Abstract
Aim: Recently, it has been established that most of the pleiotropic effects of high-density lipoprotein (HDL) are attributed to sphingosine 1-phosphate (S1P), which rides on HDL via apolipoprotein M (ApoM). In subjects with diabetes mellitus, both the pleiotropic effects of HDL and its role in reverse cholesterol transport are reported to be impaired. To elucidate the mechanisms underlying the impaired pleiotropic effects of HDL in subjects with diabetes, from the aspects of S1P and ApoM.
Methods: The incubation of HDL in a high-glucose condition resulted in the dimerization of ApoM. Moreover, the treatment of HDL with methylglyoxal resulted in the modulation of the ApoM structure, as suggested by the results of western blot analysis, isoelectric focusing electrophoresis, and two-dimensional gel electrophoresis, which was reversed by treatment with anti-glycation reagents.
Results: The glycation of HDL resulted in impaired binding of the glycated HDL to S1P, and the S1P on glycated HDL degraded faster. In the case of human subjects, on the other hand, although both the serum ApoM levels and the ApoM content in HDL were lower in subjects with diabetes, we did not observe the polymerization of ApoM.
Conclusions: Modulation of the quantity and quality of ApoM might explain, at least in part, the impaired functions of HDL in subjects with diabetes mellitus. ApoM might be a useful target for laboratory testing and/or the treatment of diabetes mellitus.
Keywords: Sphingosine 1-phosphate, HDL, Apolipoprotein M, Glycation
Abbreviations
AG:aminoguanidine, Arg:L-arginine, ALT-711:alagebrium chloride, ApoM:apolipoprotein M, BMOE:bismaleimidoethane, DSS:disuccinimidyl suberate, HDL:high-density lipoprotein, IEF:isoelectric focusing electrophoresis, LC-SDA:succinimidyl 44’-azipentanoate, MG:methylglyoxal, S1P:sphingosine 1-phosphate, PNGase F:peptide-N-glycosidase F
Introduction
The inverse correlation between the serum levels of high-density lipoprotein (HDL) and the risk of atherosclerosis is well established. Along with its important role in reverse cholesterol transport, HDL has been reported to exert many pleiotropic effects, such as antiapoptotic, anti-inflammatory, and vasoprotective effects 1) . The relatively low levels of HDL in subjects with diabetes may explain, at least in part, the high prevalence of atherosclerotic diseases in these subjects 2) . In addition to the quantitative abnormality of HDL in subjects with diabetes, a series of studies has also shown impairments of the pleiotropic effects of HDL, especially its anti-inflammatory and antioxidant effects, in patients with diabetes 3 - 5) .
Recently, many of these pleiotropic effects of HDL have been shown to be attributable to the sphingosine 1-phosphate (S1P) distributed on HDL 6) . Two-thirds of the plasma S1P is distributed on HDL; most of the remaining is distributed on albumin 7) . S1P exerts potent bioactivities through the mediation of S1P receptors expressed on the cell membrane, such as antiapoptotic effects 8) , cell proliferative effects 9) , vasorelaxant effects, and beneficial effects on the maintenance of the vascular permeability 10 , 11) , some of which overlap with the pleiotropic effects of HDL. In 2011, Christoffersen et al. reported that apolipoprotein M (ApoM) is the main carrier of S1P on HDL 12) . We and others have demonstrated that ApoM is also an important modulator of S1P metabolism and functions 13 - 22) .
Considering the impaired pleiotropic effects of HDL in subjects with diabetes and the contribution of ApoM to the functions of HDL through S1P, the modulation of ApoM might partly explain the impaired functions of HDL in these subjects. Some studies have shown that plasma ApoM concentrations are lower in subjects with type 2 diabetes mellitus 23 - 26) . In addition to these quantitative changes in serum ApoM levels, it was recently shown that the glycation of HDL can lower the S1P content on HDL and that the supplementation of S1P on glycated HDL restored the anti-inflammatory effects of HDL; however, the mechanism for the reduction in S1P content on glycated HDL has not yet been elucidated 27) .
Regarding the association between the glycation of HDL and ApoM, western blot analyses of human ApoM showed that it is the ApoM on HDL that is glycated and that this glycation might be specific to human ApoM (Supplemental Fig. III in the paper published previously by us) 28) .
Therefore, in this study, we investigated the possibility that the impaired capacity of HDL to carry S1P under high-glucose conditions and/or as a result of glycation may be mediated by modulating the quantity and quality of ApoM on HDL, and that such a modulation might explain, at least in part, the decreased anti-atherosclerotic effects of HDL in subjects with diabetes.
Aim
In this study, we attempted to elucidate the mechanisms underlying the impaired pleiotropic effects of HDL in subjects with diabetes, from the aspects of S1P and ApoM.
Methods
Separation and Preparation of Lipoproteins
Blood samples were collected from the study participants, including healthy human volunteers and patients with diabetes, after they provided written informed consent for participation in this study. We separated the HDL fractions using a standard ultracentrifugation method. HDL was then dialyzed against phosphate buffered saline (PBS) for 48 hours before it was used for the in vitro experiments. This study was conducted with the approval by the Institutional Research Ethics Committee of the Faculty of Medicine, the University of Tokyo (10266 and 11158). The characteristics of the subjects are described in Table 1 .
Table 1. Clinical characteristics of the subjects.
| DM patients ( n = 36) | Healthy volunteers ( n = 22) | |
|---|---|---|
| Age (years) | 66.2±14.5 | 27.4±2.4 |
| Female, n (%) | 13 (36%) | 9 (41%) |
| HbA1c, % | 7.8±1.5 | not assessed |
| T-Cho, mg/dL | 167.6±54.3 | 186.3±35.7 |
| TG, mg/dL | 208.1±422.9 | 94.0±76.7 |
| Serum ApoA1, mg/dL | 124.3±39.2 | 146.0±19.1 |
Data are presented as the means±S.D.
Glycation of HDL and Anti-Glycation of HDL
We incubated HDL at the concentration of 1 mg/mL with glucose or methylglyoxal (MG; Sigma-Aldrich Co., St. Louis, MO, USA) at various concentrations (glucose: 100 and 500 mg/dL; MG: 0.06, 0.6, 1.6, 6, 16, 60 and 160 mM) at 37℃ for 24 or 96 hours. After treatment with MG, glycated HDL was dialyzed against sterile PBS at 4℃ for 48 hours. Naive HDL was prepared as the control in the same manner, except for the treatment with MG.
The enzymatic deglycosylation of HDL was performed using peptide-N-glycosidase F (PNGase F), neuraminidase, and o-glycosidase (New England Biolabs, Inc., Ipswich, MA), in accordance with the manufacturer’s protocol. To prevent glycation, we incubated HDL with aminoguanidine (AG; guanidine hydrochloride, Tokyo Kasei Co., Tokyo), L-arginine (Arg, Sigma-Aldrich Co., St. Louis, MO, USA), or alagebrium chloride (ALT-711, CHEMOS GmbH & Co., KG, Germany) prior to its treatment with MG.
Cross-Linking Reactions
HDL was solubilized in PBS to a final concentration of 1.0 mg/mL. We allowed the cross-linking reactions to take place by the addition of 1 mM disuccinimidyl suberate (DSS), 0.2 mM bismaleimidoethane (BMOE), and 0.5 mM succinimidyl 4,4’-azipentanoate (LC-SDA, Sigma-Aldrich Co., St. Louis, MO, USA), in accordance with the manufacturer’s protocol. We also performed a similar experiment with a dilution series of DSS to 31.3 µM.
Investigation of the Capacity of HDL to Bind C 17 S1P
We prepared 1 mg/mL of HDL obtained from healthy volunteers, as described above, and 10 mg/mL of albumin solution containing 1 µM of C 17 S1P (Avanti Polar Lipids, Birmingham, AL). We mixed 1 µM C 17 S1P bound to 10 mg/mL albumin with 0.5 mg/mL glycated HDL. After incubation for 30 minutes at 37℃, the samples were separated into HDL and albumin fractions by ultracentrifugation again. Then, the C 17 S1P levels were measured.
Cell Experiments
To examine the modulation of ApoM by glucose in the medium, HepG2 cells, which were obtained from ATCC, were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in an incubator under 5% CO 2 . Then, after the cells were washed twice with PBS, the medium was replaced with DMEM containing 0, 100, 200, or 500 mg/dL of glucose. After another 24 hours, the media and the cells were collected for western blot analyses or real-time polymerase chain reaction (PCR) analyses.
To examine the modulation of the degradation of S1P on HDL, human embryonic kidney (HEK293) cells, obtained from ATCC, were cultured to confluence in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Then, after the cells were washed with PBS, the medium was replaced with serum-free DMEM containing dialyzed native HDL or HDL glycated with 6 mM MG, bound to C 17 S1P at the final concentration of 1 µM. After 2 hours, the medium was collected and the concentration of C 17 S1P was measured.
Real-Time PCR
Total RNAs extracted from HepG2 cells with the GenElute Mammalian Total RNA Miniprep kit (Sigma-Aldrich Co., St. Louis, MO, USA) were subjected to reverse transcription with Superscript II enzyme (Invitrogen, Massachusetts, USA). Quantitative PCR was performed using an ABI 7300 Real-Time PCR System (Applied Biosystems, Forster City, CA, USA) for human ApoM (Hs01597780_g1) and β-actin (Hs01060665_g1). The expression levels of ApoM were adjusted to those of endogenous β-actin mRNA used as the control.
Western Blot Analysis, Isoelectric Focusing Electrophoresis, and Two-Dimensional Gel Electrophoresis
Cell lysates were prepared with RIPA lysis buffer. We performed western blot analyses with 10 µg of the cell lysates or HDL fraction, using the standard method. The following antibodies were used: anti-human ApoM serum prepared as described in a previous article 13) , anti-apoA-I antibody (sc-30089; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-ApoE antibody (AB947; Chemicon International Inc., Temecula, CA, USA), anti-ApoC-II antibody (178428; Calbiochem, San Diego, CA, USA), anti-ApoA-II antibody (ab92478; Abcam, Cambridge, UK), and anti-β actin antibody (MBL, Nagoya, Japan).
We performed isoelectric focusing electrophoresis (IEF) with 10 µg of HDL, using Novex Pre-Cast vertical IEF gels (pH 3–7) (Invitrogen, Massachusetts, USA). The electrophoresis was performed in an Xcell SureLock TM Mini-Cell unit (Invitrogen, Massachusetts, USA) for 1, 1, and 0.5 hour, with a voltage limit of 100, 200, and 500 V, respectively.
We performed two-dimensional gel electrophoresis to further investigate the physical modulation of ApoM by the glycation of HDL using Ettan IPGphor3 (GE Healthcare, city, US state code), in accordance with the manufacturer’s protocol. Briefly, IPG gel strips (7 cm, pH range 4–7, Bio-Rad, city, US state code, USA) were rehydrated for 1 hour after overlaying them with mineral oil. This was followed by active rehydration (50 V) of 7-cm strips for 12 hours at 20°C. For re-swelling of the dry IPG strips, rehydration and isoelectric focusing were performed at 20℃ under the following conditions: at 50 V for 12 hours (active rehydration), 250 V for 30 minutes, 1,000 V for 1 hour, and 10,000 V for 5 hours. When the isoelectric focusing was completed, equilibration of the IPG strips was performed. The two-dimensional separation was performed by SDS-PAGE using 10–20% gradient gels (Thermo Fisher Scientific, Waltham, MA, USA) sealed with 0.5% (w/v) agarose. The samples were electrophoresed at 200 V for 30 minutes until the bromophenol blue moved to 0.5 cm above the lower margin of the gel, and then subjected to western blot, as described above.
Measurement of S1P and C 17 -S1P
The contents of S1P and C 17 -S1P in the plasma, serum, and medium were determined by two-step lipid extraction, followed by high performance liquid chromatography separation, as described and validated previously 28) .
Measurement of ApoA1 and ApoM
ApoA1 (SEKISUI, Tokyo, Japan) concentrations were determined using a modified enzymatic assay, in a biochemical automatic analyzer (BM6030 [JEOL, Tokyo, Japan]). ApoM levels in the serum or on HDL were measured by ELISA, as described and validated previously 17) .
Statistical Analysis
The results are expressed as the means±SD. Differences between two groups were evaluated by the Student t -test and those among more than three groups were assessed by one-way analysis of variance, followed by the Tukey–Kramer test as post-hoc analysis. P values less than 0.05 were deemed to indicate statistical significance.
Results
Treatment with a High Glucose Concentration Resulted in Dimerization of ApoM in HDL, but did not Affect the Capacity of HDL to Bind S1P
First, we investigated whether the incubation of HDL with various concentrations of glucose (0, 100, 200, or 500 mg/dL) for 24 or 96 hours modulated the bands of ApoM on the western blots. We observed that depending on the glucose concentration and the duration of incubation, the ApoM bands appeared at around 50 kDa, as well as the innate ApoM bands at 25 kDa ( Fig.1A ) . These results suggest that ApoM might dimerize when HDL is placed under a high-glucose condition for a long duration. When we investigated the C 17 S1P-binding capacity of HDL following its incubation under a high-glucose condition, however, no significant modulation of the C 17 S1P-binding capacity of HDL was observed ( Fig.1B ) .
Fig.1. Modulations of ApoM following the incubation of HDL and HepG2 cells under a high-glucose condition .
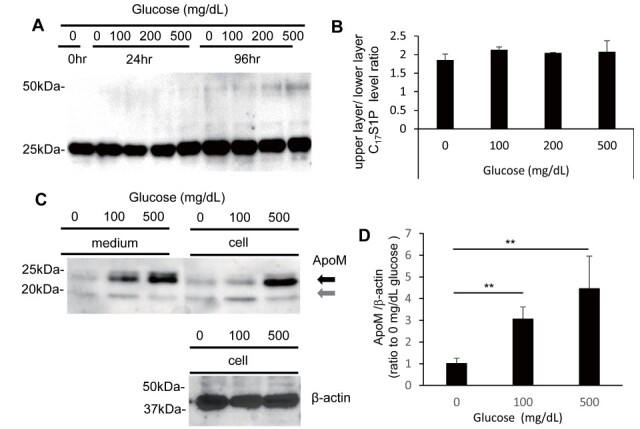
(A, B) HDL was incubated under various glucose conditions for 24 or 96 hours. (A) Western blot analyses were performed for ApoM. (B) The binding capacities of HDL incubated under various glucose conditions for 96 hours were evaluated by the pattern of redistribution of C 17 S1P between HDL and albumin ( n =4).
(C, D) HepG2 cells were cultured in media containing various concentrations of glucose. (C) Western blot analysis for ApoM in medium or cell lysate. (D) Real-time PCR for ApoM. β-actin was used as the control in both assays ( n =6). ** P <0.01.
Treatment with High Glucose Concentrations Increased the Expression of ApoM in HepG2 Cells
Since plasma ApoM is derived mainly from hepatocytes, we next investigated whether the concentration of glucose in the medium might affect the expression and glycation levels of ApoM in HepG2 cells, a cell line derived from human hepatocellular carcinoma. The medium and cellular ApoM levels were higher in the case of the HepG2 cells incubated in a medium containing 500 mg/dL of glucose; the patterns of the cellular ApoM bands suggested that the glycated form of ApoM (bands marked with a black arrow) decreased and a non- or less glycated form of ApoM appeared (bands marked with a gray arrow) in the HepG2 cells when they were incubated in a medium containing 0 or 100 mg/dL of glucose ( Fig.1C ) . The ApoM mRNA expression levels were also higher in the HepG2 cells incubated in a medium containing 500 mg/dL of glucose ( Fig.1D ) .
Treatment with MG Resulted in Polymerization of ApoM in HDL
Next, we treated HDL with various concentrations of MG (0.06, 0.6, and 6 mM) that are commonly used to investigate the effects of glycation. Consistent with the results of the experiments performed using high-glucose conditions ( Fig.1A ) , we observed that the extra bands, other than those of the innate ApoM bands, appeared at around 50, 75, and 100 kDa, depending on the concentration of MG ( Fig.2A and B ) , suggesting the possible polymerization of ApoM.
Fig.2. Modulations of ApoM in HDL glycated with methylglyoxal .
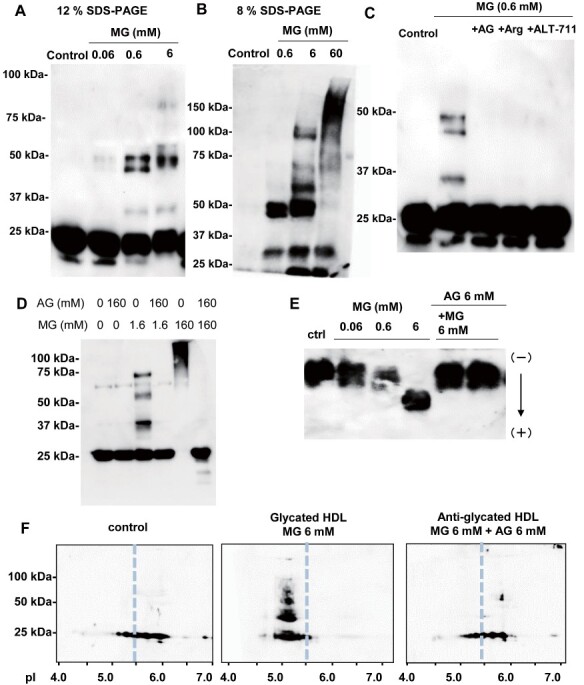
HDL was incubated with methylglyoxal (MG) at various concentrations (0, 0.06, 0.6, and 6 mM) after or without pretreatment with aminoguanidine (AG), L-arginine (Arg), or N-phenacyl-4,5-dimethylthiazolium bromide (ALT-711). (A–C) Western blot analyses for ApoM with 12% SDS-PAGE (A, C) and with 8% SDS-PAGE (B). (D) Western blot analyses for ApoA-I. (E) Isoelectric focusing electrophoresis for ApoM. (F) Two-dimensional electrophoresis for ApoM.
When we treated HDL with MG in the presence of AG, Arg, or ALT-711, which are anti-glycation reagents, the bands at higher molecular weights of ApoM disappeared ( Fig.2C ) . Regarding ApoA-I, we also observed that ApoA1 bands appeared at higher molecular weight when glycated HDL and these bands were diminished by treatment with AG ( Fig.2D ) . We also performed western blot analyses with glycated HDL for ApoA-II, ApoC-II, and ApoE, and observed that ApoA-II and ApoE bands appeared at around 50 kDa ( Supplemental Fig.1C, E ) , ApoA-I, ApoA-II, ApoC-II, and ApoE bands at around 75 kDa ( Supplemental Fig.1G–J ) , and ApoA-I, ApoA-II, and ApoE bands at around 100 kDa ( Supplemental Fig.1G, H, J ) . Considering that the molecular weights of ApoA-I, Apo A-II, Apo C-II, and ApoE are 28, 17, 9, and 34 kDa, respectively, we could not exclude the possibility that some of the ApoM molecules might form polymers with these apolipoproteins. We also observed the extra band between 25 and 37 kDa, while we did not find the corresponding bands in the western blots using antibodies against apolipoproteins other than ApoM ( Supplemental Fig.1 ) .
Supplemental Fig.1. Glycation of apolipoproteins in HDL .
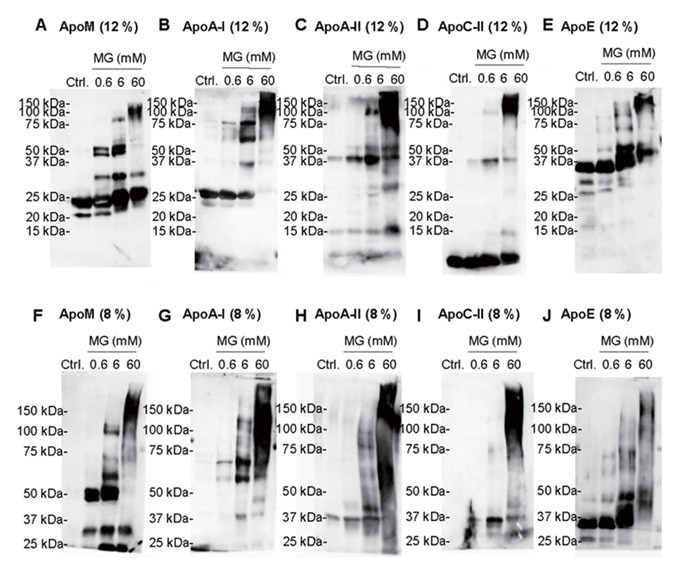
HDL was incubated with MG at various concentrations (0 mM, 0.6 mM, 6 mM, and 60 mM). Western blot analyses were performed with a 12% SDS-PAGE for ApoM (A), ApoA-I (B), ApoA- Ⅱ (C), ApoC-Ⅱ (D), ApoE (E) and with 8% SDS-PAGE analyses for ApoM (F), ApoA-I (G), ApoA-Ⅱ (H), ApoC-Ⅱ (I), ApoE (J). Ctrl.: control
To further investigate the modulations of the physical characteristics of ApoM on glycated HDL, we performed IEF and two-dimensional electrophoresis (2DE) analyses. We observed that the treatment with MG shifted the ApoM bands to the anode side, while the presence of AG reversed this modulation ( Fig.2E ) . Concordantly, on 2DE analyses, the ApoM bands were shifted to higher molecular-weight positions when HDL was treated with MG and the treatment together with AG reversed these modulations ( Fig.2F ) .
ApoM Polymers were not Cleaved by Sugar Chain–Eliminating Enzymes
To investigate how ApoM polymers were produced, we treated glycated HDL with three kinds of enzymes eliminating sugar chains: PNGase F, glucosidase, and neuraminidase. When we treated glycated HDL with PNGase F, we observed that the molecular weight of a naive ApoM band was shifted from 25 to 20 kDa and ApoM bands at higher molecular weight were also shifted to lower molecular-weight sites by around 5 kDa, demonstrating that a naive ApoM itself is glycated to some extent ( Fig.3A ) . We also observed that polymerization was not diminished when we treated glycated HDL with glucosidase and/or neuraminidase ( Fig.3B ) . These results suggested that the polymerization of ApoM might not occur by the addition of sugar chains, although the sugar chains were linked to ApoM in a manner that could be dissociated with PNGase F.
Fig.3. Effects of sugar chain decomposition enzymes or cross-linkers on the bands of ApoM on the western blots .
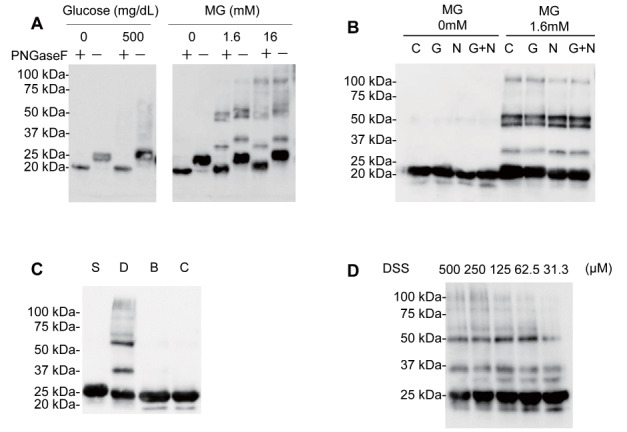
(A, B) Glycated HDL was incubated with the sugar chain decomposition enzymes, PNGase F or glycosidase (G) and/or neuraminidase (N), followed by western blot analysis for ApoM. (C, D) HDL was incubated with vehicle (C) or succinimidyl 4,4’-azipentanoate (LC-SDA, S), disuccinimidyl suberate (DSS, D), or bismaleimidoethane (BMOE, B), followed by western blot analysis for ApoM.
As MG is reported to possess crosslinking activities, we treated HDL with chemical cross-linkers, such as DSS, BMOE, and LC-SDA. When we treated HDL with these cross-linkers, we observed that ApoM polymers were formed only when we treated HDL with 1 mM DSS ( Fig.3C ) . When we treated HDL with a diluted series of DSS (500, 250, 125, 62.5, and 31.3 µM), higher molecular bands of ApoM were detected in a DSS concentration–dependent manner ( Fig.3D ) . The molecular weights of the ApoM bands that appeared with DSS were similar to those of HDL glycated with MG, suggesting that MG polymerized ApoM by its chemical activities exerted by DSS.
Glycation with MG Attenuated the Capacity of HDL to Bind S1P
Next, we investigated whether the glycation of HDL with MG would affect the metabolism of S1P. First, we investigated the modulation of S1P contents in HDL by the treatment with MG and observed that the S1P contents were lower in HDL glycated with MG at 60 mM ( Fig.4A ) . However, the treatment with MG degraded S1P in a standard solution, suggesting that the decreased contents of S1P in HDL glycated with MG might not be due to the glycation of ApoM, but to the physical degradation of S1P by MG ( Fig.4B ) . But when we investigated the capacity of HDL to bind C 17 S1P, we observed that the binding capacity was significantly lower in HDL glycated with MG 0.6 or 6.0 mM ( Fig.4C ) . ApoM reportedly retarded the degradation of S1P. Therefore, we also incubated 1 µM C 17 S1P bound to native HDL or glycated HDL on confluent HEK293 cells for 2 hours and observed that the C 17 S1P levels on glycated HDL were significantly lower ( Fig.4D ) . These results suggested that the ability of ApoM to retard the degradation of S1P was attenuated when HDL is glycated.
Fig.4. Modulation of the S1P-binding capacity of glycated HDL .
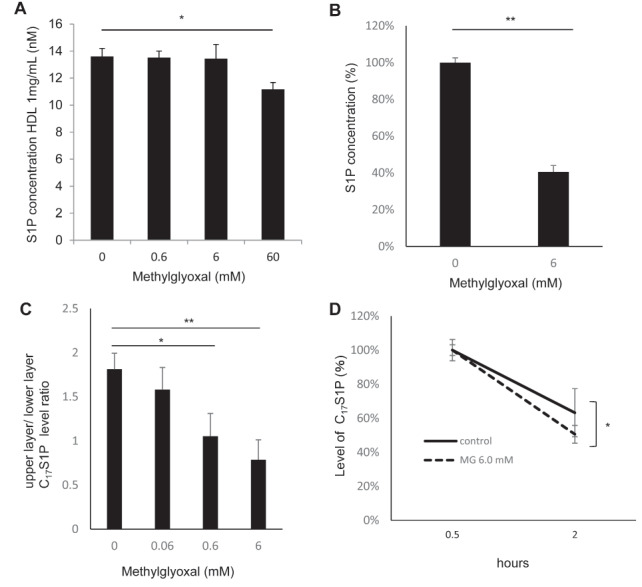
(A) Modulation of the contents of S1P in HDL treated or not treated with methylglyoxal (MG) ( n =4). (B) Modulation of the S1P levels in standard solutions treated or not treated with 6 mM of MG ( n =3). (C) The binding capacities of HDL treated with various concentrations of MG for 24 hours were evaluated by the pattern of redistribution of C 17 S1P between HDL and albumin ( n =3–4). (D) Time-course of C 17 S1P levels in HDL treated or not treated with 6 mM of MG incubated on HEK293 cells ( n =4). The results are presented as the ratio of C 17 S1P levels on HDL treated and not treated with MG to each C17S1P level at 0.5 hour. * P <0.01, ** P <0.05.
ApoM Levels in Subjects with Diabetes
Lastly, we measured ApoM levels in sera and HDL in the subjects with diabetes and healthy volunteers. Both serum ApoM levels and ApoM levels in HDL were significantly lower in the subjects with diabetes than in the healthy subject ( Fig.5A, B ) , while ApoM levels in HDL were not different when adjusted to cholesterol levels in HDL ( Fig.5C ) . Regarding ApoM polymers, we did not detect ApoM polymers in the subjects with diabetes ( Fig.5D ) .
Fig.5. ApoM levels determined by ELISA and ApoM bands detected on western blots in subjects with diabetes mellitus and healthy volunteers .
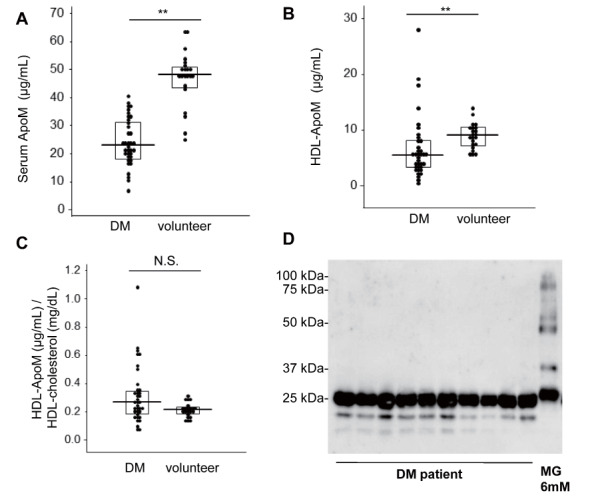
Sera were collected from healthy volunteers ( n =22) and subjects with diabetes ( n =36), and portions of the samples were used to separate HDL fractions. (A, B) ApoM levels in the sera or HDL fractions determined with ELISA. (C) ApoM levels adjusted to the total cholesterol levels in HDL. (D) Western blot analyses for ApoM, using 10 mg of separated HDL. ** P <0.01.
Discussion
Most of the pleiotropic effects of HDL, such as antiapoptosis, cell proliferation, and vasorelaxant effects, are attributed to S1P bound to ApoM on HDL. In the present study, we investigated the possibility that not only the quantity but also the quality of ApoM may be deficient in subjects with diabetes, although we could demonstrate the association between diabetes and the modulation of the quality of ApoM in the in vitro experiments conducted with glycated HDL.
Incubation under high-glucose conditions increased the expression of ApoM in HepG2 cells; on the other hand, the serum ApoM and HDL-linked ApoM levels were lower in the subjects with diabetes mellitus ( Figs.1C, 1D and 5A, 5B ) . Till date, several studies have investigated the association between the serum levels of ApoM and diabetes mellitus. Previous studies have reported lower plasma ApoM levels in subjects with diabetes, and the results of our present study were consistent with these reports 23 - 26) . On the other hand, another previous report indicated that in an in-vitro experiment, incubation under high-glucose conditions, decreased the expression of ApoM in HepG2 cells 29) ; our present results were not consistent with this report. The reasons for this discrepancy remain unknown, although the media used to culture the HepG2 cells were different and only the mRNA levels, not the protein levels of ApoM, were investigated in the report 29) . However, as metabolic factors other than glucose, such as insulin, leptin, and lipoprotein metabolism 28 , 30 - 35) , can affect the homeostasis of ApoM in humans, further studies are necessary to investigate the mechanisms that regulate ApoM.
At present, this is the first study that investigated the association between diabetes and the quality of ApoM. Many of the long-term complications of diabetes have been proposed to be associated with the process of protein glycation 36 - 38) . Early-stage glycation involves the nucleophilic attack of a reducing sugar with primary amine groups on proteins to form a Schiff base and then create a more stable Amadori product. Then, further oxidation, dehydration, and cross-linking steps can occur and produce reactive dicarbonyl compounds, such as MG. The highly reactive sites on the proteins have been proposed to create advanced glycation end products (AGEs) 39) . Regarding HDL, previous reports revealed the decrease of HDL-paraoxonase activity in MG-treated HDL 40) and HDL modified with MG accelerated its degradation and impaired its functionality in vivo 41) . In the present study, we hypothesized that the modulation of apoM might also be involved in the impaired functions of glycated HDL. When we treated HDL with MG, ApoM formed polymers, maybe due to the cross-linking reaction ( Figs.2 and 3 ) . We also performed western blot analyses with glycated HDL for ApoA-I, ApoA-II, ApoC-II, and ApoE, and observed that the bands of these apolipoproteins were detected at molecular weights similar to ApoM, suggesting that ApoM might form polymers not only with ApoM itself but also with these apolipoproteins other than ApoM riding on HDL. Regarding the band between 25 and 37 kDa, we did not find any corresponding bands in the western blots using antibodies against apolipoproteins other than ApoM. Considering that these bands could be detected in an experiment using PNGase F ( Fig.3A ) and a cross-linking experiment ( Fig.3D ) , these bands might be formed by ApoM binding with some other components of HDL or because ApoM itself might be modulated by MG irreversibly.
As a result of polymerization, we observed that the binding capacity to S1P was reduced and therefore, the protective ability against the degradation of S1P was also attenuated ( Fig.4C, D ) . These results suggest that the glycation of ApoM might lower the S1P contents on HDL and attenuate the pleiotropic effects of HDL. ApoM is known to have a S1P binding pocket 12) . The phosphate moiety of S1P interacts directly with the side chains of Arg98, Arg116, and Trp100 of ApoM and several amino acids of S1P are proposed to be hydrogen-bonded to Glu136, Tyr102, and Arg143 of ApoM 42) . Considering that MG is known to be cross-linked with Cys, Lys, and Arg, and that ApoM consists of 188 amino acids, including four Cys, eight Lys, and eight Arg amino acid residues, the affinity of ApoM to S1P might be reduced by the modulation of these amino acids of ApoM by MG.
Contrary to our expectations, ApoM polymers were not detected in sera from subjects with diabetes ( Fig.5D ) . Although we could not confirm whether ApoM polymers really exist in human subjects at present, one possible mechanism is that ApoM polymers might be removed quickly from circulation by the receptors for AGEs after their formation. Another possible reason is a technical reason, for example, the glycation of ApoM might occur in a different way from in vitro experiments using MG and we could not detect it with the antibody we used in the present study. Further studies are necessary to investigate whether the polymerization of ApoM might occur in human subjects under high-glucose conditions.
Conclusion
In this study, we demonstrated that ApoM may be deficient in both quantity and quality in patients with diabetes mellitus, and that as a result, the binding capacity of ApoM to S1P might be impaired. Since many of these pleiotropic effects of HDL are attributable to the S1P on HDL, the glycation of ApoM, together with the decreased ApoM levels, lowers the pleiotropic activities of HDL, at least partly, through the decreased capacity to carry S1P. These findings suggest the potential usefulness of ApoM as a target for laboratory testing and/or treatment medication in subjects with diabetes mellitus.
Acknowledgements
None.
Funding
This work was supported by Leading Advanced Projects for medical innovation (LEAP) from AMED, a Grant-in-Aid for Scientific Research on Innovative Areas 15H05906 (Y.Y.), JSPS KAKENHI Grant Numbers 16H06236 and 20H03573 (M.K.), Research Fund of MITSUKOSHI Health and Welfare Foundation 2017 (M.K.), Japan Heart Foundation & Astellas Grant for Research on Atherosclerosis Update (M.K.), and MSD Life Science Foundation, Public Interest Incorporated Foundation (M.K.).
Coi
T. Shimizu is an employee of Sekisui Medical Co., Ltd.
References
- 1).Brewer HB, Jr.: Clinical review: The evolving role of HDL in the treatment of high-risk patients with cardiovascular disease. J Clin Endocrinol Metab, 2011; 96: 1246-1257 [DOI] [PubMed] [Google Scholar]
- 2).Nigro J, Osman N, Dart AM and Little PJ: Insulin resistance and atherosclerosis. Endocr Rev, 2006; 27: 242-259 [DOI] [PubMed] [Google Scholar]
- 3).Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, Ferrannini E and Reddy ST: Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes, 2011; 60: 2617-2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Ebtehaj S, Gruppen EG, Parvizi M, Tietge UJF and Dullaart RPF: The anti-inflammatory function of HDL is impaired in type 2 diabetes: role of hyperglycemia, paraoxonase-1 and low grade inflammation. Cardiovasc Diabetol, 2017; 16: 132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Lemmers RFH, van Hoek M, Lieverse AG, Verhoeven AJM, Sijbrands EJG and Mulder MT: The anti-inflammatory function of high-density lipoprotein in type II diabetes: A systematic review. J Clin Lipidol, 2017; 11: 712-724 e715 [DOI] [PubMed] [Google Scholar]
- 6).Kurano M and Yatomi Y: Sphingosine 1-Phosphate and Atherosclerosis. J Atheroscler Thromb, 2018; 25: 16-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Okajima F: Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta, 2002; 1582: 132-137 [DOI] [PubMed] [Google Scholar]
- 8).Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S and Spiegel S: Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature, 1996; 381: 800-803 [DOI] [PubMed] [Google Scholar]
- 9).Takabe K and Spiegel S: Export of sphingosine-1-phosphate and cancer progression. J Lipid Res, 2014; 55: 1839-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Christensen PM, Liu CH, Swendeman SL, Obinata H, Qvortrup K, Nielsen LB, Hla T, Di Lorenzo A and Christoffersen C: Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J, 2016; 30: 2351-2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Burg N, Swendeman S, Worgall S, Hla T and Salmon JE: Sphingosine 1-Phosphate Receptor 1 Signaling Maintains Endothelial Cell Barrier Function and Protects Against Immune Complex-Induced Vascular Injury. Arthritis Rheumatol, 2018; 70: 1879-1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB and Dahlback B: Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A, 2011; 108: 9613-9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Kurano M, Tsukamoto K, Ohkawa R, Hara M, Iino J, Kageyama Y, Ikeda H and Yatomi Y: Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis, 2013; 229: 102-109 [DOI] [PubMed] [Google Scholar]
- 14).Liu M, Seo J, Allegood J, Bi X, Zhu X, Boudyguina E, Gebre AK, Avni D, Shah D, Sorci-Thomas MG, Thomas MJ, Shelness GS, Spiegel S and Parks JS: Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein. J Biol Chem, 2014; 289: 2801-2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Swendeman SL, Xiong Y, Cantalupo A, Yuan H, Burg N, Hisano Y, Cartier A, Liu CH, Engelbrecht E, Blaho V, Zhang Y, Yanagida K, Galvani S, Obinata H, Salmon JE, Sanchez T, Di Lorenzo A and Hla T: An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci Signal, 2017; 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Wilkerson BA, Grass GD, Wing SB, Argraves WS and Argraves KM: Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J Biol Chem, 2012; 287: 44645-44653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Kurano M, Hara M, Tsuneyama K, Sakoda H, Shimizu T, Tsukamoto K, Ikeda H and Yatomi Y: Induction of insulin secretion by apolipoprotein M, a carrier for sphingosine 1-phosphate. Biochim Biophys Acta, 2014; 1841: 1217-1226 [DOI] [PubMed] [Google Scholar]
- 18).Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, Dahlback B, Kono M, Proia RL, Smith JD and Hla T: HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal, 2015; 8: ra79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M, Proia RL, Steinman L, Han MH and Hla T: HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature, 2015; 523: 342-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Takahashi C, Kurano M, Nishikawa M, Kano K, Dohi T, Miyauchi K, Daida H, Shimizu T, Aoki J and Yatomi Y: Vehicle-dependent Effects of Sphingosine 1-phosphate on Plasminogen Activator Inhibitor-1 Expression. J Atheroscler Thromb, 2017; 24: 954-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Kurano M, Tsuneyama K, Morimoto Y, Shimizu T, Jona M, Kassai H, Nakao K, Aiba A and Yatomi Y: Apolipoprotein M Protects Lipopolysaccharide-Treated Mice from Death and Organ Injury. Thromb Haemost, 2018; 118: 1021-1035 [DOI] [PubMed] [Google Scholar]
- 22).Kurano M, Tsuneyama K, Morimoto Y, Nishikawa M and Yatomi Y: Apolipoprotein M suppresses the phenotypes of IgA nephropathy in hyper-IgA mice. FASEB J, 2019; 33: 5181-5195 [DOI] [PubMed] [Google Scholar]
- 23).Plomgaard P, Dullaart RP, de Vries R, Groen AK, Dahlback B and Nielsen LB: Apolipoprotein M predicts pre-beta-HDL formation: studies in type 2 diabetic and nondiabetic subjects. J Intern Med, 2009; 266: 258-267 [DOI] [PubMed] [Google Scholar]
- 24).Memon AA, Bennet L, Zoller B, Wang X, Palmer K, Dahlback B, Sundquist J and Sundquist K: The association between apolipoprotein M and insulin resistance varies with country of birth. Nutr Metab Cardiovasc Dis, 2014; 24: 1174-1180 [DOI] [PubMed] [Google Scholar]
- 25).Zhang P, Gao J, Pu C, Feng G, Wang L, Huang L, Tao Q and Zhang Y: Effects of hyperlipidaemia on plasma apolipoprotein M levels in patients with type 2 diabetes mellitus: an independent case-control study. Lipids Health Dis, 2016; 15: 158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Kurano M, Tsukamoto K, Shimizu T, Kassai H, Nakao K, Aiba A, Hara M and Yatomi Y: Protection Against Insulin Resistance by Apolipoprotein M/Sphingosine 1-Phosphate. Diabetes, 2020; [DOI] [PubMed] [Google Scholar]
- 27).Brinck JW, Thomas A, Lauer E, Jornayvaz FR, Brulhart-Meynet MC, Prost JC, Pataky Z, Lofgren P, Hoffstedt J, Eriksson M, Pramfalk C, Morel S, Kwak BR, van Eck M, James RW and Frias MA: Diabetes Mellitus Is Associated With Reduced High-Density Lipoprotein Sphingosine-1-Phosphate Content and Impaired High-Density Lipoprotein Cardiac Cell Protection. Arterioscler Thromb Vasc Biol, 2016; 36: 817-824 [DOI] [PubMed] [Google Scholar]
- 28).Kurano M, Hara M, Ikeda H, Tsukamoto K and Yatomi Y: Involvement of CETP (Cholesteryl Ester Transfer Protein) in the Shift of Sphingosine-1-Phosphate Among Lipoproteins and in the Modulation of its Functions. Arterioscler Thromb Vasc Biol, 2017; 37: 506-514 [DOI] [PubMed] [Google Scholar]
- 29).Zhang X, Jiang B, Luo G, Nilsson-Ehle P and Xu N: Hyperglycemia down-regulates apolipoprotein M expression in vivo and in vitro. Biochim Biophys Acta, 2007; 1771: 879-882 [DOI] [PubMed] [Google Scholar]
- 30).Nojiri T, Kurano M, Tokuhara Y, Ohkubo S, Hara M, Ikeda H, Tsukamoto K and Yatomi Y: Modulation of sphingosine-1-phosphate and apolipoprotein M levels in the plasma, liver and kidneys in streptozotocin-induced diabetic mice. J Diabetes Investig, 2014; 5: 639-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Luo G, Hurtig M, Zhang X, Nilsson-Ehle P and Xu N: Leptin inhibits apolipoprotein M transcription and secretion in human hepatoma cell line, HepG2 cells. Biochim Biophys Acta, 2005; 1734: 198-202 [DOI] [PubMed] [Google Scholar]
- 32).Kurano M, Hara M, Nojiri T, Ikeda H, Tsukamoto K and Yatomi Y: Resveratrol exerts a biphasic effect on apolipoprotein M. Br J Pharmacol, 2016; 173: 222-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Kurano M, Ikeda H, Iso ON, Hara M, Tsukamoto K and Yatomi Y: Regulation of the metabolism of apolipoprotein M and sphingosine 1-phosphate by hepatic PPARgamma activity. Biochem J, 2018; 475: 2009-2024 [DOI] [PubMed] [Google Scholar]
- 34).Kurano M, Tsukamoto K, Hara M, Ohkawa R, Ikeda H and Yatomi Y: LDL receptor and ApoE are involved in the clearance of ApoM-associated sphingosine 1-phosphate. J Biol Chem, 2015; 290: 2477-2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Christoffersen C, Benn M, Christensen PM, Gordts PL, Roebroek AJ, Frikke-Schmidt R, Tybjaerg-Hansen A, Dahlback B and Nielsen LB: The plasma concentration of HDL-associated apoM is influenced by LDL receptor-mediated clearance of apoB-containing particles. J Lipid Res, 2012; 53: 2198-2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Hanssen KF and Laakso M: Increased serum levels of methylglyoxal-derived hydroimidazolone-AGE are associated with increased cardiovascular disease mortality in nondiabetic women. Atherosclerosis, 2009; 205: 590-594 [DOI] [PubMed] [Google Scholar]
- 37).Ahmed N: Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract, 2005; 67: 3-21 [DOI] [PubMed] [Google Scholar]
- 38).Agalou S, Ahmed N, Babaei-Jadidi R, Dawnay A and Thornalley PJ: Profound mishandling of protein glycation degradation products in uremia and dialysis. J Am Soc Nephrol, 2005; 16: 1471-1485 [DOI] [PubMed] [Google Scholar]
- 39).Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, Koke M and Hage DS: Review: Glycation of human serum albumin. Clin Chim Acta, 2013; 425: 64-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Bacchetti T, Masciangelo S, Armeni T, Bicchiega V and Ferretti G: Glycation of human high density lipoprotein by methylglyoxal: effect on HDL-paraoxonase activity. Metabolism, 2014; 63: 307-311 [DOI] [PubMed] [Google Scholar]
- 41).Godfrey L, Yamada-Fowler N, Smith J, Thornalley PJ and Rabbani N: Arginine-directed glycation and decreased HDL plasma concentration and functionality. Nutr Diabetes, 2014; 4: e134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Zhang H, Pluhackova K, Jiang Z and Bockmann RA: Binding Characteristics of Sphingosine-1-Phosphate to ApoM hints to Assisted Release Mechanism via the ApoM Calyx-Opening. Sci Rep, 2016; 6: 30655 [DOI] [PMC free article] [PubMed] [Google Scholar]


