Abstract
Objective:
Well-defined germ-line mutations in the PTCH1 gene are associated with syndromic multiple basal cell carcinomas (BCCs). Here, we used whole exome sequencing (WES) to identify the role of patched-1 in patients with multiple, unusually large BCCs.
Methods:
A 72-year old patient presenting with numerous BCCs progressing to large ulcerating lesions was enrolled. WES was used to identify the pathogenic gene locus.
Results:
Genetic work-up by WES identified a homozygous PTCH1 nonsense mutation in the tumor tissue but not present in her blood cells or in non-lesional skin. In addition, heterozygous missense mutations were identified in three cancer-associated genes (EPHB2, RET, and GALNT12) in blood cells as well as in lesional and non-lesional skin. We also tested systemic immune therapy as a potentially beneficial approach to treat patients with numerous large BCCs on scatted areas of involvement. A rapid and sustained response to nivolumab was noted, suggesting that it is an efficacious drug for long-term therapeutic outcome.
Conclusion:
PTCH1, EPHB2, RET, and GALNT12 may potentially contribute to the synergistic oncogene driven malignant transformation manifesting as multiple, unusually large BCCs.
Keywords: immune therapy, malignant transformation gene-susceptibility, non-syndromic basal cell carcinoma, PTCH1, skin neoplasms
Introduction
Well-defined germ-line mutations in the PTCH1 gene are associated with syndromic multiple basal cell carcinomas (BCCs) or Gorlin syndrome.1 DNA isolated from the blood cells and non-sun exposed normal skin of our patient showed germ-line mutations of GALNT12, RET, and EPHB2 (Tables 1 and 2). GALNT12 is reported to be associated with increased susceptibility for colorectal carcinoma.2-3RET is a known proto-oncogene reported in association with multiple endocrine neoplasia type 2, familial medullary thyroid carcinoma, lung carcinoma, pheochromocytoma, and other sporadic carcinomas.4–6RET gene fusions caused by chromosomal rearrangements resulting in RET onco-protein have been associated with papillary thyroid carcinoma.7 Moreover, sequence variants in EPHB2, another oncogene, were initially reported in prostate and brain carcinomas,8 but recently also in association with colorectal carcinoma9 and squamous cell carcinoma.10 Interestingly, high expression of oncogenes GALNT12 and EPHB2 is reported to be associated with favorable prognosis and survival in various malignancies, including follicular lymphoma and colorectal carcinoma. These studies have also suggested an association between lower expression of GALNT12 and EPHB2 oncogenes and poor prognosis in such patients.9,11-12 In the current study, we performed genetic tests on a female patient with numerous BCCs to identify the role of patched-1 in genetic mechanisms of this unusual presentation of the disease.
Table 1.
Documented cancer susceptibility and oncogenicity of mutations in EPHB2, RET and GALNT12
| Gene | Associated disorders |
|---|---|
| EPHB2 | Prostate cancer, brain cancer |
| RET | Familial medullary thyroid carcinoma, Pheochromocytoma, Hereditary cancer-predisposing syndrome, Hirschsprung disease, Renal dysplasia, Multiple endocrine neoplasia types 1, 2a, 2b, and 4 |
| GALNT12 | Hereditary cancer-predisposing syndrome |
Table 2.
The GRCh38 coordinates of mutations in the present case with multiple large basal cell carcinomas
| GRCh38 coordinates | Mutant Allele Proportion Tissue Origin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dbSNP ID | Chr | Position | Ref | Alt | Gene | Consequence | Amino Acid Change | 1000 genomes MAF | ExAC MAF | Blood | Normal | Lesion |
| rs28936395 | 1 | 22906853 | G | A | EPHB2 | nonsynonymous SNV | NM_004442:p.D679N (exon11) | 0.001198 | 0.0033 | 47% | 46% | 24% |
| rs77724903 | 10 | 43118460 | A | T | RET | nonsynonymous SNV | NM_020630:p.Y791F (exon13) | 0.0002 | 0.0016 | 50% | 49% | 47% |
| rs145236923 | 9 | 98831947 | G | A | GALNT12 | nonsynonymous SNV | NM_024642:p.D303N (exon4) | 0.000799 | 0.0011 | 47% | 42% | 3% |
Chr, chromosome; Ref, reference sequence; Alt, alteration; MAF, minor allele frequency.
Material and methods
Patient
A 72-year-old woman with numerous primary BCCs involving non-sun exposed as well as sun-exposed areas of skin (Fig. 1) was enrolled in the study after the informed consent for performance of the genetic tests was provided.
Figure 1.
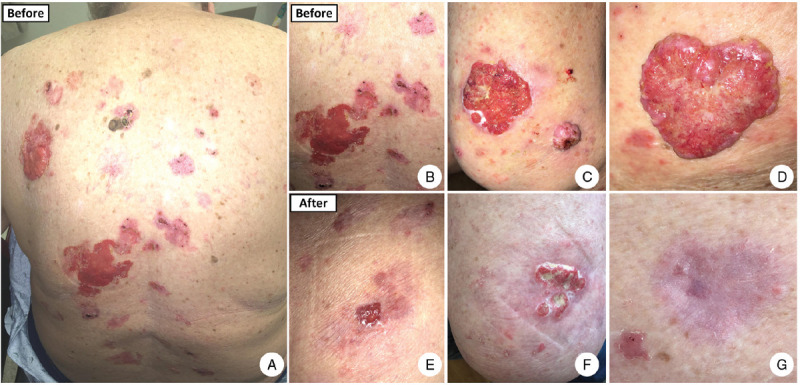
Clinical presentation of the patient with numerous basal cell carcinomas before and after treatment with nivolumab. A–D: There are numerous large, exophytic, and ulcerating BCCs involving mainly non-sun exposed areas of the skin (A), including mid and lower back (B), bilateral thighs (C), and buttock (D), as well as sun-exposed areas including lower legs, forearms, and hands (not shown). Head and neck including face were largely spared. E–G: Almost all large and ulcerating tumors have regressed mostly or completely after treatment with nivolumab. There are few small and thin residual lesions that are gradually resolving after treatment.
Genetic tests
A peripheral blood sample, as well as skin biopsies from normal and tumor tissue were obtained from the patient, and DNA was extracted. These three DNA samples were sequenced on the Illumina NextSeq using the Illumina TruSeq Whole Exome Sequencing kit. Sequencing was performed in paired-end mode with 150-base reads. The resulting paired-end reads were aligned to the human genome version GRCh38, using the BWA mem aligner. Alignment metrics indicated satisfactory coverage averaging above 100× for all three types of samples. Picard tools software was used to flag duplicates, and the genome analysis toolkit was used for base quality score recalibration; both steps are necessary to remove bias from subsequent variant calling. The FreeBayes Bayesian genetic variant detector was used to identify base substitutions and short insertion/deletion sequences in the samples. The resulting set of variants was annotated with ANNOVAR software. The presence and absence of variants and associated variant frequencies were reviewed across all three samples with aims of identifying (1) germline mutations in the genes with known roles in cancer etiology, and (2) somatic mutations or mosaicism in tumor sample with known roles in cancer development.
Results
Clinical report of the patient
The first BCC appeared when the patient was in her late 50s, and thereafter she developed about 10 new lesions per year. In her mid-60s, she noticed a sudden increase in appearance of new lesions as well as rapid increase in the size and volume of the existing tumors. Although at the beginning, few early lesions were treated surgically, later on she was no longer deemed to be a candidate for surgical interventions due to the size and number of tumors. When presented, she was suffering from pain and bleeding of numerous ulcerating lesions with major impact on her quality of life.
On physical examination, numerous skin tumors were observed mostly involving non-sun exposed areas (Fig. 1). Biopsies from several representative lesions confirmed the diagnosis of BCC as the underlying malignancy (Fig. 2), while only one lesion showed squamous cell carcinoma. On careful examination, no other skin findings, such as palmar pits, suggestive of Gorlin syndrome, were noted. Furthermore, whole body positron emission tomography/computed tomography scan failed to show any metastatic disease or extracutaneous findings suggestive of Gorlin syndrome (Fig. 2). Genetic profiling of DNA isolated from her blood cells and non-sun exposed normal as well as lesional skin identified mutations that can predispose to carcinomatous changes as detailed below in the Results and Discussion sections (Fig. 3). Given the extensive nature of her disease and possible underlying genetic predisposition, systemic treatment, including nivolumab, was considered.
Figure 2.
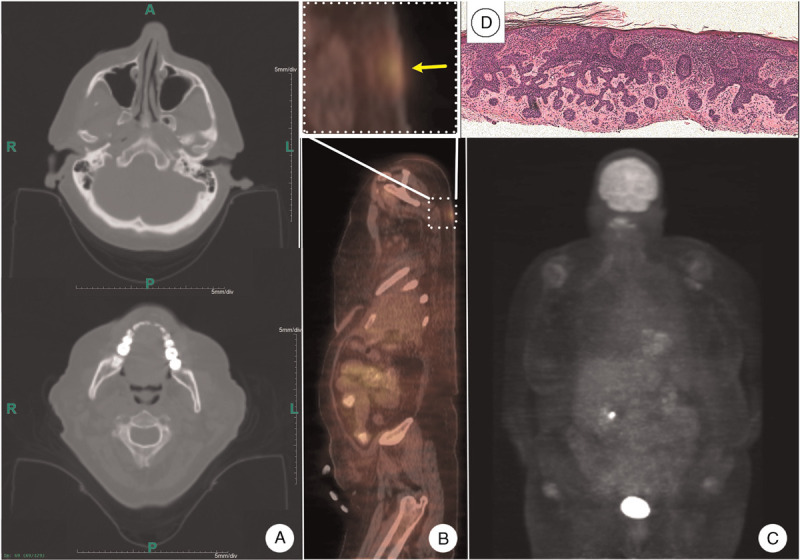
Imaging studies and histopathology. A: Head and neck CT scan failed to show any skeletal or brain stem abnormalities suggestive of Gorlin syndrome. B: Whole body PET/CT showed no evidence of metastasis (lower panel). Large cutaneous tumor (yellow arrow) showed increased metabolic activity (upper panel). C: Brain and other internal organs showed physiologic distribution of fluorodeoxyglucose. D: Histomicrograph of the biopsy from the lesion shown in section proved to be a BCC (hematoxylin-eosin stain). BCC: Basal cell carcinoma; CT: Computed tomography; PET: Positron emission tomography.
Figure 3.
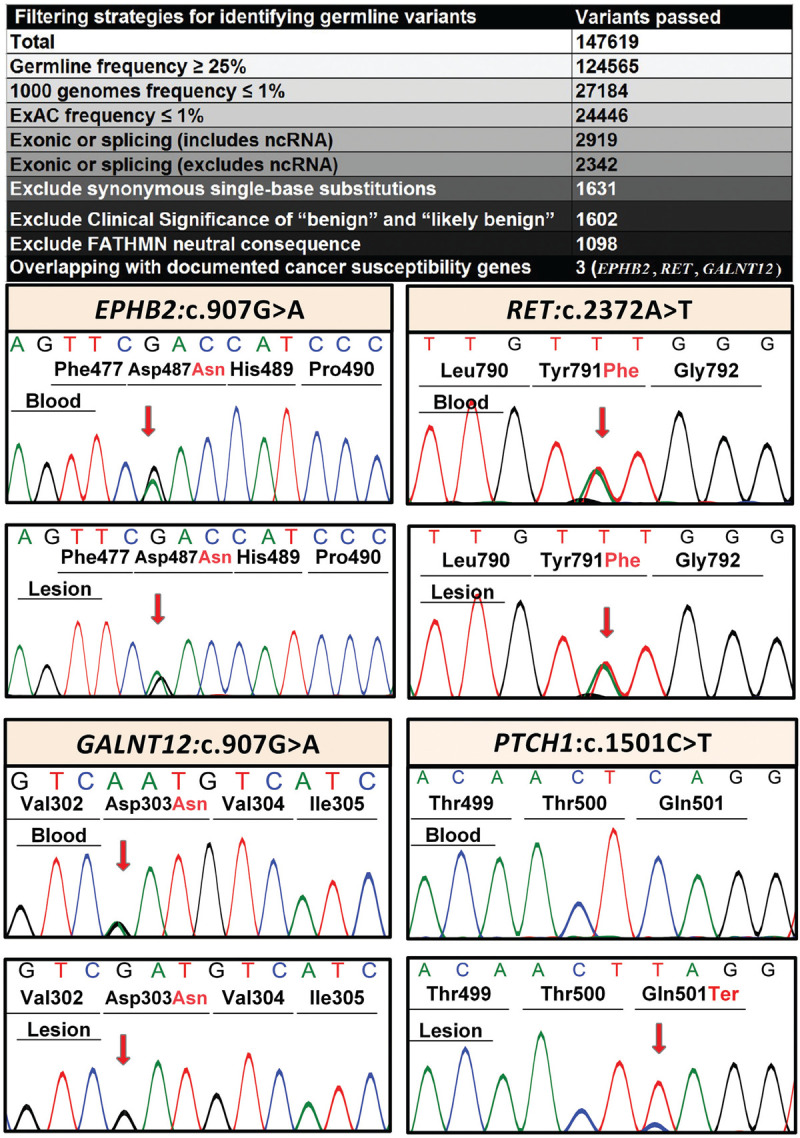
Identification of sequence variants in cancer susceptibility genes by whole exome sequencing. Filtering strategy for finding germ-line oncogene variants in DNA isolated from blood cells of the proband identified three candidate genes, including GALNT12, RET, and EPHB2 (upper panel). Sanger sequencing confirmed the heterozygous state of these variants in blood and in lesional skin (lower panel). In addition, whole exome sequencing showed the presence of a homozygous PTCH1 variant in the tumor sample while the blood cells and normal skin were negative for this variant.
PD-L1 and PD-1 protein expression has been reported in BCC and tumor infiltrating lymphocytes (TIL). In addition, anti-PD-1 inhibitors have shown to have activity in the treatment of advanced BCC.13 In a sample of the patient's BCC tumor, low positive PD-1 immuno histochemistry in TIL was reported by Foundation One Medicine. Therefore, nivolumab was started as an option given the complexity and the extent of her disease. Moreover, the long lasting effect of immune-modulating therapy approach was thought to help treat the existing lesions and hypothetically prevent recurrence once the treatment is discontinued.
Treatment was initiated with nivolumab 240 mg every 2 weeks. To enhance durable response, low-dose radiation was applied to one of the ulcerating tumors on her left back to elicit a neo-antigen response. The outcome proved successful with interim decrease in size and number of tumors on each subsequent visit (Fig. 1). However, after the ninth infusion, she developed grade 1 hepatitis and grade 2 colitis. Nivolumab was discontinued and the adverse events were successfully managed with a rapid course of tapering prednisone. Despite discontinuation of treatment, a year later the patient still exhibited a durable response showing interval improvement on each visit. All large ulcerating lesions had resolved and only a few small, thin BCCs remained (Fig. 1). Most importantly, no new lesions had been noticed since the first infusion of nivolumab.
Sequencing results
A total of 147,619 variants were annotated and subjected to bioinformatics filtering by steps shown in Fig. 3, and sequence variants in three documented cancer susceptibility genes were identified. These heterozygous germline variants, which were found both in the blood DNA as well as in non-lesional and lesional skin, included EPHB2: NM_004442: c.2035G>A, p.D679N; RET: NM_020630: c.2372A>T; p.Y791F; and GALNT12: NM_024642: c.907G>A, p.D303N. These sequence variants were confirmed by Sanger sequencing (Fig. 3). The proportions of mutant alleles of EPHB2 and RET variants were high in all three types of DNA analyzed, while the GALNT12 variant was present in lesional DNA at very low level (Tables 1 and 2). The p.D679N variant in EPHB2 was previously associated with primary tumor of prostate cancer in 2 out of 95 primary and metastatic specimens.8 This variant resides in kinase domain of the EPHB2 protein which is essential for receptor signaling and is the most frequently targeted region for mutations. Next, the variant p.Y791F in RET has been reported in one patient out of 271 patients who presented with nonsyndromic pheochromocytoma without a family history of the disease.14 The third variant, p.D303N in GALNT12, has been previously reported in colorectal cancer and this mutation was shown to reduce the enzymatic activity of N-acetylgalactosaminyl transferase by 37%. In addition, the patient was found to have a homozygous, previously unreported sequence variant in exon 10 of PTCH1: NM_000264; c.1501C>T; p.Q501X. This mutation was present in tumor tissue only, and not in DNA isolated from blood cells or normal skin (Fig. 3). This sequence variant was absent in 119,654 alleles in the control population (ExAC.broadinstitute.org), and 1000 Genomes (www.1000genomes.org).
Discussion
In the case presented here, the presence of GALNT12 and EPHB2 was high in blood and normal non-sun exposed skin but drops to significantly lower level of presence in multiple samples of large, exophytic, and ulcerating BCCs from the same patient. Whether such findings reflect a parallel poor prognosis or the potential to advance the lesions to large exophytic and ulcerating tumors mainly in non-sun-exposed areas as seen in the present case, cannot be ascertained only on the basis of the one patient presented here.
It is plausible that patients who present with numerous skin carcinomas, but proven not to have Gorlin syndrome, may have a similar or another type of hereditary susceptibility to germ-line oncogene mutations that may involve areas as limited as a dermatome or the whole skin, depending on when such mutations occurred during embryonic development. This is in contrast with most patients in general population, who present with one or a few solitary basal cell carcinomas involving mainly sun-exposed areas of skin. Recently, such genetic susceptibility has been reported with the ACTRT1 gene encoding an inhibitor of hedgehog signaling to suppress BCC. In our patient, no mutations in this gene were detected.
In published research from our group, the percentage of UV signature in idiopathic sun-induced SCC tumors ranged between 60% to 85%.15 In comparison, a BCC tumor sample from the shoulder of our patient, showed only 50% UV signature. There are published reports of >90% UV-specific p53 mutations in idiopathic cutaneous SCCs. In the same report, specific UV-induced mutations were found only in 50% of sporadic BCCs. These observations can potentially question the impact of cumulative UV irradiation as the main underlying driver etiology in formation of BCCs. When it comes to patients with numerous BCCs, especially in non-sun exposed areas, the UV causality in relation to BCC can be further questioned.16 In this context, new reports of BCC-associated genes, such as PTPN14 and LATS1 have recently been introduced in the literature.17
One limitation is that the subject of the study was only one patient and we need to pursue these findings in additional cases with similar presentation. Also, we studied only two representative tumors among the numerous ones. Moreover, for control we used non-sun exposed normal skin, while in the future, we also plan to study adjacent normal skin as control.
Given the implication of such genetic predisposition, the patients could benefit from broader surveillance of skin as well as other organs at risk, including, but not limited to colon. Our patient had a recent normal colonoscopy as well as an updated normal mammography. Currently, she has full skin surveillance every 3–6 months. Extending genetic investigation to direct family members, especially offspring, can be helpful and potentially lifesaving. Accordingly, treatment options with durable response, such as immune modulating therapies, can be the preferred treatment method in skin carcinoma patients with underlying inherited genetic susceptibility for malignant transformation even in the absence of metastases. To the best of our knowledge, this is the first report of systemic immune therapy, nivolumab, given for treatment of BCC in general or specifically applied to a patient with BCCs with inherent susceptibility for malignancy transformation in the absence of metastasis.
Acknowledgments
The authors thank the patient and her family for participation in this study. Carol Kelly assisted in manuscript preparation.
Source of funding
The study was supported by NIH R01 IA143810, and the Department of Dermatology and Cutaneous Biology, Thomas Jefferson University Institutional funds.
References
- [1].Guarneri B, Borgia F, Cannavò SP, et al. Multiple familial basal cell carcinomas including a case of segmental manifestation. Dermatology 2000;200(4):299–302. doi:10.1159/000018391. [DOI] [PubMed] [Google Scholar]
- [2].Guda K, Moinova H, He J, et al. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc Natl Acad Sci U S A 2009;106(31):12921–12925. doi:10.1073/pnas.0901454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clarke E, Green RC, Green JS, et al. Inherited deleterious variants in GALNT12 are associated with CRC susceptibility. Hum Mutat 2012;33(7):1056–1058. doi:10.1002/humu.22088. [DOI] [PubMed] [Google Scholar]
- [4].Huang SC, Koch CA, Vortmeyer AO, et al. Duplication of the mutant RET allele in trisomy 10 or loss of the wild-type allele in multiple endocrine neoplasia type 2-associated pheochromocytomas. Cancer Res 2000;60(22):6223–6226. [PubMed] [Google Scholar]
- [5].Huang SC, Torres-Cruz J, Pack SD, et al. Amplification and overexpression of mutant RET in multiple endocrine neoplasia type 2-associated medullary thyroid carcinoma. J Clin Endocrinol Metab 2003;88(1):459–463. doi:10.1210/jc.2002-021254. [DOI] [PubMed] [Google Scholar]
- [6].Eng C, Crossey PA, Mulligan LM, et al. Mutations in the RET proto-oncogene and the von Hippel-Lindau disease tumour suppressor gene in sporadic and syndromic phaeochromocytomas. J Med Genet 1995;32(12):934–937. doi:10.1136/jmg.32.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008;93(3):682–687. doi:10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- [8].Huusko P, Ponciano-Jackson D, Wolf M, et al. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nat Genet 2004;36(9):979–983. doi:10.1038/ng1408. [DOI] [PubMed] [Google Scholar]
- [9].Jang BG, Kim HS, Chang WY, et al. Prognostic significance of EPHB2 expression in colorectal cancer progression. J Pathol Transl Med 2018;52(5):298–306. doi:10.4132/jptm.2018.06.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farshchian M, Nissinen L, Siljamäki E, et al. EphB2 promotes progression of cutaneous squamous cell carcinoma. J Invest Dermatol 2015;135(7):1882–1892. doi:10.1038/jid.2015.104. [DOI] [PubMed] [Google Scholar]
- [11].Jubb AM, Zhong F, Bheddah S, et al. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res 2005;11(14):5181–5187. doi:10.1158/1078-0432.CCR-05-0143. [DOI] [PubMed] [Google Scholar]
- [12].Gibson TM, Wang SS, Cerhan JR, et al. Inherited genetic variation and overall survival following follicular lymphoma. Am J Hematol 2012;87(7):724–726. doi:10.1002/ajh.23184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lipson EJ, Lilo MT, Ogurtsova A, et al. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer 2017;5:23. doi:10.1186/s40425-017-0228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 2002;346(19):1459–1466. doi:10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- [15].Cho RJ, Alexandrov LB, den Breems NY, et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci Transl Med 2018;10(455). doi:10.1126/scitranslmed.aas9668. [DOI] [PubMed] [Google Scholar]
- [16].Reichrath J, Rass K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: an update. Adv Exp Med Biol 2014;810:208–233. doi:10.1007/978-1-4939-0437-2_12. [DOI] [PubMed] [Google Scholar]
- [17].Pellegrini C, Maturo MG, Di Nardo L, et al. Understanding the molecular genetics of basal cell carcinoma. Int J Mol Sci 2017;18(11). doi:10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]


