Abstract
A combination of vermicast and sawdust mixed medium is commonly used in horticulture, but the added benefit of microbial inoculation and mechanism of nutrient availability are unknown. This study was done to determine nutrient mineralization and nutrient release patterns of different combinations or a mix of vermicast-sawdust growing media amended with or without Trichoderma viride (105 spores/g). The mixed-media treatments were (1) 80% vermicast+20% sawdust; (2) 60% vermicast+40% sawdust; (3) 40% vermicast+60% sawdust; (4) 20% vermicast+80% sawdust; and (5) sawdust alone (control). Total dissolved solids, electric conductivity and salinity increased with each sampling time following submergence in deionized. Nutrients released from media without T. viride were significantly higher than the corresponding media with added T. viride. Overall, the starting total nitrogen of the different media did not change during the incubation period, but nitrate-nitrogen was reduced to a negligible amount by the end of day 30 of incubation. A repeated measures analysis showed a significant effect of Time*T. viride*Treatment on total dissolved solids. Redundancy analysis demonstrated a positive and strong association between media composed of ≥40% vermicast and ≤60% sawdust with or without T. viride and mineral nutrients released, electrical conductivity, total dissolved solids and salinity. These findings suggest that fast-growing plants may benefit from 40% to 60% vermicast added to 40% to 60% sawdust without T. viride while slow-growing plants can benefit from the same mixed medium combined with the addition of T. viride. Further investigation is underway to assess microbial dynamics in the mixed media and their influence on plant growth.
Introduction
Plant growth and development are largely dependent on available nutrients, temperature, heat, water, pests and diseases incidences, which may directly or indirectly influence plant uptake and utilization of nutrients. Plants obtain essential mineral elements for their growth from the soil, organic amendments or synthetic chemical fertilizers [1, 2]. The availability of these mineral elements and their uptake efficiency by plants is influenced by several biological and biochemical processes including biotransformation through mineralization or immobilization and fixation [3]. Nutrients can also be immobilized or rendered unavailable due to microbial use, chelation and interference with other competing nutrients through cation or anionic exchange processes [3, 4]. For instance, a C/N ratio ≥ 30:1 suggests immobilization of mineral N by decomposing microbes that absorb mineral N in the form of ammonium or nitrates leading to reductions in available N for plant uptake [3, 5]. Moreover, nutrient availability and mineralization efficiency are increased when its concentration exceeds the needs of microbial decomposers for biosynthesis or storage [6].
In sustainable agricultural systems, natural amendments such as vermicast, a pure worm excreta, is widely used to improve the quality and health of growing medium and to meet the nutritional needs of plants [7]. A greenhouse pot experiment revealed that application of vermicast alone (100%) or 75% vermicast with added Mycorrhizal fungi were toxic to plants due to high chemical nutrients concentration compared to the addition of 25% or 50% vermicast [8]. Vermicast is rich in beneficial microorganisms, essential nutrients, humic and non-humic substances and growth-promoting hormones with desirable physical properties [9–11]. Additionally, the large surface area of vermicast granules provides more microsites for microbial activity and nutrients retention [12, 13], which could lead to slow nutrient release for an extensive period. However, literature information on vermicast nutrient mineralization and pattern of release over a pre-determined period is limited. The understanding of these patterns is important if vermicast is to be used extensively in sustainable agricultural systems as a nutrient supplement for plants.
The use of sawdust as a growing medium substrate has attracted the interest of many researchers and the greenhouse industry due to its ability to improve growing medium properties such as enhanced porosity and water retention of the growing medium [14]. However, the high C/N ratio and potential toxic compounds could adversely affect microbial community activities, nutrients availability and plant growth. These effects can be reduced by thermal treatment of sawdust which involves heating wood to high temperatures between 160°-250°C. For instance, the content of potentially phytotoxic hemicellulosic sugar i.e., arabinose, xylose, mannose and galactose in oak (Quercus spp.) sawdust was reduced by thermal treatment compared with non-thermally treated sawdust, which has higher glucose, P, K, Ca and Mg contents [15]. Additionally, thermally treated oak sawdust had higher porosity and water-holding capacity than non-thermally treated samples. Thermal treatment of sawdust also reduced lignin, hemicellulose and cellulose crystallinity. Earlier studies revealed that foliar application of untreated sawdust mixed with vermicast extract (1:10 v/v; 1000 mg/l) increased N uptake in Syngonium spp. [16]. These findings suggested that an appropriate proportion of vermicast-sawdust mixed medium can significantly improve growing medium biological, physical and chemical properties.
Trichoderma viride is a free-living fungus that has beneficial association with organic and inorganic compounds in the media including humic substances found in vermicast [17]. T. viride is ubiquitous in different soils and root ecosystems and has the potential to improve growing medium nutrients availability by mineralization of carbon and nitrogen during composting and, to protect plants from pathogens by activating immunity-mediated genes through the canonical defence signals i.e., jasmonic acid and salicylic acid pathways [18]. However, the significance of T. viride abundance in a sawdust based growing medium has not been reported. Therefore, we postulate that vermicast-Trichoderma-sawdust mixed medium can be a holistic approach to provide essential plant nutrients and increase plant resilience to pathogens. Abbey et al. [19] determined nutrient-release from dehydrated vermicast using water quality indices i.e., electrical conductivity (EC), total dissolved solids (TDS) and salinity. Based on our preliminary study, we hypothesized that vermicast-sawdust mix media with the addition of T. viride will enhance mineralization and nutrient availability. Therefore, the objective of this study was to determine mineralization and nutrient release patterns in different proportions of vermicast-sawdust media with or without the addition of T. viride.
Materials and methods
Experimental location and materials
Mineralization and nutrient release studies were performed in the Compost and Biostimulant Laboratory in the Department of Plant, Food, and Environmental Sciences, Dalhousie University, Faculty of Agriculture, Truro, Canada from March 2018 to September 2019. Vermicast is the waste product produced by red wiggler worms (Eisenia fetida) was obtained from Pagonis Live Bait, ON, Canada; and thermally treated (>120°C and 100% relative humidity) oak (Quercus spp.), maple (Acer spp.) and aspen (Populus tremuloides) tree sawdust were supplied by Thermal Wood Canada, NB, Canada. Promix-BX (Premier Horticulture Inc., Quakertown, USA), a general-purpose peat-based substrate consisting of 75–85% sphagnum peat moss, horticultural-grade perlite and vermiculite, dolomitic and calcitic limestone, a wetting agent was purchased from Halifax Seed Inc., Halifax, NS, Canada. T. viride was isolated from municipal solid waste obtained from Fundy compost, Nova Scotia, Canada and characterized by ITS region gene sequencing.
Experimental design and media preparation
The experiment was arranged in a 5×2 factorial completely randomized design with three replications. The two factors consisted of: (1) five levels of vermicast-sawdust mixed media (weight/weight): (i) 80% vermicast + 20% sawdust, (ii) 60% vermicast + 40% sawdust, (iii) 40% vermicast + 60% sawdust, (iv) 20% vermicast + 80% sawdust, and (v) sawdust alone (control); and (2) two levels of T. viride: (i) without (A) and (ii) with 105 spores/g of T. viride (B). The fungal inoculum was prepared by adding 5 ml of pure cultured T. viride to 400 ml of sterilized deionized water and thoroughly mixed. The treatment combinations were coded as A1-A5 and B1-B5 as presented in Table 1.
Table 1. Mixed proportions of vermicast, sawdust and Trichoderma viride growing media.
| Growing medium code | Vermicast (%) | Sawdust (%) | T. viride |
|---|---|---|---|
| A1a | 80 | 20 | Absent |
| A2 | 60 | 40 | Absent |
| A3 | 40 | 60 | Absent |
| A4 | 20 | 80 | Absent |
| A5 | 0 | 100 | Absent |
| B1b | 80 | 20 | Present |
| B2 | 60 | 40 | Present |
| B3 | 40 | 60 | Present |
| B4 | 20 | 80 | Present |
| B5 | 0 | 100 | Present |
aA is Growing media with no T. viride.
bB is growing media with T. viride.
Media incubation and nutrient mineralization
The individual growing media was incubated in the dark at room temperature for 90 days. Aliquots (200 g) of samples from each media was taken at the beginning of day 1 (i.e., just before incubation and denoted as time 0), this was followed by another sampling on 30, 60- and 90-days post-incubation. The samples were further coded as A1-1 to A5-4 and B1-1 to B5-4. A1-1 or B1-1 represented sample A1 or B1 taken on the first sampling point (i.e., beginning of incubation), and A5-4 or B5-4 represented A5 or B5 on the 4th sampling point (i.e., 90 days after incubation). A total of 160 samples of mixed media were collected every 30 days during the incubation period and kept in a plastic bag, sealed and stored in a -20°C freezer (Whirlpool, Mississauga, ON, CA) to conduct the mineral nutrients analyses. The frozen samples were thawed at room temperature for 24 hr and treated as saturated greenhouse soil paste according to standard laboratory protocol [20] of the Nova Scotia Department of Agriculture Laboratory Services in Truro, NS. Complete micro-and macro-nutrients including total N, nitrate (NO3-), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), boron (B), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), sodium (Na), chlorine (Cl), sulphate (SO42-) and aluminum (Al) were analyzed using the AOAC-968.08 inductively coupled plasma mass spectrometer (ICP-MS) method [20].
Nutrient release
The nutrient release method described by Abbey et al. [19] with some modification was used. Briefly, 20-g samples of each mixed media were placed in a rosin press nylon bag (i.e., 2.5 cm x 10.2 cm of mesh-size 160 μm) and submerged into a clean glass column (i.e., 35-cm height and 10-cm inner diameter) containing 500 ml deionized water. The bags were weighed down with washed pebbles. To determine nutrients released into the deionized water, triplicate samples of the nutrient solution was collected in a 2.5-cm depth sample cup and total dissolved solids (TDS), electric conductivity (EC), salinity and pH were measured using ExStik® EC500 instrument (Extech Instruments Corporation, NH, USA). These chemical indices were recorded every 15 min up to 30 min after submergence; followed by every 30 min up to 1.5 hr; every 1 hr up to 3 hr; 3 hr; 5.5 hr; 9 hr; 12 hr; and then every 24 hr for 7 days.
Statistical analysis
All data collected were subjected to two-way analysis of variance (ANOVA) using Minitab version 19.1 (Minitab Inc., PA, USA). Treatment means were separated and compared using Fisher’s least significant difference (LSD) method at α = 0.05. Student t-test was also used to compare media quality among the two groups i.e., media without (A) versus media with added T. viride (B). A nonlinear regression model was used to explain the pattern of nutrient release. The nonlinear regression model was:
where θ represents a parameter vector that has more than one value (θ1, θ2, θ3,…, θk); yi is each response i.e., i = 1, … n; where n and xi represent the two independent variables. Repeated measures analysis was also carried out to examine simple factor effects (main effects) and interaction effects, and to reduce variability among treatments. All statistical analyses were performed using Minitab and two-dimensional redundancy analysis using XLSTAT version 19.1 (Addinsoft, NY, USA).
Results and discussion
Group A media (i.e., A1—A5) with no T. viride showed rapid initial release of nutrients as determined by the EC, TDS and salinity of the deionized water (Fig 1A–1C). The EC, TDS and salinity for A1—A4 rose steadily up to the 8th-hr followed by a rapid decline. The water quality parameters increased sharply again and maintained a gentle rise to 178.5 hrs except for A5, which levelled off after the first 8 hrs (Fig 1A–1C). However, the EC, TDS and salinity of group A growing media followed similar patterns i.e., A1 > A2 = A3 > A4 > A5 (Fig 1A–1C).
Fig 1. Electric conductivity (A), total dissolved solids (B), salinity (C) and pH (D) of vermicast-sawdust mixed media without addition of Trichoderma viride (group A).
A1 (blue line) is 80%vermicast+20%sawdust; A2 (brown line) is 60%vermicast+40%sawdust; A3 (black line) is 40%vermicast+60%sawdust; A4 (green line) is 20%vermicast+ 80%sawdust; and A5 (red line) is sawdust alone with n = 3 per experimental replicate. Error bars represent standard error.
These similar patterns in water quality properties can be attributed to the existing relationship among TDS, EC and salinity. Besides, the pH of the deionized water rose sharply from the beginning up to the 22.5th-hr for A1 and A2 followed by a sudden dip at the 34.5th- hr. For A3 and A4, the pH of the deionized water dipped at the 13.5th-hr before rising for 9 hr and dipping again at the 34.5th-hr. All the pH values for treatments A1—A4 rose gently after the 34.5th-hr. On the contrary, the pH of the sawdust alone treatment (A5) fluctuated for the first 85 hr with a sharp rise from a pH of 6.8 to a peak pH of 7.4 at the 58.5th-hr. The pH of A5 then dropped significantly by approximately 10% at the 96th-hr where it remained constant until the end of the experiment (Fig 1D). These variations can be ascribed to high hydrogen ion concentration in the sawdust alone, which made the solution more acidic compared to a mixture of sawdust and vermicast medium [21]. Overall, the pattern for pH was A1 > A2 > A3 = A4 >A5, which was like the patterns for the TDS, EC and salinity. This pattern can be positively associated with variations in the proportion of vermicast in the mixed media. The higher the proportion of vermicast, the higher the values of the four water quality parameters measured in the mixed media combinations.
The overall pattern of the EC, TDS and salinity for treatment B was somewhat like that of treatment A except for treatment B3, which was different from treatment B4 (Fig 2A–2C) unlike A3, which was similar to A4 (Fig 1A–1C). Moreover, the initial nutrient release activities for treatment B were different from that of treatment A. Overall, EC, TDS, and salinity levels of treatment B increased sharply from the beginning up to the 3rd-hr before declining for the next 3 hr after which it rose again gently and remained at a constant rate from the 13.5th-hr until the end of the experiment. The pattern for treatment B water quality parameters was B1 > B2 > B3 > B4 > B5. Comparatively, A1 and B1 recorded the highest EC, TDS and salinity values, which also corresponded to the highest proportion of added vermicast. It was also found that nutrient release measured by the water quality parameters were higher for treatment A media than those of treatment B media (Figs 1A–1C and 2A–2C). Therefore, the EC, TDS and salinity values suggested that the addition of T. viride reduced media nutrient release. The individual media within each group also differed in their nutrient release characteristics except for A3 and A4, which were similar.
Fig 2. Electric conductivity (A), total dissolved solids (B), salinity (C) and pH (D) of vermicast-sawdust mixed media with addition of Trichoderma viride (group B).
B1 (blue line) is 80%vermicast+20%sawdust; B2 (brown line) is 60%vermicast+40%sawdust; B3 (black line) is 40%vermicast+60%sawdust; B4 (green line) is 20%vermicast+ 80%sawdust; and B5 (red line) is sawdust alone with n = 3 per experimental replicate. Error bars represent standard error.
The pH for all the T. viride amended growing media of treatment B (Fig 2D) followed a similar trend and fell within the range of values for treatment A media, except for the obvious fluctuations prior to the 82.5th-hr (Fig 1D). Nevertheless, the pH of B5 was consistently reduced until it stabilized after 58 hrs. The pH of B1—B4 increased sharply before declining at the 22nd-hr but rose gently again with minimum fluctuations after 12 hr. The pattern of treatment B media pH was B1 = B2 = B3 > B4 > B5 (Fig 2D). Overall, the rate of change in water quality indices for treatment B was consistently slower compared to those of treatment A. This can be attributed to the presence of T. viride in treatment B growing media which for some reason, reduced the rate of nutrient released into solution. For most plants, the optimum pH is between 6.0 and 7.8. Usually, 65% of the applied nutrients are available for plants at growing medium pH levels below 5.9, while 35% may be available below pH of 5.5 [22]. As such, all the mixed media except treatment A5 and B5 were suitable for growing most plants. Consistently, pH was not altered by the addition of T. viride in vermicast amended media since vermicast is capable of buffering growing medium pH [23, 24]. Without vermicast, the pH of treatment A5 was found to be moderately higher than that of B5.
An independent 2-sample t-test analysis revealed no significant (P>0.05) differences amongst EC, TDS and salinity values between A1 versus B1, A2 versus B2 or A3 versus B3 (Table 2). However, there was a non-significant (P>0.05) pattern of B1 > A1, A2 > B2 and A3 > B3. On the other hand, EC, TDS and salinity for A4 and A5 were all increased significantly (P<0.05) by an average of 36% and 46.7% compared to B4 and B5, respectively. The average pH for treatment A media were significantly (P<0.05) higher than the average for treatment B media (Table 2). The significant differences in pH and the variations in the proportion of vermicast in the different mixed media influenced the composition of nutrients released and the water quality parameters.
Table 2. Two-sample independent t-test for comparing electrical conductivity (EC), total dissolved solids (TDS), salinity and pH for vermicast-sawdust mixed media without (A) and with (B) Trichoderma viride in the nutrient release experiments.
| Growing Medium | pH | EC | TDS | Salinity |
|---|---|---|---|---|
| A1 | 7.41±0.16 | 122.4±43.9 | 84.6±31.5 | 57.2±20.8 |
| B1 | 7.21±0.23 | 125.4±56.7 | 88.6±39.8 | 59.4±26.9 |
| P-value | 0.003 | ns | ns | ns |
| A2 | 7.37±0.13 | 101.8±38.0 | 71.0±26.2 | 47.5±17.7 |
| B2 | 7.18±0.23 | 88.3±41.1 | 61.9±28.7 | 41.3±19.5 |
| P-value | 0.002 | ns | ns | ns |
| A3 | 7.35±0.12 | 88.7±43.2 | 62.0±30.2 | 41.6±20.2 |
| B3 | 7.16±0.21 | 73.0±33.2 | 51.4±23.2 | 34.7±16.1 |
| P-value | 0.002 | ns | ns | ns |
| A4 | 7.30±0.10 | 67.4±25.4 | 47.0±17.9 | 31.4±12.1 |
| B4 | 7.09±0.19 | 49.5±21.9 | 34.6±15.3 | 23.0±10.2 |
| P-value | <0.001 | 0.026 | 0.028 | 0.027 |
| A5 | 7.00±0.20 | 44.07±9.37 | 30.99±6.80 | 20.46±4.48 |
| B5 | 6.72±0.16 | 30.30±10.50 | 21.00±7.22 | 13.91±4.93 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Values are means ± standard errors and n = 3 per experimental replicate.
ns, no significant difference at P>0.05.
A1, B1 are 80%vermicast+20%sawdust; A2, B2 are 60%vermicast+40%sawdust; A3, B3 are 40%vermicast+60%sawdust; A4, B4 are 20%vermicast+ 80%sawdust; and A5, B5 are sawdust alone.
Therefore, it can be said that the presence of T. viride might have slowed the release of nutrients probably due to the immobilization of available nutrients. Generally, a considerable portion of nutrients is utilized by microbes for growth, which influenced the chemical properties of the media. The incorporated elemental nutrients become available after the death and degradation of microbial cells [25, 26]. Additionally, Trichoderma spp are known to secret organic acids, which decreases soil pH and chelates mineral elements, thus rendering them unavailable for plant uptake [25]. Hence, the mixed media without T. viride, particularly A1, A2 and A3 will likely be more effective than the media amended with T. viride.
It was found that unstructured covariance was appropriate for EC, TDS, salinity and pH, and was used in the covariance analysis employed. Multiple means comparison was performed when the repeated measures analysis (RMA) (Proc MIXED) showed a significant (P<0.0001) difference (Table 3). The RMA results confirmed that the interaction effect of Time*Trichoderma*Treatment for TDS was significantly different (P = 0.01). Overall, the multiple means comparison result showed that for each sampling time, the addition of T. viride increased the values of the water quality parameters for A1 and B1; both of which had the highest proportion of vermicast (S1 Table). For instance, 34.5 hr, with T. viride, A1 and B1 combination had the highest TDS least-squares mean of 10.18. This could be due to increased nutrient availability with vermicast application and promotion of mineral solubilization by which Trichoderma regulate plant nutrient and growth as reported in previous studies [26, 27].
Table 3. Repeated measures analysis (RMA) for total dissolved solids, electrical conductivity, salinity and pH for vermicast-sawdust mixed media without (A) and with (B) Trichoderma viride in the nutrient release experiments.
| RMA outcome | Num DFa | Den DF | F-Value | P-value |
|---|---|---|---|---|
| Total dissolved solids | ||||
| Time | 12 | 390 | 361.46 | <0.0001 |
| T. viride | 1 | 390 | 150.27 | <0.0001 |
| Time*T. viride | 12 | 390 | 3.57 | <0.0001 |
| Treatment | 4 | 390 | 414.98 | <0.0001 |
| Time*Treatment | 48 | 390 | 13.94 | <0.0001 |
| T. viride*Treatment | 4 | 390 | 8.48 | <0.0001 |
| Time*T. viride*Treatment | 48 | 390 | 1.59 | 0.0102 |
| Electrical conductivity | ||||
| Time | 12 | 390 | 228.08 | <0.0001 |
| T. viride | 1 | 390 | 75.97 | <0.0001 |
| Time*T. viride | 12 | 390 | 1.57 | 0.0978 |
| Treatment | 4 | 390 | 279.63 | <0.0001 |
| Time*Treatment | 48 | 390 | 16.26 | <0.0001 |
| T. viride*Treatment | 4 | 390 | 2.23 | 0.0655 |
| Time*T. viride*Treatment | 48 | 390 | 1.25 | 0.1345 |
| Salinity | ||||
| Time | 12 | 390 | 231.42 | <0.0001 |
| T. viride | 1 | 390 | 70.31 | <0.0001 |
| Time*T. viride | 12 | 390 | 2.01 | 0.0225 |
| Treatment | 4 | 390 | 303.74 | <0.0001 |
| Time*Treatment | 48 | 390 | 16.76 | <0.0001 |
| T. viride*Treatment | 4 | 390 | 3.56 | 0.0073 |
| Time*T. viride*Treatment | 48 | 390 | 1.25 | 0.1325 |
| pH | ||||
| Time | 12 | 390 | 70.08 | <0.0001 |
| T. viride | 1 | 390 | 3052.65 | <0.0001 |
| Time*T. viride | 12 | 390 | 17.46 | <0.0001 |
| Treatment | 4 | 390 | 434.12 | <0.0001 |
| Time*Treatment | 48 | 390 | 27.18 | <0.0001 |
| T. viride*Treatment | 4 | 390 | 0.61 | 0.6530 |
| Time*T. viride*Treatment | 48 | 390 | 3.83 | <0.0001 |
aDF is degrees of freedom; Num DF is numerator DF = k–1; Den DF is denominator DF = N–k; n = 3 per experimental replicate.
However, the lowest TDS least-squares mean was at 0.5 hr, with T. viride, A5 and B5. The RMA for EC showed a significant (P<0.0001) 2-way interaction of Time *Treatment, and a significant (P<0.0001) main effect of T. viride (Table 3). For T. viride main effect on EC, it was confirmed that the media treatments without T. viride (i.e., group A) had better performance than when T. viride was added (Table 3). The results indicated that at 34.5 hr, A1 and B1 combination had the highest EC least-squares means (i.e., 141.14). The lowest EC least-squares mean was observed at 0.25 hr, and in A5 and B5 (i.e., 22.67) (S2 Table).
For salinity, all the 2-way analysis showed a significant interactive effect of Time*T. viride (P = 0.0225), Time*Treatment (P<0.0001) and T. viride*Treatment (P = 0.0073) as presented in S3–S5 Tables. The Time*T. viride interaction on salinity revealed that incubation at 34.5 hr, with T. viride combination had the highest salinity least squares mean (i.e., 43.40), while the lowest salinity least-squares mean was at 0 hr, with T. viride (i.e., 22.67) (S3 Table). For Time*Treatment interaction on salinity, it was found that at 34.5 hr, A1 and B1 combinations had the highest salinity least squares mean (i.e., 66.51) and the lowest salinity least-squares mean was at 0.25 hr, A5 and B5 (i.e., 10.41) (S4 Table). For T. viride*Treatment interaction on salinity, it was found that media without T. viride, A1 and B1 combinations had the highest salinity least square means (i.e., 47.31), whiles the lowest salinity least-squares mean was observed in T. viride, A5 and B5 (i.e., 12.11) combinations (S5 Table). Salinity is a major factor affecting media quality and use for crop production. High media salinity results in increased ion toxicity, which is possibly caused by the rapid dissociation of soluble salts [28]. The increase in salts with high vermicast contained media could be attributed to high soluble salt concentration in vermicast as revealed by Joshi and Kelkar [29], They reported that vermicast contains higher soluble salts with higher cation exchangeable properties compared to natural soils. The RMA for pH showed a significant (P<0.0001) 3-way i.e., Time*T. viride*Treatment interaction effect (Table 3). The effect of such interaction on pH showed that 22.5 hrs without T. viride, A1 and B1 combinations had the highest pH least squares mean (i.e., 7.51). However, the least pH least-squares mean (i.e., 6.62) was recorded in A5 and B5 at 34.5 hr and in A2 and B2 at 2 hr (S6 and S7 Tables). Samples of the incubated mixed media were collected at different times for the determination of nutrients mineralization in the growing media with or without T. viride. It was evident that the mineralization and composition of mineral nutrients in the different growing media varied with the time of incubation (Figs 3 and 4).
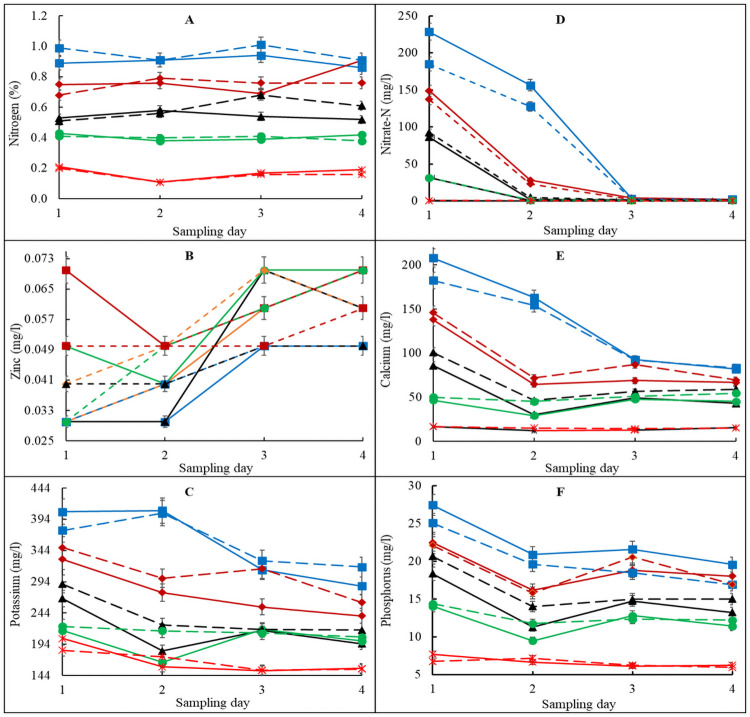
Fig 3. Nitrogen (A), zinc (B), potassium (C), nitrate-nitrogen (D), calcium (E) and phosphorus (F) availability in vermicast-sawdust mixed media without (solid lines) and with (broken lines) addition of Trichoderma viride during a 90-day incubation period.
A1, B1 (blue line) is 80%vermicast+20%sawdust; A2, B2 (brown line) is 60%vermicast+40%sawdust; A3, B3 (black line) is 40%vermicast+60%sawdust; A4, B4 (green line) is 20%vermicast+ 80%sawdust; and A5, B5 (red line) is sawdust alone. Day 0 (point 1); day 30 (point 2); day 60 (point 3) and day 90 (point 4) with n = 3 per experimental replicate. Error bars represent standard error.
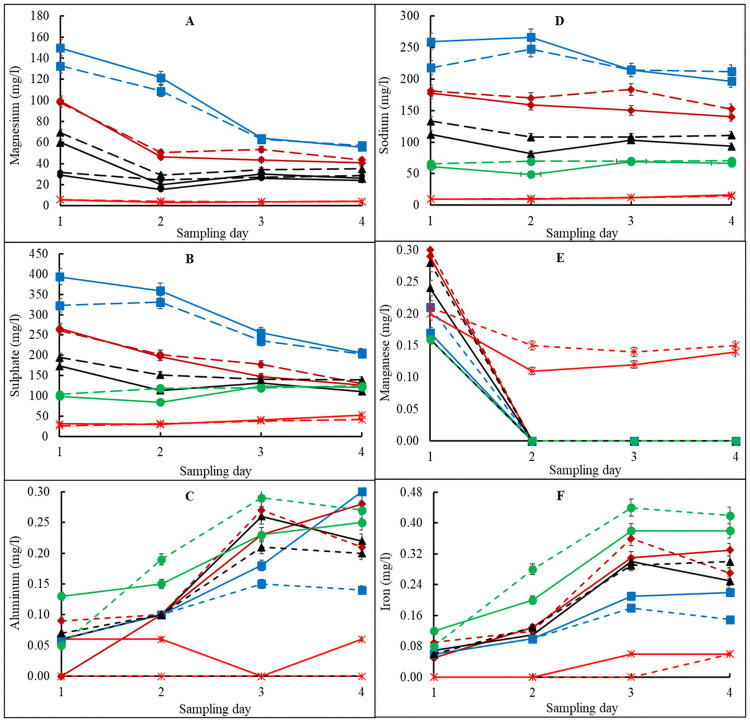
Fig 4. Magnesium (A), sulphate (B), aluminum (C), sodium (D), manganese (E) and iron (F) availability in vermicast-sawdust mixed media without (solid lines) and with (broken lines) addition of Trichoderma viride during a 90-day incubation period.
A1, B1 (blue line) is 80%vermicast+20%sawdust; A2, B2 (brown line) is 60%vermicast+40%sawdust; A3, B3 (black line) is 40%vermicast+60%sawdust; A4, B4 (green line) is 20%vermicast+ 80%sawdust; and A5, B5 (red line) is sawdust alone. Day 0 (point 1); day 30 (point 2); day 60 (point 3) and day 90 (point 4) with n = 3 per experimental replicate. Error bars represent standard error.
Vermicast is inherently nutrient-rich and commonly used as a growing medium amendment. As such, the growing media with high proportions of vermicast, particularly A1 and B1 had the highest nutrients concentration followed by A2 and B2, A3 and B3, A4 and B4 and the least in A5 and B5. Overall, total N did not change during the incubation period (Fig 3). However, NO3—N which is a component of total N was reduced to a negligible amount at the 3rd sampling date on day 30 of incubation (Fig 3). These observations suggested N immobilization occurred through bioconversion of NO3—N to organic forms by microbial populations present in the growing media. It seemed T. viride did alter N and most of the nutrients in the growing media when group A media was compared to group B media. Zn concentration fluctuated with its reductions in some of the growing media before rising from day 30 and then remained constant in A1, B1 and B2, but increased in A4 and B3 or reduced in A2, B4 and B5 after day 60 (Fig 3). The concentrations of Ca, K and P for A1, A2 and A3; and for B1, B2 and B3 declined at different rates. However, P remained constant after the 2nd sampling date but changes in Ca and K delayed until the 3rd sampling date (Fig 3).
Significant reductions in the concentrations of Mg and SO42- in A1 and B1 occurred followed by moderate reductions in A2 and B2 and slight reductions in A3 and B3 (Fig 4A and 4B).
In general, the patterns of Al and Fe concentrations were similar in groups A and B (Fig 4C and 4F). There were also moderate reductions in the concentrations of Na in treatment A1 and B1 but negligible changes in all the other growing media compositions. This high Na concentration with increased proportion of vermicast was consistent with earlier finding [30], which can potentially affect root nutrient uptake and plant performance (Fig 4D). There was a sharp decline in Mn concentrations in all the growing media except for A5 and B5, which had the highest and stable concentration of Mn (Fig 4E). There was a gentle rise in Al and Fe up to the 3rd sampling point on day 60 before levelling off. In both cases, the concentrations of Al and Fe were moderately higher in B1 than in A1, but they were similar on day 90. It was obvious that concentrations of mineral nutrients in A5 and B5 were the least except for Mn. A4 and B4 were the second worse in mineral nutrients concentration. A5 and B5 were composed of sawdust alone without and with T. viride, respectively. As a result of the low inherent nutrient concentration, changes in mineral nutrients in A5 and B5 during the 90 days of incubation period were negligible except for Zn.
The findings further confirmed that EC was the best estimator for N and K as treatments with lower EC were also associated with lower N and K concentrations (Table 4). Martínez-Suller et al. [31] reported a high positive correlation between EC and nutrient concentration, which was confirmed by Abbey et al. [19].
Table 4. Mineral nutrients concentration of vermicast-sawdust mixed media without (A) and with (B) addition of Trichoderma viride during a 90-day incubation period.
| Growing Medium | N (%) | EC (μS/cm) | Ca (mg/l) | K (mg/l) | Mg (mg/l) | P (mg/l) | Na (mg/l) | SO42- (mg/l) | Cl- (mg/l) | B (mg/l) |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 0.9a | 3400a | 136.4a | 353.6a | 97.8a | 22.4a | 234.3a | 303.7a | 405.5a | ND |
| A2 | 0.8b | 2400b | 84.7b | 274.9b | 57.5b | 18.9a | 156.8b | 184.3b | 310.0b | ND |
| A3 | 0.5c | 1600bc | 52.3bc | 215.3bc | 34.1bc | 14.4b | 97.6c | 132.7bc | 286.0b | 0.10b |
| A4 | 0.4d | 1100cd | 42.3bc | 199.4c | 23.8bc | 12.0b | 61.8d | 107.1cd | 150.3c | 0.13b |
| A5 | 0.2e | 600d | 14.4c | 166.9c | 4.3c | 6.7c | 12.1e | 38.6d | 24.0d | 0.23a |
| B1 | 1.0a | 3500a | 128.2a | 356.3a | 90.5a | 20.0a | 223.1a | 273.1a | 453.3a | ND |
| B2 | 0.8b | 2500b | 93.7ab | 306.0b | 61.4ab | 18.9ab | 171.9b | 193.1b | 405.5a | 0.10c |
| B3 | 0.6c | 1600c | 65.7b | 237.2c | 42.0bc | 16.2bc | 115.2c | 156.7bc | 286.0b | 0.11bc |
| B4 | 0.4d | 1100cd | 50.3bc | 213.9c | 27.9cd | 12.7c | 69.1d | 116.5c | 107.2c | 0.12b |
| B5 | 0.2e | 500d | 15.5c | 166.3d | 4.6d | 6.6d | 11.8e | 34.4d | 19.0d | 0.25a |
Means that do not share the same letter are significantly different at P<0.05. ND, data were not detected; n = 3 per experimental replicate.
A1, B1 are 80%vermicast+20%sawdust; A2, B2 are 60%vermicast+40%sawdust; A3, B3 are 40%vermicast+60%sawdust; A4, B4 are 20%vermicast+ 80%sawdust; and A5, B5 are sawdust alone.
EC is electric conductivity; N is nitrogen; Ca, calcium; K is potassium; Mg is magnesium; P is phosphorus; Na is sodium; SO42- is sulphate; Cl- is chloride; and B is boron.
The deionized water used could conduct electricity due to the presence of charged ions i.e., cations such as Ca2+, K+ and Mg2+ and anions such as NO3-, SO42- and Cl- released into solution from the growing media treatments. As such, the ionic concentration of mineral elements and compounds as determined by the EC measurements was used to estimate the mineral nutrients content of the growing media. Student t-test confirmed that differences in nutrients concentration between growing medium with T. viride (group B) and those without T. viride (group A) were not significant (P>0.05). However, there were significant (P<0.05) differences in mineral nutrients within each group (Table 5) and when the nutrients were released (Figs 3 and 4).
Table 5. Least-squares mean of electric conductivity for T. viride main effect for vermicast-sawdust mixed media without (A) and with (B) Trichoderma viride in a nutrient release experiment.
| Effect | Estimate | P-value |
|---|---|---|
| Without T. viride | 68.4188 | <0.0001 |
| With T. viride | 56.4762 | <0.0001 |
n = 3 per experimental replicate.
All the analysed mineral nutrients were significantly (P<0.05) highest in A1 and B1 followed by A2 and B2. The least nutrient values were recorded by A5 and B5. Nonetheless, boron (B) was highest in A5 and B5 but was not detected in A1, A2 and B1. Notably, the more the vermicast and the less the incubation time, the better the nutrient status of the growing medium and the more mineral nutrients were available for plant use. Accordingly, the mineral nutrients concentrations of the growing media followed the trend A1, B1 > A2, B2 > A3, B3 > A4, B4 > A5, B5 with an overall average of A > B.
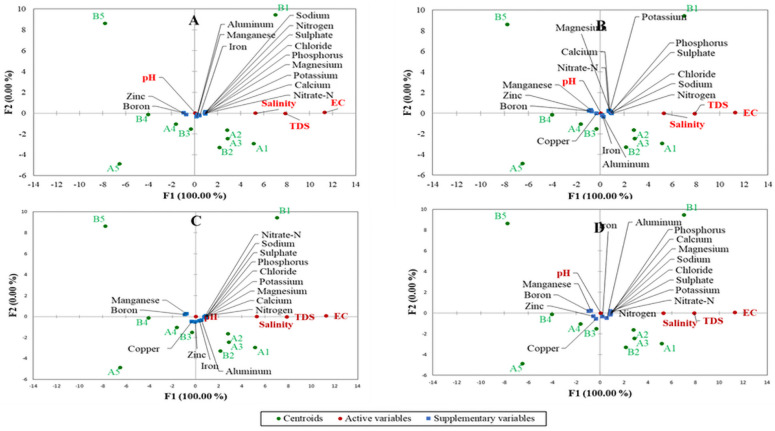
A two-dimension redundancy analysis (RDA) was used to further delineate the association amongst the growing media treatments for mineralization and nutrient release (Fig 5A–5D). The RDA showed projections of the variables in the factors space denoted by F1 and F2, which explained 100% of the total variations in the dataset. The RDA explained the existing relationship or otherwise amongst the different growing media, water quality parameters and mineral nutrients. Before incubation for the mineralization experiment, A1, B1, A2, B2, and A3 had a positive and strong relationship with EC, TDS and salinity. These five media were associated with high concentrations of most of the mineral nutrients except for Zn and B, which were high in the other media treatments (Fig 5A). After 30 days of incubation, Mn and Cu were altered and were less associated with A1, A2, A3, and B2 (Fig 5B).
Fig 5. Two-dimension redundancy analyses of growing media nutrient availability and nutrient release as determined using electric conductivity (EC), total dissolved solids (TDS), salinity and pH.
A1, B1: 80%vermicast+20%sawdust; A2, B2: 60%vermicast+40%sawdust; A3, B3: 40%vermicast+60%sawdust; A4, B4: 20%vermicast+ 80%sawdust; and A5, B5: sawdust alone. n = 3 per experimental replicate.
The mineral nutrients distribution on day 60 was like day 1 at the beginning of the incubation, but only Zn and B had a positively high association with B3, A4, B4, A5, and B5 (Fig 5A and 5C). Furthermore, the distribution of mineral nutrients did not change significantly after 90 days of incubation (Fig 5D) as compared to those of days 30 and 60 (Fig 5B and 5C). Overall, A2, A3, B2, B3, A4, and B4 were close to the centre, which suggested that the nutrient release pattern of the media was mainly influenced by the concentrations of the mineral nutrients in the individual media. Although it can be concluded that A1 and B1 had higher mineral nutrients than the other treatments (Figs 4 and 5), and the nutrients released were dependent on concentrations of the nutrients in the media.
Conclusions
The composition of essential nutrients present in the growing medium is critical to the growth and development of plants and can be estimated using TDS, EC, salinity and pH measurements. We found that nutrients mineralization and availability were higher in sawdust-based media without T. viride (i.e., treatment A) compared to those with added T. viride (i.e., treatment B). Hence, T. viride seems to reduce the rate of media nutrient release and could be used in a slow-release nutrient formulation. The reason is that mineralized nutrients at the end of the studies were similar for individual formulations of A and B. That is, A1 = B1 for the highest nutrient concentration followed by A2 = B2, A3 = B3, A4 = B4 and the least for A5 = B5 in a descending order of magnitude. The interaction of Time*Media Treatment*T. viride significantly influenced the nutrient release pattern of the media. Overall, the results showed that vermicast-sawdust mixed media can support plant growth and development. In this case, fast-growing plants may benefit from a growing medium mix of 40% vermicast and 60% sawdust or 60% vermicast and 40% sawdust without T. viride while slow-growing plants can benefit from the same mixed medium nut with added T. viride. Further investigation is in progress to assess growing media microbial dynamics and plant growth response.
Supporting information
Growing media treatment codes for the mineralization and nutrient-release pattern experiments during December 2018 to February 2019 incubation period.
(DOCX)
Determination for Time*Trichoderma viride*Treatment interaction.
(DOCX)
Determination for Time*Treatment interaction.
(DOCX)
Determination for Time*Trichoderma viride interaction.
(DOCX)
Determination for Time*Treatment interaction.
(DOCX)
Determination for Trichoderma viride*Treatment interaction.
(DOCX)
Determination for Time*Trichoderma viride*Treatment interaction.
(DOCX)
Acknowledgments
The lead author wishes to thank all her lab mates for their support and suggestions during the study.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
LA and RHT received the funding. Grant #CRDPJ 523129-17 Natural Sciences and Engineering Research Council of Canada https://www.nserc-crsng.gc.ca/onlineservices-servicesenligne/index_eng.asp No-The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bellitürk K, Adiloğlu S, Solmaz Y, Zahmacıoğlu A, Adiloğlu A. Effects of increasing doses of vermicompost applications on P and K contents of pepper (Capsicum annuum L.) and eggplant (Solanum melongena L.). J. Adv. Agric. Technol. 2017; 4(4): 372–375. [Google Scholar]
- 2.Adekiya AO, Ejue WS, Olayanju A, Dunsin O, Aboyeji CM, Aremu C, et al. Different organic manure sources and NPK fertilizer on soil chemical properties, growth, yield and quality of okra. Sci. Rep. 2020; 10(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul EA. Soil Microbiology, Ecology and Biochemistry: Academic press; 2014. [Google Scholar]
- 4.Waheed H, Ilyas N, Raja NI, Mahmood T, Ali Z. Heavy metal phyto-accumulation in leafy vegetables irrigated with municipal wastewater and human health risk repercussions. Int. J. Phytoremed. 2019; 2: 170–179. doi: 10.1080/15226514.2018.1540547 [DOI] [PubMed] [Google Scholar]
- 5.White RE. Principles and practice of soil science, The soil as a natural resource: John Wiley & Sons; 2013. [Google Scholar]
- 6.Zhang S, Zheng Q, Noll L, Hu Y, Wanek W. Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Soil Biol. Biochem. 2019; 135: 304–315. doi: 10.1016/j.soilbio.2019.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blouin M, Barrere J, Meyer N, Lartigue S, Barot S, Mathieu J. Vermicompost significantly affects plant growth. A meta-analysis. Agronomy for Sustainable Development, 2019; 39: 34. doi: 10.1007/s13593-019-0579-x [DOI] [Google Scholar]
- 8.Abbey L, Appah P. Pot-grown swiss chard and kale responses to a variable rate of manure compost in mycorrhizal fungi inoculated medium. Paper presented at the III International Symposium on Organic Greenhouse Horticulture 1164. 2016; 241–248.
- 9.Nagavallemma KP, Wani SP, Lacroix S, Padmaja VV, Vineela C, Rao MB, et al. Vermicomposting, recycling wastes into valuable organic fertilizer. 2004. Global Theme on Agroecosystems report no. 8.
- 10.Abbey L, Pham TH, Annan N, Leke-Aladekoba A, Thomas RH. Chemical composition of kale as influenced by dry vermicast, potassium humate and volcanic minerals. Food Res. Int. 2018; 107: 726–737. doi: 10.1016/j.foodres.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 11.El-Goud A, Amal K. Efficiency response of vermicompost and vermitea levels on growth and yield of eggplant (Solanum melongena L.). Alexandria Science Exchange J. 2020; 41: 69–75. [Google Scholar]
- 12.Shi-Wei Z, Fu-Zhen H. The nitrogen uptake efficiency from 15N labeled chemical fertilizer in the presence of earthworm manure (cast). Adv. Manage. Conserv. Soil Fauna 2019; 539–542. [Google Scholar]
- 13.Palaniappan S, Alagappan M, Rayar S. Influence of substrate particle size on vermicomposting of pre-processed vegetable waste. Nature Env. Poll. Technol. 2018; 17(1): 277–286. [Google Scholar]
- 14.Agboola OO, Oseni OM, Adewale OM, Shonubi O. Effect of the use of sawdust as a growth medium on the growth and yield of tomato. Ann. West Univ. Timisoara. Series of Biol. 2018; 21(1): 67–74. [Google Scholar]
- 15.Jung S-J, Kim S-H, Chung I-M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biom. Bioenerg. 2015; 83: 322–327. [Google Scholar]
- 16.Khomami MA, Padasht MN, Ajili Lahiji A, Shirinfekr A. The effect of sawdust vermicompost extract on Syngonium podophyllum growth and nutrition. J. Plant Nutr. 2019; 42(4): 410–416. doi: 10.1080/01904167.2018.1554071 [DOI] [Google Scholar]
- 17.Fiorentino N, Ventorino V, Woo SL, Pepe O, De Rosa A, Gioia L, et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018; 9: 743. doi: 10.3389/fpls.2018.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Ann. Rev. Phytopathol. 2010; 48: 21–43. doi: 10.1146/annurev-phyto-073009-114450 [DOI] [PubMed] [Google Scholar]
- 19.Abbey L, Rao SA, Hodgins LN, Briet F. Drying and rehydration of vermicasts do not affect nutrient bioavailability and seedling growth. Amer. J. Plant Nutr. Fert. Technol. 2013; 3(1): 12–21. [Google Scholar]
- 20.AOAC. Metals and other elements in plants and pet foods, inductively coupled plasma spectroscopic method, AOAC official method 968.08. 2003; Official methods of analysis. 17th ed. Association of Official Analytical Chemists.
- 21.Geffert A, Geffertova J, Dudiak M. Direct method of measuring the pH value of wood. Forests 2019; 10(10): 852. [Google Scholar]
- 22.Neina D. The role of soil pH in plant nutrition and soil remediation. Appl. Env. Soil Scie. 2019; 1–9. doi: 10.1155/2019/5794869 [DOI] [Google Scholar]
- 23.Kwon YT, Lee CW, Yun JH. Development of vermicast from sludge and powdered oyster shell. J. Cleaner Prod. 2009; 17(7): 708–711. [Google Scholar]
- 24.Singh J. Role of earthworm in sustainable agriculture. In Sustainable Food Systems from Agriculture to Industry; Academic Press; 2018. pp. 83–122. [Google Scholar]
- 25.de Santiago A, Quintero JM, Avilés M, Delgado A. Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant and Soil 2011; 342(1): 97–104. [Google Scholar]
- 26.Li RX, Cai F, Pang G, Shen QR, Li R, Chen W. Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS One 2015; 10(6): e0130081. doi: 10.1371/journal.pone.0130081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiquee S, Shafawati SN, Naher L. Effective composting of empty fruit bunches using potential Trichoderma strains. Biotechnol. Rep. 2017; 13: 1–7. doi: 10.1016/j.btre.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butcher K, Wick AF, DeSutter T, Chatterjee A, Harmon J. Soil salinity: a threat to global food security. Agron. J. 2016; 108(6): 2189–2200. [Google Scholar]
- 29.Joshi NV, Kelkar BV. The role of earthworms in soil fertility. Indian J. Agric. Sci. 1952; 22: 189–196. [Google Scholar]
- 30.Chakraborty S, Paul N, Chaudhuri PS. Earthworm casting activities under bamboo plantations of West Tripura, India and their impact on soil physicochemical properties. Current Sci. 119(7): 2020; 1–9. [Google Scholar]
- 31.Martinez-Suller L, Azzellino A, Provolo G. Analysis of livestock slurries from farms across Northern Italy: Relationship between indicators and nutrient content. Biosyst. Eng. 2008; 99(4): 540–552. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growing media treatment codes for the mineralization and nutrient-release pattern experiments during December 2018 to February 2019 incubation period.
(DOCX)
Determination for Time*Trichoderma viride*Treatment interaction.
(DOCX)
Determination for Time*Treatment interaction.
(DOCX)
Determination for Time*Trichoderma viride interaction.
(DOCX)
Determination for Time*Treatment interaction.
(DOCX)
Determination for Trichoderma viride*Treatment interaction.
(DOCX)
Determination for Time*Trichoderma viride*Treatment interaction.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.