and invasion were depressed by restoring miR-628-5p, hinting that miR-628-5p was a tumor-suppressor in GBM. Moreover, this finding was consistent with previous data [10,11,30,31]. The promoting role of miR-628-5p in colony formation was similarly revealed in gastric cancer cells [8]. All these data suggested miR-628-5p as a tumor suppressor in multiple cancers, including in gliomas.
In this study, we identified a hsa_circ_0006168/miR-628-5p/IGF1R ceRNA axis. IGF1R could be targeted by a few of miRNAs in gliomas, such as miR-432, miR-422a and miR-503 [32–34]. Here, we proposed IGF1R as a new target gene for miR-628-5p in GBM cells. In function, restoring IGF1R could contribute to cell proliferation, colony formation, migration, invasion, and EMT of GBM cells in vitro, indicating that IGF1R was an oncogene in GBM aggressiveness. IGF1R and its ligands such as IGF1 were expressed in high-grade gliomas [35], and both contributed to glioma cell proliferation, migration and invasion [33]. Even though IGFR1 overexpression has been widely demonstrated to be associated with tumor cell development including GBM [35], there seemed no clear and solid evidence illuminating whether the oncogenic role of IGFR is independent or dependent on its ligands.
It was reported that about 85% of GBM cases showed an overregulation of the Ras/Erk and PI3K/AKT pathways [36], which were two major IGF1R signaling transduction pathways [37]. However, IGF system containing IGF1, IGF1R and binding proteins was little known in gliomas. IGF1R-driven Ras/Erk pathway had been well-described in many types of human cancers, such as breast, lung and gastric cancers, as well as hepatocellular carcinoma [37,38]. And, this study might be the first document to illuminate this pathway in gliomas cells. As a result, protein expression of Ras and p-Erk1/2 were depressed by hsa_circ_0006168 interference in vitro and in vivo (Figure S1A, S1B and S1E), and the in vitro result was similar to the that of Ras antagonist (salirasib [39]) treatment; besides, miR-628-5p downregulation and IGF1R reinforcement could diminish the inhibitory effect of hsa_circ_0006168 silencing on Ras and p-Erk1/2 expression (Figure S1C and S1D), suggesting hsa_circ_0006168/miR-628-5p/IGF1R axis might contribute to the activation of Ras/Erk signaling pathway.
In conclusion, we demonstrated a tumor-suppressive role of hsa_circ_0006168 knockdown in human GBM cell proliferation and motility, and hsa_circ_0006168 functioned as ceRNA for miR-628-5p in regulating IGF1R/Ras/Erk signaling pathway. This finding could define hsa_circ_0006168 as a potential circRNA-based therapeutic and IGF1R-targeting therapy in GBM.
Supplementary Material
Acknowledgments
Not Applicable
Funding Statement
This work was supported by The mechanism study of FOXC2 regulated the transcription level of EphB4/EphrinB2 in the promotion of glioma (NSFC 81702487) and The mechanism study of CXCL5 regulated Notch1 signaling pathway in the promotion of glioma stem cell proliferation and angiogenesis (NSFC 81602207)
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Mazurek M, Grochowski C, Litak J, et al. Recent trends of microRNA significance in pediatric population glioblastoma and current knowledge of micro RNA function in glioblastoma multiforme. Int J Mol Sci. 2020;21(9):3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alexander BM, Cloughesy TF.. Adult Glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. [DOI] [PubMed] [Google Scholar]
- [4].Mesti T, Ocvirk J. Malignant gliomas: old and new systemic treatment approaches. Radiol Oncol. 2016;50(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mercatelli N, Galardi S, Ciafre SA. MicroRNAs as multifaceted players in glioblastoma multiforme. Int Rev Cell Mol Biol. 2017;333:269–323. [DOI] [PubMed] [Google Scholar]
- [6].Huang SW, Ali ND, Zhong L, et al. MicroRNAs as biomarkers for human glioblastoma: progress and potential. Acta Pharmacol Sin. 2018;39(9):1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pottoo FH, Javed MN, Rahman JU, et al. Targeted delivery of miRNA based therapeuticals in the clinical management of Glioblastoma Multiforme. Semin Cancer Biol. 2020;69:391–398. [DOI] [PubMed] [Google Scholar]
- [8].Chen Y, Wu Y, Yu S, et al. Deficiency of microRNA-628-5p promotes the progression of gastric cancer by upregulating PIN1. Cell Death Dis. 2020;122(5):559. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou L, Jiao X, Peng X, et al. MicroRNA-628-5p inhibits invasion and migration of human pancreatic ductal adenocarcinoma via suppression of the AKT/NF-kappa B pathway. J Cell Physiol. 2020;235(11):8141–8154. [DOI] [PubMed] [Google Scholar]
- [10].Xie P, Wang Y, Liao Y, et al. MicroRNA-628-5p inhibits cell proliferation in glioma by targeting DDX59. J Cell Biochem. 2019;120(10):17293–17302. [DOI] [PubMed] [Google Scholar]
- [11].Li Y, Xu J, Chen H, et al. Comprehensive analysis of the functional microRNA–mRNA regulatory network identifies miRNA signatures associated with glioma malignant progression. Nucleic Acids Res. 2013;41(22):e203. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu C, Zhang Z, Tang H, et al. Crosstalk between IGF-1R and other tumor promoting pathways. Curr Pharm Des. 2014;20(17):2912–2921. [DOI] [PubMed] [Google Scholar]
- [13].Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gong Y, Ma Y, Sinyuk M, et al. Insulin-mediated signaling promotes proliferation and survival of glioblastoma through Akt activation. Neuro Oncol. 2016;18(1):48–57. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maris C, D’Haene N, Trepant AL, et al. IGF-IR: a new prognostic biomarker for human glioblastoma. Br J Cancer. 2015;113(5):729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Imperial R, Toor OM, Hussain A, et al. Comprehensive pancancer genomic analysis reveals (RTK)-RAS-RAF-MEK as a key dysregulated pathway in cancer: its clinical implications. Semin Cancer Biol. 2019;54:14–28. [DOI] [PubMed] [Google Scholar]
- [17].Lei K, Bai H, Wei Z, et al. The mechanism and function of circular RNAs in human diseases. Exp Cell Res. 2018;368(2):147–158. [DOI] [PubMed] [Google Scholar]
- [18].Hao Z, Hu S, Liu Z, et al. Circular RNAs: functions and prospects in glioma. J Mol Neurosci. 2019;67(1):72–81. [DOI] [PubMed] [Google Scholar]
- [19].Yuan Y, Jiaoming L, Xiang W, et al. Analyzing the interactions of mRNAs, miRNAs, lncRNAs and circRNAs to predict competing endogenous RNA networks in glioblastoma. J Neurooncol. 2018;137(3):493–502. [DOI] [PubMed] [Google Scholar]
- [20].Wei Y, Lu C, Zhou P, et al. EIF4A3-induced circular RNA ASAP1(circASAP1) promotes tumorigenesis and temozolomide resistance of glioblastoma via NRAS/MEK1/ERK1/2 signaling. Neuro Oncol. 2020;23:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paulmurugan R, Malhotra M, Massoud TF. The protean world of non-coding RNAs in glioblastoma. J Mol Med. 2019;97(7):909–925. [DOI] [PubMed] [Google Scholar]
- [22].Chen A, Zhong L, Ju K, et al. <p>Plasmatic circRNA predicting the occurrence of human glioblastoma. Cancer Manag Res. 2020;12:2917–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu F, Cheng C, Qin H, et al. A novel circular RNA circENTPD7 contributes to glioblastoma progression by targeting ROS1. Cancer Cell Int. 2020;20(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xia X, Li X, Li F, et al. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol Cancer. 2019;18(1):131. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu Y, Deng X, Xiao G, et al. circ_0001730 promotes proliferation and invasion via the miR-326/Wnt7B axis in glioma cells. Epigenomics. 2019;11(11):1335–1352. [DOI] [PubMed] [Google Scholar]
- [26].Shi Y, Guo Z, Fang N, et al. hsa_circ_0006168 sponges miR-100 and regulates mTOR to promote the proliferation, migration and invasion of esophageal squamous cell carcinoma. Biomed Pharmacothe. 2019;117:109151. [DOI] [PubMed] [Google Scholar]
- [27].Xie ZF, Li HT, Xie SH, et al. Circular RNA hsa_circ_0006168 contributes to cell proliferation, migration and invasion in esophageal cancer by regulating miR-384/RBBP7 axis via activation of S6K/S6 pathway. Eur Rev Med Pharmacol Sci. 2020;24(1):151–163. [DOI] [PubMed] [Google Scholar]
- [28].Meel MH, Schaper SA, Kaspers GJL, et al. Signaling pathways and mesenchymal transition in pediatric high-grade glioma. Cell Mol Life Sci. 2018;75(5):871–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Majc B, Sever T, Zaric M, et al. Epithelial-to-mesenchymal transition as the driver of changing carcinoma and glioblastoma microenvironment. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research. 2020;1867(10):118782. [DOI] [PubMed] [Google Scholar]
- [30].Yan Y, Wang Y, Liu Y, et al. <p>Long non-coding RNA AGAP2-AS1/miR-628-5p/PTN axis modulates proliferation, migration, invasion, and apoptosis of glioma cells. Cancer Manag Res. 2020;12:6059–6068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Chen WL, Jiang L, Wang JS, et al. Circ-0001801 contributes to cell proliferation, migration, invasion and epithelial to mesenchymal transition (EMT) in glioblastoma by regulating miR-628-5p/HMGB3 axis. Eur Rev Med Pharmacol Sci. 2019;23(24):10874–10885. [DOI] [PubMed] [Google Scholar]
- [32].Chen X, Zhang X, Sun S, et al. MicroRNA432 inhibits the aggressiveness of glioblastoma multiforme by directly targeting IGF1R. Int J Mol Med. 2020;45(2):597–606. [DOI] [PubMed] [Google Scholar]
- [33].Wang H, Tang C, Na M, et al. miR-422a inhibits glioma proliferation and invasion by targeting IGF1 and IGF1R. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. 2017;25(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang Y, Chen X, Lian H, et al. MicroRNA-503 acts as a tumor suppressor in glioblastoma for multiple antitumor effects by targeting IGF-1R. Oncol Rep. 2014;31(3):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Simpson AD, Soo YWJ, Rieunier G, et al. Type 1 IGF receptor associates with adverse outcome and cellular radioresistance in paediatric high-grade glioma. Br J Cancer. 2020;122(5):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Escamilla-Ramirez A, Castillo-Rodriguez RA, Zavala-Vega S, et al. Autophagy as a potential therapy for malignant glioma. Pharmaceuticals. 2020;13(7):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cevenini A, Orru S, Mancini A, et al. Molecular signatures of the insulin-like growth factor 1-mediated epithelial-mesenchymal transition in breast, lung and gastric cancers. Int J Mol Sci. 2018;19(8):2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu Y, Huang J, Ma L, et al. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016;371(2):171–181. [DOI] [PubMed] [Google Scholar]
- [39].Rotblat B, Ehrlich M, Haklai R, et al. The Ras inhibitor farnesylthiosalicylic acid (Salirasib) disrupts the spatiotemporal localization of active Ras: a potential treatment for cancer. Methods Enzymol. 2008;439:467–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


