Abstract
Background:
Pain is a major concern among patients with advanced cancer and their family caregivers. Evidence suggests that pain coping skills training interventions can improve outcomes, however they have rarely been tested in this population.
Aim:
To test the efficacy of a caregiver-guided pain coping skills training intervention. The primary outcome was caregiver self-efficacy for helping the patient manage pain.
Design:
A randomized controlled trial compared the intervention to an enhanced treatment-as-usual control. Dyads in both conditions received pain education, and those in the intervention received three sessions of pain coping skills training. Caregiver outcomes (self-efficacy; caregiver strain, caregiving satisfaction, psychological distress) and patient outcomes (self-efficacy, pain intensity and interference, psychological distress) were collected at baseline and post-intervention.
Setting/participants:
202 patients with stage III-IV cancer and pain and their family caregivers were enrolled from four outpatient oncology clinics and a free-standing hospice/palliative care organization.
Results:
Compared to those in the control arm, caregivers in the intervention reported significant increases in caregiving satisfaction (p<.01) and decreased anxiety (p=.04). In both conditions, caregivers reported improvements in self-efficacy, and patients reported improvements in self-efficacy, pain severity and interference, and psychological distress.
Conclusions:
This is the first study to test a pain coping skills intervention targeted to patients and caregivers facing advanced cancer. Findings suggest that pain education provides benefits for patients and caregivers, and coping skills training may be beneficial for caregivers. Further research is needed to optimize the benefits of education and pain coping skills training for improving cancer pain outcomes.
Keywords: cancer, pain, family caregivers, behavioral medicine, clinical trial
Introduction
Despite advances in treating pain, the prevalence of pain among cancer patients remains high, particularly with advanced disease. Almost two-thirds of patients with advanced cancer report pain, and half report pain of moderate to severe intensity. (1) Pain is a major source of suffering for patients and their family caregivers. It is a complex, multidimensional experience that impedes patients’ ability to perform valued activities and increases the risk of psychological distress for patients and caregivers.(2, 3) Patients with advanced cancer often view pain as a reminder of disease progression and death.(4) Pain is also a dynamic experience, fluctuating in frequency, intensity, and sensory qualities. The unpredictable and complex nature of pain can make it challenging for patients and caregivers to manage, leading to a perceived lack of control.(5)
Medication remains the mainstay in managing cancer pain. Despite its potential efficacy, pain medication has drawbacks including side effects which negatively impact patients’ quality of life. Medical approaches also fail to address psychosocial and behavioral factors (e.g., psychological distress, social support, inactivity) that both impact and are impacted by pain. Finally, pain medication often does not address patients’ primary goals which include performing valued tasks and activities, maintaining important relationships, and preserving a sense of control and independence.(6)
Pain coping skills training interventions address many of these limitations by teaching patients cognitive and behavioral skills (e.g., relaxation, imagery, activity pacing) that can help reduce pain, and psychological distress, and increase engagement in meaningful activities. One of the main intervention targets is improving self-efficacy (e.g., confidence) for managing pain, which is associated with improvements in pain-related outcomes and a greater sense of control. While the focus of the intervention is typically on the patient, pain coping skills can also be targeted to patient-caregiver dyads. In caregiver-assisted protocols, caregivers are conceptualized as coaches, learning skills alongside the patient and helping the patient practice and apply skills in challenging situations. Caregivers are also encouraged to use the skills to manage their own psychological distress, which is often considerable. (7, 8)
While evidence supports the efficacy of pain coping skills interventions for improving pain and pain-related outcomes in patients with cancer, these interventions have rarely been tested in patients with advanced disease.(4) There have also been few studies testing protocols targeted to patient-caregiver dyads. We previously designed a novel caregiver-guided pain coping skills training intervention for hospice-eligible cancer patients.(9) Findings from a small pilot study indicated that the intervention led to improvements in caregiver self-efficacy for helping the patient manage pain, caregiver strain, patient pain, and patient quality of life.
The current study builds on and extends these findings by conducting a larger multi-site trial of the caregiver-assisted pain coping skills intervention targeting patients with advanced cancer receiving outpatient treatment. Design of the study was based on the biopsychosocial model of cancer pain in which improvements in self-efficacy are critical to improved outcomes in other domains. (10) For patients with advanced disease, caregivers are often highly involved in pain management efforts as pain becomes more severe, and many caregivers experience high levels of distress themselves. Thus, in this context, we believed it was particularly important to target caregiver self-efficacy to improve other patient and caregiver outcomes.(11)
Methods
Hypotheses
Our primary hypothesis was that the intervention would lead to significant improvements in caregivers’ self-efficacy immediately following the intervention. Secondary hypotheses were that the intervention would lead to improvements in caregiver adjustment (psychological distress, caregiver strain, and caregiving satisfaction), and improvements in patient outcomes including pain severity and interference, self-efficacy for pain management, and psychological distress.
Design
We conducted a randomized clinical trial in which patient-caregiver dyads were randomly assigned with 1:1 allocation to either: 1) Caregiver-guided pain coping skills training (“intervention”), or 2) Enhanced treatment-as-usual (“control). Table 1 provides an overview of study activities.
Table 1.
Schedule of enrollment, interventions, and assessments
| STUDY PERIOD | |||||||
|---|---|---|---|---|---|---|---|
| Pre-Intervention | Intervention* | Post-intervention** | |||||
| Activity | Enrollment | Baseline assessment (T1) | Randomization | Session 1 | Session 2 | Session 3 | Outcome Assessment (T2) |
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Medical data collection | X | ||||||
| Assessment | |||||||
| Demographics | X | ||||||
| Caregiver self- efficacy | X | X | |||||
| Caregiver strain | X | X | |||||
| Caregiving satisfaction | X | X | |||||
| Caregiver psychological distress | X | X | |||||
| Patient self-efficacy | X | X | |||||
| Patient pain severity/interference | X | X | |||||
| Patient psychological distress | X | X | |||||
| Randomization | X | ||||||
| Intervention | |||||||
| (a) Caregiver-assisted pain coping skills training (intervention) | X | X | X | ||||
| (b) Enhanced treatment-as-usual (control) | |||||||
The intervention period was designed to last three weeks but was extended as necessary to accommodate the patients’ health issues and patient and caregiver availability
The T2 assessment was administered following completing of the intervention sessions for patients and caregivers in the intervention arm, and approximately 4 weeks following the T1 assessment for those in the control arm.
Participants and setting
Participants were recruited between October, 2015 and July, 2018 at outpatient oncology clinics at four university medical centers and a free-standing hospice and palliative care organization. Patient inclusion criteria included (a) clinical diagnosis of Stage IV solid or hematologic malignancy, or Stage III unresectable gastrointestinal cancer; (b) worst pain in the past two weeks ≥ 4 (e.g., moderate pain)(12); and (c) identified caregiver (e.g., person who provides practical and emotional support.)(13) Exclusion criteria were life expectancy < one month, Palliative Performance Scale rating < 40 (e.g., mainly in bed), and treatment with radiation therapy that significantly affected pain. Patients and caregivers had to be age ≥ 18 years and able to read/speak English. Original eligibility criteria included primary oncologist’s verification that they would not be surprised if the patient died within the year (i.e. “the surprise question”).(14) This was eliminated due to the low frequency with which oncologists endorsed this question, even for patients with diagnoses typically associated with poor prognosis. Also, while original criteria specified a diagnosis of Stage IV cancer, we expanded inclusion to patients with Stage III unresectable gastrointestinal cancers as they commonly have significant pain and poor prognosis.(15)
Intervention and Control Conditions
Caregiver-Guided Pain Coping Skills Training.
The intervention has been described in detail previously.(15) Briefly, patient-caregiver dyads received three weekly 60-minute sessions conducted by licensed doctoral and master’s level therapists. Sessions were conducted with dyads in their homes via videoconference. Therapists received initial training in the protocol and followed a detailed treatment manual. Audio recordings of the sessions were reviewed by investigators who provided feedback during biweekly supervision. Sessions were supplemented with written materials (see Appendix), an educational videotape (16), and audio recordings of relaxation and imagery exercises. Table 2 lists session content. At each session, the therapist reviewed the dyad’s use of the coping skills, reinforced practice of the skills, elicited positive and negative reactions, and helped the dyad problem-solve to address challenges to practice and/or application.
Table 2.
Content of Caregiver-Guided Pain Coping Skills Sessions
| Session | Topics/Skills* |
|---|---|
| 1 | • Introduction to pain coping skills training as a method to help patients and their caregivers better manage cancer pain • Overview of program and materials • Watch and discuss segment of educational video, “Relieving Cancer Pain,” that provides information on topics related to the medical management of cancer pain (e.g., types of treatment, common side effects, and communication with health care providers) • Relaxation exercise |
| 2 | • Communication skills: guidelines for effective speaking and listening39 • Brief relaxation mini-practices • Pleasant imagery |
| 3 | • Activity-rest cycle • Pleasant activity scheduling • Coping skills review and maintenance plan |
For each skill, the therapist (a) provided a rationale for the skill’s use in reducing pain, stress, and distress; (b) used a behavioral rehearsal procedure to teach each skill (e.g., providing instruction in the skill, having the dyad practice the skill together, and providing feedback on their practice); and (c) provided recommendations for home practice, including how the caregiver could effectively coach the patient in using the skill.
Control.
Given that patients had clinically significant pain, we wanted to ensure they had access to standard educational material about pain management. Thus, we provided them with the same educational video on cancer pain used in the intervention, and links to websites with accurate, up-to-date information on cancer pain (e.g., American Cancer Society, National Cancer Institute). At the time of enrollment, we encouraged them to utilize these resources but did not track their use.
Outcome Measures
Participants completed paper assessments, and data were entered by study staff into a Research Electronic Data Capture (REDCap) database located on a secure, encrypted network. Patient medical data were collected from the medical record at enrollment. Sociodemographic data were collected from patients and caregivers at baseline. Participants completed the following measures at baseline (T1) and post-intervention (T2).
Caregiver outcomes
Caregivers’ Self-Efficacy in Pain Management (Primary Outcome) was assessed using a standardized 10-item measure (e.g. “How certain are you that you can help the patient control his/her pain?”) Multiple prior studies support the scale’s reliability and validity.(9, 11, 17–19) The total score was used.
Caregiver Strain was measured using the 13-item Caregiver Strain Index (CSI) (20) which assesses a variety of stressors commonly experienced by caregivers. Prior studies have supported the internal consistency of the CSI.(2, 7, 9, 20)
Caregiving Satisfaction was measured using the Caregiving Satisfaction scale of the Caregiver Appraisal measure (21) which assesses benefits associated with caregiving (e.g., feeling closer to the patient, being happy that the patient is cared for by family). The scale has demonstrated good test-retest reliability and internal consistency.(21)
Psychological Distress was measured using (a) the Center for Epidemiology Studies Depression Scale –10, a validated scale assessing affective, cognitive, motivational, and physiologic areas of depressive symptomatology (22); and (b) the trait anxiety version of the State Trait Anxiety Inventory which was developed as a tool for investigating anxiety in non-psychiatric adults and has demonstrated good psychometric properties.(23)
Patient outcomes
Patient Self-Efficacy for Pain Management was assessed using a standard self-efficacy scale similar to that used with caregivers.(24) Multiple prior studies provide strong support for the reliability and validity of this scale.(9, 17–19, 24, 25)
Pain Intensity and Interference were measured using three items from the Brief Pain Inventory (26) (usual and worst pain intensity, and pain interference), rated on 0 to 10 scales. The BPI has demonstrated validity (26) and is used widely in cancer pain research studies.
Psychological Distress was measured using the Hospital Anxiety and Depression Scale (27), a 14-item instrument that assesses anxiety and depression as two dimensions. This scale has been used to assess psychological distress in cancer patients.(28),(29, 30)
Sample Size
Our original power calculation projected a sample size of 236 dyads, with 20% attrition, to achieve 80% power to detect a small-to-moderate effect size of 0.4 in caregiver self-efficacy. Due to challenges in enrollment (see (15)), we revised our target sample to 214 dyads. With 20% attrition, the power is approximately 74% to detect an effect size of 0.4.
Sampling, recruitment, and randomization procedures
All eligible patients received a brief summary of the study by their health care provider. If they agreed, the site research coordinator met with the patient and caregiver to provide further information, obtain written consent, and administer the baseline assessment (T1).
Patient-caregiver dyads were then randomly assigned to either the intervention or control arm. Randomization was stratified by study site and patient sex and occurred in blocks of four, guaranteeing that after every four assignments the study arms had equal numbers. Randomization assignments were generated by REDCap (31) and accessible to research staff only after participants completed T1. At this time, the research coordinator retrieved the randomization assignment and informed the dyad. Research staff involved in collecting outcome data were blinded to randomization status.
Patients and caregivers completed T2 assessments following completion of the intervention sessions (intervention arm) or 4 weeks following baseline (control arm). We loaned dyads tablet computers with free internet access for use during the intervention phase of the study. Participants were each paid $25 for completing each assessment.
Statistical Analyses
Data were analyzed using repeated measure mixed models (32) with an unstructured covariance to reflect the correlation of the pre-post measurements and was implemented using the SAS MIXED procedure. This model uses all available data and assumes that missing assessments are missing at random and only dependent on observed data. Within group means were estimated and the pre-post difference as the change in these means. The effect of treatment condition would be demonstrated by significant differences (alpha=0.05) in the pre-post changes across treatment conditions. Effect size for the within and between group change was calculated relative to the standard deviation of the pre/post change. Several sensitivity analyses were performed to check assumptions; results of these analyses were very similar and the conclusions remained the same. To examine the missing-at-random assumption, a procedure for Brown’s Protective Estimate was implemented (33). To examine the impact of variation in the timing of post intervention assessments, mixed effect growth curve models (32) were used with piecewise linear splines to model the average change over time and two random effects (intercept and slope) to model the between subject variation.
Due to the relatively high rate of withdrawals due to death or deterioration in health which is common in palliative care RCTs, we also conducted palliative-modified intent-to-treat analyses as recommended by Currow et al. (34). These secondary analyses excluded participants who dropped out of the study prior to the T2 assessment due to declining health or death (15 intervention, 6 control). Results of these analyses were very similar to those from the intent-to-treat analyses and the conclusions remained the same, except for caregiver anxiety (see Results).
Ethical issues and permissions
Study procedures received approval from Institutional Review Boards at their respective sites (see Table 3).
Table 3.
Recruitment sites and Institutional Board Approval
| Site | Site PI | IRB | Reference number | Initial Approval Date |
|---|---|---|---|---|
| Duke University | Laura Porter | Duke University Health System (DUHS) IRB | Pro00057512 | 10/27/14 |
| Four Seasons Compassion for Life | Janet Bull | Margaret R. Pardee Memorial Hospital IRB | n/a | 6/3/15 |
| University of North Carolina at Chapel Hill | Laura Hanson | Office of Human Research Ethics, UNC Chapel Hill | 15–1047 | 7/30/15 |
| University of Pittsburgh | Jennifer Steel | University of Pittsburgh Medical Campus (UPMC) IRB | PRO15040600 | 7/20/15 |
| University of Colorado at Denver | Stacy Fischer | COMIRB (Colorado Multiple Institutional Review Board) | 16–0829 | 4/26/16 |
Results
Among 1257 individuals contacted for screening, 629 were eligible and 226 of those eligible enrolled (Figure 1). The main reasons for exclusion were not meeting the pain criteria and not having a caregiver. The main reasons for refusal were patient lack of interest and lack of time. 24 dyads dropped out prior to randomization, most due to death or declining health. 202 dyads were randomized; 101 were assigned to intervention and 101 were assigned to the control arm. Participant characteristics are shown in Table 4.
Figure 1.
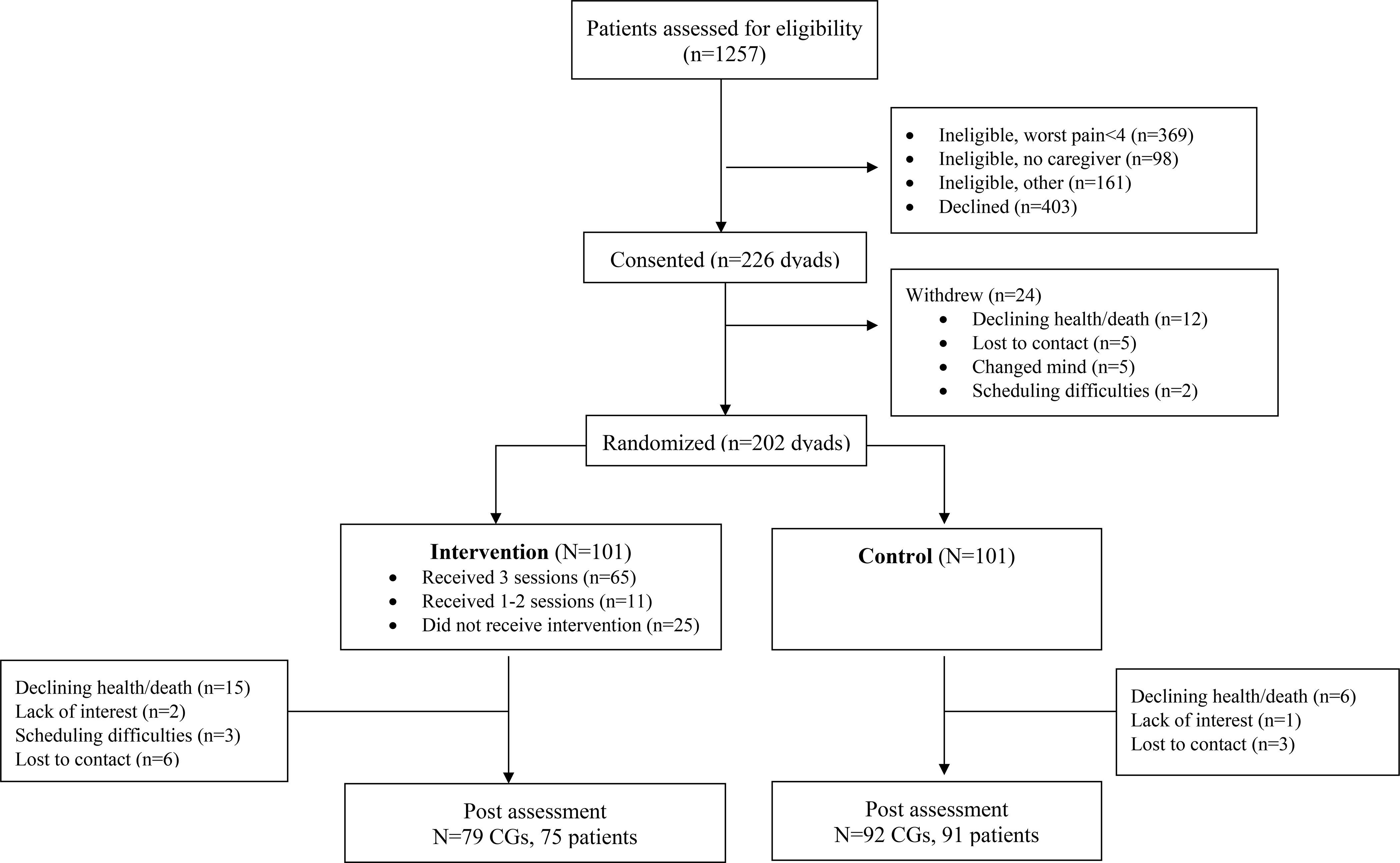
Study Consort Diagram
Table 4.
Participant characteristics
| Intervention | Control | |||
|---|---|---|---|---|
| Patients N=101 | Caregivers N=101 | Patients N=101 | Caregivers N=101 | |
| Age, M(SD) | 62.84 (10.34) | 57.11 (12.83) | 62.18 (12.44) | 58.19 (12.61) |
| Gender, n (%) | ||||
| Female | 42 (41.6%) | 73 (72.3%) | 41 (40.6%) | 69 (68.3%) |
| Male | 59 (58.4%) | 28 (27.7%) | 60 (59.4%) | 32 (31.7%) |
| Race, n (%) | ||||
| Black of African American | 15 (16.0%) | 17 (18.9%) | 16 (16.5%) | 16 (17.0%) |
| White | 78 (83.0%) | 72 (80.0%) | 81 (83.5%) | 77 (81.9%) |
| Other/unknown | 1 (1.1%) | 1 (1.1%) | 0 (0.0%) | 1 (1.1%) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 2 (2.0%) | 4 (2.0%) | 1 (1.0%) | 4 (2.0%) |
| Not Hispanic or Latino | 99 (98.0%) | 198 (98.0%) | 100 (99.0%) | 198 (98.0%) |
| Relationship to patient, n (%) | ||||
| Spouse/partner | 69 (68.3%) | 77 (76.2%) | ||
| Adult child | 16 (15.8%) | 8 (7.9%) | ||
| Sibling | 6 (5.9%) | 6 (5.9%) | ||
| Other/unknown | 10 (9.9%) | 10 (9.9%) | ||
| Cancer type, n (%) | ||||
| Blood | 1 (1.0%) | 3 (3.0%) | ||
| Bone | 5 (5.0%) | 2 (2.0%) | ||
| Breast | 7 (6.9%) | 5 (5.0%) | ||
| Gastrointestinal | 32 (31.7%) | 27 (26.7%) | ||
| Lung | 15 (14.8%) | 24 (23.8%) | ||
| Prostate | 19 (18.8%) | 13 (12.9%) | ||
| Other/multiple | 22 (21.8%) | 27 (26.7%) | ||
Among those assigned to the intervention, 25 (24.8%) dyads did not receive any intervention sessions, primarily due to the patient’s declining health and/or hospitalization, or scheduling difficulties. Sixty-five (64.4%) of the dyads assigned to the intervention completed all three sessions. Seventy-nine intervention dyads (78.2%) and 92 control dyads (91.1%) completed T2. Primary reasons for dropout in both arms included patient death or declining health. The interval between T1 and T2 was 76.59 days (SD=58.18) for intervention participants and 36.67 (SD=20.49) days for control participants. The longer interval in the intervention arm was a result of difficulties in scheduling intervention sessions, primarily due to patient illness/hospitalization and/or conflicts with the caregiver’s schedule.
Caregiver outcomes (Table 5).
Table 5.
Baseline and post measures: Estimates, standard errors, estimated changes, within-group effect sizes, and between group effect sizes
| Intervention Group | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Estimate (SE) | Post Estimate (SE) | Estimated change (95% CI) | Effect size | Baseline Estimate (SE) | Post Estimate (SE) | Estimated change (95% CI) | Effect size | Between group effect size | |
| Caregiver measures | |||||||||
| Self-efficacy | 59.09 (1.90) | 64.21 (1.89) | 5.13 (1.12, 9.13) | 0.27 | 58.42 (1.90) | 61.80 (1.76) | 3.38 (−0.39, 7.15) | 0.18 | 0.09 |
| Depression | 7.93 (0.52) | 7.82 (0.56) | −0.12 (−0.96, 0.73) | −0.03 | 8.26 (0.52) | 8.92 (0.54) | 0.67 (−0.12, 1.45) | 0.17 | −0.20 |
| Anxiety | 38.28 (0.97) | 37.37 (1.12) | −0.91 (−2.59, 0.77) | −0.12 | 38.46 (0.97) | 39.82 (1.08) | 1.36 (−0.22, 2.94) | 0.18 | −0.30+ |
| Caregiver strain | 5.47 (0.33) | 5.40 (0.36) | −0.06 (−0.62, 0.49) | −0.03 | 6.13 (0.33) | 5.72 (0.35) | −0.41 (−0.92, 0.10) | −0.16 | 0.14 |
| Caregiving satisfaction | 4.56 (0.04) | 4.60 (0.05) | 0.05 (−0.04, 0.13) | 0.12 | 4.7 (0.04) | 4.6 (0.05) | −0.11 (−0.19, −0.03) | −0.29 | 0.41** |
| Patient measures | |||||||||
| Pain Severity | 4.55 (0.18) | 3.94 (0.23) | −0.60 (−1.02, −0.19) | −0.32 | 4.20 (0.18) | 3.70 (0.22) | −0.50 (−0.88, −0.12) | −0.27 | −0.06 |
| Pain Interference | 4.90 (0.22) | 3.88 (0.24) | −1.02 (−1.55, −0.49) | −0.42 | 4.64 (0.22) | 3.57 (0.22) | −1.06 (−1.55, −0.57) | −0.44 | 0.02 |
| Self-efficacy | 56.16 (1.63) | 62.47 (1.70) | 6.31 (2.81, 9.91) | 0.39 | 56.84 (1.63) | 63.62 (1.58) | 6.78 (3.51, 10.04) | 0.42 | −0.03 |
| Psychological distress | 14.49 (0.60) | 13.23 (0.66) | −1.26 (−2.30, −0.21) | −0.27 | 14.18 (0.60) | 12.73 (0.62) | −1.44 (−2.40, −0.48) | −0.31 | 0.04 |
Notes:
p=0.06
p<0.01.
Caregivers in both arms reported small improvements in self-efficacy for helping the patient manage pain (primary outcome); the between-group difference was non-significant. Similarly, caregivers in both arms reported decreases in caregiver strain with no significant between-group differences. Caregivers in the intervention reported an increase in satisfaction, while caregivers in the control arm reported decreased satisfaction, a difference that was significant (p<.01). Caregivers in the intervention reported decreases in depression and anxiety while those in the control arm reported increases. The between-group difference in anxiety approached significance (p=.06) in intent-to-treat analyses and was significant in palliative-modified intent-to-treat analyses (p=.04).
Patient outcomes.
Patients in both arms reported decreases in pain severity, pain interference and psychological distress, and increases in self-efficacy. There were no significant between-group effects. (Table 5)
Discussion
Main findings.
Contrary to our hypothesis, caregivers in the pain coping skills intervention did not report improvements in self-efficacy for helping the patient manage pain compared to those in the control condition. However, the intervention did lead to significant improvements in caregiving satisfaction and decreased caregiver anxiety. Both conditions led to similar improvements in caregiver depression, and patient outcomes including pain severity and interference, self-efficacy for pain management, and psychological distress.
Strengths/limitations.
To our knowledge, this is the first multi-site randomized controlled trial testing a pain coping skills intervention targeted to patients and caregivers facing advanced cancer, a population that experiences a high burden of pain and pain-related symptoms. Participants were recruited from five institutions in three different geographic regions of the United States (Southeast, Northeast, West), increasing the generalizability of the findings.
Limitations include between group differences in the timing of T2 data collection which was due to challenges in delivering intervention sessions in the context of patients’ declining health. Sessions were often delayed because patients became more ill and/or were hospitalized, leading to extensions in the intervention period beyond three weeks to accommodate participants’ needs. While this enabled some participants to complete the study, the increased T1-T2 interval in the intervention versus control arm may have masked some treatment effects. Symptoms and psychological distress typically worsen over time in advanced cancer, and the T1-T2 interval was twice as long for intervention patients versus control. The extended intervention period likely also led to increased attrition in the intervention group due declining health or death.
Additional limitations include the uptake, with 36% of eligible participants enrolling in the study. While this is lower than some psychosocial intervention studies in cancer, it is similar to that in other studies that require the participation of a caregiver (7). Finally, because the study was conducted in the United States, findings may not generalize to countries whose health care systems have different standard approaches for managing cancer pain.
What this study adds.
The pain coping skills intervention tested in this study was modeled after that piloted by Keefe et al.(9) which led to improvements in caregiver self-efficacy for helping the patient manage pain, caregiver strain, and patient pain. Several differences in the study designs may account for the differing findings. First, the control condition in the pilot study was standard care without educational materials; patients in this condition reported increases in pain over time. Also, the pilot intervention was conducted in person in participants’ homes which facilitated session completion. Intervention effects in the current study were likely dampened by the fact that not all dyads received all three intervention sessions. The current intervention was designed to be accessible in that it was brief and delivered by videoconference, with considerable flexibility in the delivery of sessions. However, future studies may consider conducting home-based sessions to increase participant engagement. Engaging patients closer to the time of diagnosis may also help prevent attrition due to declining health.
The benefits of the control condition were unexpected given that dyads in this arm were given standard educational information about cancer pain management without any personalized guidance or instruction. In addition, improvements occurred in the control arm within a shorter follow-up time period. The improvements in pain interference (effect size=−0.44) and self-efficacy (effect size=0.42) are particularly notable and likely clinically significant. A recent systematic review of patient-based educational interventions for cancer pain management found improvements in pain were seen in less than one-third of studies and less than 20% of patients. (35) More effective educational interventions typically include at least some one-on-one training with a health care professional.(35) The video provided to participants in both arms provided basic information on topics related to the medical management of pain, primarily via cancer patients with pain talking about their experiences with treatment. At the time dyads received study materials, study staff encouraged participants to view the video and access other informational resources provided. However, we did not collect data regarding the degree to which dyads accessed the materials or applied them to cope with pain-related challenges, thus it is not clear how improvements reported by dyads in both arms might be related to exposure to the educational materials. We also did not systematically assess changes in pain medication use which could have influenced outcomes. Future studies might benefit from increased attention to pain medication use as well as use of qualitative interviews with participants to better understand unanticipated findings.
Prior research indicates that training in pain coping skills training improves outcomes in patients with cancer (4) and other pain conditions (36). This trial provides the first evidence that structured education and training has the potential to benefit patients and caregivers struggling with pain due to advanced cancer. While it is likely that factors driven by severity of illness, including attrition and timing of T2 data collection, contribute to the overall null finding for patients, it is also possible that the educational materials in the control arm provided unanticipated benefits. Overall, our findings suggest that a caregiver-assisted pain coping skills intervention may provide benefits for caregivers of patients with advanced cancer. Additional research is needed to determine how best to deliver interventions to optimize cancer pain outcomes in advanced cancer for patients and caregivers alike.
Supplementary Material
Key Statements:
What is already known about the topic?
Pain is one of the most frequent and distressing symptoms of cancer, particularly for patients with advanced disease.
Family caregivers are adversely impacted by the patient’s pain and often highly involved in pain management efforts.
Evidence suggests that behavioral pain coping skills training interventions can improve pain-related outcomes, however these interventions have rarely been tested in patients with advanced cancer or included family caregivers.
What this paper adds?
We tested the efficacy of a caregiver-assisted pain coping skills intervention that combined structured pain education with skills training among patients with advanced cancer and pain and their family caregivers.
Compared to a control condition that provided patients and caregivers with information about pain management only, the pain coping skills intervention led to improvements in caregiving satisfaction and caregiver anxiety.
Both pain coping skills training and the control condition led to improvements in pain-related outcomes for patients and caregivers.
Implications for practice, theory or policy
Structured education and pain coping skills training may benefit patients and caregivers facing advanced cancer.
Acknowledgements
This project was supported by the Palliative Care Research Cooperative Group funded by the National Institute of Nursing Research U24NR014637.
The authors thank Jessyka Glatz, Barbara Walukas, Kathy Ramadanovic, Emily Patterson, and Tina Gremore for their contributions to the study, and the patients and caregivers for their time and effort.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Nursing Research (grant number NR015348)
Footnotes
Declaration of Conflicting Interests
The authors declare that there are no conflicts of interest.
Trial registration: ClinicalTrials.gov NCT02430467, Caregiver-Guided Pain Management Training in Palliative Care
References
- 1.Van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symp Manag. 2016;51(6):1070–90. [DOI] [PubMed] [Google Scholar]
- 2.Miaskowski C, Kragness L, Dibble S, Wallhagen M. Differences in mood states, health status, and caregiver strain between family caregivers of oncology outpatients with and without cancer-related pain. J Pain Symptom Manage. 1997;13(3):138–47. [DOI] [PubMed] [Google Scholar]
- 3.Hodges L, Humphris GM, Macfarlane G A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Social Science & Medicine. 2005;60(1):1–12. [DOI] [PubMed] [Google Scholar]
- 4.Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32(16):1703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hackett J, Godfrey M, Bennett M. Patient and caregiver perspectives on managing pain in advanced cancer: A qualitative longitudinal study. Palliative Medicine. 2016;30(8):711–19. [DOI] [PubMed] [Google Scholar]
- 6.Gibbins J, Bhatia R, Forbes K, Reid CM. What do patients with advanced incurable cancer want from the management of their pain? A qualitative study. Palliative Medicine. 2014;28(1):71–8. [DOI] [PubMed] [Google Scholar]
- 7.Porter LS, Keefe FJ, Garst J, Baucom DH, McBride CM, McKee DC, et al. Caregiver-Assisted Coping Skills Training for Lung Cancer: Results of a Randomized Clinical Trial. Journal of Pain and Symptom Management. 2011;41(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keefe FJ, Blumenthal J, Baucom D, Affleck G, Waugh R, Caldwell DS, et al. Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: a randomized controlled study. Pain. 2004;110(3):539–49. [DOI] [PubMed] [Google Scholar]
- 9.Keefe FJ, Ahles TA, Sutton L, Dalton J, Baucom D, Pope MS, et al. Partner-guided cancer pain management at the end of life: a preliminary study. Journal of Pain & Symptom Management. 2005;29(3):263–72. [DOI] [PubMed] [Google Scholar]
- 10.Sutton LM, Porter LS, Keefe FJ. Cancer pain at the end of life: a biopsychosocial perspective. Pain. 2002;99(1–2):5–10. [DOI] [PubMed] [Google Scholar]
- 11.Keefe FJ, Ahles TA, Porter LS, Sutton LM, McBride CM, Pope MS, et al. The self-efficacy of family caregivers for helping cancer patients manage pain at end-of-life. Pain. 2003;103(1–2):157–62. [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS, Ryan KM Pain assessment: Global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 13.Van Ryn M, Sanders S, Kahn K, van Houtven C, Griffin JM, Martin M, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue?. Psychooncology. 2011;20:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss AH, Lunney JR, Culp S, Auber M, Kurian S, Rogers J, et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13(7):837–40. [DOI] [PubMed] [Google Scholar]
- 15.Porter LS, Samsa G, Steel JL, Hanson LC, LeBlanc TW, Bull J, et al. Caregiver-guided pain coping skills training for patients with advanced cancer: Background, design, and challenges for the CaringPals study. Clinical Trials. 2019;16(3):263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syrjala KL, Rupert J, DuPen A, Abrams J. Relieving cancer pain (Videotape). Seattle: Fred Hutchinson Cancer Research Center; 1997. [Google Scholar]
- 17.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C, Anderson KO, et al. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–84. [DOI] [PubMed] [Google Scholar]
- 18.Porter LS, Keefe FJ, Garst J, McBride CM, Baucom DH. Self-efficacy for symptom management in patients with lung cancer and their informal caregivers: Associations with symptoms and distress. [Journal Article In Press at Pain]. In press 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somers TJ, Kurakula PC, Criscione-Schreiber L, Keefe FJ, Clowse ME. Self-efficacy and pain catastrophizing in systemic lupus erythematosus: relationship to pain, stiffness, fatigue, and psychological distress. Arthritis Care Res. 2012;64(9):1334–40. [DOI] [PubMed] [Google Scholar]
- 20.Robinson BC. Validation of a Caregiver Strain Index. J Gerontol. 1983;38(3):344–8. [DOI] [PubMed] [Google Scholar]
- 21.Lawton MP, Kleban MH, Moss M, Rovine M, Glicksman A. Measuring caregiving appraisal. J Gerontol. 1989;44(3):P61–71. [DOI] [PubMed] [Google Scholar]
- 22.Van Dam NT, Earleywine M. Validation of the Center for Epidemiologic Studies Depression Scale--Revised (CESD-R): pragmatic depression assessment in the general population. Psychiatry Research. 2011;186(1):128–32. [DOI] [PubMed] [Google Scholar]
- 23.Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 24.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR, Lorig K, et al. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis & Rheumatism. 1989;32(1):37–44. [DOI] [PubMed] [Google Scholar]
- 25.Keefe FJ, Lefebvre JC, Maixner W, Salley AN Jr., Caldwell DS, Keefe FJ, et al. Self-efficacy for arthritis pain: relationship to perception of thermal laboratory pain stimuli. Arthritis Care & Research. 1997;10(3):177–84. [DOI] [PubMed] [Google Scholar]
- 26.Daut RL, Cleeland CS, Flanery RC Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. [DOI] [PubMed] [Google Scholar]
- 27.Carroll B, Kathol R, Noyes R, et al. Screening for depression and anxiety in cancer patients using the Hospital Anxiety and Depression Scale. Gen Hosp Psychiatry. 1993;15:69–74. [DOI] [PubMed] [Google Scholar]
- 28.Butow PN, Price MA, Bell ML, Webb PM, Defazio A, The Australian Ovarian Cancer Study G, et al. Caring for women with ovarian cancer in the last year of life: A longitudinal study of caregiver quality of life, distress and unmet needs. Gynecologic oncology. 2014;132(3):690–7. [DOI] [PubMed] [Google Scholar]
- 29.Moorey S, Greer S, Watson M, et al. The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. Br J Psychiatry. 1991;158:2555–259. [DOI] [PubMed] [Google Scholar]
- 30.Hopwood P, Howell A, Maguire P. Screening for psychiatric morbidity in patients with advanced breast cancer: validation of two self-report questionnaires. Br J Cancer. 1991;64:353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JR. Research electronic data capture (REDCap) - a metdata-drive methodology and workflow process for providing translational research informatics support. J BIomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little RJ, Rubin DB. Statistical Analysis with Missing Data. New York: John Wiley and Sons; 1987. [Google Scholar]
- 33.Little RJ. A class of pattern-mixture models for normal incomplete data. Biometrika. 1994;81:471–83. [Google Scholar]
- 34.Currow DC, Plummer JL, Kutner JS, Samsa GP, Abernethy AP. Analyzing Phase III Studies in Hospice/Palliative Care. A Solution That Sits Between Intention-to-Treat and Per Protocol Analyses: The Palliative-Modified ITT Analysis. J Pain Symptom Manage. 2012;44(4):595–603. [DOI] [PubMed] [Google Scholar]
- 35.Oldenmenger W, Geerling J, Mostovaya I, Vissers K, de Graeff A, Reyners A, et al. A systematic review of the effectiveness of patient-based educational interventions to improve cancer-related pain. Cancer Treatment Reviews. 2018;63:96–103. [DOI] [PubMed] [Google Scholar]
- 36.Turk DC, Swanson KS, Tunks ER. Psychological approaches in the treatment of chronic pain patients--when pills, scalpels, and needles are not enough. Canadian journal of psychiatry. 2008;53(4):213–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


