Abstract
Plant species from Annonaceae are commonly used in traditional medicine to treat various cancer types. This study aimed to investigate the antiproliferative potential of an alkaloid and acetogenin-rich fraction from the fruit peel of Annona crassiflora in HepG2 cells. A liquid-liquid fractionation was carried out on the ethanol extract of A. crassiflora fruit peel in order to obtain an alkaloid and acetogenin-rich fraction (AF-Ac). Cytotoxicity, proliferation and migration were evaluated in the HepG2 cells, as well as the proliferating cell nuclear antigen (PCNA), vinculin and epidermal growth factor receptor (EGFR) expression. In addition, intracellular Ca2+ was determined using Fluo4-AM and fluorescence microscopy. First, 9 aporphine alkaloids and 4 acetogenins that had not yet been identified in the fruit peel of A. crassiflora were found in AF-Ac. The treatment with 50 μg/mL AF-Ac reduced HepG2 cell viability, proliferation and migration (p < 0.001), which is in accordance with the reduced expression of PCNA and EGFR levels (p < 0.05). Furthermore, AF-Ac increased intracellular Ca2+ in the HepG2 cells, mobilizing intracellular calcium stores, which might be involved in the anti-migration and anti-proliferation capacities of AF-Ac. Our results support the growth-inhibitory potential of AF-Ac on HepG2 cells and suggest that this effect is triggered, at least in part, by PCNA and EGFR modulation and mobilization of intracellular Ca2+. This study showed biological activities not yet described for A. crassiflora fruit peel, which provide new possibilities for further in vivo studies to assess the antitumoral potential of A. crassiflora, especially its fruit peel.
Introduction
Cancer represents one of the main challenges for medicine being one of the most critical problems of public health in the world. Hepatocellular carcinoma (HCC) is the seventh most frequently occurring cancer and the fourth most common cause of cancer mortality, with over half a million new cases diagnosed annually worldwide [1]. Hepatitis B and C virus and excessive alcohol consumption are important risk factors for HCC [2]. In addition to its high incidence, this tumor is usually diagnosed at advanced stages, which hampers effective treatment. Recently, the intracellular Ca2+ signaling was related to the development of HCC from different etiologies, increasing cell proliferation and reducing cell death, being a promissing target for new therapies [3]. Guimaraes, Machado [4] also showed that intracellular Ca2+ is important during the primary tumor establishment and keys steps for the metastasis formation, as angiogenesis and migration, trough upregulation of CXCL10 and vinculin, a well-known angiostatic factor and focal adhesion protein, respectively. Therefore, the search of new agents capable of controlling the development of hepatocellular tumor is important to reduce the mortality caused by this disease.
Thus, over the past decade, numerous studies have shown that compounds derived from plants are potentially interesting for therapeutic interventions in various cancer types due to their great diversity of chemical structures, and better drug-like properties compared to the synthetic compounds [5–7]. Examples include plant-derived alkaloids, specifically aporphine alkaloids, which had previously demonstrated antitumor effects in different cancer cell models [7–10]. Acetogenins, a class of polyketide compounds found in plants of the Annonaceae family, have also been reported to possess apoptosis-inducing effects [11]. Annona crassiflora Mart., an Annonaceae species native to the Brazilian Savanna, where it is known as araticum, might be a potential source of acetogenins and aporphine alkaloids [12–14]. Different parts of this species such as bark, leaf, fruit and seed have been widely used in folk medicine for the treatment of inflammation, microbial infections, malaria, veneral diseases, snakebites, diarrhea, and as cancer chemopreventive agents [15–17].
Recently, methanolic extracts of leaves and seeds of A. crassiflora have shown in vitro antiproliferative properties in leukemia, glioblastoma, lung and ovarian cancer cell lines [18]. Furthermore, a study done by Silva, Alves (19) showed that a hexane fraction from the crude extract of A. crassiflora leaf had cytotoxic effect on cervical cancer cells by acting through DNA damage, apoptosis via intrinsic pathway and mitochondrial membrane depolarization [19]. However, scientific reports demonstrating antitumoral activities of the fruit peel of this species are still limited.
Previously, a pre-purification of the ethanol extract of A. crassiflora fruit peel was conducted, resulting in an alkaloid (CH2Cl2 fraction)-enriched fraction [12]. From the CH2Cl2 fraction, stephalagine, an aporphine alkaloid, was isolated and characterized [13]. It is worth mentioning here that the only biological activities described for this alkaloid are its antinociceptive feature [12] and potential inhibitory effect against pancreatic lipase [13]. In this context, in this study, we first identified the main alkaloids and acetogenins present in the alkaloid and acetogenin-rich fraction from A. crassiflora fruit peel, named here as AF-Ac. Then, we evaluated the antiproliferative potential of AF-Ac in HepG2 cells, exploring the possible involvement of the proliferating cell nuclear antigen (PCNA), vinculin and epidermal growth factor receptor (EGFR), as well as the intracellular calcium (Ca2+) signaling.
Materials and methods
Reagents
Ethanol (>98%), n-hexane (99%), dichloromethane (99.5%), ethyl acetate (99.5%), n-butanol (≥99.5%), methanol (≥99.8%), hydrochloric acid (37%), ammonium hydroxide (30%) and formic acid (98%) were purchased from Vetec Quimica Fina Ltda (Duque de Caxias, Rio de Janeiro, Brazil). Fluo-4/AM was purchased from Invitrogen (Eugene, USA). Enhanced chemiluminescence (ECL-plus Western Blotting Detection System) and peroxidase conjugated antibodies were purchased from Amersham Biosciences (Buckinghamshire, UK). All other reagents and standards were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Milli-Q Academic Water Purification System (Millipore Corp., Billerica, MA) was used to obatin the ionexchanged water. All reagents were of analytical grade.
Plant material and alkaloid and acetogenin-rich fraction
A. crassiflora fruits were collected in the northern region of Minas Gerais State, Brazil, in March 2017. Voucher specimens (leaf, branch and fruit) (HUFU68467) were deposited in the herbarium of the Federal University of Uberlandia. The peels were quickly removed from the fresh fruits and crushed, and the obtained powder was stored at -20°C until the moment of extraction. The dried and powdered peels (1.0 kg) were extracted for three days by maceration with 6 L of 98% ethanol at 25°C. After filtration, ethanol was removed under reduced pressure using a rotary evaporator (Bunchi Rotavapor R-210, Switzerland) at 40°C. This process was repeated until the last extract turned colorless (54.2 g, 5.42%). The alkaloid and acetogenin-rich fraction (AF-Ac) was obtained by a liquid-liquid extraction [12]. Briefly, the ethanol extract (10.0 g) was diluted in methanol:water (9:1, v/v, 200 mL), filtered and extracted using n-hexane (4 × 200 mL, 0.17 g), dichloromethane (4 × 200 mL, 0.31 g), ethyl acetate (4 x 200 mL, 2.71 g) and n-butanol (4 x 200 mL, 2.65 g). Additionally, an aqueous fraction (0.61 g) was obtained. All solvents were completely removed under reduced pressure at 40°C using a rotary evaporator, and the remaining water was removed by lyophilization (L101, Liobras, SP, Brazil). The dichloromethane fraction, named here as alkaloid and acetogenin-rich fraction (AF-Ac), was selected to perform the experiments, since previous studies have showed that alkaloids and acetogenins are detected and concentrated in this fraction [12, 20]. AF-Ac was maintained at -20˚C until use.
Ultra-High-Performance Liquid Chromatography—Electrospray Ionization-tandem Mass Spectrometry (UHPLC-ESI/MSn)
The UHPLC-ESI/MSn analysis of AF-Ac was done on an Agilent Q-TOF (model 6520) apparatus (Agilent, Santa Clara, CA, USA), operating in the positive mode. Methanol:water (4:1) was used as solvent system and the AF-Ac infused at the source at 200 μL/h. The parameters of chromatography were: Agilent Zorbax model 50 x 2.1 mm column, particles of 1.8 μm and pore diameter of 110 Å, mobile phase: water (0.1% formic acid, v/v) (A) and methanol (B). The gradient solvent system for B was: 2% (0 min); 98% (0–15 min); 100% (15–17 min); 2% (17–18 min); 2% (18–22 min), 0.35 mL/min and detection at 280 and 360 nm. The parameters of ionization were: 58 psi nebulizer pressure, 8 L/min N2 at 220°C, and 4.5 kVa energy in the capillary. Sequential mass spectrometry (MS/MS) analyses were done with different collision energies (5–30 eV). The peaks and spectra were processed using the Agilent’s MassHunter Qualitative Analysis (B07.00) software and tentatively identified by comparing its retention time (Rt), error values (ppm) and mass spectrum with reported data [21].
Cell culture
Human hepatocellular carcinoma cell line HepG2 was obtained from the American Type Culture Collection (ATCC HB-8065). HepG2 cells were cultured at 37°C in 5% CO2 in DMEM (GIBCOTM, Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 25 mM glucose, 1 mM sodium pyruvate, 50 units/mL penicillin, and 85 μM streptomycin. Prior to addition of the treatments, cells were grown to 80–90% confluency and synchronized by incubating in serum-free medium (100% DMEM) for 24 h. The human peripheral blood mononuclear cells (PBMC) were purified using Histopaque-1077. All experimental procedures were carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and were approved by the Institutional Review Board of the Federal University of Uberlandia (no. 1.908.151) The informed written consent was obtained from all subjects. Briefly, in conical tube 3 mL of EDTA-anticoagulated whole blood from three healthy volunteers was carefully layered onto 3 mL of Histopaque-1077 and then centrifuged at 400 xg for 30 min. PBMC were collected in plasma/Hitopaque-1077 interface and washed with 10 mL of Hank’s Balanced Salt Solution without calcium. Cells were suspended in RPMI-1640 supplemented with 10% of fetal bovine serum (Gibco), 2 mM L-glutamine, 100 U/mL penicillin and 0.17 mM streptomycin. Semi-confluent (80% to 90%) cell cultures were used in all studies. The HepG2 cells were plated and then, 24 h later the AF-Ac treatment was done.The cells were then incubated with various concentrations (0–500 μg/mL) of AF-Ac for 24 and/or 48 h. Control group consisted of cells without addition of AF-Ac incubated only with vehicle (medium containing 0.05% DMSO). After 24 and/or 48 h, the cells and medium were collected. Protein contents in cells and medium were quantified by Bradford method [22].
Cell viability
HepG2 and PBMC cells were seeded in 96-well microplate at 0.2 × 106 cells/well and treated with AF-Ac (diluted in DMEM medium containing 0.05% DMSO for HepG2 cells or diluted in RPMI-1640 medium containing 0.05% DMSO for PBMC cells) or vehicle (control, DMEM medium containing 0.05% DMSO for HepG2 cells; RPMI-1640 medium containing 0.05% DMSO for PBMC cells) for 24 h. Then, 100 μL of 12 mM (3-(4,5-dimethylthiazolyl-2)−2,5-diphenyltetrazolium bromide) solution was incubated with the supernatant at 37°C for 2 h in 5% CO2. Next, dimethyl sulfoxide (DMSO) was added and the cell viability was analyzed by absorbance of the purple formazan from viable cells at 570 nm (Molecular Devices, Menlo Park, CA, USA).
Cellular proliferation assay
HepG2 cells were grown in 24 well-plates. FBS was removed for overnight and then the cells were treated with 50 μg/mL AF-Ac or vehicle (control, DMEM medium containing 0.05% DMSO). In vitro cell proliferation assay was assessed by manual counting in Neubauer chamber using optic microscopy at 6, 12, 24 and 48 h, as previously described [23].
Migration assay
HepG2 cells were grown in 12-well plates and treated with 50 μg/mL AF-Ac or vehicle (control, DMEM medium containing 0.05% DMSO) for 48 h. Migration assay was performed as previously described [24]. The wound was achieved by scratching a pipette tip across the cell monolayer (approximately 1.3 mm in width); 1 μM hydroxyurea was added to prevent the proliferation [4]. The wound area was measured using the Northern Eclipse (Empix, Mississauga, Canada) software, and the percentage of wound closure at each time point was derived by the formula: (1 –[current wound size/initial wound size]) × 100.
Western blot analyses
HepG2 cell lysates in SDS-sample buffer containing an additional 100 mM Tris-HCl pH 8.0 and 25% glycerol were boiled for 5 min and equal amounts of total protein (25 μg/mL) were separated by 12% SDS-PAGE gel. To better take advantage of the western blot, in which triplicates of each sample are present, the whole membranes were cut into strips for the different antibodies tested. The blots were cut prior to hybridization with antibodies. Images of all blots as they are, and all replicates performed are shown in S1 Raw images. For protein detection, specific primary antibodies against proliferating cell nuclear antigen PCNA (mouse, 1:1,000;), vinculin (mouse, 1:1,000, Cell Signaling Technology), epidermal growth factor receptor EGFR (mouse, 1:1,000 Santa Cruz Biotechnology, Dallas, TX) and β-actin, as a protein loading control (mouse, 1:1,000; Santa Cruz Biotechnology, Dallas, TX), were used. The primary antibody incubation proceeded for 2 h at room temperature. After being washed, blots were incubated with horseradish peroxidase-conjugated specific secondary antibody (anti-mouse or anti-rabbit, 1:5,000; Sigma-Aldrich) at room temperature for 1 h. Immune detection was carried out using enhanced chemiluminescence (ECL plus; Amersham Biosciences) [25]. Western blot digital images (8-bit) were used for densitometric analysis using ImageJ (National Institutes of Health, Bethesda, MD).
Immunofluorescence
Confocal microscopy examination of immunofluorescence in HepG2 cells was performed as described [26]. Cells were seeded onto 6-well culture dishes and incubated with 50 μg/mL AF-Ac or vehicle (control, DMEM medium containing 0.05% DMSO) for 24 h. Then, cells were fixed with 4% paraformaldehyde, permeabilized with PBS 1X/Triton 0.5% and blocked with PBS (10% BSA, 0.5% Triton 0.5% and 5% goat serum) for 1 h. Cells were incubated with anti-EGFR antibody (anti-mouse, 1:100; Abcam, MA, USA) for 2 h at room temperature, followed by incubation with anti-mouse secondary antibody conjugated with Alexa 488 (1:500; Life Technologies) for 1 h. Isotype control was used to assess non-specific binding under the same experimental conditions. Images were obtained using a Zeiss LSM 510 confocal microscope (Thornwood, NY, USA) equipped with a 63×/1.4 NA objective with excitation laser at 488 nm and emission bandpass filter at 505–550 nm.
Detection of Ca2+ signals
Intracellular Ca2+ was monitored in individual cells by time lapse confocal microscopy, as described previously [27]. Briefly, HepG2 cells were incubated with Fluo-4/AM (6 μM) for 30 min at 37°C in 5% CO2 in HEPES buffer with or without 10 mM EGTA. Then, coverslips containing cells were transferred to a perfusion chamber on the stage of the Zeiss LSM510 confocal imaging system equipped with a Kr-Ar laser. Nuclear and cytosolic Ca2+ signals were monitored in individual cells during stimulation with 50 μg/mL AF-Ac using a ×63, 1.4 NA objective lens. Fluo-4/AM was excited at 488 nm and observed at 505–550 nm. Changes in fluorescence were normalized by the initial fluorescence (F0) and were expressed as (F/F0) × 100. During the 600 s for the calcium signaling experiments, the cells were perfused with HEPES solution without fetal bovine serum, grown factor and molecules that can themselves alter the calcium signaling.
Statistical analysis
The graphics and statistical analyzes were done using SigmaPlot (Systat Software, Point Richmond, USA) and Prism (GraphPad Software, San Diego, USA). The data were expressed as mean ± SD and the statistical significance was tested using Student’s t test, one-way or two-way ANOVA followed by Dunnett or Bonferroni test. p value < 0.05 was taken to indicate statistical significance.
Results
Identification of alkaloids and acetogenins by Ultra-High-Performance Liquid Chromatography-Electrospray Ionization-tandem Mass Spectrometry (UHPLC-ESI/MSn)
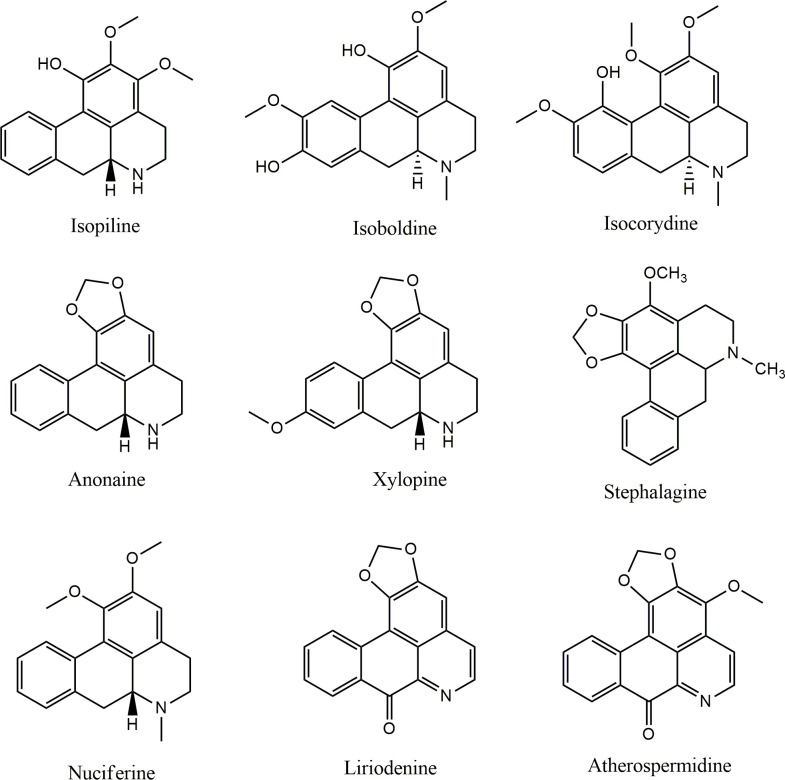
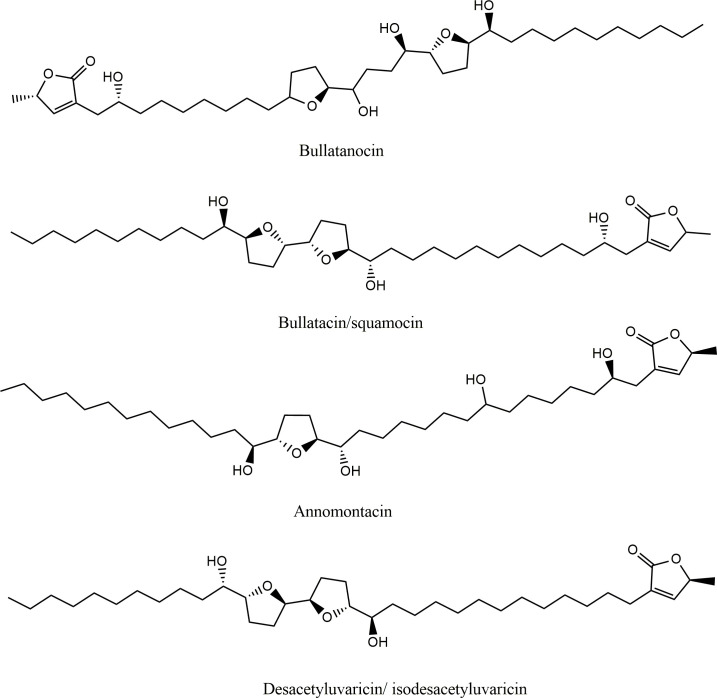
The alkaloid and acetogenin profile of AF-Ac was performed by UHPLC-ESI–MSn. The presence of ions m/z attributed to alkaloids and acetogenins was confirmed in the positive mode by high resolution “zoom scan” analysis. Isopiline, isoboldine, isocorydine, anonaine, nuciferine, xylopine, stephalagine, liriodenine and atherospermidine were the alkaloids found in AF-Ac [13, 28–33], whereas bullatanocin, bullatacin/squamocin, annomontacin and desacetyluvaricin/ isodesacetyluvaricin were the acetogenins found in AF-Ac [34] (Fig 1 and Table 1). The chemical structures of the alkaloids and acetogenins identified in the AF-Ac fraction are shown in Figs 2 and 3, respectively. The sequential mass spectra can be found as online (S1–S13 Figs).
Fig 1. Chromatogram of the alkaloid and acetogenin-rich fraction from Annona crassiflora fruit peel (AF-Ac) by HPLC-ESI-MS/MS (positive mode).
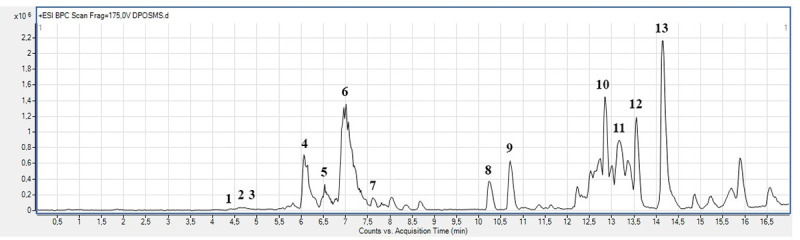
Table 1. Alkaloids and acetogenins identified in the alkaloid and acetogenin-rich fraction from Annona crassiflora fruit peel (AF-Ac) by UHPLC-ESI/MSn (positive mode).
| Peak | Tentative identificationa(alkaloid) | Retention time (min) | Formula [M+H]+ | Mass calculated for [M+H]+ | m/z of [M+H]+ | Error (ppm) | m/z of fragments | References |
| 1 | Isopiline | 4.4 | C18H20NO3+ | 298.1437 | 298.1448 | 3.68 | 281, 270, 266, 250 | [28] |
| 2 | Isoboldine | 4.6 | C19H22NO4+ | 328.1518 | 328.1525 | 2.13 | 297, 265, 178, 151 | [29] |
| 3 | Isocorydine | 4.7 | C20H24NO4+ | 342.1699 | 342.1699 | 0.00 | 311, 296, 279, 265 | [30] |
| 4 | Anonaine | 6.0 | C17H16NO2+ | 266.1166 | 266.1169 | 1.13 | 249, 234, 219, 191 | [31] |
| 5 | Xylopine | 6.5 | C18H18NO3+ | 296.1227 | 296.1228 | 0.34 | 281, 249, 221, 206 | [32] |
| 6 | Stephalagine | 7.0 | C19H20NO3+ | 310.1472 | 310.1470 | 0.96 | 279, 264, 234, 178 | [13] |
| 7 | Nuciferine | 7.6 | C19H22NO2+ | 296.1320 | 296.1323 | 1.01 | 279, 264, 249, 234 | [28] |
| 8 | Liriodenine | 10.2 | C17H10NO3+ | 276.0653 | 276.0655 | 0.72 | 259, 251, 248, 232 | [31] |
| 9 | Atherospermidine | 10.7 | C18H12NO4+ | 306.0758 | 306.0758 | 0.00 | 291, 263, 251, 235 | [33] |
| Tentative identification†(acetogenin) | Retention time (min) | Formula [M+Na]+ | Mass calculated for [M+Na]+ | m/z of [M+Na]+ | Error (ppm) | m/z of fragments | References | |
| 10 | Bullatanocin | 12.8 | C37H66O8Na+ | 661.465 | 661.4662 | 1.81 | 639 [M+H]+, 621 [M+H-H2O]+,585 [M+H-3H2O]+, 567 [M+H-4H2O]+,549 [M+Na-112]+, 531 [M+NA-6H2O]+ | [34] |
| 11 | Bullatacin/squamocin | 13.3 | C37H66O7Na+ | 645.4701 | 645.4707 | 0.92 | 569 [M+H-3H2O]+, 551 [M+H-4H2O]+,533 [M+H-5H2O]+, 523 [M+Na-112]+ | [34] |
| 12 | Annomontacin | 13.5 | C37H68O7Na+ | 647.4857 | 647.4855 | -0.30 | 607 [M+H-H2O]+, 589 [M+H-2H2O]+,535 [M+Na-112]+| | [34] |
| 13 | Desacetyluvaricin/ isodesacetyluvaricin | 14.3 | C37H66O6Na+ | 629.4752 | 629.4750 | -0.31 | 589 [M+H-H2O]+, 571 [M+H-2H2O]+,553 [M+H-3H2O]+, 535 [M+H-4H2O]+ | [34] |
aTentative identification of compounds was based on published literature of Annona species.
Fig 2. Chemical structures of the aporphine alkaloids identified in the AF-Ac fraction from A. crassiflora fruit peel.
Fig 3. Chemical structures of the acetogenins identified in the AF-Ac fraction from A. crassiflora fruit peel.
AF-Ac reduces HepG2 cell viability
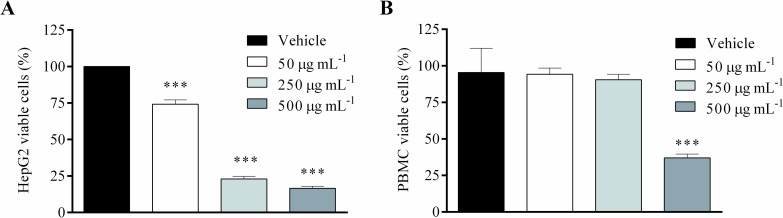
Fig 4 shows cell viability of HepG2 and PBMC cells treated with different concentrations of AF-Ac for 24 h. AF-Ac was able to reduce HepG2 cell viability at 50, 250 and 500 μg/mL, compared to untreated control cells (cells treated only with vehicle) (25.7 ± 2.8, 77.0 ± 1.8 and 83.3 ± 1.3% of reductions, respectively, p < 0.001) (Fig 4A). However, AF-Ac was cytotoxicity for PBMC cells only at 500 μg/mL (Fig 4B). As was observed with PBMC cells, the AF-Ac fraction at a concentration of 50 μg/mL did not affect the cell viability of fibroblasts treated for 24 h (S14 Fig).
Fig 4.
Cell viability of HepG2 (A) and PBMC (B) cells treated with the alkaloid and acetogenin-rich fraction of Annona crassiflora fruit peel (AF-Ac) or vehicle (control, cells treated with DMEM medium containing 0.05% DMSO for HepG2 cells or RPMI-1640 containing 0.05% DMSO for PBMC cells). Results (mean ± SD, n = 3) expressed as the percentage of viable cells compared to the vehicle group. Significance levels are indicated by ***p < 0.001 when compared to control (one-way ANOVA and Dunnett as posttest).
AF-Ac reduces HepG2 cell proliferation
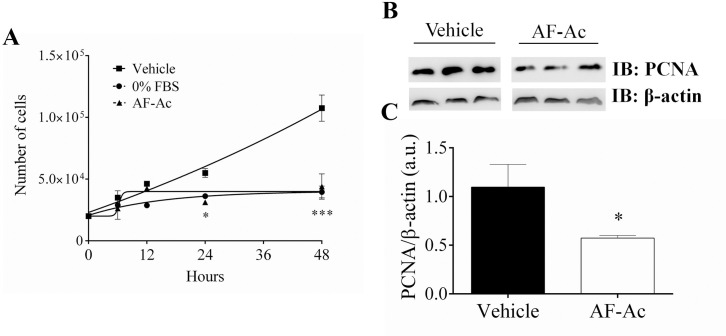
We investigated whether AF-Ac presents antiproliferative effect in HepG2 cells since it reduced its viability. Incubation of HepG2 cells with 50 μg/mL AF-Ac for 48 h led to a reduction in cell proliferation (75.2 ± 10.5%, p < 0.001) (Fig 5A). This result was similar to cells in medium with 0% fetal bovine serum. After 24 h incubation with AF-Ac, the expression of PCNA, a marker of cell proliferation, in HepG2 cells was analyzed by Western blotting. In accordance with the cell proliferation assay, AF-Ac at the dose of 50 μg/mL decreased PCNA expression (p < 0.05) (Fig 5B and 5C).
Fig 5.
Cell growth assay of HepG2 cells at 6, 12, 24 and 48 h after stimulation with 50 μg/mL AF-Ac or vehicle (control, cells treated with DMEM medium containing 0.05% DMSO), triplicate in 3 individual experiments (A). Representative immunoblotting of total HepG2 cell lysates probed with anti-PCNA and anti-β-actin, used as protein loading control (B). Immunoblotting densitometry analysis. Results show β-actin normalized proteins expression (n = 3 individual experiments/group) (C). Values are expressed as mean ± SD. Significance levels are indicated by *p < 0.05 (unpaired t-test) and ***p < 0.001 (two-way ANOVA followed by Bonferroni’s post hoc test) when compared to the vehicle group. Each protein was analyzed in cropped membranes of different Western blots along with other proteins. Full-length blots are presented in S1 Raw images.
AF-Ac reduces HepG2 cell migration
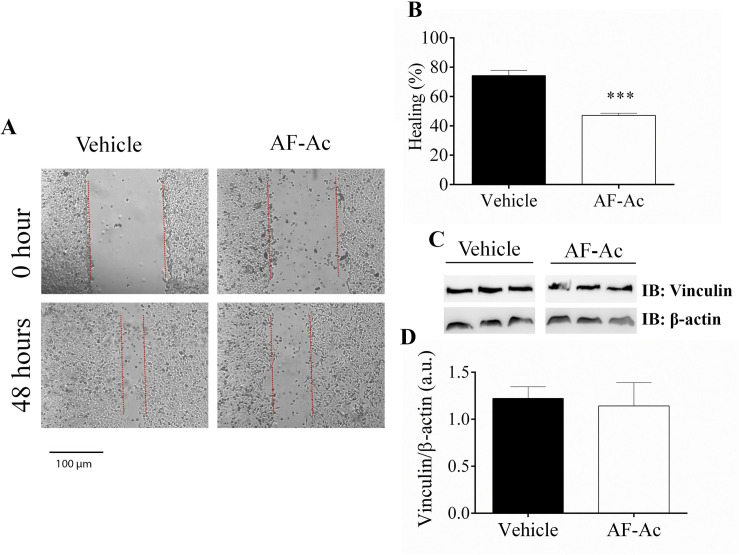
Following these observations, we investigated the influence of AF-Ac on the migration of HepG2 cells. Thus, a scratch assay was made in the presence or absence of AF-Ac (Fig 6A). After 48 h, AF-Ac decreased the healing process (47.1 ± 1.5% of healing) when compared with untreated cells (74.2 ± 3.5% of healing) (p < 0.001) (Fig 6B). In order to check if the reduced healing observed during stimulation with AF-Ac is due to alterations on the focal adhesion points, we performed immunoblotting for vinculin, a focal adhesion protein that plays a central role in cell shape and motility that have been related with cell cancer migration and its expression is regulated by Ca2+ signaling [4, 25]. However, vinculin levels were not affected in HepG2 cells treated with 50 μg/mL AF-Ac (Fig 6C and 6D).
Fig 6. Representative image of in vitro wound healing assay performed with HepG2 cells.
Images were selected from a representative well 48 h after stimulation with 50 μg/mL AF-Ac or vehicle (control, cells treated with DMEM medium containing 0.05% DMSO). Scale bar = 100 μm (A). Average of wound healing closure 48 h after stimulation with AF-Ac (n = 5 wells/group, for each time point). Results represent % of initial wound area (0 h) (B). Representative cropped immunoblotting of total HepG2 cell lysates probed with anti-vinculin and anti-β-actin, used as protein loading control (C). Immunoblotting densitometry analysis. Results show β-actin normalized proteins expression (n = 3 individual experiments/group) (D). Values are expressed as mean ± SD. Significance levels are indicated by ***p < 0.001 (unpaired t-test) when compared to the vehicle group. Each protein was analyzed in cropped membranes of different Western blots along with other proteins. Full-length blots are presented in S1 Raw images.
Ac reduces EGFR in HepG2 cells
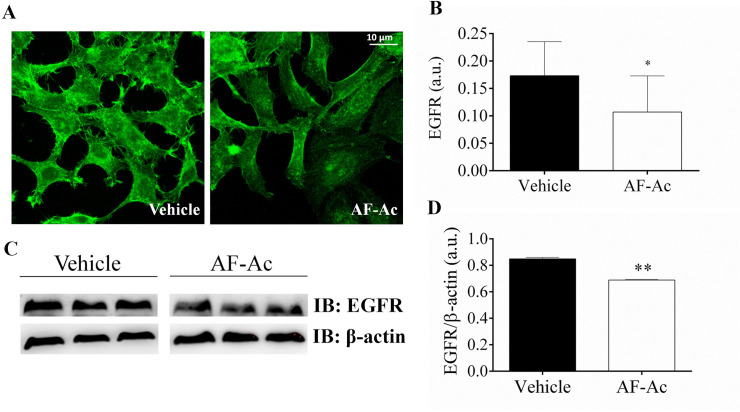
EGFR is known to play an important role in the regulation of cell proliferation in HepG2 cells and it is an important receptor that trigger Ca2+ signaling in hepatic cells [27, 35]. As revealed by immunofluorescence assay (Fig 7A and 7B), EFGR levels were reduced in HepG2 cells treated with 50 μg/mL AF-Ac (p < 0.05). This result is in accordance with data obtained by immunoblotting assay, with HepG2 cells treated with 50 μg/mL AF-Ac presenting decreased expression of EGFR (p < 0.01) (Fig 7C and 7D).
Fig 7. Representative immunofluorescence images of HepG2 cells treated with 50 μg/mL AF-Ac or vehicle (control, cells treated with DMEM medium containing 0.05% DMSO) labeled with specific anti-EGFR (green) antibody.
Scale bar = 10 μm (A). Average number of EGFR–positive regions for the cell types analyzed are shown as EGFR/1000 μm2 on each respective graph (n = 20 cells/group) (B). Representative cropped immunoblotting of total HepG2 cell lysates probed with anti-EGFR and anti-β-actin, used as protein loading control (C). Immunoblotting densitometry analysis. Results show β-actin normalized proteins expression (n = 3 individual experiments/group) (D). Values are expressed as mean ± SD. Significance levels are indicated by **p < 0.01 (unpaired t-test). Each protein was analyzed in cropped membranes of different Western blots along with other proteins. Full-length blots are presented in S1 Raw images.
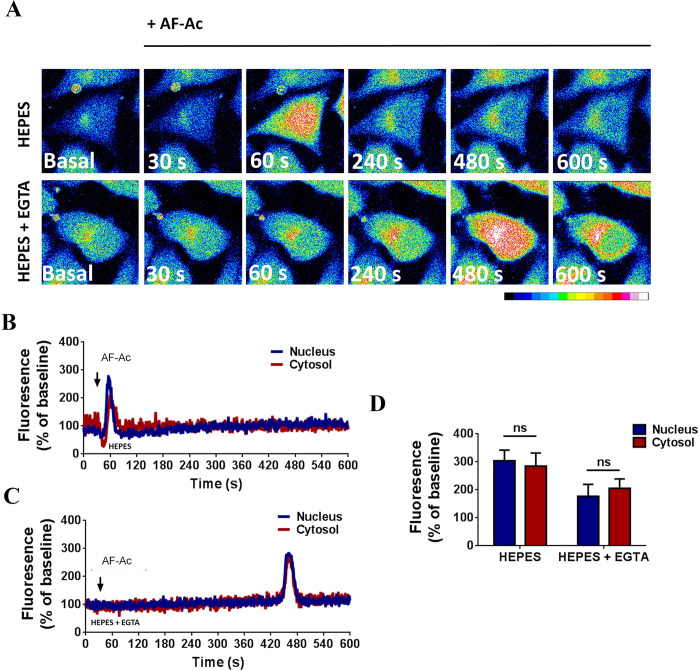
AF-Ac increases intracellular Ca2+ in HepG2 cells
HepG2 cells were loaded with fluo4/AM and assayed for Ca2+ signals during AF-Ac stimulation. Fig 8A shows that there was an increase in intracellular-free Ca2+ when HepG2 cells were exposed to 50 μg/mL AF-Ac. After 60 s intracellular Ca2+ rose to peak levels, as shown by the increase in fluorescence (Fig 8B). We also performed experiments in the absence of extracellular Ca2+ to explore the relative contribution of intracellular Ca2+ pools to the overall response induced by AF-Ac. Thus, 10 mM EGTA was added to the Ca2+-free HEPES buffer, chelating extracellular-free Ca2+ levels. AF-Ac triggered Ca2+ wave in HepG2 cells with a peak at 480 s after AF-Ac exposure (Fig 8C). No difference was observed between Ca2+ levels from nucleus and cytosol (Fig 8D).
Fig 8. Confocal serial images of HepG2 cells, loaded with fluo-4/AM and stimulated with 50 μg/mL AF-Ac for 30, 60, 240, 360, 480 and 600 s.
Dashed yellow regions represent the nuclear region. Images were pseudocolored according to the scale shown at the bottom. Scale bar = 10 μM (A). Representative time course of nuclear and cytosol fluorescence levels of HepG2 cells, in the presence (B) or absent (C) of EGTA, stimulated with AF-Ac. Black arrow indicates initial AF-Ac stimulation and fluorescence level is expressed as % of basal fluorescence. Average nuclear and cytosol fluorescence peaks (n = 20 cells) of each cell group and condition throughout the time-course; fluorescence level is expressed as % of basal fluorescence (D). Values are expressed as mean ± SD. ns = p > 0.05 (unpaired t-test).
Discussion
The search for natural agents capable of controlling tumor growth and presenting low toxicity on normal healthy cells has gained prominence in the treatment of cancer [5, 6, 36, 37]. Whole plants or herbal extracts/fractions have been used rather than isolated molecules due to their more affordable access and synergistic interaction between the compounds that may increase the biological effects [38]. Numerous alkaloids from medicinal plants and herbs have showed antiproliferative and anticancer effects on a wide category of cancers both in vitro and in vivo [6]. Another example is the Annonaceous acetogenins, which have been identified as cancer growth inhibitors and/or apoptotic agents [11]. In the present study, we showed the anticancer potential of an alkaloid and acetogenin-rich fraction from A. crassiflora fruit peel, named here as AF-Ac, by evaluating its antiproliferative properties in human liver carcinoma cells (HepG2) in vitro.
First, we performed an ethanolic extraction of the fruit peel of A. crassiflora and followed this with a liquid-liquid fractionation of the crude extract to obtain an alkaloid and acetogenin-rich fraction. Our findings indicated that the dichloromethane fraction had alkaloids and acetogenins, which was confirmed by UHPLC-ESI/MSn. Interestingly, all the alkaloids found in AF-Ac are aporphine alkaloids, such as annonaine, isopoline, isoboldine, isocorydine, liriodenine, stephalagine, nuciferine, atherospermidine and xylopine. Until now, stephalagine was the only alkaloid isolated and characterized in A. crassiflora fruit peel [13]. The retention time, exact mass and MS/MS spectra of stephalagine showed in the present study corroborates the data reported by Justino, Barbosa (12). In addition, acetogenins such as bullatanocin, bullatacin/squamocin, annomontacin and desacetyluvaricin were identified for the first time in the fruit peel of A. crassiflora.
Annonaceous acetogenins, more specifically bis-tetrahydrofuranic (THF) acetogenins like that found in AF-Ac, have shown cytotoxicity in HepG2 cells through the induction of cell-cycle arrest and induction of the apoptotic mitochondrial pathway involving complexation with Ca2+ [39–41]. Previous studies have also reported that aporphine alkaloids, such as liriodenine, anonaine, xylopine and isoboldine that were detected in the fruit peel of A. crassiflora, have prominent cytotoxic effects on HepG2 cells [8, 42]. The anti-tumor activities of liriodenine are related to the G1 cell cycle arrest, nitric oxide (NO)-mediated activation of p53 expression and DNA synthesis repress. The cytotoxic effects of xylopine and isoboldine are associated with their ability to arrest G2/M cycle [43, 44]. DNA damage associated with increased intracellular NO and ROS, glutathione depletion, disruptive mitochondrial transmembrane potential, activation of caspases 3, 7, 8 and 9, and up-regulation of p53 and Bax expression have been reported for anonaine [42]. Furthermore, isocorydine, another aporphine alkaloid found in A. crassiflora fruit peel, has also shown anti-tumor properties by decreasing the viability of hepatocellular carcinoma (HCC) and HepG2 cells [45, 46].
AF-Ac at the dose of 50 μg/mL effectively decreased HepG2 cell viability and reduced cell proliferation. It is worth mentioning that the AF-Ac at 50 μg/mL was not cytotoxic for PBMC and fibroblast cells. The objective of using PBMC and fibroblast cells as controls is to demonstrate that the AF-Ac fraction is not cytotoxic to healthy cells, since these human non-cancer cells are potentially useful models for cell viability testing using plant extracts [47–50]. Also, PBMC cells represent the whole metabolic status and an excellent model for assessing the differences or changes associated with pathophysiological conditions [47, 51]. Additionally, a study conducted by our research group also showed no cytotoxicity of the AF-Ac fraction in Vero cells [13].
Consistent with the results of MTT and proliferation assays, AF-Ac decreased the expression of PCNA in the HepG2 cells. PCNA is a cell nuclear protein whose expression is correlated with DNA replication, regulating the transition from G1 phase to S phase, and is connected with the proliferation of tumor cells [52]. The antiproliferative potential of some alkaloids and Annonaceous acetogenins has been associated with their capacity to reduce and/or down-regulate the PCNA expression [53–56]. A study done by Long and Li (56) showed the reduction of PCNA expression by an alkaloidal fraction from aerial parts of Oxytropis ochrocephala in mice hepatocellular carcinoma. However, the potential PCNA-lowering mechanism of these bioactive molecules still needs detailed investigations.
As well as the PCNA, EGFR also plays an important role in the regulation of cell proliferation [57]. EGFR overexpression might contribute to deregulated cellular processes, such as uncontrolled proliferation, invasion, DNA synthesis, angiogenesis, cell motility and inhibition of apoptosis, which makes it a molecular target for tumor therapy [57]. In the present study, AF-Ac reduced the expression of EGFR in the HepG2 cells, as showed by Western blot and immunofluorescence assays. Isocorydine, an aporphine alkaloid found in AF-Ac, has previously shown cytotoxic effects in HepG2 cells and, by a docking analysis, had inhibitory activity against EGFR [46]. Dicentrine, another aporphine alkaloid, has been shown to exert cytotoxic activity towards cancer cells by binding to EGFR [9, 36]. In addition, acetogenins influences EGFR signaling to induce cell cycle arrest and inhibit cytotoxic cell survival [58]. These findings indicate that EGFR and PCNA signaling pathways might play a role in mediating the antiproliferative activity of AF-Ac on HepG2 cells. Overproduction of ROS is also an important event related to cell death and cancer [59]. Preliminary data from our research group showed an increase in intracellular ROS production in HepG2 cells treated with the AF-Ac fraction (S15 Fig), which also be involved in the anti-proliferative effects of AF-Ac.
Studies have demonstrated that alkaloids and acetogenins may inhibit cell migration and metastasis of cancer cells [60–62]. Here, we showed the capacity of AF-Ac to decrease cell migration of HepG2 cells without vinculin overexpression. The capacity of tumor cells to migrate is essential for many physiological processes including tumor invasion, angiogenesis and metastasis [63]. The filamentous (F)-actin-binding protein vinculin is required for cell polarization and migration, having a key role on the formation of focal adhesion points [64]. Thus, cells with reduced expression of vinculin become less adherent and more motile [65]. Therefore, AF-Ac might be acting on other targets involved with cell migration than vinculin expression.
Finally we investigated whether AF-Ac alters intracellular Ca2+ in the HepG2 cells, since nuclear Ca2+ was previously found to negatively regulate cell motility, invasion and proliferation [4, 66]. Of note, confocal analysis showed that AF-Ac increased intracellular Ca2+ through a process that involved Ca2+ influx which requires external calcium, when Ca2+ was present in the external media. In the absence of external Ca2+, exposure of HepG2 cells to AF-Ac also mobilized intracellular Ca2+. This suggests that the alkaloid and acetogenin-rich fraction induces a mobilization of intracellular Ca2+ stores. Ca2+ is a ubiquitous second messenger that regulates a wide range of activities in cells, such as secretion, contraction, metabolism, gene transcription, apoptosis and proliferation [67]. Studies have showed that nuclear Ca2+ buffering reduced cell proliferation in hepatocellular carcinoma cells by stopping cell cycle progression, modulating the promoter region activity of genes involved in cell proliferation and/or preventing the upregulation of the tyrosine kinase receptor [23, 26, 68]. In addition, nuclear Ca2+ buffering may turn cells more rigid and less motile due to the reduction of membrane fluctuations [4].
Although studies have showed that the mentioned aporphine alkaloids reduce Ca2+ influx [12, 69, 70], AF-Ac induced increases in cytosolic and nuclear Ca2+ in the HepG2 cells. It is well known that acetogenins induce an increase of cytosolic and mitochondrial Ca2+ in several cancer cells [41], which might explain the intracellular Ca2+ increase observed in the HepG2 treated with AF-Ac. The mechanism underlying the cytotoxicity and antiproliferative effect of Annonaceous acetogenins is modulated by the chelation of THF moieties with Ca2+ to form hydrophobic complexes, which may induce sustained increases in intracellular and mitochondrial Ca2+ concentrations, resulting in decrease of mitochondrial membrane potential that leads to the release of apoptotic initiators [41, 71]. Thus, the chelating ability of the A. crassiflora acetogenins with Ca2+ might contribute, at least in part, to the anti-migration and anti-proliferative capacities of AF-Ac in the HepG2 cells.
Conclusions
In summary, our results have established the antiproliferative properties of AF-Ac on HepG2 cells and suggest that this effect is mediated, at least in part, by reducing PCNA and EGFR expression with a mobilization of intracellular Ca2. Although the biochemical mechanisms involved in the antiproliferative effect of the alkaloids and acetogenins from A. crassiflora on HepG2 cells were not fully explored, this study is the first to identify the alkaloids and acetogenins present in the fruit peel of A. crassiflora and to demonstrate its antitumoral potential. Furthermore, the biological activities exercised by the AF-Ac fraction were observed in concentrations below the cytotoxic level. Thus, the use of this alkaloid and acetogenin-rich fraction in further in vivo assays is justified.
Supporting information
Six samples of each treatment group were evenly distributed in two gels resulting in 3 samples/gel/group. It was necessary to crop the membranes in order to incubate them with different antibodies since different proteins were analyzed in each western blot. Vinculin (124 kDa MW), β-actin (42 kDa MW) and PCNA (36 kDa MW) were analyzed in the same western blot by cutting the membrane in three (A). The top part was used to blot vinculin antibodies, the middle part was used to blot β-actin antibodies and the bottom part for PCNA antibodies. EGFR (180 kDa MW) and β-actin (42 kDa MW) were analyzed in the same western blot by cutting the membrane in two (B). The top part was used to blot EGFR antibodies and the bottom part for β-actin antibodies. The original blots show results of vinculin, β-actin, PCNA and EGFR expression of HepG2 cells treated with vehicle (control, showed in the three first lanes, included in the present study), crude ethanol extract from A. crassiflora fruit peel (EtOH, not included in the present study), n-butanol fraction from A. crassiflora fruit peel (BuOH, not included in the present study) and alkaloid and acetogenin-rich fraction from A. crassiflora fruit peel (AF-Ac, showed in the last three lanes, included in the present study). The corresponding MW (KD) markers are shown to the left of the Western blot image.
(PDF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Results (mean ± SD, n = 3) expressed as the percentage of viable cells compared to the vehicle group. Significance levels are indicated by *p < 0.05 and ***p < 0.001 when compared to control (one-way ANOVA and Dunnett as posttest). The fibroblast cells (NIH/3T3) were grown in RPMI-1640 medium supplemented with fetal bovine serum 10% (FBS), 2 mM glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin, at 37°C and CO2 5%. 3 x 104 cells were plated in 96-well plates and treated with different concentrations of the AF-Ac fraction or vehicle and incubated for 24 h at 37°C and 5% CO2. Then, 100 μL of 5 mg/mL (3-(4,5-dimethylthiazolyl-2)−2,5-diphenyltetrazolium bromide) solution was incubated with the supernatant at 37°C for 2 h in 5% CO2. Next, dimethyl sulfoxide (DMSO) was added and the cell viability was analyzed by absorbance of the purple formazan from viable cells at 570 nm (Molecular Devices, Menlo Park, CA, USA).
(TIF)
Time-lapse of HepG2 cells labeled with dihydroethidium (DHE) and exposed to the AF-Ac fraction (A). Quantification of the ROS production during 9 minutes after AF-Ac treatment (B). The values are expressed in the percentage of the control. HepG2 cells were labeled with 5 μM DHE. The cells were placed at the confocal stage and perfused with HEPES solution as control and HEPES + AF-AC fraction for 10 min. The fluorescence intensity was measured using Image J and the values are expressed in percentage of the control.
(TIF)
Acknowledgments
The authors gratefully acknowledge the Institute of Biotechnology of the Federal University of Uberlândia and the Department of Physiology and Biophysics and the Liver Center of the Federal University of Minas Gerais for infrastructural support, Mário M. Martins and Paula Souza Santos for technical assistance in LC-MS analysis, and Luiz Ricardo Goulart for supervision.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
A.B.J., R.M.F., A.F., A.C.M.L.F. and R.R.F. received graduate fellowships from Coordination for the Improvement of Higher Education Personnel (CAPES, https://www.gov.br/capes/pt-br), A.L.S. received post-doctoral fellowships from CAPES and M.F.L. is a grant recipient of National Council for Scientific and Technological Development (CNPq, https://www.gov.br/cnpq/pt-br). FSE received financial support of CNPq/FAPEMIG from resources of the INCT-TeraNano – UFU (FAPEMIG, https://fapemig.br/pt/).
References
- 1.Petrick JL, McGlynn KA. The Changing Epidemiology of Primary Liver Cancer. Current Epidemiology Reports. 2019;6(2):104–11. doi: 10.1007/s40471-019-00188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomaa A-I, Khan S-A, Toledano M-B, Waked I, Taylor-Robinson S-D. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World journal of gastroenterology. 2008;14(27):4300–8. doi: 10.3748/wjg.14.4300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerra MT, Florentino RM, Franca A, Lima Filho AC, Dos Santos ML, Fonseca RC, et al. Expression of the type 3 InsP(3) receptor is a final common event in the development of hepatocellular carcinoma. Gut. 2019;68(9):1676–87. Epub 2019/07/19. doi: 10.1136/gutjnl-2018-317811 ; PubMed Central PMCID: PMC7087395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guimaraes E, Machado R, Fonseca MC, Franca A, Carvalho C, Araujo ESAC, et al. Inositol 1, 4, 5-trisphosphate-dependent nuclear calcium signals regulate angiogenesis and cell motility in triple negative breast cancer. PLoS One. 2017;12(4):e0175041. Epub 2017/04/05. doi: 10.1371/journal.pone.0175041 ; PubMed Central PMCID: PMC5380351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Mesquita ML, de Paula JE, Pessoa C, de Moraes MO, Costa-Lotufo LV, Grougnet R, et al. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. Journal of ethnopharmacology. 2009;123(3):439–45. Epub 2009/06/09. doi: 10.1016/j.jep.2009.03.018 . [DOI] [PubMed] [Google Scholar]
- 6.Mondal A, Gandhi A, Fimognari C, Atanasov AG, Bishayee A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. European journal of pharmacology. 2019;858:172472. doi: 10.1016/j.ejphar.2019.172472 [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Liu J, Di D, Li M, Fen Y. Structural and mechanistic bases of the anticancer activity of natural aporphinoid alkaloids. Curr Top Med Chem. 2013;13(17):2116–26. Epub 2013/08/28. doi: 10.2174/15680266113139990147 . [DOI] [PubMed] [Google Scholar]
- 8.Hsieh TJ, Liu TZ, Chern CL, Tsao DA, Lu FJ, Syu YH, et al. Liriodenine inhibits the proliferation of human hepatoma cell lines by blocking cell cycle progression and nitric oxide-mediated activation of p53 expression. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2005;43(7):1117–26. Epub 2005/04/19. doi: 10.1016/j.fct.2005.03.002 . [DOI] [PubMed] [Google Scholar]
- 9.Kondo Y, Imai Y, Hojo H, Endo T, Nozoe S. Suppression of tumor cell growth and mitogen response by aporphine alkaloids, dicentrine, glaucine, corydine, and apomorphine. J Pharmacobiodyn. 1990;13(7):426–31. Epub 1990/07/01. doi: 10.1248/bpb1978.13.426 . [DOI] [PubMed] [Google Scholar]
- 10.Liu CM, Kao CL, Wu HM, Li WJ, Huang CT, Li HT, et al. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules. 2014;19(11):17829–38. Epub 2014/11/06. doi: 10.3390/molecules191117829 ; PubMed Central PMCID: PMC6271390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobo-Herrera N, Pérez-Plasencia C, Castro-Torres VA, Martínez-Vázquez M, González-Esquinca AR, Zentella-Dehesa A. Selective Acetogenins and Their Potential as Anticancer Agents. Frontiers in Pharmacology. 2019;10(783). doi: 10.3389/fphar.2019.00783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Justino AB, Barbosa MF, Neves TV, Silva HCG, Brum EdS, Fialho MFP, et al. Stephalagine, an aporphine alkaloid from Annona crassiflora fruit peel, induces antinociceptive effects by TRPA1 and TRPV1 channels modulation in mice. Bioorganic Chemistry. 2020;96:103562. doi: 10.1016/j.bioorg.2019.103562 [DOI] [PubMed] [Google Scholar]
- 13.Pereira MN, Justino AB, Martins MM, Peixoto LG, Vilela DD, Santos PS, et al. Stephalagine, an alkaloid with pancreatic lipase inhibitory activity isolated from the fruit peel of Annona crassiflora Mart. Industrial Crops and Products. 2017;97:324–9. 10.1016/j.indcrop.2016.12.038. [DOI] [Google Scholar]
- 14.Pimenta LP, Garcia GM, Goncalves SG, Dionisio BL, Braga EM, Mosqueira VC. In vivo antimalarial efficacy of acetogenins, alkaloids and flavonoids enriched fractions from Annona crassiflora Mart. Natural product research. 2014;28(16):1254–9. Epub 2014/04/01. doi: 10.1080/14786419.2014.900496 . [DOI] [PubMed] [Google Scholar]
- 15.Vilar JB, Ferreira FL, Ferri PH, Guillo LA, Chen Chen L. Assessment of the mutagenic, antimutagenic and cytotoxic activities of ethanolic extract of araticum (Annona crassiflora Mart. 1841) by micronucleus test in mice. Braz J Biol. 2008;68(1):141–7. doi: 10.1590/s1519-69842008000100020 . [DOI] [PubMed] [Google Scholar]
- 16.Silva JJ, Cerdeira CD, Chavasco JM, Cintra AB, Silva CB, Mendonca AN, et al. In vitro screening antibacterial activity of Bidens pilosa Linne and Annona crassiflora Mart. against oxacillin resistant Staphylococcus aureus (ORSA) from the aerial environment at the dental clinic. Rev Inst Med Trop Sao Paulo. 2014;56(4):333–40. doi: 10.1590/s0036-46652014000400011 ; PubMed Central PMCID: PMC4131820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer—an extension of the work of Jonathan Hartwell. Journal of ethnopharmacology. 2000;73(3):347–77. Epub 2000/11/25. doi: 10.1016/s0378-8741(00)00341-x . [DOI] [PubMed] [Google Scholar]
- 18.Formagio AS, Vieira MC, Volobuff CR, Silva MS, Matos AI, Cardoso CA, et al. In vitro biological screening of the anticholinesterase and antiproliferative activities of medicinal plants belonging to Annonaceae. Braz J Med Biol Res. 2015;48(4):308–15. doi: 10.1590/1414-431X20144127 ; PubMed Central PMCID: PMC4418360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva VAO, Alves ALV, Rosa MN, Silva LRV, Melendez ME, Cury FP, et al. Hexane partition from Annona crassiflora Mart. promotes cytotoxity and apoptosis on human cervical cancer cell lines. Investigational new drugs. 2019;37(4):602–15. doi: 10.1007/s10637-018-0657-y [DOI] [PubMed] [Google Scholar]
- 20.Pereira MN, Justino AB, Martins MM, Peixoto LG, Vilela DD, Santos PS, et al. Stephalagine, an alkaloid with pancreatic lipase inhibitory activity isolated from the fruit peel of Annona crassiflora Mart. Industrial Crops and Products. 2017. doi: 10.1016/j.indcrop.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justino AB, Miranda NC, Franco RR, Martins MM, Silva NMD, Espindola FS. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against alpha-amylase, alpha-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;100:83–92. Epub 2018/02/10. doi: 10.1016/j.biopha.2018.01.172 . [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999 . [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, et al. Nucleoplasmic calcium is required for cell proliferation. The Journal of biological chemistry. 2007;282(23):17061–8. Epub 04/09. doi: 10.1074/jbc.M700490200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–33. Epub 2007/04/05. doi: 10.1038/nprot.2007.30 . [DOI] [PubMed] [Google Scholar]
- 25.Alvarenga EC, Fonseca MC, Carvalho CC, Florentino RM, França A, Matias E, et al. Angiotensin Converting Enzyme Regulates Cell Proliferation and Migration. PLoS One. 2016;11(12):e0165371. Epub 2016/12/20. doi: 10.1371/journal.pone.0165371 ; PubMed Central PMCID: PMC5167550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaya MJ, Oliveira AG, Guimaraes ES, Casteluber MC, Carvalho SM, Andrade LM, et al. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014;59(1):274–83. Epub 2013/07/11. doi: 10.1002/hep.26609 ; PubMed Central PMCID: PMC3823683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca MdC, França A, Florentino RM, Fonseca RC, Lima Filho ACM, Vidigal PTV, et al. Cholesterol-enriched membrane microdomains are needed for insulin signaling and proliferation in hepatic cells. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2018;315(1):G80–G94. doi: 10.1152/ajpgi.00008.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lima BR, da Silva FM, Soares ER, de Almeida RA, da Silva-Filho FA, Barison A, et al. Integrative Approach Based on Leaf Spray Mass Spectrometry, HPLC‑DAD‑MS/MS, and NMR for Comprehensive Characterization of Isoquinoline‑Derived Alkaloids in Leaves of Onychopetalum amazonicum RE Fr. J Braz Chem Soc. 2019:1–11. [Google Scholar]
- 29.Costa EV, Sampaio MFC, Salvador MJ, Nepel A, Barison A. Chemical constituents from the stem bark of Annona pickelii (Annonaceae). Química Nova. 2015;38(6):769–76. [Google Scholar]
- 30.Santos MdFC, Fontes JEN, Dutra LM, Bomfim LM, Costa CO, Moraes VR, et al. Alkaloids from leaves of Guatteria pogonopus (Annonaceae) and their cytotoxicities. Química Nova. 2018;41(8):884–90. [Google Scholar]
- 31.Silva F, Koolen HHF, Almeida R, Souza A, Pinheiro M, Costa E. Desreplicação de alcaloides aporfínicos e oxoaporfínicos de Unonopsis guatterioides por ESI-IT-MS. Quím nova. 2012;35(5):S1–S5. [Google Scholar]
- 32.Galarce-Bustos O, Pavon J, Henriquez-Aedo K, Aranda M. Detection and identification of acetylcholinesterase inhibitors in Annona cherimola Mill. by effect-directed analysis using thin-layer chromatography-bioassay-mass spectrometry. 2019;30(6):679–86. doi: 10.1002/pca.2843 . [DOI] [PubMed] [Google Scholar]
- 33.Aldulaimi AKO, Azziz S, Bakri YM, Nafiah MA, Awang K, Aowda S, et al. Alkaloids from Alphonsea Elliptica Barks and their biological activities. J Global Pharma Technol. 2018;10(08):270–5. [Google Scholar]
- 34.Avula B, Bae JY, Majrashi T, Wu TY, Wang YH, Wang M, et al. Targeted and non-targeted analysis of annonaceous alkaloids and acetogenins from Asimina and Annona species using UHPLC-QToF-MS. Journal of pharmaceutical and biomedical analysis. 2018;159:548–66. Epub 2018/08/06. doi: 10.1016/j.jpba.2018.07.030 . [DOI] [PubMed] [Google Scholar]
- 35.De Angelis Campos AC, Rodrigues MA, de Andrade C, de Goes AM, Nathanson MH, Gomes DA. Epidermal growth factor receptors destined for the nucleus are internalized via a clathrin-dependent pathway. Biochemical and biophysical research communications. 2011;412(2):341–6. Epub 2011/08/09. doi: 10.1016/j.bbrc.2011.07.100 ; PubMed Central PMCID: PMC3572844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konkimalla VB, Efferth T. Inhibition of epidermal growth factor receptor over-expressing cancer cells by the aphorphine-type isoquinoline alkaloid, dicentrine. Biochemical pharmacology. 2010;79(8):1092–9. doi: 10.1016/j.bcp.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 37.Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. Journal of natural products. 2016;79(3):629–61. Epub 2016/02/09. doi: 10.1021/acs.jnatprod.5b01055 . [DOI] [PubMed] [Google Scholar]
- 38.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2009;16(2–3):97–110. Epub 2009/02/13. doi: 10.1016/j.phymed.2008.12.018 . [DOI] [PubMed] [Google Scholar]
- 39.de Pedro N, Cautain B, Melguizo A, Vicente F, Genilloud O, Pelaez F, et al. Mitochondrial complex I inhibitors, acetogenins, induce HepG2 cell death through the induction of the complete apoptotic mitochondrial pathway. Journal of bioenergetics and biomembranes. 2013;45(1–2):153–64. Epub 2012/11/28. doi: 10.1007/s10863-012-9489-1 . [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Chen JW, Zhai JH, Wang Y, Wang SL, Li X. Antitumor activity and toxicity relationship of annonaceous acetogenins. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2013;58:394–400. Epub 2013/05/29. doi: 10.1016/j.fct.2013.05.028 . [DOI] [PubMed] [Google Scholar]
- 41.Juang SH, Chiang CY, Liang FP, Chan HH, Yang JS, Wang SH, et al. Mechanistic Study of Tetrahydrofuran- acetogenins In Triggering Endoplasmic Reticulum Stress Response-apotoposis in Human Nasopharyngeal Carcinoma. Scientific reports. 2016;6:39251. Epub 2016/12/22. doi: 10.1038/srep39251 ; PubMed Central PMCID: PMC5175284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H-T, Wu H-M, Chen H-L, Liu C-M, Chen C-Y. The pharmacological activities of (-)-anonaine. Molecules. 2013;18(7):8257–63. doi: 10.3390/molecules18078257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menezes LRA, Costa CODS, Rodrigues ACBdC, Santo FRdE, Nepel A, Dutra LM, et al. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules. 2016;21(7):890. doi: 10.3390/molecules21070890 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez-Arce E, Cancino P, Arias-Calderón M, Silva-Matus P, Saldías M. Oxoisoaporphines and Aporphines: Versatile Molecules with Anticancer Effects. Molecules. 2019;25(1):108. doi: 10.3390/molecules25010108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu P, Sun H, Zhang L, Hou H, Zhang L, Zhao F, et al. Isocorydine targets the drug-resistant cellular side population through PDCD4-related apoptosis in hepatocellular carcinoma. Molecular medicine (Cambridge, Mass). 2012;18(1):1136–46. doi: 10.2119/molmed.2012.00055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Q, Li RX, Xin AY, Liu JX, Li WG, Di DL. [Research on anticancer activity of isocorydine and its derivatives]. Zhongguo Zhong Yao Za Zhi. 2017;42(16):3152–8. Epub 2017/11/25. doi: 10.19540/j.cnki.cjcmm.20170512.008 . [DOI] [PubMed] [Google Scholar]
- 47.Liew CC, Ma J, Tang HC, Zheng R, Dempsey AA. The peripheral blood transcriptome dynamically reflects system wide biology: a potential diagnostic tool. The Journal of laboratory and clinical medicine. 2006;147(3):126–32. Epub 2006/03/01. doi: 10.1016/j.lab.2005.10.005 . [DOI] [PubMed] [Google Scholar]
- 48.Jantová S, Topoľská D, Janošková M, Pánik M, Milata V. Study of the cytotoxic/toxic potential of the novel anticancer selenodiazoloquinolone on fibroblast cells and 3D skin model. Interdiscip Toxicol. 2016;9(3–4):106–12. Epub 05/17. doi: 10.1515/intox-2016-0014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Senousy HH, Abd Ellatif S, Ali S. Assessment of the antioxidant and anticancer potential of different isolated strains of cyanobacteria and microalgae from soil and agriculture drain water. Environmental Science and Pollution Research. 2020;27(15):18463–74. doi: 10.1007/s11356-020-08332-z [DOI] [PubMed] [Google Scholar]
- 50.El-Garawani IM, El-Sabbagh SM, Abbas NH, Ahmed HS, Eissa OA, Abo-Atya DM, et al. A newly isolated strain of Halomonas sp. (HA1) exerts anticancer potential via induction of apoptosis and G2/M arrest in hepatocellular carcinoma (HepG2) cell line. Scientific reports. 2020;10(1):14076. doi: 10.1038/s41598-020-70945-8 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Burczynski ME, Dorner AJ. Transcriptional profiling of peripheral blood cells in clinical pharmacogenomic studies. Pharmacogenomics. 2006;7(2):187–202. Epub 2006/03/07. doi: 10.2217/14622416.7.2.187 . [DOI] [PubMed] [Google Scholar]
- 52.Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Annals of botany. 2011;107(7):1127–40. Epub 12/17. doi: 10.1093/aob/mcq243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Fan J, Ai G, Liu J, Luo N, Li C, et al. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol Res. 2019;52(1):37. Epub 2019/07/20. doi: 10.1186/s40659-019-0243-6 ; PubMed Central PMCID: PMC6637630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Z, Xiao Y, Liu X, Zhang Z, Xiao F, Bi Y. Matrine reduces the proliferation of A549 cells via the p53/p21/PCNA/eIF4E signaling pathway. Molecular medicine reports. 2017;15(5):2415–22. Epub 2017/04/28. doi: 10.3892/mmr.2017.6331 ; PubMed Central PMCID: PMC5428535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zorofchian Moghadamtousi S, Rouhollahi E, Karimian H, Fadaeinasab M, Firoozinia M, Ameen Abdulla M, et al. The chemopotential effect of Annona muricata leaves against azoxymethane-induced colonic aberrant crypt foci in rats and the apoptotic effect of Acetogenin Annomuricin E in HT-29 cells: a bioassay-guided approach. PLoS One. 2015;10(4):e0122288–e. doi: 10.1371/journal.pone.0122288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long L, Li Q. The effect of alkaloid from Oxytropis ochrocephala on growth inhibition and expression of PCNA and p53 in mice bearing H22 Hepatocellular Carcinoma. Yakugaku Zasshi. 2005;125(8):665–70. Epub 2005/08/05. doi: 10.1248/yakushi.125.665 . [DOI] [PubMed] [Google Scholar]
- 57.Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Molecular oncology. 2018;12(1):3–20. Epub 11/27. doi: 10.1002/1878-0261.12155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qazi AK, Siddiqui JA, Jahan R, Chaudhary S, Walker LA, Sayed Z, et al. Emerging therapeutic potential of graviola and its constituents in cancers. Carcinogenesis. 2018;39(4):522–33. Epub 2018/02/21. doi: 10.1093/carcin/bgy024 ; PubMed Central PMCID: PMC5888937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivanova D, Zhelev Z, Aoki I, Bakalova R, Higashi T. Overproduction of reactive oxygen species—obligatory or not for induction of apoptosis by anticancer drugs. Chin J Cancer Res. 2016;28(4):383–96. doi: 10.21147/j.issn.1000-9604.2016.04.01 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, He Y, Zhang D, Cai Y, Zhang C, Zhang P, et al. In vitro anticancer effects of two novel phenanthroindolizidine alkaloid compounds on human colon and liver cancer cells. Molecular medicine reports. 2017;16(3):2595–603. Epub 06/29. doi: 10.3892/mmr.2017.6879 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Decker CC, Zechner L, Krstin S, Wink M. In vitro wound healing of tumor cells: inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacology and Toxicology. 2019;20(1):4. doi: 10.1186/s40360-018-0284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yiallouris A, Patrikios I, Johnson EO, Sereti E, Dimas K, De Ford C, et al. Annonacin promotes selective cancer cell death via NKA-dependent and SERCA-dependent pathways. Cell Death Dis. 2018;9(7):764–. doi: 10.1038/s41419-018-0772-x . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Small JV, Geiger B, Kaverina I, Bershadsky A. How do microtubules guide migrating cells? Nature Reviews Molecular Cell Biology. 2002;3(12):957–64. doi: 10.1038/nrm971 [DOI] [PubMed] [Google Scholar]
- 64.Thievessen I, Fakhri N, Steinwachs J, Kraus V, McIsaac RS, Gao L, et al. Vinculin is required for cell polarization, migration, and extracellular matrix remodeling in 3D collagen. Faseb j. 2015;29(11):4555–67. Epub 2015/07/22. doi: 10.1096/fj.14-268235 ; PubMed Central PMCID: PMC4608908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu W, Coll JL, Adamson ED. Rescue of the mutant phenotype by reexpression of full-length vinculin in null F9 cells; effects on cell locomotion by domain deleted vinculin. J Cell Sci. 1998;111 (Pt 11):1535–44. Epub 1998/05/15. . [DOI] [PubMed] [Google Scholar]
- 66.Resende RR, Andrade LM, Oliveira AG, Guimarães ES, Guatimosim S, Leite MF. Nucleoplasmic calcium signaling and cell proliferation: calcium signaling in the nucleus. Cell Commun Signal. 2013;11(1):14–. doi: 10.1186/1478-811X-11-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. Epub 2001/06/20. doi: 10.1038/35036035 . [DOI] [PubMed] [Google Scholar]
- 68.Andrade V, Guerra M, Jardim C, Melo F, Silva W, Ortega JM, et al. Nucleoplasmic calcium regulates cell proliferation through legumain. J Hepatol. 2011;55(3):626–35. Epub 2011/01/18. doi: 10.1016/j.jhep.2010.12.022 ; PubMed Central PMCID: PMC3158841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sotnikova R, Kettmann V, Kostalova D, Taborska E. Relaxant properties of some aporphine alkaloids from Mahonia aquifolium. Methods and findings in experimental and clinical pharmacology. 1997;19(9):589–97. Epub 1998/03/21. . [PubMed] [Google Scholar]
- 70.Ivorra MD, Martinez F, Serrano A, D’Ocon P. Different mechanism of relaxation induced by aporphine alkaloids in rat uterus. The Journal of pharmacy and pharmacology. 1993;45(5):439–43. Epub 1993/05/01. doi: 10.1111/j.2042-7158.1993.tb05572.x . [DOI] [PubMed] [Google Scholar]
- 71.Liaw CC, Liao WY, Chen CS, Jao SC, Wu YC, Shen CN, et al. The calcium-chelating capability of tetrahydrofuranic moieties modulates the cytotoxicity of annonaceous acetogenins. Angewandte Chemie (International ed in English). 2011;50(34):7885–91. Epub 2011/07/12. doi: 10.1002/anie.201100717 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Six samples of each treatment group were evenly distributed in two gels resulting in 3 samples/gel/group. It was necessary to crop the membranes in order to incubate them with different antibodies since different proteins were analyzed in each western blot. Vinculin (124 kDa MW), β-actin (42 kDa MW) and PCNA (36 kDa MW) were analyzed in the same western blot by cutting the membrane in three (A). The top part was used to blot vinculin antibodies, the middle part was used to blot β-actin antibodies and the bottom part for PCNA antibodies. EGFR (180 kDa MW) and β-actin (42 kDa MW) were analyzed in the same western blot by cutting the membrane in two (B). The top part was used to blot EGFR antibodies and the bottom part for β-actin antibodies. The original blots show results of vinculin, β-actin, PCNA and EGFR expression of HepG2 cells treated with vehicle (control, showed in the three first lanes, included in the present study), crude ethanol extract from A. crassiflora fruit peel (EtOH, not included in the present study), n-butanol fraction from A. crassiflora fruit peel (BuOH, not included in the present study) and alkaloid and acetogenin-rich fraction from A. crassiflora fruit peel (AF-Ac, showed in the last three lanes, included in the present study). The corresponding MW (KD) markers are shown to the left of the Western blot image.
(PDF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Results (mean ± SD, n = 3) expressed as the percentage of viable cells compared to the vehicle group. Significance levels are indicated by *p < 0.05 and ***p < 0.001 when compared to control (one-way ANOVA and Dunnett as posttest). The fibroblast cells (NIH/3T3) were grown in RPMI-1640 medium supplemented with fetal bovine serum 10% (FBS), 2 mM glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin, at 37°C and CO2 5%. 3 x 104 cells were plated in 96-well plates and treated with different concentrations of the AF-Ac fraction or vehicle and incubated for 24 h at 37°C and 5% CO2. Then, 100 μL of 5 mg/mL (3-(4,5-dimethylthiazolyl-2)−2,5-diphenyltetrazolium bromide) solution was incubated with the supernatant at 37°C for 2 h in 5% CO2. Next, dimethyl sulfoxide (DMSO) was added and the cell viability was analyzed by absorbance of the purple formazan from viable cells at 570 nm (Molecular Devices, Menlo Park, CA, USA).
(TIF)
Time-lapse of HepG2 cells labeled with dihydroethidium (DHE) and exposed to the AF-Ac fraction (A). Quantification of the ROS production during 9 minutes after AF-Ac treatment (B). The values are expressed in the percentage of the control. HepG2 cells were labeled with 5 μM DHE. The cells were placed at the confocal stage and perfused with HEPES solution as control and HEPES + AF-AC fraction for 10 min. The fluorescence intensity was measured using Image J and the values are expressed in percentage of the control.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.