Abstract
COVID-19 is considered as the third human coronavirus and has a high potential for transmission. Fast public health interventions through antibodies, anti-virals or novel vaccine strategies to control the virus and disease transmission have been extremely followed. SARS-CoV-2 shares about 79% genomic similarity with SARS-CoV and approximately 50% with MERS-CoV. Based on these similarities, prior knowledge in treating SARS-CoV and MERS-CoV can be used as the basis of majority of the alternatives for controlling SARS-CoV-2. Immunotherapy is an effective strategy for clinical treatment of infectious diseases such as SARS-CoV-2. Passive antibody therapy, which decreases the virus replication and disease severity, is assessed as an effective therapeutic approach to control SARS-CoV-2 epidemics. The close similarity between SARS-CoV-2 genome with the SARS-CoV genome caused both coronaviruses to bind to the same angiotensin-converting enzyme 2 (ACE2) receptors that found in the human lung. There are several strategies to develop SARS-CoV-2 vaccines, which the majority of them are based on those developed previously for SARS-CoV. The interaction between the spike (S) protein of SARS-CoV-2 and ACE2 on the host cell surface leads to the initiation of SARS-CoV-2 infection. S protein, which is the main inducer of neutralizing antibodies, has been targeted by most of these strategies. Vaccines that induce an immune response against the S protein to inhibit its binding with the host ACE2 receptor, can be considered as effective vaccines against SARS-CoV-2. Here, we aimed to review frontier therapeutics and vaccination strategies for SARS-CoV-2 (COVID-19).
Keywords: COVID-19, Immunotherapy, ACE2, S protein, Vaccines
Introduction
Coronaviruses (CoVs) are classified into the family Coronaviridae, subfamily Coronavirinae, and the order Nidovirales (1). The family Coronaviridae is categorized into Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus that each of them is classified into lineage subgroups (2). Human coronaviruses (HCoVs), as a main group of coronaviruses, are recognized as respiratory pathogens related to respiratory and intestinal infections with various severities from the usual cold to pneumonia, and bronchiolitis. hCoV-229E, OC43, NL63, HKU1, and other human coronaviruses generally induce mild infection in humans (3). While Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) can induce intense respiratory illness and casualties (4). Mutations, high rate of nucleotide substitution, potential to infect new host, and cross-species transmission result in a fast evolution in HCoVs (5).
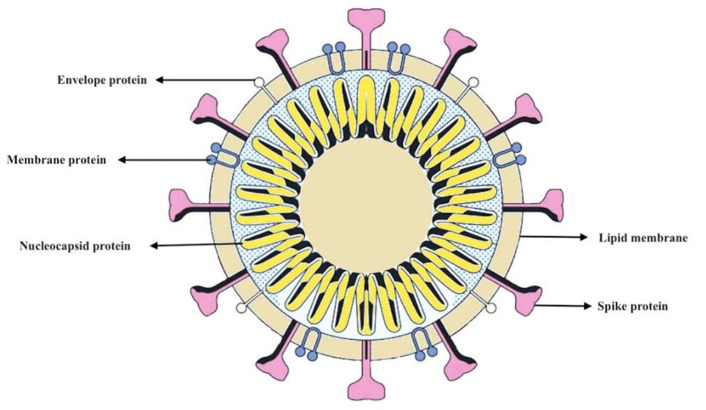
In structural point of view, they are large enveloped viruses with a positive sense, single-stranded RNA genome of about 26 to 33 kb that infect a wide range of hosts (6). A helical capsid and an envelope surround the genome, and the spike protein creates considerable protrusions in the envelope in the figure of a crown. It leads to the coronal appearance of the virus. Corona is a Latin word that means crown (7, 8). The surface spike protein (S), the membrane glycoprotein (M), and the envelope protein (E) are three MERS-CoV proteins, expressed on the envelope of the virus (Fig. 1). Viral entry by attaching to and merging with the host cell membrane occur through the S protein. MERS-CoV host cell receptors are dipeptidyl peptidase-4 (DPP4) (9, 10).
Fig. 1:
Diagram of coronavirus structure depicts surface proteins
Outbreak of another coronavirus, which induced respiratory associated illness, was reported in China at the end of 2019. It is the sister virus of SARS-CoV, so it has been named “SARS-CoV-2”. Its genome sequenced completely and although there are similarities with genome composition of SARS-CoV and MERS-CoV, they are distinguishable (1, 11). WHO announced a global health emergency, because of the continuing threat of coronavirus, briskly distributed to other countries. Although many nations are trying to perform preventive and control strategies, there is no report for both vaccines and drug to treat coronavirus infections (12, 13). Despite this, there have been numerous attempts to develop vaccines against human CoV infections in recent decades, but their considerable sequence diversity leads to the degree of cross-protection rendered by these vaccines is a limiting factor (14). Prior experiences in treating SARS- and MERS-CoV are considered as the foundation of majority of the therapeutic alternatives, which are accessible for controlling SARS-CoV-2.
Therapeutic methods for SARS-CoV-2
In order to control the virus and disease transmission, fast public health interferences through antibodies, anti-virals or novel vaccine strategies are extremely crucial. An effective strategy for clinical treatment of infectious disease is immunotherapy. A novel era in prevention of infectious disease that curbs lots of drawbacks related to serum therapy and intravenous immunoglobulin’s preparations in terms of specificity, purity, low risk of blood-borne pathogen pollution and safety is utilize of monoclonal antibodies. To curb SARS-CoV-2 epidemics, passive antibody therapy is evaluated. Monoclonal antibodies are adaptable type of pharmaceuticals, successfully utilized by pharmaceutical industry. Antibodies, required for passive immunotherapy, are possible to obtain from the blood of the infected patients or can be produced in the laboratory. The virus replication and disease severity can be decreased through passive immunization of antibodies, which can identify epitopic regions in the foreign virus particle. These antibodies have the potential to offer an effective therapeutic treatment with an extremely particular treatment against specific disease (15, 16).
In recent years, lot of monoclonal antibodies against viruses have been developed, furthermore several are in clinical pipeline (17, 18). In mice that were exposed to fatal MERS-CoV, considerable protection was induced through passive immunization with poorly and potently neutralizing antibodies. A set of monoclonal antibodies, which functionally target specific domains in MERS-CoV S protein, were used in a clinical experiment. These monoclonal antibodies bind to six specific epitope regions, which have interaction with the receptor binding, membrane fusion, and sialic acid-binding sites, that illustrate the three significant entry functions of MERS-CoV S protein (19, 20).
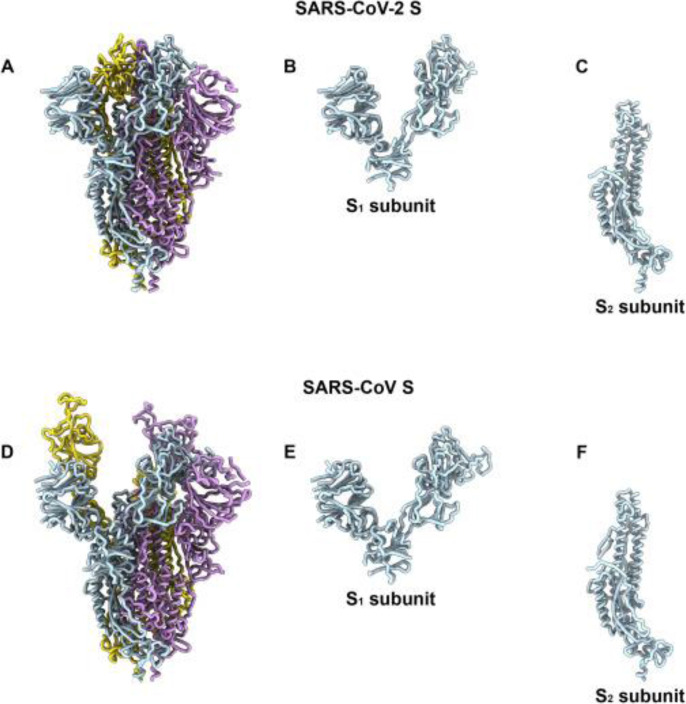
The interaction between the receptor binding domain, located in the S protein, and target receptor on the host cell surface, such as angiotensin-converting enzyme 2 (ACE2) for SARS-CoV and dipeptidyl peptidase-4 (DPP4) for MERS-CoV, leads to the initiation of CoV infection (21). Utilize of broad-spectrum anti-viral drugs or determine therapeutic molecules that can directly disturb any levels of the viral lifecycle or the receptor proteins on the host cell membrane to prevent the virus binding, and consequently blocking the attachment virus and its entrance. We can achieve this by utilizing peptidic fusion inhibitors, anti-SARS-CoV-2 neutralizing monoclonal antibodies, anti-ACE2 monoclonal antibodies and protease inhibitors. The main antigenic component, which causes host immune response, is the spike protein (Fig. 2). In addition, the spike protein located on the viral membrane has an essential influence on virus entry (22). For this reason, to develop effective therapeutics and increase humoral protection against coronavirus infection through targeting various S protein epitopes and functions, a novel approach can be represented by using these antibodies. In the receptor-binding domain (RBD) of S1 subunit of spike protein, the receptor-binding motif is located that has an interaction with the cell receptor and mediates the virus attachment with the host cells (23). The cross-neutralization capacity of SARS-CoV RBD-specific neutralizing monoclonal antibodies widely relies on the similarity between their RBDs. SARS-CoV RBD specific antibodies can cross-neutralize SARS-like (SL) CoVs, i.e., bat-SL-CoV strain WIV1 RBD which had 8 different amino acids with SARS-CoV, though not bat-SL-CoV strain SHC014 which had 24 different amino acids (25). Appropriate RBD-specific monoclonal antibodies can be recognized and assessed in clinical experiments through a comparative analysis of SARS-CoV-2 RBD with SARS-CoV. To recognize monoclonal antibodies specific and effective for SARS-CoV-2, regeneron is trying. Therefore, the therapies for SARS-CoV are possible to extrapolate to utilize for SARS-CoV-2. The specific neutralizing monoclonal antibodies that target RBD in spike protein or specific antibodies that bind to ACE2 could effectively inhibit the virus binding to its cellular receptor, and consequently prevent its entry into the cell.
Fig. 2:
SARS-CoV-2 and SARS-CoV S Structures (24)
Since SARS-CoV and SARS-CoV-2 utilize the same host cell surface receptor, potential blocking agents or strategies that examined to prevent SARS entry can be assessed against SARS-CoV-2. A group of human monoclonal antibodies, which target the RBD region of S protein of SARS-CoV, are demonstrated by Coughlin and Prabhakar (26).
Different monoclonal antibodies may combine to identify various epitopes on the viral surface. This combination can be evaluated to neutralize broad range of isolates containing escape mutants. Consequently, we can utilize the best candidates for passive immunotherapy. Monoclonal antibody combination represents more potent anti-virus activity which can enhance the efficacy of the treatment and prevent the viral escape (27, 28).
Since produce extensive monoclonal antibodies is labor-intensive, expensive and time-consuming, there is an urgent need to reduce the production costs of monoclonal antibody. Suitable expression system such as mammalian, yeast or plant can be used for cloning and expressing the sequences of monoclonal antibodies, which are effective against SARS-CoV. However, this method should be investigated. Interestingly, to produce monoclonal antibodies in a short time, plant expression system can be recognized, which has an affordable cost. This is an important advantage that should be evaluated particularly during epidemic situation (29–32).
In addition, combination therapy with monoclonal antibodies and the drug remdesivir may be considered as an ideal therapeutic option for SARS-CoV-2 (33). However, before confirming the effectiveness of this combination therapy, more assessment is needed. To inhibit virus replication, fully human antibodies (such as human single-chain antibodies; Hu-scFvs) or humanizednanobodies (single-domain antibodies, sdAb, VH/VHH), can be produced. These fully human antibodies and humanized-nanobodies can traverse across the membrane of the virus-infected cells (trans bodies) and bind to or interfere with biological activities of replicating virus proteins, and consequently inhibit the replication of virus. Some examples consist of trans bodies to influenza virus, hepatitis C virus, Ebola virus, and Dengue virus (34). Therefore, trans bodies can be produced to CoV intracellular proteins, for instance, the papain-like proteases (PLpro), cysteine-like protease (3CLpro) or other non-structural proteins (nsps). These are essential for CoV replication and transcription for safe, nonimmunogenic, widely effective passive immunization of CoV exposed subjects and treatment of individuals infected. In neutralizing the virus and prevent further infection, immunotherapy through transferring the serum of recovered individuals to infected patients can be effective. There are evidences and experiences in treating other viral infections like influenza, SARS, MERS and Ebola, which reveal that the early administration of plasma of recovered individuals or hyper-immune immunoglobulin from patients that includes considerable antibody titers, probably can decrease the viral load and disease fatality (35, 36). Nevertheless, before considering that plasma of recovered individuals as a therapeutic option, the main challenges like accessibility of enough donors, clinical condition, viral kinetics, and host interactions of SARS-CoV-2, should be considered.
SARS-CoV-2 vaccine strategies
There are several strategies to develop CoV vaccines. A classical strategy for viral vaccinations is live-attenuated or inactive whole virus vaccines. Whole virus vaccines have innate immunogenicity and can stimulate toll-like receptors (TLRs) consisting TLR 3, TLR 7/8, and TLR 9, considered as important benefits. However, to approve live virus vaccine safety, extensive extra examining is needed. This matter is particularly considered for coronavirus vaccines, based on the findings of enhanced infectivity following immunization with live or killed whole virus SARS coronavirus vaccines (37).
An industry newsletter reported that Johnson & Johnson is one of the few multinational companies initiating SARS-CoV-2 vaccines. They are using Janssen’s AdVac® adenoviral vector and manufacturing in their PER.C6® cell line technology, similar to their Ebola vaccine platform. Moreover, researchers at the University of Hong Kong have developed a live influenza vaccine, which expresses SARS-CoV-2 proteins. Ultimately, a “codon deoptimization” technology to attenuate viruses has been developed by Codagenix. This technology is investigating SARS-CoV-2 vaccine strategies (38).
Among different strategies to develop CoV vaccines, most of them target the surface-exposed spike (S) glycoprotein or S protein as the main inducer of neutralizing antibodies. Many S-protein-based strategies have been tried to develop CoV vaccines. For example, utilize of full-length S protein or S1-receptor-binding domain (RBD) and expression in virus-like particles (VLP), DNA, or viral vectors (14, 19, 39, 40).
Subunit vaccines are included one or more immunogenic units, derived from a pathogen (41). For both SARS-CoV and SARS-CoV-2, subunit vaccines depend on inducing an immune response against the S protein to prevent its binding with the host ACE2 receptor (37). S1 and S2 are two subunits of the S protein molecule, which have their own responsibilities. The S1 subunit has an RBD that interacts with ACE2. The S2 subunit has an important effect on merging between the virus and host cell membranes in order to transferring viral RNA into the cytoplasm for replication (42). For this reason, S-proteinbased vaccines must stimulate antibodies, which block both viral receptor binding and virus genome uncoating. The immunodominant region is constituted by the C-terminal domain of the S1 subunit of porcine Deltacoronavirus, in addition the immune response to this region demonstrates the most potent neutralizing result (43). During infection with SARS-CoV, the S protein has an important effect on the initiation of protective immunity through inducing neutralizing-antibodies and T-cell responses (42). Enhanced infection and eosinophilic infiltration are induced by some full-length S proteins in SARS. For this reason, full-length or suitable portions of the S protein can be considered as an effective candidate CoV vaccine composition. S protein immunogenicity or the binding of it to the ACE2 receptor, which is essential for virus to accessibility to the host cell, are not affected by other structural proteins (44, 45).
The University of Queensland is synthesizing viral surface proteins, under funding from the Coalition for Epidemic Preparedness (CEPI), to represent them without difficulty to the immune system. In addition, immunogenic virus-like nanoparticles, relied on recombinant expression of the S protein, have been developed and manufactured by Novavax (46). Clover Biopharmaceuticals through utilizing their patented Trimer-Tag® technology, is developing a subunit vaccine included of a trimerized SARS-CoV-2 S protein (38).
Recombinant proteins that consist of RBD and the recombinant vectors that encode RBD are possible to be used for developing the effective SARS-CoV vaccines, based on the exclusive potential of RBD to induce neutralizing antibodies (47). A subunit vaccine, consisted of RBD of the SARS-CoV S protein, has developed and examined by a consortium guided by Texas Children’s Hospital Center for Vaccine Development at Baylor College of Medicine (37, 48, 49). The SARS-CoV RBD vaccine induces a peak of protective immunity on the similar virus challenge when formulated on alum. RBD-based vaccine can minimize host immunopotentiation, considered as a benefit for this vaccine (37). Since the SARS-CoV and SARS-CoV-2 RBDs have more than 80% amino acid similarity and bind to the same ACE2 receptor, make it possible to develop either protein as a subunit vaccine.
By applying intranasally of recombinant adenovirus-based vaccine expressing MERS-CoV S protein into BALB/c mice, systemic IgG, secretory IgA, and lung resident memory T-cell responses are induced and long-lasting neutralizing immunity to MERS spike pseudotyped virus is provided. As a result, the vaccine might be effective in the protection against MERS-CoV (45). Moreover, to express MERS-CoV S protein, rabies virus (RV), which is a viral vector, and Grampositive enhancer matrix (GEM), which is a bacterial vector, have been utilized. In BALB/c mice, the immune responses to these vaccine candidates were investigated for cellular and humoral immune responses. Considerably higher levels of cellular immunity and earlier antibody responses were induced by RV based vaccine in comparison to the GEM particle vector (50). Because of the similarity in T-cell epitopes of SARS and MERSCoV, there is an expectancy of developing a universal CoV vaccine. In fact, the potential for cross reactivity between CoVs was confirmed, based on this similarity (51).
Moreover, vaccines developed for SARS-CoV are possible to reveal crossreactivity to SARS-CoV-2 (52). On full-length S protein sequences of SARS-CoV-2 and SARS-CoV, the comparative evaluation was carried out and recognized that the essential CoV vaccine target is the most variable residues located in the S1 subunit of S protein (53). These findings suggest that the particular neutralizing antibodies, which are effective against the SARS-CoV, may not be effective against the SARS-CoV-2. There are critical mutations in the S protein of SARS-CoV-2 in comparison to SARS-CoV, they will still act as a feasible target for vaccine development (54).
In order to recognize epitopes for inclusion in SARS-CoV-2 vaccine candidates, it is possible to utilize immuno-informatics strategy. To identify considerable cytotoxic T lymphocyte (CTL) and B-cell epitopes in SARS-CoV-2 S protein, immuno-informatics was used recently. Through utilizing molecular dynamics simulations, the interactions between these epitopes and their corresponding MHC class I molecules were investigated, and the CTL epitopes bind with MHC class I peptide-binding grooves through multiple contacts, so demonstrating their potential for producing immune responses (55). These epitopes might have the principal features to be a component of SARS-CoV-2 vaccine candidates. As possible immunoprotective targets, which stimulate T-cell and neutralizing antibody responses, the nucleocapsid (N) protein, as well as the potential B-cell epitopes of the E protein of MERS-CoV, has been proposed (56, 57). To inactivate the exonuclease influences of non-structural protein 14 (nsp14) or to eliminate the envelope protein in SARS in live-attenuated vaccines, reverse genetic approaches have been utilized (14). Avian live virus IBV vaccine (strain H), which is a vaccine for avian infectious bronchitis virus (IBV) as a chicken CoV, may be effective for SARS (58). Strain H presents an immunization relies on neutralizing antibody production as well as other immune responses. As a result, if the safety of avian IBV vaccine confirms through assessing, it can be regarded as another alternative for SARS-CoV-2 (59).
There is cooperation between researchers at Rocky Mountain Laboratories and Oxford University, to develop a chimpanzee adenovirusvectored SARS-CoV-2 vaccine candidate. The Coalition for Epidemic Preparedness Innovations (CEPI) recently announced the initiation of three programs aimed to develop SARS-CoV-2 vaccines by utilizing constituted vaccine platforms (60–62). Two of these programs are continuances of earlier cooperation.
There are advanced nucleic acid vaccine platforms for SARS-CoV-2 by several major biotechs. For instance, a DNA vaccine is developing by Inovio Pharmaceuticals, on the other hand, RNA vaccine platforms are developing by Moderna Therapeutics and Curevac. In 1993, immunizing with DNA in mice represented protective immunity against influenza, which was the first idea of immunizing with DNA. In 2018, for developing DNA vaccine candidates for MERS, there was cooperation between CEPI and Inovio. This vaccine, which is under development, to transporting synthetic genes into cells for translation into antigenic proteins, uses DNA Medicines’ platform. Then, T-cell and antibody responses are induced through antigenic proteins. Since 2019, cooperation between CEPI and The University of Queensland has started to develop the molecular clamp vaccine platform against multiple viral pathogens consisting MERS-CoV. The operation of the vaccine platform is in a way that viral surface proteins are generated to get bind to the host cells and attach them into shape. It assists to identify antigens by the immune system (60–62).
Except these programs, funding has presented by CEPI to Moderna for comparing mRNA therapeutics and vaccines. They collaborate with Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), which is a section of the National Institutes of Health (NIH), with the aim of design and produce mRNA vaccine (63). A vaccine candidate is developing by NIAID-VRC scientists, which is expressing SARS-CoV-2 S protein in the mRNA vaccine platform technology. This vaccine undergoes clinical experiments in a few months. Table 1 illustrates some of the main SARS-CoV-2 clinical vaccines and therapies under development, including biocentury.com (38).
Table 1:
Some of the main SARS-CoV-2 vaccines under development (38)
| Compound | Sponsor | Modality | Type | Phase (COVID-19) |
|---|---|---|---|---|
| Anti-SARS-CoV-2 polyclonal hyperimmune immunoglobulin | CSL; Takeda; Biotest; BPL; LFB; Octapharma | Biologic | Therapy | Preclin |
| Anti-SARS-CoV-2 hyperimmune globulin | Grifols | Antibody | Therapy | Preclin |
| CORVax12 | OncoSec | DNA vaccine | Vaccine | IND |
| CoroFlu | FluGen / Bharat Biotech | Viral vaccine | Vaccine | Preclin |
| CV-15 (iCP-NI) | Cellivery Therapeutics | Peptide | Therapy | Preclin |
| EIDD-2801 | Emory University; Ridgeback Biotherapeutics | Small molecule | Therapy | Preclin |
| ExpreS2-CoV | ExpreS2ion Biotech Holding; AdaptVac; University of Tübingen; Leiden University Medical Center; University of Copenhagen; Wageningen University | Protein-based | Vaccine | Preclin |
| Ii-Key-SARS-2 | Generex Biotechnology; EpiVax | Protein-based | Vaccine | Preclin |
| ISR50 | ISR | Small molecule | Therapy | Preclin |
| LUNAR-COV19 | Arcturus Therapeutics; Duke-NUS Medical School | RNA vaccine | Vaccine | Preclin |
| NCP112 | NovaCell Technology | Peptide | Therapy | Preclin |
| Neumifil | Pneumagen | Protein | Therapy | Preclin |
| PRTX007 | Primmune Therapeutics | Small molecule | Therapy | Preclin |
| Unnamed | Baylor College of Medicine; University of Texas Medical Branch; New York Blood Center; Fudan University | Protein-based | Vaccine | Preclin |
| WP1122 | Moleculin Biotech | Small molecule | Therapy | Preclin |
| Kainos small molecule antivirals | Kainos Medicine | Small molecule | Therapy | Preclin |
| Ad26 SARS-CoV-2 | Johnson & Johnson; Beth Israel Deaconess Medical Center; BARDA | Viral vector | Vaccine | Preclin |
| AdCOVID | Altimmune; University of Alabama | Viral vector | Vaccine | Preclin |
| BNT162 | BioNTech; Pfizer; Fosun Pharma | RNA vaccine | Vaccine | Preclin |
| COVID-19 S-Trimer Vaccine | Sichuan Clover Biopharmaceuticals; Dynavax | Protein-based | Vaccine | Preclin |
| COVID-HIG | Emergent BioSolutions; BARDA | Antibody | Therapy | Preclin |
| COVID-EIG | Emergent BioSolutions | Antibody | Therapy | Preclin |
| Coronavirus VLP | Mitsubishi Tanabe (Medicago) | Vaccine | Vaccine | Preclin |
| DPX-COVID-19 | IMV | Protein-based | Vaccine | Preclin |
| IBIO-200 | iBio; Texas A&M University | Protein-based | Vaccine | Preclin |
| ISR50 | ISR | Small molecule | Therapy | Preclin |
| NI007 | Neurimmune; Ethris | Antibody; RNA | Therapy | Preclin |
| rCIG | GigaGen | Antibody | Therapy | Preclin |
| SAB-185 | SAB Biotherapeutics; BARDA | Antibody | Therapy | Preclin |
| STI-4398 | Sorrento Therapeutics | Fusion protein | Therapy | Preclin |
| STI-6991 | Sorrento Therapeutics | Cellular vaccine | Vaccine | Preclin |
| TAK-888 | Takeda Pharmaceutical | Antibody | Therapy | Preclin |
| TNX-1800 | Tonix Pharmaceuticals; Southern Research Institute | Engineered live attenuated virus | Vaccine | Preclin |
| VIR-7831 | Vir Biotechnology; GlaxoSmithKline | Antibody | Therapy | Preclin |
| VIR-7832 | Vir Biotechnology; GlaxoSmithKline | Antibody | Therapy | Preclin |
Conclusion
SARS-CoV-2 has been distributed rapidly, while neither an effective anti-viral nor a vaccine in order to treat this infection is available until now. There is an urgent demand to a successful vaccine against SARS-CoV-2 to prevent the spread of this virus. Specific preventive and therapeutic intervention strategies consisting vaccines, monoclonal antibodies, peptides, interferon therapies and small-molecule drugs to combat SARS-CoV-2 has been developing; however examine their effectiveness in vitro/in vivo might need some months. In addition, it widely relies on the outcomes of the clinical experiments. Although this virus is recently recognized, the clinical and genetic features demonstrate similarity with SARS-CoV.
Due to their similarities, it is possible to use the prior knowledge and modify the existing vaccines or therapeutic models, which were developed against different coronaviruses to target the unique aspects of SARS-CoV-2.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
Thanks to guidance and advice from “Clinical Research Development Unit of Baqiyatallah Hospital”.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Gorbalenya AE. (2020). Severe acute respiratory syndrome-related coronavirus–The species and its viruses, a statement of the Coronavirus Study Group. Cold Spring Harbor Laboratory. www.biorxiv.org [Google Scholar]
- 2.Mubarak A, Alturaiki W, Hemida MG. (2019). Middle east respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res, 2019: 6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J, Li F, Shi Z-L. (2019). Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol, 17 (3): 181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlos WG, Dela Cruz CS, et al. (2020). Novel wuhan (2019-nCoV) coronavirus. Am J Respir Crit Care Med, 201(4): P7–P8. [DOI] [PubMed] [Google Scholar]
- 5.Vijgen L, Keyaerts E, Moës E, et al. (2005). Development of one-step, real-time, quantitative reverse transcriptase PCR assays for absolute quantitation of human coronaviruses OC43 and 229E. J Clin Microbiol, 43 (11): 5452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R, Zhao X, Li J, et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 395(10224): 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss SR, Leibowitz JL. (2011). Coronavirus pathogenesis. In: Advances in Virus Research. Ed Maramorosch K, Shatkin AJ, Murphy AF. Elsevier, 32 Jamestown Road, London, NW1 7BY, UK. 85–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F. (2016). Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol, 3: 237–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia S, Liu Q, Wang Q, et al. (2014). Middle East respiratory syndrome coronavirus (MERS-CoV) entry inhibitors targeting spike protein. Virus Res, 194 (2014): 200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu G, Hu Y, Wang Q, et al. (2013). Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature, 500 (7461): 227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Qahtani AA, Lyroni K, Aznaourova M, et al. (2017). Middle east respiratory syndrome corona virus spike glycoprotein suppresses macrophage responses via DPP4-mediated induction of IRAK-M and PPARγ. Oncotarget, 8 (6): 9053–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H. (2020). Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends, 14 (1): 69–71. [DOI] [PubMed] [Google Scholar]
- 13.Pillaiyar T, Meenakshisundaram S, Manickam M. (2020). Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov Today, 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed]
- 14.Graham RL, Donaldson EF, Baric RS. (2013). A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol, 11 (12): 836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sui J, Li W, Roberts A, et al. (2005). Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J Virol, 79 (10): 5900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marasco WA, Sui J. (2007). The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol, 25 (12): 1421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group TPIW, Team M-NPIS. (2016). A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med, 375 (15): 1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caskey M, Klein F, Lorenzi JC, et al. (2015). Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature, 522 (7557): 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widjaja I, Wang C, van Haperen R, et al. (2019). Towards a solution to MERS: protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerg Microbes Infect, 8 (1): 516–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goo J, Jeong Y, Park Y-S, et al. (2020). Characterization of novel monoclonal antibodies against MERS-coronavirus spike protein. Virus Res, 278:197863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandekar AA, Perlman S. (2005). Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol, 5 (12): 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du L, Yang Y, Zhou Y, et al. (2017). MERSCoV spike protein: a key target for antivirals. Expert Opin Ther Targets, 21 (2): 131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Z, Xu Y, Bao L, et al. (2019). From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses, 11 (1): 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls AC, Park Y-J, Tortorici MA, et al. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 181 (2): 281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng L-P, Ge X-Y, Peng C, et al. (2017). Cross-neutralization of SARS coronavirus-specific antibodies against bat SARS-like coronaviruses. Sci China Life Sci, 60 (12): 1399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coughlin MM, Prabhakar BS. (2012). Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev Med Virol, 22 (1): 2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder HW, Jr, Cavacini L. (2010). Structure and function of immunoglobulins. J Allergy Clin Immunol, 125 (2): S41–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparrow E, Friede M, Sheikh M, et al. (2017). Therapeutic antibodies for infectious diseases. Bull World Health Org, 95 (3): 235–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demurtas OC, Massa S, Illiano E, et al. (2016). Antigen production in plant to tackle infectious diseases flare up: the case of SARS. Front Plant Sci, 7: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiatt A, Whaley K, Zeitlin L. (2015). Plant-derived monoclonal antibodies for prevention and treatment of infectious disease. In: Antibodies for Infectious Diseases: Ed, Crowe James E., Jr., Rappuoli Diana Boraschi Rino. Microbiol Spectr, mBio press, Washington, D.C., USA. 413–25. [DOI] [PubMed] [Google Scholar]
- 31.Sainsbury F. (2020). Innovation in plant-based transient protein expression for infectious disease prevention and preparedness. Curr Opin Biotechnol, 61: 110–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanmugaraj B, Malla A, Phoolcharoen W. (2020). Emergence of Novel Coronavirus 2019-nCoV: Need for Rapid Vaccine and Biologics Development. Pathogens, 9 (2): 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. (2020). New coronavirus threat galvanizes scientists. Science, 367 (6477): 492–3 [DOI] [PubMed] [Google Scholar]
- 34.Seesuay W, Jittavisutthikul S, Sae-Lim N, et al. (2018). Human transbodies that interfere with the functions of Ebola virus VP35 protein in genome replication and transcription and innate immune antagonism. Emerg Microbes Infect, 7(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mupapa K, Massamba M, Kibadi K, et al. (1999). Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis, 179 Suppl 1:S18–23. [DOI] [PubMed] [Google Scholar]
- 36.Arabi Y, Balkhy H, Hajeer AH, et al. (2015). Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus, 19;4:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang S, Bottazzi ME, Du L, et al. (2012). Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expert Rev Vaccines, 11 (12): 1405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W-H, Strych U, Hotez PJ, et al. (2020). The SARS-CoV-2 Vaccine Pipeline: an Overview. Curr Trop Med Rep: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z-y, Kong W-p, Huang Y, et al. (2004). A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature, 428 (6982): 561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji W, Wang W, Zhao X, et al. (2020). Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol, 92 (4): 433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindewolf C, Menachery VD. (2019). Middle east respiratory syndrome vaccine candidates: cautious optimism. Viruses, 17;11(1).: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du L, He Y, Zhou Y, et al. (2009). The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol, 7 (3): 226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen R, Fu J, Hu J, et al. (2020). Identification of the immunodominant neutralizing regions in the spike glycoprotein of porcine deltacoronavirus. Virus Res, 276: 197834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchholz UJ, Bukreyev A, Yang L, et al. (2004). Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A, 101 (26): 9804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim MH, Kim HJ, Chang J. (2019). Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus. PLoS One, 14(7):e0220196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman CM, Liu YV, Mu H, et al. (2014). Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine, 32 (26): 3169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang S, He Y, Liu S. (2005). SARS vaccine development. Emerg Infect Dis, 11 (7): 1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W-H, Chag SM, Poongavanam MV, et al. (2017). Optimization of the production process and characterization of the yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1), a SARS vaccine candidate. J Pharm Sci, 106 (8): 1961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W-H, Du L, Chag SM, et al. (2014). Yeast-expressed recombinant protein of the receptor-binding domain in SARS-CoV spike protein with deglycosylated forms as a SARS vaccine candidate. Hum Vaccin Immunother, 10 (3): 648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li E, Yan F, Huang P, Xu S, Li G, Liu C, et al. (2020). Characterization of the immune response of MERS-CoV vaccine candidates derived from two different vectors in mice. Viruses, 12 (1): 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu WJ, Zhao M, Liu K, Xu K, Wong G, Tan W, Gao GF. (2017). T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral Res, 137 (2017): 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang S, Du L, Shi Z. (2020). An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect, 9 (1): 275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu F, Du L, Ojcius DM, et al. (2020). Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect, 22 (2): 74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W, Morse JS, Lalonde T, et al. (2020). Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem, 21 (5): 730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baruah V, Bose S. (2020). Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J Med Virol, 92 (5): 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi J, Zhang J, Li S, et al. (2015). Epitope-based vaccine target screening against highly pathogenic MERS-CoV: an in silico approach applied to emerging infectious diseases. PLoS One, 10 (12): e0144475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie Q, He X, Yang F, Liu X, Li Y, Liu Y, et al. (2017). Analysis of the genome sequence and prediction of B-cell epitopes of the envelope protein of Middle East respiratory syndrome-coronavirus. IEEE/ACM Trans Comput Biol Bioinform. 15 (4): 1344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bijlenga G. (2005). Proposal for vaccination against SARS coronavirus using avian infectious bronchitis virus strain H from The Netherlands. J Infect, 51 (3): 263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, Liu Y. (2020). Potential interventions for novel coronavirus in China: A systematic review. J Med Virol, 92 (5): 479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz DA, Graham AL. (2020). Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-ncov infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses, 12 (2): 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang J, Wang MX, Ang IYH, et al. (2020). Potential rapid diagnostics, vaccine and therapeutics for 2019 novel Coronavirus (2019-ncoV): a systematic review. J Clin Med, 9 (3): 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rutschman AS. (2020). The Intellectual Property of Vaccines: Takeaways from Recent Infectious Disease Outbreaks. Mich Law Rev, 2020: 1–13. [Google Scholar]
- 63.Challener CA. (2020). Can Vaccine Development Be Safely Accelerated. Pharm Technol, 44 (4): 22–26. [Google Scholar]